Abstract
Significance: Oxygen and circadian rhythmicity are essential in a myriad of physiological processes to maintain homeostasis, from blood pressure and sleep/wake cycles, down to cellular signaling pathways that play critical roles in health and disease. If the human body or cells experience significant stress, their ability to regulate internal systems, including redox levels and circadian rhythms, may become impaired. At cellular as well as organismal levels, impairment in redox regulation and circadian rhythms may lead to a number of adverse effects, including the manifestation of a variety of diseases such as heart diseases, neurodegenerative conditions, and cancer. Recent Advances: Researchers have come to an understanding as to the basics of the circadian rhythm mechanism, as well as the importance of the numerous species of oxidative stress components. The effects of oxidative stress and dysregulated circadian rhythms have been a subject of intense investigations since they were first discovered, and recent investigations into the molecular mechanisms linking the two have started to elucidate the bases of their connection. Critical Issues: While much is known about the mechanics and importance of oxidative stress systems and circadian rhythms, the front where they interact has had very little research focused on it. This review discusses the idea that these two systems are together intricately involved in the healthy body, as well as in disease. Future Directions: We believe that for a more efficacious management of diseases that have both circadian rhythm and oxidative stress components in their pathogenesis, targeting both systems in tandem would be far more successful. Antioxid. Redox Signal. 19, 192–208
Introduction
Rhythmicity and oxygen are essential to life, from the beating of the heart for pumping oxygenated blood, to the rhythmic breathing of air by the lungs. Circadian rhythms are intimately involved in living systems at environmental, organismal, and cellular levels. Circadian rhythms, by definition, are physical, mental, and behavioral changes that follow an ∼24-h cycle. This cycle is generally slightly longer than 24 h (an average of 24.2 h in sighted humans, and 24.5 h in blind humans), but can vary from person to person (48, 142). It is believed that the development of circadian rhythms is a response to the earth's rotation, both around its axis and around the sun, which dictates daily light and temperature changes (111). Similarly important are the role of oxygen in our universe and a myriad of biological processes. The balance of oxygen and its related compounds is one of the most crucial parts of regulating life on the Earth, especially in humans. It is also an immensely intricate system involving numerous enzymes, biologically active compounds, and signaling molecules. Deregulation of normal circadian rhythms as well as the various biological activities of oxygen can compromise the quality or even the very existence of life, and there is much evidence that these two fundamental processes are intimately linked.
The toxic effects of oxygen were first appreciated in 1954 with Gershman's free-radical theory, suggesting that oxygen toxicity may happen due to partially reduced forms of oxygen (59). Commoner et al., in the same year, suggested the presence of free radicals in a variety of biological materials (34). These new ideas triggered a surge of scientific research into the idea that although a necessary part of life, oxygen may not always be beneficial. The cell has evolved an intricate web of energy synthesis and signaling mechanisms that are dependent on oxygen and its more reactive forms, reactive oxygen species (ROS). Intense research has been done on ROS, their beneficial and detrimental effects on the organism, as well as the efforts mounted by the cell to counteract them. Interestingly, many of these efforts, including the production of antioxidants and protective enzymes, have been reported to be regulated or expressed in rhythmic fashions. Thus, oxidative stress seems to have a circadian rhythm connection. This gives rise to a myriad of questions. When an oxidative-stress-linked disorder manifests, is it because of a dysregulation of the protective mechanism, or just because of the increased ROS and their detrimental effects? Would targeting dysregulated circadian rhythm components help in the overall management of oxidative-stress-mediated diseases and conditions? Would restoring rhythmicity in the protective enzymes and antioxidants cure or mitigate the disease? It is important to consider, discuss, and investigate the connection between oxidative stress and circadian rhythms, especially in regard to human health and disease. In light of these questions, we offer support for the idea that oxidative stress and circadian rhythms are intertwined, both in the maintenance of homeostasis, as well as in certain diseases.
Oxidative Stress: The Disease Connection
ROS, such as superoxide radicals (O2−), peroxides (ROOR), and hydroxyl radicals (OH−), are byproducts of normal cellular metabolism, mainly in the mitochondria. These molecules can benefit the cell by playing critical roles in cellular defense and other important cellular processes (19, 181). One such role that many of the ROS have, especially O2− and hydrogen peroxide (H2O2), is in cellular signaling controlling a variety of biological processes. The ROS have many features that make them excellent signaling molecules, and have been shown to be involved in many pathways, from kinase activation to insulin action [reviewed in Refs. (8, 52)]. However, a tight regulation of ROS is needed, as they may inflict serious detrimental effects if left unchecked. To place checks and balances on ROS, the cellular machinery has a sophisticated system to neutralize them before they become problematic. This includes producing protective enzymes (e.g., catalases [CATs], superoxide dismutases [SODs], and glutathione peroxidases [GPxs]) and physiologically generated small-molecule antioxidants (e.g., Vitamins C and E, glutathione [GSH], and uric acid) (70, 108). If these protective measures are not enough, due to exposure to environmental stresses (such as ultraviolet [UV] light, chemical pollutants, or heat) or due to other physiological reasons (poor or inappropriate diet, life style, etc.), the cell enters a state of oxidative stress. When this happens, it can be disastrous for the cells, causing DNA damage, lipid peroxidation, oxidation of amino acids, and ultimately death of the cell or disease in the host (181). Reactive nitrogen species (RNS), including nitric oxide (NO) and peroxynitrite (ONOO−), also play an important role in oxidative stress. NO is produced in normal cells as an integral part of cellular signaling and is a mediator of cellular damage [reviewed in Ref. (175)]. NO can diffuse easily through cells, which makes it especially useful as a biological messenger, and although it is only mildly reactive, its chemical properties make it a very potent intermediate messenger for production of more reactive RNS [reviewed in Refs. (25, 124)], as well as other biological processes such as vasodilation (175). Excess NO is normally inactivated by various cellular responses, including in the blood by a reaction with oxyhemoglobin to form nitrate (124). However, since NO is poorly reactive, it has been suggested that much of the cellular damage that occurs during oxidative stress comes from the oxidation products of NO, especially peroxynitrite (12, 181). This compound is formed from the reaction of O2− and NO, is a particularly active oxidative molecule, and has been implicated in several diseases and disorders, including hypertension, arthritis, and cancer [reviewed in Refs. (124, 181)].
As mentioned above, these reactive species can be produced in the mitochondria as a byproduct of metabolism or by environmental stressors such as exposure to UV light or exposure to chemical pollutants. Since it is difficult to directly measure the very short-lived ROS, much research has focused on studying the presence of the antioxidants and other enzymes produced by the body that work against the oxidant radicals (70). Interestingly, the cellular concentrations or activity levels of many of these antioxidants and protective small molecules (such as SOD, GPx, melatonin, and several others discussed in the next section) have been found to have circadian rhythmicity (37, 107, 134). This suggests an importance of both oxidative stress and the circadian rhythm in human diseases.
Circadian Rhythms and Zeitgebers: The Tempo of Life
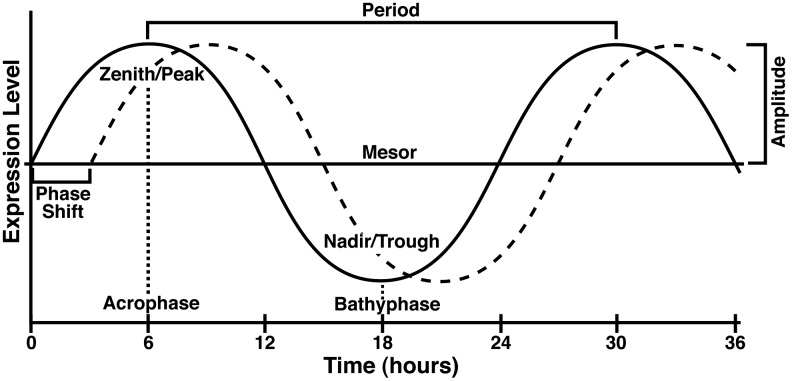
Circadian rhythms are the natural patterns of physical, mental, and behavioral changes in living organisms. For a pattern to be considered circadian, it must have five features, including (i) the ability to become entrained (synchronized), (ii) the persistence of the cycle after the removal of external stimuli, (iii) the ability to shift the phase of the cycle, (iv) a period of around 24 h, and (v) the ability to maintain its period independent of temperature (13, 50). To determine if the expression pattern is circadian, data are traditionally fit to a cosine curve (the cosinor method) and displayed as a graph such as that shown in Figure 1. If the levels do not fit the regularly recurring wave, the pattern is most likely not circadian.
FIG. 1.
Circadian curve and terminology. A model curve representing a circadian rhythm with a period of 24 h. Zenith or Peak=the maximum level of gene expression over the course of the oscillation. Acrophase=the time at which the curve reaches the Zenith/Peak. Nadir or Trough=the minimum level of gene expression over the course of the oscillation. Bathy phase=the time at which the curve reaches the Nadir/Trough. Period=the time required to complete one cycle, that is, the distance from one peak to the next. Midline estimating statistic of rhythm (Mesor)=the value halfway between the highest and lowest values of the cosine curve of best fit to the data. Amplitude=the distance from the mesor value to the Zenith/Peak. Phase shift=displacement of a curve along the time axis.
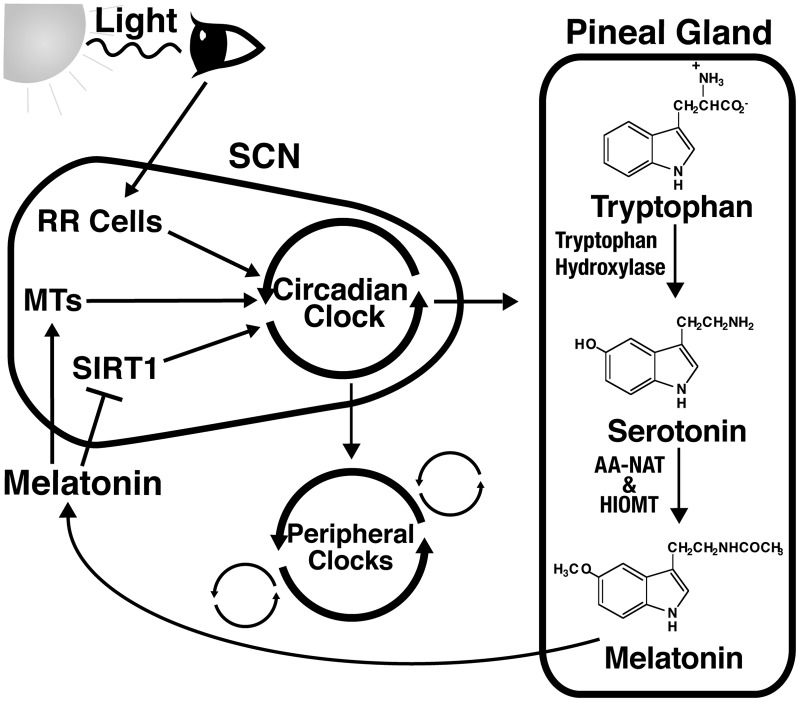
The cyclic pattern in the expression of circadian proteins and other rhythmic elements is dependent on a number of external cues or zeitgebers, including light exposure, feeding patterns, exercise, and temperature change (62, 183). The strongest zeitgeber, light, initiates synchronization of rhythms when the retinorecipient cells within the suprachiasmatic nucleus (SCN) in the hypothalamus region of the brain receive the light impulses from the retina (62). The SCN has been found to be the main regulator of circadian rhythms, which then sends signals to peripheral cells throughout the body, causing that rhythm to be passed on to the proper cells and tissues (53). However, the SCN can be stimulated by zeitgebers other than light, especially by serotonin and melatonin, although these are thought to be internal feedback regulators as opposed to primary circadian rhythm initiators (112, 113, 128). Melatonin has also been found to be one of the signaling molecules used by the body to synchronize certain peripheral cells. However, it is believed that many more molecules and hormones, including insulin and glucocorticoids, may be involved in this process (145, 154). These concepts are outlined in Figure 2.
FIG. 2.
Circadian rhythms are regulated by more than one zeitgeber. Light is presumably the strongest zeitgeber that acts directly on the suprachiasmatic nucleus (SCN). It is sensed by the retina, which signals to retinorecipient cells (RR Cells) in the SCN region of the hypothalamus. This entrains the circadian clock within the SCN, which in turn signals to peripheral clocks throughout the body. However, a number of other endogenous and exogenous cues are capable of entraining circadian rhythms. For example, the circadian clock also stimulates production of melatonin in the pineal gland, which can also act as a zeitgeber, entraining peripheral clocks. Melatonin can also bind to melatonin receptors (MTs) in the SCN or inhibit SIRT1 expression and activity, both of which can also affect circadian rhythms, thereby acting as a zeitgeber itself.
It has been estimated that the body uses these cues to synchronize the oscillatory expression of up to 10% of genes, which thereby regulate many different physiological and biochemical processes (146). Many of these circadian genes are important in basic cellular processes, such as regulation of cellular division, proliferation, and DNA damage repair (17, 146). Physiologically, many processes have been found to have circadian rhythmicity, such as sleep/wake cycles, body temperatures, and T-cell immune responses (94, 138, 144). As expected, a dysregulation of these rhythms may trigger a variety of adverse effects in the cell and living system. Below, we have described the roles and interaction of circadian rhythms and oxidative stress in biological systems and as well as discussed the consequences of their dysregulation.
Circadian Rhythms: Molecular Clockwork
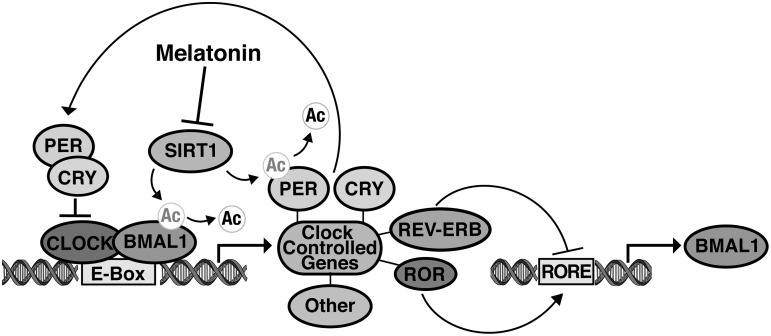
While the central pacemaker is located in the SCN region of the hypothalamus, each peripheral cell in the body contains its own clock that adjusts its rhythm in response to entrainment signals (116, 190, 200, 205). A molecular mechanism consisting of a transcriptional–translational feedback loop is responsible for this time-keeping capability. The positive branch of this loop consists of the proteins aryl hydrocarbon receptor nuclear translocator-like (ARNTL), also known as BMAL1, and circadian locomotor output cycles kaput protein (CLOCK) (4, 58, 93). The negative branch of the loop consists of period homologs 1–3 (Periods [PER]1–3), and cryptochrome 1 and 2 (CRY1 and 2) (98, 205). As shown in Figure 3, the heterodimerization of BMAL1 and CLOCK or its homolog NPAS2 starts the circadian cycle. Once dimerized, BMAL1 and CLOCK bind to E-box elements in the promoters of their target genes, initiating transcription of various rhythmic proteins (58). Two groups of these transcriptional targets are the PERS and the CRYS. The PER and CRY proteins then form a second heterodimer that translocates into the nucleus and interferes with the activity of BMAL1 and CLOCK at the promoter sites, completing the cycle (156).
FIG. 3.
The roles of SIRT1 and melatonin in the molecular mechanism of the circadian clock. The basic circadian mechanism consists of a transcriptional-translational feedback loop. Circadian locomotor output cycles kaput protein (CLOCK) and BMAL1 form a heterodimer in the nucleus and bind to E-box elements in the promoters of their target genes. Known target genes include the Periods (PER) and cryptochromes (CRY), which form a heterodimer, enter the nucleus, and interfere with CLOCK-BMAL1 activity. BMAL1 acetylation is required for the recruitment of CRY, and SIRT1 disrupts this recruitment through deacetylation of BMAL1. SIRT1 also deacetylates PER2, targeting it for degradation by the proteasome. Melatonin is capable of inhibiting SIRT1 expression and activity, thereby preventing BMAL1 and PER2 deacetylation. An auxiliary loop-regulating BMAL1 expression is controlled by two groups of clock-controlled proteins, the REV-ERBs, and the RAR-related orphan receptors (RORs). The RORs promote BMAL1 expression by binding to the retinoic acid-related orphan receptor-response elements (RORE) site in the BMAL1 promoter, while the REV-ERBs inhibit BMAL1 transcription through competitive binding to the same site. Note that while both REV-ERBα and β are clock-controlled proteins, RORα expression oscillates only in the SCN; RORγ expression oscillates only in peripheral tissues; and it is currently unknown if RORβ expression is clock controlled.
The regulation of BMAL1, CLOCK, and NPAS2 transcription adds a second regulatory loop to the core circadian mechanism. The orphan nuclear receptors REV-ERB α and β repress BMAL1 transcription through binding to retinoic acid-related orphan receptor-response elements (ROREs) in the BMAL1 promoter (66, 130). Recent studies have shown that REV-ERBα also represses both CLOCK and NPAS2 transcription (41, 69). Competing with the REV-ERBs for RORE-binding sites in the BMAL1 promoter are RAR-related orphan receptors (RORs) α, β, and γ (also known as ROR a, b, and c), all of which activate BMAL1 transcription (2, 66, 150). RORα has also been shown to activate NPAS2 transcription, but not the transcription of CLOCK (41). Like the PERs and the CRYs, the REV-ERBs and the RORs are clock-controlled (2, 3, 66, 150, 177), thereby forming a second, stabilizing feedback loop. However, the status of each individual ROR as a clock-controlled element depends on whether circadian regulation is being studied in the master regulator in the SCN, or in peripheral tissues, as RORα expression oscillates only in the SCN; RORγ expression oscillates only in peripheral tissue; and the circadian status of RORβ expression is currently unknown (66, 150).
Interestingly, this regulation of BMAL1, CLOCK, and NPAS2 expression is affected by the redox state of REV-ERBβ. REV-ERBβ activity is facilitated by heme binding that allows for the recruitment and binding of a histone deacetylase (HDAC), which when complexed with REV-ERBβ yields its active form. The reduced dithiol state of REV-ERBβ binds heme more tightly than the oxidized form of REV-ERBβ (69). Since ROS oxidize thiol groups, oxidative stress is predicted to reduce REV-ERBβ activity, thereby allowing for increased BMAL1, CLOCK, and NPAS2 transcription and disrupting circadian rhythms. However, this has not yet been directly tested.
A number of post-translational modifications are also known to regulate the core mechanism. Briefly, phosphorylation by CKIɛ facilitates cytoplasmic sequestration of the PER1 and 2 proteins. Nuclear export of PER1 and PER2 is facilitated by a nuclear export signal, which is activated upon phosphorylation (186). PER1 also contains a nuclear localization signal that is masked upon phosphorylation at the adjacent CKIɛ-binding site (185). CLOCK's nuclear localization is dependent on its binding to BMAL1 and subsequent phosphorylation, and this localization oscillates with circadian rhythmicity (96). The acetylation status of at least two of the core circadian proteins is important in regulation of this system. During a normal circadian cycle, CLOCK acetylates its heterodimerization partner, BMAL1, at its Lys537 residue, allowing for the recruitment of CRY1 in the negative limb of the cycle (73). The class III HDAC SIRT1 then deacetylates BMAL1, allowing for the system to reset for a new cycle (118).
Overall, the regulation of circadian rhythms is a very complex system with many details still to be explored. Despite the clear role of the transcriptional–translational feedback loops in the regulation of these patterns, a recent study indicates that there is a significant contribution to normal clock function outside of the core feedback loops (119). In this study, the authors have shown that human red blood cells display circadian oscillation in peroxiredoxin (Prx)-redox cycles in spite of the fact that they lack nuclei, and are therefore incapable of the transcription necessary to sustain the transcriptional–translational feedback loops of the core circadian mechanism (119). While much remains to be explored concerning the intricacies of circadian rhythm control by external cues, these findings suggest that they play a more significant role than previously understood.
Rhythmicity of the Cellular Antioxidant System: Patterns of Protection
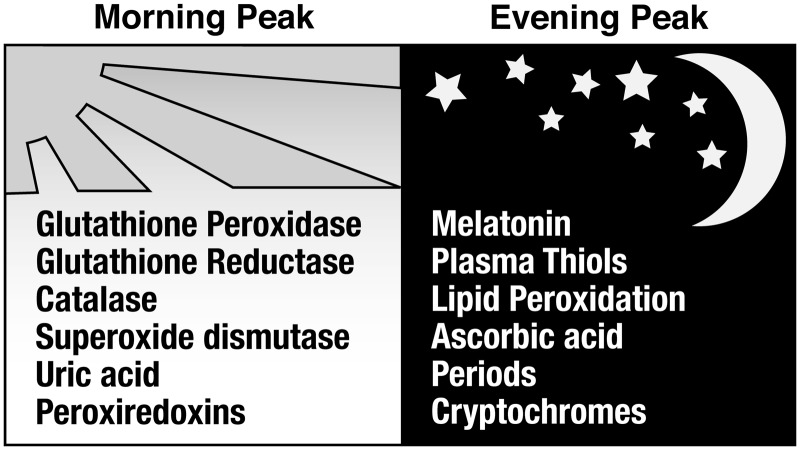
A growing collection of data suggests that the circadian regulation of protein expression plays a significant role in the cellular response to oxidative stress. Several studies have shown evidence of differences in DNA damage, lipid peroxidation, and protein oxidation at different times of the day, thus indicating circadian oscillations of oxidative stress responses (51, 90, 97, 100, 176). These oscillations relate directly to the daily rhythm of antioxidant expression and protective enzyme activity levels (Fig. 4). This rhythmicity in antioxidant levels may be exploited for a more precise targeting of the ROS, thereby offering better protection for the cells. One of the biggest causes of oxidative damage in the cell is the O2− molecule. As elucidated below and in Figure 5, many of the enzymes used to transform it into less-reactive species are rhythmically expressed.
FIG. 4.
Circadian rhythms and oxidative stress components oscillate in humans. Many antioxidants and enzymes that protect the cell from oxidative stress exhibit daily cycles in their expression or activity levels. Levels of byproducts of oxidative stress, such as those indicating DNA damage, protein damage, or lipid peroxidation, also oscillate with circadian rhythmicity. Some of these are shown along with the time of day at which they are most highly expressed in humans. In addition, the peak time of expression for the circadian period and CRY proteins are listed for comparison. Those that peak in the morning include glutathione peroxidase (GPx) (159), glutathione reductase (GR) (159), catalase (159), superoxide dismutase (SOD) (159), uric acid (159), and peroxiredoxins (Prxs) (119). Peaks in the evening have been observed in melatonin (103), plasma thiols (18), lipid peroxidation (18, 159), ascorbic acid (159), Period 1 and 2 (143), and the CRYs (143).
FIG. 5.
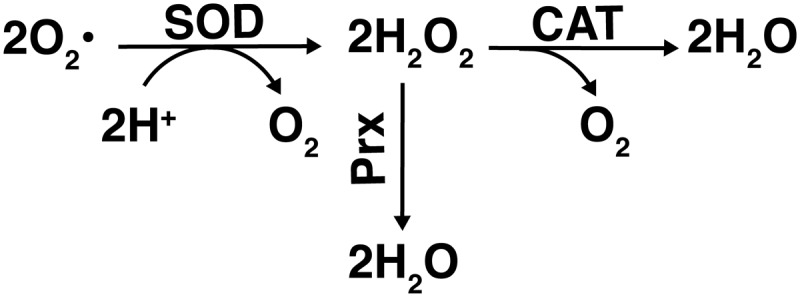
Superoxide enzymatic decomposition. The decomposition of superoxide (O2•) to hydrogen peroxide (H2O2) is catalyzed by SOD, utilizing two hydrogen atoms and releasing oxygen. H2O2 is further decomposed to water in reactions catalyzed by Prx, catalases (CAT), or peroxidases such as GPx, as depicted in Figure 3.
One key group of antioxidant enzymes that oscillate with circadian rhythmicity is SODs. These enzymes protect against oxidative damage by catalyzing the dismutation of O2− into O2 and H2O2. In eukaryotes, there are two main types of SODs: copper/zinc (Cu/Zn), found cytoplasmically and extracellularly, and manganese (Mn), found mitochondrially (127). Mn SOD is thought to be especially important in maintaining and influencing the redox status of the cell, since it is the form present in the mitochondria where most ROS are produced. Also, it can directly influence the flux of certain ROS in some instances [reviewed in Ref. (21)]. Daily rhythmicity in SOD activity was first reported by Diaz-Munoz et al. in 1985. They found that in the rat cerebral cortex, SOD activity peaks in the dark phase, coinciding with the peak level of malondialdehyde, which is a product of lipid peroxidation (43). Later studies showed that both Cu/Zn and Mn SOD expression levels were shown to oscillate with daily rhythmicity in the rat intestine, lung, and cerebellum (107). In addition, PER2 knockout mice have shown a decrease in the amplitude of oscillations in Cu/Zn SOD expression and activity in liver homogenates (84). Double knockout of PER1 and PER2 resulted in a significant phase shift in Cu/Zn SOD expression, with levels peaking 4 h earlier than in wild-type mice (84). Thus, there is a direct link between the known molecular mechanism of circadian oscillation and SOD levels.
Once SOD converts O2− into O2 and H2O2, CAT is one enzyme that offers further protection by catalyzing the decomposition of H2O2 to H2O and O2 (149). The rhythmicity of CAT activity was established over 25 years ago, and has been studied in many model organisms, as well as in humans (38, 149). CAT activity oscillation has been shown to peak in the middle of the dark phase in the liver and kidneys of nocturnal mice (149). However, in diurnal humans, the peak occurs in the beginning of the light phase as detected in plasma samples (160), illustrating the difference in circadian oscillation between nocturnal and diurnal species that would be expected due to their opposing patterns of sleep/wake cycles and the differences in their feeding patterns and light exposure.
Another group of enzymes that regulate the effects of oxidative stress on the cell by removing peroxides is the Prxs. In Syrian hamsters, Prxs have been shown to oscillate with circadian rhythmicity in both the SCN region of the brain, as well as in the peripheral tissue of the liver, although the two rhythms are not in sync with each other (176). As mentioned earlier, Prxs have also been studied in mature red blood cells (as they do not have a nucleus or most other organelles, including mitochondria), and O'Neill and Reddy found that there was still rhythmicity of the Prxs, in the absence of external cues (119). This suggests that more intricate molecular clocks may be present in some parts of the body. Finally, circadian oscillation is highly involved in the regulation of the GSH system, which is pictured in Figure 6. GSH is a powerful antioxidant that neutralizes ROS in a process catalyzed by one of the 4 selenium-dependant GPx proteins, thereby converting GSH to the oxidized state of glutathione disulfide (GSSG). Glutathione reductase (GR) then catalyzes the reaction in which GSSG is reduced to GSH, allowing for additional neutralization of ROS (105). An additional component of the GSH system, the glutathione S-transferases (GSTs), is a group of enzymes separated into three major classes in mammals (cytosolic, mitochondrial, and microsomal), with at least 18 different isoforms expressed in humans. They play important roles in oxidative stress defense through the inactivation of cytotoxic and mutagenic byproducts of the process, including α,β-unsaturated aldehydes, quinones, epoxides, and hydroperoxides (6, 71).
FIG. 6.
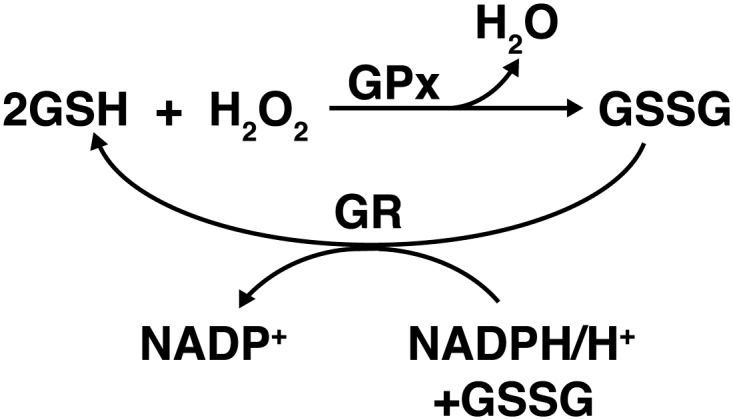
The GSH system. Two GSH molecules react with H2O2 in a reaction catalyzed by GPx. The reaction decomposes H2O2 into H2O and the oxidized form of GSH, glutathione disulfide (GSSG). GSSG is then reduced to GSH in a reaction catalyzed by GR, which utilizes NADPH as a hydrogen donor.
Circadian variation in GSH expression was first reported in rat blood as early as 1967 (26). Since then, GSH circadian oscillation has been confirmed in many studies across several different species, including rat, mouse, crayfish, and human (6, 11, 51, 82). Further studies have shown that GSSG also shows daily patterns of expression, as well as the enzymes GPx, GR, and GST (1, 6, 11, 37, 42, 100, 109). With the exception of one study in the mouse liver (81), rodent studies in liver and brain tissue have shown an acrophase (time of peak expression) for GSH expression during the middle of the light phase (42, 43, 179), while humans show an acrophase at the end of the night (6). This further illustrates the expected differences in circadian oscillation between nocturnal and diurnal species, similar to that found in CAT activity. In the rat cerebral cortex, this peak in GSH expression correlates with the GPx acrophase, and both peak in the opposite phase to lipid peroxidation, which is highest during the dark phase (43). This suggests that the peak in lipid peroxidation is partly due to the low levels of protective enzymes at that point in the day.
Oxidative Stress and Circadian Rhythms: Possible Links and Applications
In addition to the oscillation of protective enzymes that directly connects circadian rhythms to oxidative stress defense mechanisms, there are several other ways in which the two are closely linked. In 2008, Krishnan et al. demonstrated that the rhythmicity of oxidative damage extends past the lipid peroxidation mentioned above to include cases of protein oxidation as well (97). The authors studied H2O2-induced oxidative stress in Drosophila melanogaster at different time points over a 24-h period (97). They found that there was a significantly higher mortality level in flies treated during the day than in those treated at night (97). This higher daytime mortality was accompanied by higher levels of protein carbonyls, which are a byproduct of protein oxidation. To demonstrate that these effects were directly related to the circadian clock, the authors demonstrated that flies lacking the protein PER did not show a difference in mortality rate between daytime and night-time exposure to H2O2 (97). Interestingly, there was also no difference in mortality rate between daytime and night-time in flies that were exposed to constant light, a condition that is shown to disrupt circadian rhythms (97, 115, 121, 172). Mammalian studies in the Syrian Hamster have supported this finding, showing that continuous light greatly diminishes or abolishes the circadian rhythms that are observed under normal light/dark cycles in SOD, GR, and CAT (176).
Circadian rhythms and oxidative stress are also linked through the activity of at least two known factors that play a significant role in both processes. The first and most well known is the pineal hormone melatonin. Its circadian oscillation was first discovered in 1964 (134), and it has been studied extensively as a marker of circadian rhythms ever since. Its antioxidant properties, however, were not discovered for almost another 30 years, when Ianas et al. first published an article discussing the involvement of melatonin in the oxidative process (78). Since then, melatonin's antioxidant properties have been explored and exploited by many researchers in various fields (28, 29, 135, 173). More recently, SIRT1 has been linked to both oxidative stress and circadian rhythms as well. SIRT1 plays a role in oxidative stress through the initiation of several downstream effectors, including p53 and FOXO transcription factors (187). In 2008, two independent studies linked SIRT1 to the core circadian mechanism through the deacetylation of the proteins BMAL1 and PER2. As outlined above and in Figure 3, SIRT1 is responsible for deacetylating BMAL1, thereby resetting the clock to its original state (118). In addition, SIRT1 deacetylates PER2, targeting it for degradation (5). This prevents the heterodimerization of PER2 with the CRYs in the negative feedback portion of the core circadian mechanism. Interestingly, melatonin and SIRT1 have also been shown to interact, as melatonin is an inhibitor of both SIRT1 expression and activity (87, 89). Thus, not only is melatonin expression controlled by the circadian clock, but circadian rhythms are controlled by melatonin feedback via SIRT1 (see Figs. 2 and 3). Further linking SIRT1 to both oxidative stress and circadian rhythms is the coenzyme nicotinamide adenine dinucleotide (NAD+). The mechanism of protein deacetylation by SIRT1 involves the use of NAD+ as a cofactor, and thus SIRT1 activity is dependent upon the availability of NAD+ (74). Therefore, through its role as a coenzyme of SIRT1, NAD+ affects circadian rhythm regulation. NAD+ and its redox partner NADH are also intimately involved in redox reactions, primarily driving oxidation, while their phosphorylated forms NADP+/NADPH drive reduction reactions (74). These reactions include the reduction of GSSG to GSH as depicted in Figure 6, as well as the generation of O2− through the donation of electrons from NADPH in a reaction catalyzed by NADPH oxidase. Thus, the initiation of oxidative stress and the general redox state of the cell affects the availability of NAD+ and consequent activity of SIRT1. Finally, both NADH and NADPH have been shown to enhance DNA binding of the CLOCK:BMAL1 and NPAS2:BMAL1 heterodimers to their transcriptional targets, whereas NAD+ and NADP+ inhibit this activity (141). Thus, there is a direct link between the redox state of NAD+/NADH and NADP+/NADPH and circadian rhythms.
Although some overlap has been found between the two systems, there is still an incomplete understanding of the molecular mechanisms linking circadian rhythms and oxidative stress. However, the fact that the two are related has been exploited by researchers and physicians in the form of chronotherapy. As defined by Merriam-Webster, chronotherapy is the “administration of medication in coordination with the body's circadian rhythms to maximize effectiveness and minimize side effects.” Research suggests that applying our current knowledge of circadian rhythms to timed medical treatment yields better outcomes in a number of diseases. For example, a study of diabetic patients who took hypertensive medications at bedtime rather than upon waking resulted in significantly reduced cardiovascular morbidity and mortality rates, as well as improved ambulatory blood pressure control (72). This benefit in the timed dosing of cardiovascular medications extends to nondiabetic patients as well, and has been shown to significantly improve patient health for a variety of blood pressure medications (166). Rheumatoid arthritis, a systemic inflammatory disease with links to oxidative stress and circadian rhythms, is another disease in which a benefit has been shown when timing treatments with biological rhythms. A slow-release form of prednisone has been developed, so that the rise of proinflammatory cytokines in the morning could be counteracted (23). In cancer, chronotherapeutic treatments have been studied more extensively [reviewed in Ref. (80)]. Overall, these studies have shown that cancer treatments also show greater benefit when administered at specific times of day. The identification of circadian disruptions in a number of other diseases suggests that this timed treatment could also be useful in diabetes, asthma, arthritis, and Parkinson's disease (PD), to name a few (20, 22, 39, 91). The following section provides a brief review of several of these diseases and their relationship to oxidative stress and circadian rhythms.
Oxidative Stress and Circadian Rhythms: Partnership of the Processes
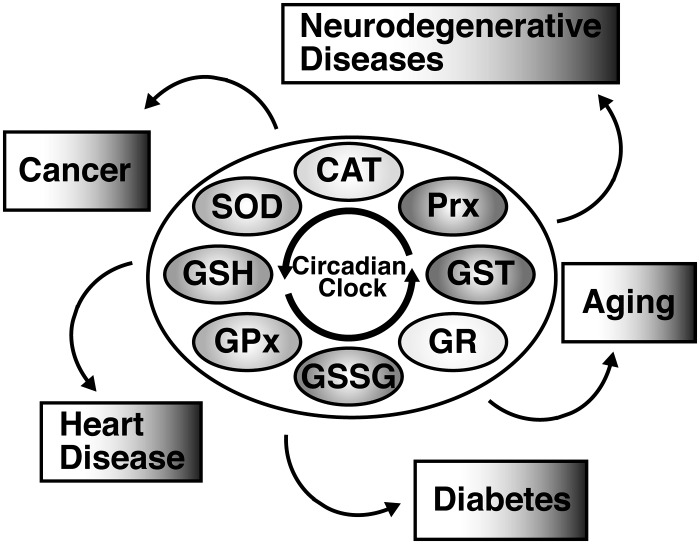
Oxidative stress and circadian rhythms have each been studied extensively in their own right, as they relate to different conditions and disorders. However, because of the links that have been found between many of their individual components at a cellular level, we feel that an evaluation of the intersections of the systems in certain diseases is merited. Here, we discuss several diseases that have been found to have strong oxidative stress and circadian rhythm links, including aging, heart disease, cancer, diabetes, and neurodegenerative disorders.
Aging
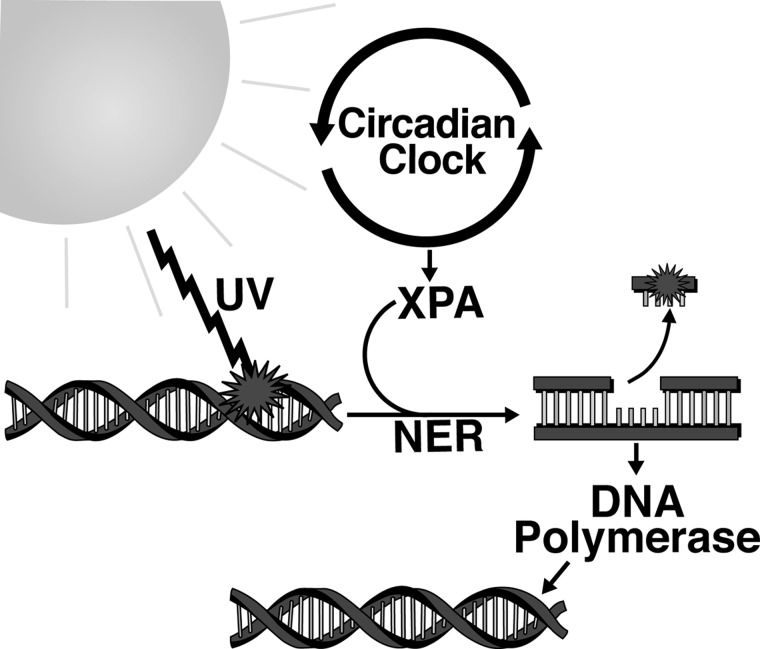
Aging is a complex process characterized by the slowing or alteration of cellular and bodily processes over time, resulting in reduced functionality of cells, increased susceptibility to disease, and ultimately the death of the organism. This is a process that happens naturally to all organisms, and can ultimately not be avoided. All of the factors involved in aging are not known, and intense research is currently ongoing in this direction. In the cell, indicators of aging can be seen in changes/damage to DNA, proteins, and other cellular components. One of the key factors that have been found to be involved in the aging process is oxidative stress. Among environmental stressors, UV radiation causes significant oxidative stress, primarily in skin cells. UV exposure from the sun or other artificial sources can lead to a buildup of DNA damage and destruction of skin cells, leading to photoaging [reviewed in Ref. (114)]. There are several ways the DNA can be repaired, depending on the exact form of damage, but only one has been found to be linked to circadian rhythms (146, 147). When UV light reaches the DNA, it can cause cyclobutane pyrimidine dimers. The system that the cell uses to repair this damage is called nucleotide excision repair (NER, Fig. 7) (147). NER involves several critical proteins, including the protein xeroderma pigmentosum A (XPA), which shows circadian rhythmicity due to its transcription and ubiquitination by other rhythmic elements in the cell, and is also a deacetylation target of SIRT1 (57, 169). This is especially important when we realize that SIRT1 activity has been found to be proportional to lifespan in several model organisms [reviewed in Refs. (35, 120, 162)].
FIG. 7.
Nucleotide-excision repair (NER) and circadian rhythms. NER is initiated upon DNA damage by ultraviolet light. Xeroderma pigmentosum A (XPA) is the rate-limiting protein in the process of NER initiation. XPA is also clock-controlled, indicating that circadian rhythms have a significant influence on the NER process. After initiation, the damaged piece of DNA is excised, and the repair process is completed when DNA polymerase fills the gap left by the excised DNA using the undamaged single strand of DNA as a template.
Calorie restriction-related longevity increases have been shown in various species, from yeast to mammals (92, 162). It is believed that the reasons for this are that a reduced amount of ROS is produced by calorie restriction, effectively lowering overall oxidative stress in the cell, and that calorie restriction increases the ability of sirtuins to moderate the stress response in the cell, thereby countering aging two ways (35, 136). In addition to the effects of restrictive diets on the oxidative stress system, a decreased calorie diet has also been shown to entrain the SCN, consequently changing the circadian rhythm of an organism (54). This may affect aging, as a direct disruption of many of the core circadian rhythm genes has been shown to reduce lifespan in transgenic mice (47, 201).
As described in the earlier section, many oxidative stress enzymes have been found to oscillate rhythmically. This rhythmicity has been found in some instances to change as an organism ages, making it less reliably circadian. Goncharova et al. have found this to be true in a group of 10 rhesus monkeys (5 older and 5 younger) (64). The monkeys were subjected to a stressor either in the morning or in the evening and found that a circadian rhythm in corticosteroids and GR was present in the erythrocytes of younger animals. However, this rhythm was mitigated in the older animals (64). This group had earlier demonstrated a similar result in erythrocyte SOD levels with the same type of monkeys (63). This phenomenon has also been observed in a study in humans where Stritesky Larssen et al. found that in a group of 66 individuals, the variations of ROS production in leukocytes oscillated with circadian rhythmicity, and had a higher antioxidant status in newborns, as opposed to older volunteers (168). This leads us to believe that both circadian rhythms and the redox state of cells are both very important in aging in all species.
Heart disease
Both circadian rhythms and oxidative stress have been studied for a long time in various diseases and disorders of the heart. Oxidative stress has been shown to play a primary role in the development of heart disease, while various cardiovascular factors have been found to have circadian rhythms, including basic blood pressure (191). At lower levels, numerous ROS have been implicated as signaling factors in angiogenesis, but higher levels are associated with cardiovascular disease (180). The intricate balance of ROS and overall reduction of oxidative stress is extraordinarily important in heart tissue, as the cells that make up the heart have been estimated to have a renewal rate of 1% or less, depending on age (15). This is considerably less than most other organs and tissues, especially the skin, which has shown to have a turnover rate of 39 days for the entire tissue (189).
Angiotensin is one hormone that has been implicated in several necessary processes, including angiogenesis, regulation of blood pressure, induction of apoptosis, and ROS production. Angiotensin enhances ROS production via increasing cellular production of NADPH oxidase, which is one of the key enzymes that produces O2− inside the cell (125). This increase in O2− appears to be an integral part of the signaling mechanism used by angiotensin to affect the vascular tissue in the fashions listed above (125). A study done in 2002 in hypertensive rats found rhythmicity in the precursor to angiotensin, angiotensinogen, suggesting that this cascade could be important in regulating oxidative stress in cells in the heart, and that the changes are made in a rhythmic fashion (117). It is well documented that sudden cardiac death and heart attacks are more common in the morning, when blood pressure starts rising after leaving its low point overnight (129). Interestingly, it has recently been found that a dysregulated blood pressure rhythm could be corrected by blocking the angiotensin II type 1 receptor, showing that this rhythmic body process is intimately involved with oxidative stress regulation, which ultimately can lead to heart disease if incorrectly regulated (56). As mentioned earlier, the knowledge that blood pressure and other cardiovascular events follow a circadian rhythm has also been exploited in therapeutics for best response, when giving certain medications, especially angiotensin receptor blockers and other antihypertensive drugs (72, 104, 192).
Interestingly, several of the players that interact in the regulation of circadian rhythms and oxidative stress also play a role in heart disease. Ota et al. demonstrated that statin treatment of human umbilical vein endothelial cells after H2O2-induced oxidative stress induction was able to increase the levels of SIRT1 and CAT (123). SIRT1 has been found to be important in revascularization, especially in tissues that have had a restricted blood supply (196). In addition, one of the forms of GST, GST-pi, was found by investigators to be critical in restoring balance to the ischemic heart, due to its nonclassical ability to bind NO and NO-containing compounds (137, 184). The rhythmicity of both the pi form and mu form of GST was found in a study done in the mouse livers in 2012 (195). As discussed previously, melatonin is an especially important hormone in circadian rhythm regulation, as well as a potent antioxidant. It has been found that melatonin has a beneficial effect of lowering blood pressure in both men and women (158). Several studies have also shown that there is a significant reduction in circulating melatonin levels in patients with heart disease as compared to control patients [reviewed in Ref. (45)]. These findings suggest that using agents to correct arrhythmic components of diseased patients, including blood pressure, can play key roles in survival after heart injury and in preventing injury and/or death.
Cancer
The expression of the core circadian rhythm genes is dysregulated in many types of cancer, as is the expression of proteins involved in oxidative stress. Table 1 provides information regarding the reported expression pattern of circadian rhythm and oxidative stress components in different cancers in humans. Interestingly, in many cancers, the antioxidants and protective enzymes involved in preventing oxidative stress still oscillate with circadian rhythmicity, but have been found to have altered levels of expression. For example, Kolanjiappan and Manoharan found that in rats with mammary tumors, there was a delay in the acrophase of SOD, CAT, GPx, and lipid peroxidation levels in blood as compared to controls (95). In addition, the mesor value (roughly, the average expression level) for lipid peroxidation was found to be increased while values for SOD, CAT, and GPx activities decreased, indicating a correlation between lowered antioxidant levels and increased lipid peroxidation in rats during mammary carcinogenesis (95). This trend of altered levels of circadian rhythmicity in oxidative stress components was also found in human gynecological malignancies and in oral cancer (106, 159).
Table 1.
Circadian Rhythms and Oxidative Stress Components in Cancer
| BMAL1 | CLOCK | PER1 | PER2 | CAT | SOD | GSH | Lipid peroxidation | References | |
|---|---|---|---|---|---|---|---|---|---|
| Ovarian | ↑ | ↓ | ↓ | ↓ | ↓ | ↑/↓ | ↑ | ↑ | (76, 85, 148, 155, 174) |
| Prostate | ↑ | ↓ | ↓ | ↓ | ↑ | ↑ | (10, 27, 87) | ||
| Colorectal | = | ↑ | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | (122, 163, 164, 188) |
| Liver | = | = | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | (14, 31, 36, 102, 139) |
| Squamous cell | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | (61, 67, 75) | |
| Gliomas | ↓ | ↓ | ↑ | ↓ | ↓ | ↑ | (131, 194, 202) | ||
| Breast | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ | (30, 99, 132, 193, 197) | ||
| NSCL | ↓ | ↓ | ↑ | ↑ | ↑ | (33, 60, 65, 79, 199) | |||
| Endometrial | ↓ | ↓ | ↓ | ↑ | (126, 198) |
Arrows indicate direction of expression or activity change of the component; = indicates no change from control.
CAT, catalase; CLOCK, circadian locomotor output cycles kaput protein; GSH, glutathione; NSCL, non-small cell lung; PER, periods.
While these studies show a significant difference in the circadian oscillation of antioxidants, protective enzymes, and consequently a difference in oxidative damage, these studies do not clearly show whether the disrupted circadian rhythm is a cause or consequence of cancer. However, a recent article by Gaddameedhi et al. shows that UVB radiation, which is a significant source of oxidative stress in the skin, causes fivefold higher frequency and a faster growth rate of skin cancer in mice when applied in the early morning rather than the early evening (57). In addition, XPA, which is a critical rate-limiting protein in the DNA damage response (shown in Fig. 7), was shown to oscillate with circadian rhythmicity in mouse skin, peaking in the early evening (57). This suggests that even when they are not disrupted, circadian rhythms in the body's defense system can play a significant role in cancer development, simply due to their impact on expression levels of protective proteins.
Recent studies in hepatocellular carcinomas suggest that melatonin treatment can reverse abnormal changes in daily oxidative stress rhythms, as well as tumor development. In the first study, rats displayed altered circadian rhythm characteristics (acrophase, amplitude, and mesor values) of lipid peroxidation, SOD, CAT, GPx, and GSH after N-nitrosodiethylamine-induced hepatocarcinogenesis (40). Treatment with melatonin decreased many of these differences. This indicates that melatonin significantly improves lipid peroxidation levels during the development of hepatocarcinomas through an increase in the levels of antioxidants and circadian phase shifts. A second study showed that melatonin treatment within this system also leads to a significantly lower tumor burden relative to untreated controls (171). Thus, melatonin is an effective tumor suppressor, possibly through its role as an antioxidant or its effects on circadian rhythm regulation, or both. It is therefore not surprising that low levels of melatonin have been associated with several types of cancer (9, 110, 151–153).
Interestingly, a number of studies in the past decade have suggested an involvement of SIRT1 in cancer (32, 77, 83, 88, 170, 203). Since SIRT1 impacts both circadian rhythms and oxidative stress, its aberrant expression could lead to disruptions in both systems. In the case of circadian rhythms, since SIRT1 deacetylation targets PER2 for degradation, this disruption could contribute to tumor development as illustrated in the case of PER2 knockout in mice (55). Mice deficient in the circadian gene PER2 develop more tumors with reduced apoptosis after exposure to gamma-radiation. This effect is accompanied by an absence of the core circadian gene induction observed in wild-type mice and deregulation of cell cycle, cellular proliferation, and apoptosis (55). Studies from our laboratory have shown that in prostate cancer, inhibition of endogenously overexpressed SIRT1 results in decreased cell growth and viability, and an increase in FOXO1 activity (88). Interestingly, when p53 is present in this system, SIRT1 inhibition yields an increase in apoptosis, versus increased senescence in p53 knockout lines (86). Thus, p53 and FOXO1 are involved in the mechanism by which SIRT1 promotes tumor growth (86, 88, 187). Further, we have demonstrated that melatonin treatment inhibits SIRT1 expression and activity, also leading to decreased cell growth and viability, which suggests a second mechanism by which melatonin can impact both oxidative stress and circadian rhythms (89). Thus, as an upstream regulator of circadian rhythms and tumor suppressors that also impact oxidative stress, SIRT1 could play a large part in the possible role of both systems in tumor development.
Neurodegenerative diseases
Many neurodegenerative conditions show the presence of plaques of different proteins in the brain, neuronal cell death, and an onset later in life. In addition, many have been linked to both increased oxidative stress and the disruption of circadian rhythms. Two of these disorders are Alzheimer's disease (AD) and PD. As both of these diseases are age related, it is no surprise that sirtuins and antioxidants are potential targets for preventing their progression (46, 178). As discussed earlier, SIRT1 plays a major role in increased longevity due to calorie restriction and is important in both oxidative stress and circadian rhythm regulation. Qin et al. determined the relative levels of SIRT1 and amyloid-β proteins in the brains of Squirrel monkeys (133). They found that monkeys kept on a 30% calorie-restricted diet had increased levels of SIRT1 protein in the temporal cortex of the brain, and that those same areas had reduced levels of two amyloid-β peptides (133). This suggests that SIRT1 may play a part in preventing the accumulation of the peptides. In addition to the role played by SIRT1 in the oxidative stress effects on AD, mitochondrial malfunction driven by increased oxidative stress has also been implicated in increasing amyloid-β plaques (157), leading to further evidence of the importance of oxidative stress and its regulation in the development of AD. This can be further confirmed by considering evidence that both the physiology and genetic expression of important circadian rhythm regulators are impacted in patients suffering from AD. In a review by Slats et al., it was pointed out that AD patients were shown to have a severely atrophied SCN, as well as a marked decrease in melatonin levels and CLOCK expression, suggesting the importance of circadian rhythms in this disease (165). Conversely, this may also suggest that some component of AD acts to decrease the effectiveness of circadian rhythm regulation.
In PD, many of these same components have been found to be important. PD is a disease centered on the degeneration of the central nervous system, with most patients displaying a significant amount of cell death in dopaminergic neurons (41). It is thought that these neurons are especially susceptible to increases in oxidative stress, as they already have high levels of free radicals due to the cellular processes used to break down dopamine. Melatonin has been shown to offer some protection to dopaminergic neurons in a mouse model of two types of the disease, as well as attenuation of increases in lipid peroxidation and messenger RNA (mRNA) expression of cytochrome P-450 2E1, which has been shown to increase ROS (161). However, it is not known whether this observed response was due to melatonin's antioxidant property or its circadian rhythm regulation abilities. To illustrate the importance of circadian regulation in PD, several studies have looked at central circadian pathway members. For example, a study done by Cai et al. looked at expression levels of key components of the circadian rhythm machinery in leukocytes over a 12-h period in patients with and without PD (24). They found decreased levels of BMAL1, but not PER1, and further described a correlation between PD severity and BMAL1 expression levels (24). Another study found that BMAL2 also had significantly lower levels of expression in patients with PD than controls, suggesting a dysregulation of molecular clock components in patients with PD (44). Thus, both of these diseases, and many neurodegenerative diseases in general, seem to have strong oxidative stress and circadian rhythm components.
Interestingly, Prxs have been suggested to be protective agents for neurological disorders, because they are key regulators in inflammatory pathways, and as we discussed earlier, they have also been shown to be rhythmic (204). Inflammation has been shown to play a role in many neurodegenerative diseases, as well as many others, including heart disease (16, 140). Intriguingly, in addition to oxidative stress regulators being important in regulating the inflammatory response, it has been suggested that circadian rhythm regulation may also be involved in the regulation of inflammation. Radogna et al. have recently discussed a role of melatonin in regulating the inflammatory response in humans, both in a circadian fashion and through antioxidant activity, which also ties in with our premise that circadian rhythms and oxidative stress are intimately linked in many human pathologies (135).
Diabetes
Diabetes represents another excellent example of a disease that has deregulations in both oxidative stress and circadian rhythm machinery. Type I diabetes has been found to result from the loss of beta-cells in the pancreas, thereby destroying the body's ability to produce insulin and regulate blood sugar on its own (7). Type II diabetes is characterized by a reduction in the body's ability to respond to insulin or by a reduced secretion of insulin, often due to a poor diet, and generally shows up later in life (68). Among other possible causes, it has been suggested that one reason for the loss of beta-cells in Type I diabetes could be the buildup of an excessive amount of NO and other reactive species due to an external stressor (7, 49). Following this idea, a study by Van Dyke et al. found that an NO oxidizer may prevent diabetes formation in a rodent model of streptozotocin-induced diabetes (182). Thus, oxidative stress in the form of NO plays a role in diabetes.
The connection between oxidative stress and circadian rhythms in diabetes has been illustrated in several different ways. A study by Kanabrocki et al. found that many byproducts of an increase in oxidative stress are regulated rhythmically in diabetics, stressing the importance of ROS levels. They found a marked circadian rhythm in DNA oxidation (in urine), uric acid (in serum), and nitric oxide (in serum) levels in nondiabetic men, but not in diabetic subjects (90). This study found that the peak levels of serum concentrations of the markers occurred in the early evening, suggesting a correlation between oxidative damage and increased metabolism. The significance of rhythmic factors in diabetes was also shown in a study that looked at mRNA expression of the core clock genes in cells isolated from patients with and without type 2 diabetes, in addition to insulin secretion (167). The authors found that PER2, PER3, and CRY2 mRNA levels in human islets were significantly lower in patients with type II diabetes than in normal controls. These levels correlate positively with insulin content, suggesting that dysregulation of the circadian clock may contribute to the pathology of Type 2 diabetes. It is therefore most likely that both increased oxidative stress (in the form of ROS and RNS) as well as a dysregulation of key circadian clock genes are instrumental in the onset and exacerbation of diabetes.
In addition to these diseases, other critical body processes and diseases are affected by both circadian rhythms and oxidative stress working in concert. Although we have discussed several here, there are still many more that have links to either circadian rhythms or oxidative stress, with no research done on the other. This could be an interesting direction for new research in diseases that show promise in using a cross-disciplinary approach. However, the diseases we have discussed appear to represent some of the most promising diseases to benefit from a concerted effort to target both oxidative stress and circadian rhythm dysregulation.
Conclusion
Oxidative stress has been a hot research topic in a number of scientific disciplines for more than five decades. Substantial research has also been done on circadian rhythms and its effects at cellular and organismal levels. Because these two important processes are so integral to the proper functioning of cellular activity, it is not a surprise that both have been implicated in many different diseases and disorders (Fig. 8). However, in-depth studies have not been done to determine the cause-and-effect association between circadian rhythm and oxidative stress-signaling components. There is a need to intensify our efforts to conduct such studies in appropriate model systems. One caveat of the current status of circadian rhythm research is that the rodent models, which are most widely used animal models, may or may not be directly relevant to humans, especially when developing human therapies. This is because mice, rats, and hamsters are nocturnal animals. However, it is conceivable that most of the findings could be phase-adjusted (101). Another important consideration is that many of the antioxidant enzymes have several isoforms or family members, many of which were discovered after the cited research was done. This makes it difficult to determine which isoform is responsible for rhythmicity, as it is likely that not all of these members are rhythmically regulated. However, the evidence suggests that at least one form of each enzyme is regulated in a circadian fashion, and it appears to be enough to affect disease pathologies. We believe that the diseases that have both circadian rhythm and oxidative stress components in their pathogenesis may benefit from a two-pronged approach of targeting both components. For example, a combinatorial approach comprised of antioxidants and circadian rhythm-resynchronizing approaches (i.e., melatonin) could be beneficial to patients with these conditions. Intense concerted and collaborative efforts of scientists from diverse discipline are needed to explore these possibilities.
FIG. 8.
Circadian rhythms, oxidative stress, and disease. Circadian rhythms have a significant impact on the oxidative stress defense system. Several key enzymes and antioxidants involved in protection from free radicals oscillate with circadian rhythmicity in expression or activity level. These include SOD, CAT, Prx, GSH, GPx, GSSG, GR, and GST. Circadian rhythms and oxidative stress are further connected by the role that they both play in several different diseases. Whether their disruption is a cause or an effect of these diseases remains to be determined, but further investigation into the connections between circadian rhythms, oxidative stress, and disease could lead to valuable new treatments in the future.
Abbreviations Used
- AD
Alzheimer's disease
- ARNTL
aryl hydrocarbon receptor nuclear translocator-like
- BMAL1
aryl hydrocarbon receptor nuclear translocator-like (also known as ARNTL)
- CAT
catalase
- CLOCK
circadian locomotor output cycles kaput protein
- CRY
cryptochrome
- GPx
glutathione peroxidase
- GR
glutathione reductase
- Cu/Zn
copper/zinc
- GSH
glutathione
- GSSG
glutathione disulfide
- GST
glutathione S-transferase
- H2O2
hydrogen peroxide
- HDAC
histone deacetylase
- NAD+
nicotinamide adenine dinucleotide
- NER
nucleotide excision repair
- NSCL
non-small cell lung
- PD
Parkinson's disease
- PER
period
- Prx
peroxiredoxin
- RNS
reactive nitrogen species
- ROR
RAR-related orphan receptors
- RORE
retinoic acid-related orphan receptor-response elements
- ROS
reactive oxygen species
- SCN
suprachiasmatic nucleus
- SOD
superoxide dismutase
- UV
ultraviolet
- XPA
xeroderma pigmentosum A
Acknowledgments
This work was partly supported by funding from the NIH (R21CA149560 and R01AR059130) and Merit Review Award (1I01BX001008-01A1) from the Biomedical Laboratory Research and Development Service of the VA Office of Research and Development. The authors would like to thank Mr. Corrie Busch for his help in the development of the figures for this article.
References
- 1.Agapito MT. Redondo I. Plaza R. Lopez-Burillo S. Recio JM. Pablos MI. Relationships between melatonin, glutathione peroxidase, glutathione reductase, and catalase. Endogenous rhythms on cerebral cortex in Gallus domesticus. Adv Exp Med Biol. 1999;460:377–381. [PubMed] [Google Scholar]
- 2.Akashi M. Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12:441–448. doi: 10.1038/nsmb925. [DOI] [PubMed] [Google Scholar]
- 3.Andre E. Conquet F. Steinmayr M. Stratton SC. Porciatti V. Becker-Andre M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17:3867–3877. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoch MP. Song E-J. Chang A-M. Vitaterna MH. Zhao Y. Wilsbacher LD. Sangoram AM. King DP. Pinto LH. Takahashi JS. Functional identification of the mouse circadian clock gene by transgenic BAC rescue. Cell. 1997;89:655–667. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asher G. Gatfield D. Stratmann M. Reinke H. Dibner C. Kreppel F. Mostoslavsky R. Alt FW. Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson HJ. Babbitt PC. Glutathione transferases are structural and functional outliers in the thioredoxin fold. Biochemistry. 2009;48:11108–1116. doi: 10.1021/bi901180v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson MA. Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 8.Bartosz G. Reactive oxygen species: destroyers or messengers? Biochem Pharmacol. 2009;77:1303–1315. doi: 10.1016/j.bcp.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch C. Bartsch BH. Fluchter SH. Attanasio A. Gupta D. Evidence for modulation of melatonin secretion in men with benign and malignant tumors of the prostate: relationship with the pituitary hormones. J Pineal Res. 1985;2:121–132. doi: 10.1111/j.1600-079x.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 10.Battisti V. Maders LDK. Bagatini MD. Reetz LGB. Chiesa J. Battisti IE. Gonçalves JF. Duarte MMF. Schetinger MRC. Morsch VM. Oxidative stress and antioxidant status in prostate cancer patients: Relation to Gleason score, treatment and bone metastasis. Biomed Pharmacother. 2011;65:516–524. doi: 10.1016/j.biopha.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Baydas G. Gursu MF. Yilmaz S. Canpolat S. Yasar A. Cikim G. Canatan H. Daily rhythm of glutathione peroxidase activity, lipid peroxidation and glutathione levels in tissues of pinealectomized rats. Neurosci Lett. 2002;323:195–198. doi: 10.1016/s0304-3940(02)00144-1. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JS. Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 13.Bell-Pedersen D. Crosthwaite SK. Lakin-Thomas PL. Merrow M. Okland M. The Neurospora circadian clock: simple or complex? Philos Trans R Soc Lond B Biol Sci. 2001;356:1697–1709. doi: 10.1098/rstb.2001.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellisola G. Casaril M. Gabrielli GB. Caraffi M. Corrocher R. Catalase activity in human hepatocellular carcinoma (HCC) Clin Biochem. 1987;20:415–417. doi: 10.1016/0009-9120(87)90007-5. [DOI] [PubMed] [Google Scholar]
- 15.Bergmann O. Bhardwaj RD. Bernard S. Zdunek S. Barnabe-Heider F. Walsh S. Zupicich J. Alkass K. Buchholz BA. Druid H. Jovinge S. Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner MY. Arbiser JL. Targeting NADPH oxidases for the treatment of cancer and inflammation. Cell Mol Life Sci. 2012;69:2435–2442. doi: 10.1007/s00018-012-1017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgs L. Beukelaers P. Vandenbosch R. Belachew S. Nguyen L. Malgrange B. Cell “circadian” cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8:832–837. doi: 10.4161/cc.8.6.7869. [DOI] [PubMed] [Google Scholar]
- 18.Bridges AB. Scott NA. McNeill GP. Pringle TH. Belch JJ. Circadian variation of white blood cell aggregation and free radical indices in men with ischaemic heart disease. Eur Heart J. 1992;13:1632–1636. doi: 10.1093/oxfordjournals.eurheartj.a060116. [DOI] [PubMed] [Google Scholar]
- 19.Brigelius-Flohe R. Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruguerolle B. Simon N. Biologic rhythms and Parkinson's disease: a chronopharmacologic approach to considering fluctuations in function. Clin Neuropharmacol. 2002;25:194–201. doi: 10.1097/00002826-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Buettner GR. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anticancer Agents Med Chem. 2011;11:341–346. doi: 10.2174/187152011795677544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burioka N. Fukuoka Y. Koyanagi S. Miyata M. Takata M. Chikumi H. Takane H. Watanabe M. Endo M. Sako T. Suyama H. Ohdo S. Shimizu E. Asthma: chronopharmacotherapy and the molecular clock. Adv Drug Deliv Rev. 2010;62:946–955. doi: 10.1016/j.addr.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Buttgereit F. Mehta D. Kirwan J. Szechinski J. Boers M. Alten RE. Supronik J. Szombati I. Romer U. Witte S. Saag KG. Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2) Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-201067. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y. Liu S. Sothern RB. Xu S. Chan P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. Eur J Neurol. 2010;17:550–554. doi: 10.1111/j.1468-1331.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 25.Calcerrada P. Peluffo G. Radi R. Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications. Curr Pharm Des. 2011;17:3905–3932. doi: 10.2174/138161211798357719. [DOI] [PubMed] [Google Scholar]
- 26.Calcutt G. Diurnal variations in rat blood glutathione levels. Naturwissenschaften. 1967;54:120. doi: 10.1007/BF00640587. [DOI] [PubMed] [Google Scholar]
- 27.Cao Q. Gery S. Dashti A. Yin D. Zhou Y. Gu J. Koeffler HP. A role for the clock gene Per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakravarty S. Rizvi SI. Day and night GSH and MDA levels in healthy adults and effects of different doses of melatonin on these parameters. Int J Cell Biol. 2011;2011:404591. doi: 10.1155/2011/404591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakravarty S. Rizvi SI. Circadian modulation of human erythrocyte plasma membrane redox system by melatonin. Neurosci Lett. 2012;518:32–35. doi: 10.1016/j.neulet.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 30.Chen ST. Choo KB. Hou MF. Yeh KT. Kuo SJ. Chang JG. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 31.Chen XL. Zhou L. Yang J. Shen FK. Zhao SP. Wang YL. Hepatocellular carcinoma-associated protein markers investigated by MALDI-TOF MS. Mol Med Report. 2010;3:589–596. doi: 10.3892/mmr_00000302. [DOI] [PubMed] [Google Scholar]
- 32.Choi HN. Bae JS. Jamiyandorj U. Noh SJ. Park HS. Jang KY. Chung MJ. Kang MJ. Lee DG. Moon WS. Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep. 2011;26:503–510. doi: 10.3892/or.2011.1301. [DOI] [PubMed] [Google Scholar]
- 33.Chung-man Ho J. Zheng S. Comhair SA. Farver C. Erzurum SC. Differential expression of manganese superoxide dismutase and catalase in lung cancer. Cancer Res. 2001;61:8578–8585. [PubMed] [Google Scholar]
- 34.Commoner B. Townsend J. Pake GE. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 35.Corbi G. Conti V. Scapagnini G. Filippelli A. Ferrara N. Role of sirtuins, calorie restriction and physical activity in aging. Front Biosci (Elite Ed) 2012;4:768–778. doi: 10.2741/417. [DOI] [PubMed] [Google Scholar]
- 36.Corrocher R. Casaril M. Bellisola G. Gabrielli GB. Nicoli N. Guidi GC. De Sandre G. Severe impairment of antioxidant system in human hepatoma. Cancer. 1986;58:1658–1662. doi: 10.1002/1097-0142(19861015)58:8<1658::aid-cncr2820580814>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Coto-Montes A. Boga JA. Tomas-Zapico C. Rodriguez-Colunga MJ. Martinez-Fraga J. Tolivia-Cadrecha D. Menendez G. Hardeland R. Tolivia D. Physiological oxidative stress model: Syrian hamster Harderian gland-sex differences in antioxidant enzymes. Free Radic Biol Med. 2001;30:785–792. doi: 10.1016/s0891-5849(01)00468-3. [DOI] [PubMed] [Google Scholar]
- 38.Coto-Montes A. Tomas-Zapico C. Rodriguez-Colunga MJ. Tolivia-Cadrecha D. Martinez-Fraga J. Hardeland R. Tolivia D. Effects of the circadian mutation ‘tau’ on the Harderian glands of Syrian hamsters. J Cell Biochem. 2001;83:426–434. doi: 10.1002/jcb.1240. [DOI] [PubMed] [Google Scholar]
- 39.Cutolo M. Chronobiology and the treatment of rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:312–318. doi: 10.1097/BOR.0b013e3283521c78. [DOI] [PubMed] [Google Scholar]
- 40.Dakshayani KB. Subramanian P. Essa MM. Effect of melatonin on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats with reference to biochemical circadian rhythms. Toxicol Mech Methods. 2007;17:67–75. doi: 10.1080/15376520500195798. [DOI] [PubMed] [Google Scholar]
- 41.Dauer W. Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 42.Davies MH. Bozigian HP. Merrick BA. Birt DF. Schnell RC. Circadian variations in glutathione-S-transferase and glutathione peroxidase activities in the mouse. Toxicol Lett. 1983;19:23–27. doi: 10.1016/0378-4274(83)90257-6. [DOI] [PubMed] [Google Scholar]
- 43.Diaz-Munoz M. Hernandez-Munoz R. Suarez J. Chagoya de Sanchez V. Day-night cycle of lipid peroxidation in rat cerebral cortex and their relationship to the glutathione cycle and superoxide dismutase activity. Neuroscience. 1985;16:859–863. doi: 10.1016/0306-4522(85)90100-9. [DOI] [PubMed] [Google Scholar]
- 44.Ding H. Liu S. Yuan Y. Lin Q. Chan P. Cai Y. Decreased expression of Bmal2 in patients with Parkinson's disease. Neurosci Lett. 2011;499:186–188. doi: 10.1016/j.neulet.2011.05.058. [DOI] [PubMed] [Google Scholar]
- 45.Dominguez-Rodriguez A. Abreu-Gonzalez P. Avanzas P. The role of melatonin in acute myocardial infarction. Front Biosci. 2012;17:2433–2441. doi: 10.2741/4063. [DOI] [PubMed] [Google Scholar]
- 46.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci. 2012;33:494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Dubrovsky YV. Samsa WE. Kondratov RV. Deficiency of circadian protein CLOCK reduces lifespan and increases age-related cataract development in mice. Aging (Albany NY) 2010;2:936–944. doi: 10.18632/aging.100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffy JF. Wright KP., Jr Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 49.Duran-Reyes G. Pascoe-Lira D. Vilar-Rojas C. Medina-Navarro R. Diaz-Flores M. Ortega-Camarillo C. Garcia-Macedo R. Cruz M. Rodriguez JK. Diabetogenic effect of STZ diminishes with the loss of nitric oxide: role of ultraviolet light and carboxy-PTIO. Pharmacology. 2004;71:17–24. doi: 10.1159/000076258. [DOI] [PubMed] [Google Scholar]
- 50.Edmunds LN., Jr. Laval-Martin DL. Goto K. Cell division cycles and circadian clocks. Modeling a metabolic oscillator in the algal flagellate Euglena. Ann N Y Acad Sci. 1987;503:459–475. doi: 10.1111/j.1749-6632.1987.tb40630.x. [DOI] [PubMed] [Google Scholar]
- 51.Fanjul-Moles ML. Prieto-Sagredo J. Lopez DS. Bartolo-Orozco R. Cruz-Rosas H. Crayfish Procambarus clarkii retina and nervous system exhibit antioxidant circadian rhythms coupled with metabolic and luminous daily cycles. Photochem Photobiol. 2009;85:78–87. doi: 10.1111/j.1751-1097.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- 52.Forman HJ. Maiorino M. Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Froy O. Chapnik N. Miskin R. The suprachiasmatic nuclei are involved in determining circadian rhythms during restricted feeding. Neuroscience. 2008;155:1152–1159. doi: 10.1016/j.neuroscience.2008.06.060. [DOI] [PubMed] [Google Scholar]
- 54.Froy O. Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fu L. Pelicano H. Liu J. Huang P. Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 56.Fukuda M. Yamanaka T. Mizuno M. Motokawa M. Shirasawa Y. Miyagi S. Nishio T. Yoshida A. Kimura G. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J Hypertens. 2008;26:583–588. doi: 10.1097/HJH.0b013e3282f2fded. [DOI] [PubMed] [Google Scholar]
- 57.Gaddameedhi S. Selby CP. Kaufmann WK. Smart RC. Sancar A. Control of skin cancer by the circadian rhythm. Proc Natl Acad Sci U S A. 2011;108:18790–18795. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gekakis N. Staknis D. Nguyen HB. Davis FC. Wilsbacher LD. King DP. Takahashi JS. Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 59.Gerschman R. Gilbert DL. Nye SW. Dwyer P. Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 60.Gery S. Komatsu N. Kawamata N. Miller CW. Desmond J. Virk RK. Marchevsky A. Mckenna R. Taguchi H. Koeffler HP. Epigenetic silencing of the candidate tumor suppressor gene Per1 in non–small cell lung cancer. Clin Cancer Res. 2007;13:1399–1404. doi: 10.1158/1078-0432.CCR-06-1730. [DOI] [PubMed] [Google Scholar]
- 61.Gokul S. Patil VS. Jailkhani R. Hallikeri K. Kattappagari KK. Oxidant-antioxidant status in blood and tumor tissue of oral squamous cell carcinoma patients. Oral Dis. 2010;16:29–33. doi: 10.1111/j.1601-0825.2009.01598.x. [DOI] [PubMed] [Google Scholar]
- 62.Golombek DA. Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 63.Goncharova ND. Shmaliy AV. Bogatyrenko TN. Koltover VK. Correlation between activity of antioxidant enzymes and circadian rhythms of corticosteroids in Macaca mulatta monkeys of different age. Exp Gerontol. 2006;41:778–783. doi: 10.1016/j.exger.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Goncharova ND. Shmaliy AV. Marenin VY. Smelkova SA. Lapin BA. Circadian and age-related changes in stress responsiveness of the adrenal cortex and erythrocyte antioxidant enzymes in female rhesus monkeys. J Med Primatol. 2008;37:229–238. doi: 10.1111/j.1600-0684.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 65.Gresner P. Gromadzinska J. Jablonska E. Kaczmarski J. Wasowicz W. Expression of selenoprotein-coding genes SEPP1, SEP15 and hGPX1 in non-small cell lung cancer. Lung Cancer. 2009;65:34–40. doi: 10.1016/j.lungcan.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 66.Guillaumond F. Dardente H. Giguere V. Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A. Bhatt ML. Misra MK. Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxid Med Cell Longev. 2009;2:68–72. doi: 10.4161/oxim.2.2.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta D. Krueger CB. Lastra G. Over-nutrition, obesity and insulin resistance in the development of beta-cell dysfunction. Curr Diabetes Rev. 2012;8:76–83. doi: 10.2174/157339912799424564. [DOI] [PubMed] [Google Scholar]
- 69.Gupta N. Ragsdale SW. Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor Rev-erbβ. J Bio Chem. 2011;286:4392–4403. doi: 10.1074/jbc.M110.193466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hardeland R. Coto-Montes A. Poeggeler B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol Int. 2003;20:921–962. doi: 10.1081/cbi-120025245. [DOI] [PubMed] [Google Scholar]
- 71.Hayes JD. Flanagan JU. Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 72.Hermida RC. Ayala DE. Mojón A. Fernández JR. Influence of time of day of blood pressure–lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270–1276. doi: 10.2337/dc11-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hirayama J. Sahar S. Grimaldi B. Tamaru T. Takamatsu K. Nakahata Y. Sassone-Corsi P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450:1086–1090. doi: 10.1038/nature06394. [DOI] [PubMed] [Google Scholar]
- 74.Houtkooper RH. Canto C. Wanders RJ. Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu CM. Lin SF. Lu CT. Lin PM. Yang MY. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012;33:149–155. doi: 10.1007/s13277-011-0258-2. [DOI] [PubMed] [Google Scholar]
- 76.Hu Y. Rosen DG. Zhou Y. Feng L. Yang G. Liu J. Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer. J Biol Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 77.Huffman DM. Grizzle WE. Bamman MM. Kim JS. Eltoum IA. Elgavish A. Nagy TR. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 78.Ianas O. Olinescu R. Badescu I. Melatonin involvement in oxidative processes. Endocrinologie. 1991;29:147–153. [PubMed] [Google Scholar]
- 79.Ilonen IK. Rasanen JV. Sihvo EI. Knuuttila A. Salmenkivi KM. Ahotupa MO. Kinnula VL. Salo JA. Oxidative stress in non-small cell lung cancer: role of nicotinamide adenine dinucleotide phosphate oxidase and glutathione. Acta Oncol. 2009;48:1054–1061. doi: 10.1080/02841860902824909. [DOI] [PubMed] [Google Scholar]
- 80.Innominato PF. Levi FA. Bjarnason GA. Chronotherapy and the molecular clock: clinical implications in oncology. Adv Drug Deliv Rev. 2010;62:979–1001. doi: 10.1016/j.addr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 81.Inoue N. Circadian variation of hepatic glutathione S-transferase activities in the mouse. Xenobiotica. 1999;29:43–51. doi: 10.1080/004982599238803. [DOI] [PubMed] [Google Scholar]
- 82.Jaeschke H. Wendel A. Diurnal fluctuation and pharmacological alteration of mouse organ glutathione content. Biochem Pharmacol. 1985;34:1029–1033. doi: 10.1016/0006-2952(85)90606-9. [DOI] [PubMed] [Google Scholar]
- 83.Jang KY. Kim KS. Hwang SH. Kwon KS. Kim KR. Park HS. Park BH. Chung MJ. Kang MJ. Lee DG. Moon WS. Expression and prognostic significance of SIRT1 in ovarian epithelial tumours. Pathology. 2009;41:366–371. doi: 10.1080/00313020902884451. [DOI] [PubMed] [Google Scholar]
- 84.Jang YS. Lee MH. Lee SH. Bae K. Cu/Zn superoxide dismutase is differentially regulated in period gene-mutant mice. Biochem Biophys Res Commun. 2011;409:22–27. doi: 10.1016/j.bbrc.2011.04.099. [DOI] [PubMed] [Google Scholar]
- 85.Joncourt F. Buser K. Altermatt H. Bacchi M. Oberli A. Cerny T. Multiple drug resistance parameter expression in ovarian cancer. Gynecol Oncol. 1998;70:176–182. doi: 10.1006/gyno.1998.5085. [DOI] [PubMed] [Google Scholar]
- 86.Jung-Hynes B. Ahmad N. Role of p53 in the anti-proliferative effects of Sirt1 inhibition in prostate cancer cells. Cell cycle. 2009;8:1478–1483. doi: 10.4161/cc.8.10.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung-Hynes B. Huang W. Reiter RJ. Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49:60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jung-Hynes B. Nihal M. Zhong W. Ahmad N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem. 2009;284:3823–3832. doi: 10.1074/jbc.M807869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung-Hynes B. Schmit TL. Reagan-Shaw SR. Siddiqui IA. Mukhtar H. Ahmad N. Melatonin, a novel Sirt1 inhibitor, imparts antiproliferative effects against prostate cancer in vitro in culture and in vivo in TRAMP model. J Pineal Res. 2011;50:140–149. doi: 10.1111/j.1600-079X.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanabrocki EL. Murray D. Hermida RC. Scott GS. Bremner WF. Ryan MD. Ayala DE. Third JL. Shirazi P. Nemchausky BA. Hooper DC. Circadian variation in oxidative stress markers in healthy and type II diabetic men. Chronobiol Int. 2002;19:423–439. doi: 10.1081/cbi-120002914. [DOI] [PubMed] [Google Scholar]
- 91.Kanat M. Is daytime insulin more physiologic and less atherogenic than bedtime insulin? Med Hypotheses. 2007;68:1228–1232. doi: 10.1016/j.mehy.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 92.Kemnitz JW. Calorie restriction and aging in nonhuman primates. ILAR J. 2011;52:66–77. doi: 10.1093/ilar.52.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.King DP. Zhao Y. Sangoram AM. Wilsbacher LD. Tanaka M. Antoch MP. Steeves TDL. Vitaterna MH. Kornhauser JM. Lowrey PL. Turek FW. Takahashi JS. Positional Cloning of the Mouse Circadian Clock Gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirsch S. Thijssen S. Alarcon Salvador S. Heine GH. van Bentum K. Fliser D. Sester M. Sester U. T-cell numbers and antigen-specific T-cell function follow different circadian rhythms. J Clin Immunol. 2012;32:1381–1389. doi: 10.1007/s10875-012-9730-z. [DOI] [PubMed] [Google Scholar]
- 95.Kolanjiappan K. Manoharan S. Diurnal rhythmicity of thiobarbituric acid reactive substances and antioxidants in experimental mammary carcinogenesis. Exp Oncol. 2005;27:298–302. [PubMed] [Google Scholar]
- 96.Kondratov RV. Chernov MV. Kondratova AA. Gorbacheva VY. Gudkov AV. Antoch MP. BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krishnan N. Davis AJ. Giebultowicz JM. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem Biophys Res Commun. 2008;374:299–303. doi: 10.1016/j.bbrc.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kume K. Zylka MJ. Sriram S. Shearman LP. Weaver DR. Jin X. Maywood ES. Hastings MH. Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 99.Kuo SJ. Chen ST. Yeh KT. Hou MF. Chang YS. Hsu NC. Chang JG. Disturbance of circadian gene expression in breast cancer. Virchows Arch. 2009;454:467–474. doi: 10.1007/s00428-009-0761-7. [DOI] [PubMed] [Google Scholar]
- 100.Lapenna D. De Gioia S. Mezzetti A. Porreca E. Ciofani G. Marzio L. Capani F. Di Ilio C. Cuccurullo F. Circadian variations in antioxidant defences and lipid peroxidation in the rat heart. Free Radic Res Commun. 1992;17:187–194. doi: 10.3109/10715769209068165. [DOI] [PubMed] [Google Scholar]
- 101.Leibetseder V. Humpeler S. Svoboda M. Schmid D. Thalhammer T. Zuckermann A. Marktl W. Ekmekcioglu C. Clock genes display rhythmic expression in human hearts. Chronobiol Int. 2009;26:621–636. doi: 10.1080/07420520902924939. [DOI] [PubMed] [Google Scholar]
- 102.Lin YM. Chang JH. Yeh KT. Yang MY. Liu TC. Lin SF. Su WW. Chang JG. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol Carcinog. 2008;47:925–933. doi: 10.1002/mc.20446. [DOI] [PubMed] [Google Scholar]
- 103.Lynch HJ. Wurtman RJ. Moskowitz MA. Archer MC. Ho MH. Daily rhythm in human urinary melatonin. Science. 1975;187:169–171. doi: 10.1126/science.1167425. [DOI] [PubMed] [Google Scholar]
- 104.Mallion JM. Chamontin B. Asmar R. De Leeuw PW. O'Brien E. Duprez D. O'Rourke MF. Rahn KH. Romero R. Battegay E. Hitzenberger G. Safar ME. Twenty-four-hour ambulatory blood pressure monitoring efficacy of perindopril/indapamide first-line combination in hypertensive patients: the REASON study. Am J Hypertens. 2004;17:245–251. doi: 10.1016/j.amjhyper.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 105.Mannervik B. The enzymes of glutathione metabolism: an overview. Biochem Soc Trans. 1987;15:717–718. doi: 10.1042/bst0150717. [DOI] [PubMed] [Google Scholar]
- 106.Manoharan S. Baskar AA. Manivasagam T. Subramanian P. Circadian rhythmicity of plasma lipid peroxidation and antioxidants in oral squamous cell carcinoma. Singapore Med J. 2005;46:184–188. [PubMed] [Google Scholar]
- 107.Martin V. Sainz RM. Mayo JC. Antolin I. Herrera F. Rodriguez C. Daily rhythm of gene expression in rat superoxide dismutases. Endocr Res. 2003;29:83–95. doi: 10.1081/erc-120018679. [DOI] [PubMed] [Google Scholar]
- 108.Mates JM. Perez-Gomez C. Nunez de Castro I. Antioxidant enzymes and human diseases. Clin Biochem. 1999;32:595–603. doi: 10.1016/s0009-9120(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 109.Maurice DV. Lightsey SF. Hsu KT. Rhoades JF. Comparison of glutathione S-transferase activity in the rat and birds: tissue distribution and rhythmicity in chicken (Gallus domesticus) liver. Comp Biochem Physiol B. 1991;100:471–474. doi: 10.1016/0305-0491(91)90206-s. [DOI] [PubMed] [Google Scholar]
- 110.Mazzaccoli G. Carughi S. De Cata A. La Viola M. Vendemiale G. Melatonin and cortisol serum levels in lung cancer patients at different stages of disease. Med Sci Monit. 2005;11:CR284–CR288. [PubMed] [Google Scholar]
- 111.Mazzoccoli G. Pazienza V. Vinciguerra M. Clock genes and clock-controlled genes in the regulation of metabolic rhythms. Chronobiol Int. 2012;29:227–251. doi: 10.3109/07420528.2012.658127. [DOI] [PubMed] [Google Scholar]
- 112.Meyer-Bernstein EL. Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mintz EM. Gillespie CF. Marvel CL. Huhman KL. Albers HE. Serotonergic regulation of circadian rhythms in Syrian hamsters. Neuroscience. 1997;79:563–569. doi: 10.1016/s0306-4522(96)00696-3. [DOI] [PubMed] [Google Scholar]
- 114.Moriwaki S. Takahashi Y. Photoaging and DNA repair. J Dermatol Sci. 2008;50:169–176. doi: 10.1016/j.jdermsci.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 115.Muñoz M. Peirson SN. Hankins MW. Foster RG. Long-term constant light induces constitutive elevated expression of mPER2 protein in the murine SCN: a molecular basis for Aschoff's rule? J Biol Rhythms. 2005;20:3–14. doi: 10.1177/0748730404272858. [DOI] [PubMed] [Google Scholar]
- 116.Nagoshi E. Saini C. Bauer C. Laroche T. Naef F. Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 117.Naito Y. Tsujino T. Fujioka Y. Ohyanagi M. Iwasaki T. Augmented diurnal variations of the cardiac renin-angiotensin system in hypertensive rats. Hypertension. 2002;40:827–833. doi: 10.1161/01.hyp.0000039960.66987.89. [DOI] [PubMed] [Google Scholar]
- 118.Nakahata Y. Kaluzova M. Grimaldi B. Sahar S. Hirayama J. Chen D. Guarente LP. Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O'Neill JS. Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oellerich MF. Potente M. FOXOs and sirtuins in vascular growth, maintenance, and aging. Circ Res. 2012;110:1238–1251. doi: 10.1161/CIRCRESAHA.111.246488. [DOI] [PubMed] [Google Scholar]
- 121.Ohta H. Yamazaki S. McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 122.Oshima T. Takenoshita S. Akaike M. Kunisaki C. Fujii S. Nozaki A. Numata K. Shiozawa M. Rino Y. Tanaka K. Masuda M. Imada T. Expression of circadian genes correlates with liver metastasis and outcomes in colorectal cancer. Oncol Rep. 2011;25:1439–1446. doi: 10.3892/or.2011.1207. [DOI] [PubMed] [Google Scholar]
- 123.Ota H. Eto M. Kano MR. Kahyo T. Setou M. Ogawa S. Iijima K. Akishita M. Ouchi Y. Induction of endothelial nitric oxide synthase, SIRT1, and catalase by statins inhibits endothelial senescence through the Akt pathway. Arterioscler Thromb Vasc Biol. 2010;30:2205–2211. doi: 10.1161/ATVBAHA.110.210500. [DOI] [PubMed] [Google Scholar]
- 124.Pacher P. Beckman JS. Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paravicini TM. Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 126.Pejic S. Todorovic A. Stojiljkovic V. Kasapovic J. Pajovic SB. Antioxidant enzymes and lipid peroxidation in endometrium of patients with polyps, myoma, hyperplasia and adenocarcinoma. Reprod Biol Endocrinol. 2009;7:149. doi: 10.1186/1477-7827-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Perry JJ. Shin DS. Getzoff ED. Tainer JA. The structural biochemistry of the superoxide dismutases. Biochim Biophys Acta. 2010;1804:245–262. doi: 10.1016/j.bbapap.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pfeffer M. Rauch A. Korf HW. von Gall C. The endogenous melatonin (MT) signal facilitates reentrainment of the circadian system to light-induced phase advances by acting upon MT2 receptors. Chronobiol Int. 2012;29:415–429. doi: 10.3109/07420528.2012.667859. [DOI] [PubMed] [Google Scholar]
- 129.Portaluppi F. Tiseo R. Smolensky MH. Hermida RC. Ayala DE. Fabbian F. Circadian rhythms and cardiovascular health. Sleep Med Rev. 2012;16:151–166. doi: 10.1016/j.smrv.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 130.Preitner N. Damiola F. Lopez-Molina L. Zakany J. Duboule D. Albrecht U. Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 131.Pu PY. Lan J. Shan SB. Huang EQ. Bai Y. Guo Y. Jiang DH. Study of the antioxidant enzymes in human brain tumors. J Neurooncol. 1996;29:121–128. doi: 10.1007/BF00182134. [DOI] [PubMed] [Google Scholar]
- 132.Punnonen K. Ahotupa M. Asaishi K. Hyoty M. Kudo R. Punnonen R. Antioxidant enzyme activities and oxidative stress in human breast cancer. J Cancer Res Clin Oncol. 1994;120:374–377. doi: 10.1007/BF01247464. [DOI] [PubMed] [Google Scholar]
- 133.Qin W. Chachich M. Lane M. Roth G. Bryant M. de Cabo R. Ottinger MA. Mattison J. Ingram D. Gandy S. Pasinetti GM. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- 134.Quay WB. Circadian and estrous rhythms in pineal melatonin and 5-hydroxy indole-3-acetic acid. Proc Soc Exp Biol Med. 1964;115:710–713. doi: 10.3181/00379727-115-29014. [DOI] [PubMed] [Google Scholar]
- 135.Radogna F. Diederich M. Ghibelli L. Melatonin: a pleiotropic molecule regulating inflammation. Biochem Pharmacol. 2010;80:1844–1852. doi: 10.1016/j.bcp.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 136.Raghavan A. Shah ZA. Sirtuins in neurodegenerative diseases: a biological-chemical perspective. Neurodegener Dis. 2012;9:1–10. doi: 10.1159/000329724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Raza H. Dual localization of glutathione S-transferase in the cytosol and mitochondria: implications in oxidative stress, toxicity and disease. FEBS J. 2011;278:4243–4251. doi: 10.1111/j.1742-4658.2011.08358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reinberg A. Circadian changes in the temperature of human beings. Bibl Radiol. 1975;6:128–139. [PubMed] [Google Scholar]
- 139.Rohr-Udilova N. Sieghart W. Eferl R. Stoiber D. Bjorkhem-Bergman L. Eriksson LC. Stolze K. Hayden H. Keppler B. Sagmeister S. Grasl-Kraupp B. Schulte-Hermann R. Peck-Radosavljevic M. Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology. 2012;55:1112–1121. doi: 10.1002/hep.24808. [DOI] [PubMed] [Google Scholar]
- 140.Rubio-Perez JM. Morillas-Ruiz JM. A review: inflammatory process in Alzheimer's disease, role of cytokines. Scientific World J. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rutter J. Reick M. Wu LC. McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- 142.Sack RL. Brandes RW. Kendall AR. Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 143.Sahar S. Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- 144.Sahar S. Sassone-Corsi P. Regulation of metabolism: the circadian clock dictates the time. Trends Endocrinol Metab. 2012;23:1–8. doi: 10.1016/j.tem.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saini C. Suter DM. Liani A. Gos P. Schibler U. The mammalian circadian timing system: synchronization of peripheral clocks. Cold Spring Harb Symp Quant Biol. 2011;76:39–47. doi: 10.1101/sqb.2011.76.010918. [DOI] [PubMed] [Google Scholar]
- 146.Sancar A. Lindsey-Boltz LA. Kang TH. Reardon JT. Lee JH. Ozturk N. Circadian clock control of the cellular response to DNA damage. FEBS Lett. 2010;584:2618–2625. doi: 10.1016/j.febslet.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sancar A. Lindsey-Boltz LA. Unsal-Kacmaz K. Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 148.Sanchez M. Torres JV. Tormos C. Iradi A. Muniz P. Espinosa O. Salvador A. Rodriguez-Delgado J. Fandos M. Saez GT. Impairment of antioxidant enzymes, lipid peroxidation and 8-oxo-2′-deoxyguanosine in advanced epithelial ovarian carcinoma of a Spanish community. Cancer Lett. 2006;233:28–35. doi: 10.1016/j.canlet.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 149.Sani M. Sebai H. Gadacha W. Boughattas NA. Reinberg A. Mossadok BA. Catalase activity and rhythmic patterns in mouse brain, kidney and liver. Comp Biochem Physiol B Biochem Mol Biol. 2006;145:331–337. doi: 10.1016/j.cbpb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Sato TK. Panda S. Miraglia LJ. Reyes TM. Rudic RD. McNamara P. Naik KA. FitzGerald GA. Kay SA. Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 151.Schernhammer ES. Berrino F. Krogh V. Secreto G. Micheli A. Venturelli E. Sieri S. Sempos CT. Cavalleri A. Schünemann HJ. Strano S. Muti P. Urinary 6-sulfatoxymelatonin levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2008;100:898–905. doi: 10.1093/jnci/djn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schernhammer ES. Hankinson SE. Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst. 2005;97:1084–1087. doi: 10.1093/jnci/dji190. [DOI] [PubMed] [Google Scholar]
- 153.Schernhammer ES. Hankinson SE. Urinary melatonin levels and postmenopausal breast cancer risk in the nurses' health study cohort. Cancer Epidemiol Biomarkers Prev. 2009;18:74–79. doi: 10.1158/1055-9965.EPI-08-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schibler U. Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/s0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 155.Senthil K. Aranganathan S. Nalini N. Evidence of oxidative stress in the circulation of ovarian cancer patients. Clin Chim Acta. 2004;339:27–32. doi: 10.1016/j.cccn.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 156.Shearman LP. Sriram S. Weaver DR. Maywood ES. Chaves I. Zheng B. Kume K. Lee CC. van der GTJ. Horst Hastings MH. Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 157.Silva DF. Esteves AR. Oliveira CR. Cardoso SM. Mitochondria: the common upstream driver of amyloid-beta and tau pathology in Alzheimer's disease. Curr Alzheimer Res. 2011;8:563–572. doi: 10.2174/156720511796391872. [DOI] [PubMed] [Google Scholar]
- 158.Simko F. Paulis L. Melatonin as a potential antihypertensive treatment. J Pineal Res. 2007;42:319–322. doi: 10.1111/j.1600-079X.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 159.Singh R. Singh RK. Mahdi AA. Kumar A. Tripathi AK. Rai R. Singh U. Cornelissen G. Schwartzkopff O. Halberg F. Circadian periodicity of plasma lipid peroxides and other anti-oxidants as putative markers in gynecological malignancies. In Vivo. 2003;17:593–600. [PubMed] [Google Scholar]
- 160.Singh R. Singh RK. Tripathi AK. Cornelissen G. Schwartzkopff O. Otsuka K. Halberg F. Chronomics of circulating plasma lipid peroxides and anti-oxidant enzymes and other related molecules in cirrhosis of liver. In the memory of late Shri Chetan Singh. Biomed Pharmacother. 2005;59(Suppl 1):S229–S235. doi: 10.1016/s0753-3322(05)80037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Singhal NK. Srivastava G. Patel DK. Jain SK. Singh MP. Melatonin or silymarin reduces maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. J Pineal Res. 2011;50:97–109. doi: 10.1111/j.1600-079X.2010.00819.x. [DOI] [PubMed] [Google Scholar]
- 162.Skinner C. Lin SJ. Effects of calorie restriction on life span of microorganisms. Appl Microbiol Biotechnol. 2010;88:817–828. doi: 10.1007/s00253-010-2824-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Skrzydlewska E. Stankiewicz A. Sulkowska M. Sulkowski S. Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health A. 2001;64:213–222. doi: 10.1080/15287390152543690. [DOI] [PubMed] [Google Scholar]
- 164.Skrzydlewska E. Sulkowski S. Koda M. Zalewski B. Kanczuga-Koda L. Sulkowska M. Lipid peroxidation and antioxidant status in colorectal cancer. World J Gastroenterol. 2005;11:403–406. doi: 10.3748/wjg.v11.i3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Slats D. Claassen JA. Verbeek MM. Overeem S. Reciprocal interactions between sleep, circadian rhythms and Alzheimer's disease: focus on the role of hypocretin and melatonin. Ageing Res Rev. 2012;12:188–200. doi: 10.1016/j.arr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 166.Smolensky MH. Hermida RC. Ayala DE. Tiseo R. Portaluppi F. Administration-time-dependent effects of blood pressure-lowering medications: basis for the chronotherapy of hypertension. Blood Press Monit. 2010;15:173–180. doi: 10.1097/MBP.0b013e32833c7308. [DOI] [PubMed] [Google Scholar]
- 167.Stamenkovic JA. Olsson AH. Nagorny CL. Malmgren S. Dekker-Nitert M. Ling C. Mulder H. Regulation of core clock genes in human islets. Metabolism. 2012;61:978–985. doi: 10.1016/j.metabol.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 168.Stritesky Larssen K. Lyberg T. Oxidative status—age- and circadian variations?—a study in leukocytes/plasma. Neuro Endocrinol Lett. 2006;27:445–452. [PubMed] [Google Scholar]
- 169.Stunkel W. Campbell RM. Sirtuin 1 (SIRT1): the misunderstood HDAC. J Biomol Screen. 2011;16:1153–1169. doi: 10.1177/1087057111422103. [DOI] [PubMed] [Google Scholar]
- 170.Stunkel W. Peh BK. Tan YC. Nayagam VM. Wang X. Salto-Tellez M. Ni B. Entzeroth M. Wood J. Function of the SIRT1 protein deacetylase in cancer. Biotechnol J. 2007;2:1360–1368. doi: 10.1002/biot.200700087. [DOI] [PubMed] [Google Scholar]
- 171.Subramanian P. Mirunalini S. Dakshayani KB. Pandi-Perumal SR. Trakht I. Cardinali DP. Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. J Pineal Res. 2007;43:305–312. doi: 10.1111/j.1600-079X.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 172.Sudo M. Sasahara K. Moriya T. Akiyama M. Hamada T. Shibata S. Constant light housing attenuates circadian rhythms of mPer2 mRNA AND mPER2 protein expression in the suprachiasmatic nucleus of mice. Neuroscience. 2003;121:493–499. doi: 10.1016/s0306-4522(03)00457-3. [DOI] [PubMed] [Google Scholar]
- 173.Tan DX. Reiter RJ. Manchester LC. Yan MT. El-Sawi M. Sainz RM. Mayo JC. Kohen R. Allegra M. Hardeland R. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 174.Tokunaga H. Takebayashi Y. Utsunomiya H. akahira J-I. higashimoto M. mashiko M. Ito K. Niikura H. Takenoshita S-I. Yaegashi N. Clinicopathological significance of circadian rhythm-related gene expression levels in patients with epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2008;87:1060–1070. doi: 10.1080/00016340802348286. [DOI] [PubMed] [Google Scholar]
- 175.Toledo JC., Jr. Augusto O. Connecting the chemical and biological properties of nitric oxide. Chem Res Toxicol. 2012;25:975–989. doi: 10.1021/tx300042g. [DOI] [PubMed] [Google Scholar]
- 176.Tomás-Zapico C. Coto-Montes A. Martínez-Fraga J. Rodríguez-Colunga MJ. Tolivia D. Effects of continuous light exposure on antioxidant enzymes, porphyric enzymes and cellular damage in the Harderian gland of the Syrian hamster. J Pineal Res. 2003;34:60–68. doi: 10.1034/j.1600-079x.2003.02951.x. [DOI] [PubMed] [Google Scholar]
- 177.Triqueneaux G. Thenot S. Kakizawa T. Antoch MP. Safi R. Takahashi JS. Delaunay F. Laudet V. The orphan receptor Rev-erbalpha gene is a target of the circadian clock pacemaker. J Mol Endocrinol. 2004;33:585–608. doi: 10.1677/jme.1.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Troncoso J. Navia P. Romani L. Bessieres D. Lafitte T. On the isobaric thermal expansivity of liquids. J Chem Phys. 2011;134:094502. doi: 10.1063/1.3549828. [DOI] [PubMed] [Google Scholar]
- 179.Tunon MJ. Gonzalez P. Lopez P. Salido GM. Madrid JA. Circadian rhythms in glutathione and glutathione-S transferase activity of rat liver. Arch Int Physiol Biochim Biophys. 1992;100:83–87. doi: 10.3109/13813459209035264. [DOI] [PubMed] [Google Scholar]
- 180.Ushio-Fukai M. Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Valko M. Leibfritz D. Moncol J. Cronin MT. Mazur M. Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 182.Van Dyke K. Ghareeb E. Van Dyke M. Sosa A. Hoeldtke RD. Van Thiel DH. Luminescence experiments involved in the mechanism of streptozotocin diabetes and cataract formation. Luminescence. 2008;23:386–391. doi: 10.1002/bio.1050. [DOI] [PubMed] [Google Scholar]
- 183.Van Someren EJ. Riemersma-Van Der Lek RF. Live to the rhythm, slave to the rhythm. Sleep Med Rev. 2007;11:465–484. doi: 10.1016/j.smrv.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 184.Vasieva O. The many faces of glutathione transferase pi. Curr Mol Med. 2011;11:129–139. doi: 10.2174/156652411794859278. [DOI] [PubMed] [Google Scholar]
- 185.Vielhaber E. Eide E. Rivers A. Gao Z-H. Virshup DM. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I varepsilon. Mol Cell Biol. 2000;20:4888–4899. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vielhaber EL. Duricka D. Ullman KS. Virshup DM. Nuclear export of mammalian PERIOD proteins. J Biol Chem. 2001;276:45921–45927. doi: 10.1074/jbc.M107726200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Vurusaner B. Poli G. Basaga H. Tumor suppressor genes and ROS: complex networks of interactions. Free Radic Biol Med. 2012;52:7–18. doi: 10.1016/j.freeradbiomed.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 188.Wang Y. Hua L. Lu C. Chen Z. Expression of circadian clock gene human Period2 (hPer2) in human colorectal carcinoma. World J Surg Oncol. 2011;9:166. doi: 10.1186/1477-7819-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weinstein GD. McCullough JL. Ross P. Cell proliferation in normal epidermis. J Invest Dermatol. 1984;82:623–628. doi: 10.1111/1523-1747.ep12261462. [DOI] [PubMed] [Google Scholar]
- 190.Welsh DK. Yoo S-H. Liu AC. Takahashi JS. Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.White WB. Relevance of blood pressure variation in the circadian onset of cardiovascular events. J Hypertens Suppl. 2003;21:S9–S15. doi: 10.1097/00004872-200307006-00003. [DOI] [PubMed] [Google Scholar]
- 192.White WB. Mansoor GA. Pickering TG. Vidt DG. Hutchinson HG. Johnson RB. Noveck R. Differential effects of morning and evening dosing of nisoldipine ER on circadian blood pressure and heart rate. Am J Hypertens. 1999;12:806–814. doi: 10.1016/s0895-7061(99)00044-8. [DOI] [PubMed] [Google Scholar]
- 193.Winter SL. Bosnoyan-Collins L. Pinnaduwage D. Andrulis IL. Expression of the circadian clock genes Per1 and Per2 in sporadic and familial breast tumors. Neoplasia. 2007;9:797–800. doi: 10.1593/neo.07595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xia HC. Niu ZF. Ma H. Cao SZ. Hao SC. Liu ZT. Wang F. Deregulated expression of the Per1 and Per2 in human gliomas. Can J Neurol Sci. 2010;37:365–370. doi: 10.1017/s031716710001026x. [DOI] [PubMed] [Google Scholar]
- 195.Xu YQ. Zhang D. Jin T. Cai DJ. Wu Q. Lu Y. Liu J. Klaassen CD. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One. 2012;7:e44237. doi: 10.1371/journal.pone.0044237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yamamoto T. Sadoshima J. Protection of the heart against ischemia/reperfusion by silent information regulator 1. Trends Cardiovasc Med. 2011;21:27–32. doi: 10.1016/j.tcm.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yang X. Wood PA. Ansell CM. Quiton DFT. Oh E-Y. Du-Quiton J. Hrushesky WJM. The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol Int. 2009;26:1323–1339. doi: 10.3109/07420520903431301. [DOI] [PubMed] [Google Scholar]
- 198.Yeh KT. Yang MY. Liu TC. Chen JC. Chan WL. Lin SF. Chang JG. Abnormal expression of period 1 (PER1) in endometrial carcinoma. J Pathol. 2005;206:111–120. doi: 10.1002/path.1756. [DOI] [PubMed] [Google Scholar]
- 199.Yoo DG. Song YJ. Cho EJ. Lee SK. Park JB. Yu JH. Lim SP. Kim JM. Jeon BH. Alteration of APE1/ref-1 expression in non-small cell lung cancer: the implications of impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer. 2008;60:277–284. doi: 10.1016/j.lungcan.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 200.Yoo S-H. Yamazaki S. Lowrey PL. Shimomura K. Ko CH. Buhr ED. Siepka SM. Hong H-K. Oh WJ. Yoo OJ. Menaker M. Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yu EA. Weaver DR. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging (Albany, NY) 2011;3:479–493. doi: 10.18632/aging.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zengin E. Atukeren P. Kokoglu E. Gumustas MK. Zengin U. Alterations in lipid peroxidation and antioxidant status in different types of intracranial tumors within their relative peritumoral tissues. Clin Neurol Neurosurg. 2009;111:345–351. doi: 10.1016/j.clineuro.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 203.Zhao G. Cui J. Zhang JG. Qin Q. Chen Q. Yin T. Deng SC. Liu Y. Liu L. Wang B. Tian K. Wang GB. Wang CY. SIRT1 RNAi knockdown induces apoptosis and senescence, inhibits invasion and enhances chemosensitivity in pancreatic cancer cells. Gene Ther. 2011;18:920–928. doi: 10.1038/gt.2011.81. [DOI] [PubMed] [Google Scholar]
- 204.Zhu H. Santo A. Li Y. The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood) 2012;237:143–149. doi: 10.1258/ebm.2011.011152. [DOI] [PubMed] [Google Scholar]
- 205.Zylka MJ. Shearman LP. Weaver DR. Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]