Abstract
Aims: Nuclear factor-erythroid-related factor 2 (Nrf2) is a critical transcriptional factor that is used in regulating cellular defense against oxidative stress. This study is aimed at investigating new interacting protein partners of Nrf2 using One-strep tag pull-down coupled with LTQ Orbitrap LC/MS/MS, and at examining the impact on Nr2 signaling by the newly identified IQ motif containing GTPase activating protein 1 (IQGAP1). Results: Using the One-strep tag pull-down and LTQ Orbitrap LC/MS/MS, we identified IQGAP1 as a new Nrf2 interacting partner. Direct interactions between IQGAP1 and Nrf2 proteins were verified using in vitro glutathione S-transferase (GST) pull-down, transcription/translation assays, and in vivo utilizing Nrf2 overexpressing cells. Coexpression of Dsredmono-IQGAP1 and eGFP-Nrf2 increased the stability of eGFP-Nrf2 and enhanced the expression of Nrf2-target gene heme oxygenase-1 (HO-1). To confirm the functional role of IQGAP1 on Nrf2, knock-downed IQGAP1 using siIQGAP1 attenuated the expression of endogenous Nrf2, HO-1 proteins, and Nrf2-target genes GSTpi, GCLC, and NAD(P)H: quinone oxidoreductase 1 (NQO-1). Furthermore, the stability of Nrf2 was dramatically decreased in IQGAP1-deficient mouse embryonic fibroblast (MEF) cells. Since IQGAP1 signaling could be mediated by calcium, treating the cells with calcium showed the translocation of IQGAP1/Nrf2 complex into the nucleus, suggesting that IQGAP1 may play a critical role in Nrf2 stability. Interestingly, consistent with calcium signaling for IQGAP1, treating the cells with calcium functionally enhanced Nrf2-mediated antioxidant responsive element-transcription activity and enhanced the expression of the endogenous Nrf2-target gene HO-1. Innovation: In the aggregate, our current study identifies and functionally characterizes a new Nrf2 partner protein IQGAP1, which may contribute to Nrf2's regulation of antioxidant enzymes such as HO-1. Conclusion: IQGAP1 may play a critical role in the stability and transactivation of Nrf2. Antioxid. Redox Signal. 19, 89–101.
Introduction
De-regulation of redox status can cause irreversible cellular and tissue damage and result in many diseases, including cancer. In response to oxidative and electrophilic stresses, mammalian cells have evolved sophisticated cellular protective systems, one of which includes the induction of phase II detoxifying and the antioxidative stress pathway (55). Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcription factor that is involved in this defense system, and it has been shown to possess a protective role against cancer, neurodegenerative diseases, aging, diabetes, photo-oxidative stress, cardiovascular disease, and other diseases (53). When cells are exposed to oxidative stress, the cellular defense mechanism will be triggered via the activation of Nrf2, resulting in the coordinated induction of a large number of target genes, including phase II detoxifying enzymes, such as glutathione S-transferase (GST), NAD(P)H: quinone oxidoreductase 1(NQO-1), UDP-glucuronosyl transferase (6, 40, 58), antioxidant enzymes, such as γ-glutamylcysteine synthetase (γGCS) (39) and heme oxygenase-1 (HO-1) (1), and the phase III multidrug ABC transporters, such as multidrug-resistance-associated protein 1 (MRP1) and MRP2 (18, 54). In this context, many thiol-reactive dietary or natural phytochemicals on entering the cells will also stimulate an adaptive response through the activation of the Nrf2 pathway, resulting in the coordinated modulation of Nrf2-target genes (19, 22).
Innovation.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcriptional factor in the regulation of antioxidant responsive element-mediated gene expression in response to oxidative and electrophilic stresses. The kelch-like ECHassociated protein 1 (Keap1)-Nrf2 signaling model is evolving. Keap1 appears to involve dual functions in Nrf2 inhibition and Nrf2 degradation, and the overexpression of Keap1 shows mainly Nrf2 degradation in the cell culture system. In this study, we identified and verified IQ motif containing GTPase activating protein 1(IQGAP1) as a new Nrf2 partner that binds to Nrf2, increases the stability of Nrf2, accentuates the translocation of Nrf2 into the nucleus, and enhances the transcription activation of Nrf2 and the expression of Nrf2-target genes.
Nrf2 belongs to the Cap “n” Collar family members, which possess a highly conserved basic leucine zipper (bZIP) region in their C-terminal region (32). It activates down-stream target genes through the binding to a cis-acting element called the antioxidant responsive element/electrophile response element [ARE/EpRE, 5′-(A/G)TGACNNNGC(A/G)-3] at the 5′-flanking promoter region of many phase II detoxifying enzymes, phase III transporters, and antioxidant enzymes (34, 38, 47). The biological functions of Nrf2 have been elucidated in detail using Nrf2 knock-out mouse models. These mice, in general, are more susceptible to toxic chemicals and carcinogens such as hydrogen peroxide-induced tissue injury and carcinogenic insults (7, 15, 36).
The current accepted dogma of Nrf2 signaling implies that Nrf2 is activated by the dissociation of Nrf2 from the kelch-like ECH-associated protein 1 (Keap1), an inhibitory protein of Nrf2, on exposure to activators of Nrf2. Once disrupted from Keap1 protein in the cytoplasm, Nrf2 is translocated into the nucleus; heterodimerizes with small Maf basic leucine zipper (bZIP) transcription regulators, such as MafF, MafK, and MafG; binds to the ARE sequences in the target genes (3); and transcriptionally activates those target genes. Nrf2's transactivation activity can be enhanced by some kinases, such as mitogen-activated protein kinases (MAPKs) (44), RAC3 (30) protein kinase C (PKC) (23), phosphoinositide-3-kinase (PI3K) (43), and PRKR-like endoplasmic reticulum kinase (PERK) (13). Multiple serine/threonine residues of Nrf2 have been identified as targets of MAPK-mediated phosphorylation, although these modifications may cause some slight reduction of Nrf2 in its nuclear accumulation (50). The other kinases identified that are capable of modifying and regulating the level of Nrf2 in the nucleus include GSK3β (12, 45, 46, 49) and Fyn (24, 28). However, the exact biological consequences of the phosphorylation of Nrf2 by these kinases remain to be elucidated, particularly in different healthy tissues and also in different disease states.
Nrf2 protein activity can be modulated by other proteins in addition to the kinases. Nuclear coactivator CREB binding protein (CBP) has been implicated in Nrf2 signaling (29). In addition, p21 has been shown to interact with Nrf2 and compete with Keap1 for Nrf2 binding (8). Parkinson protein 7 (DJ-1/PARK7) protects neurons against oxidative stress and cell death, and it has been shown that DJ-1 is required for Nrf2 stabilization, although the physical interaction between DJ-1 and Nrf2 or Keap1 has not been detected (11). Most recently, Keap1, a critical partner of Nrf2, has been shown to interact with proteins such as sequestosome 1 (p62/SQSTM) (33, 35) and karyopherin α6 (KPNA6) (51). The in vivo biological consequences of these interacting proteins in animals or humans remain to be studied.
As just discussed, many proteins have been implicated in Nrf2 signaling; however, the precise and the exact molecular mechanisms of Nrf2 signaling remain to be further elucidated, particularly under different cellular perturbations such as intracellular calcium flux, as we have shown previously that calcium would impact Nrf2 signaling (9, 16, 42). Hence, in this study, we embarked on a search for additional proteins as potential Nrf2 interacting partners in order to have a better and more comprehensive understanding of the molecular signaling mechanisms of Nrf2 and Nrf2's regulation of downstream target genes such as antioxidant enzyme HO-1. In this context, in the current study, we have identified and functionally characterized a new Nrf2 partner protein IQ motif containing GTPase activating protein 1 (IQGAP1), which may contribute to Nrf2 signaling and the regulation of Nrf2 downstream target genes such as antioxidant HO-1.
Results
IQGAP1 was identified as a new Nrf2-interacting protein
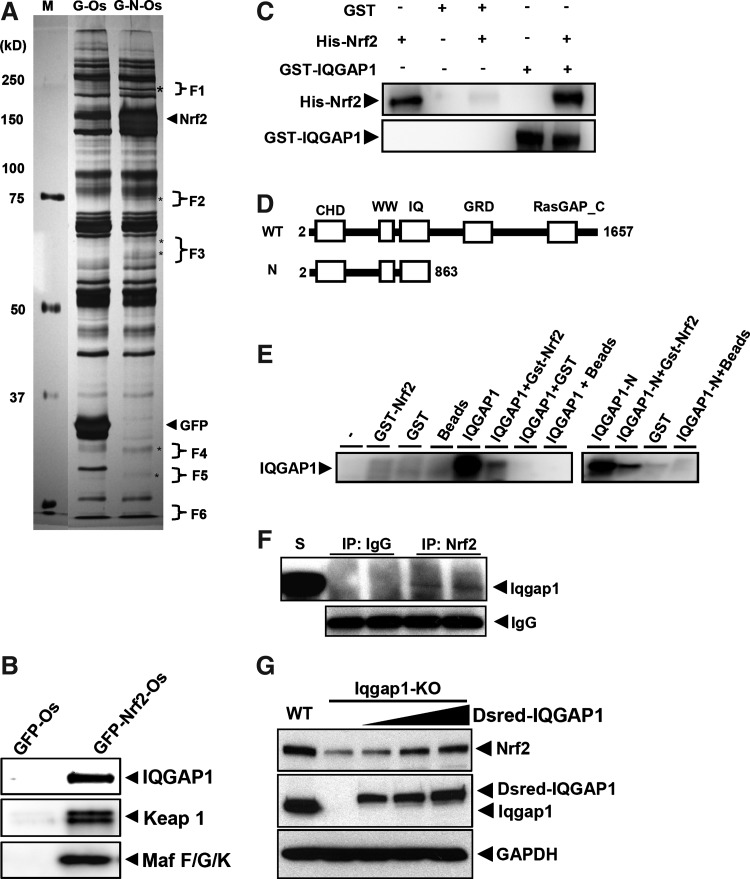
One-STrEP™ purification system was utilized in this study. The key principle of this technology is that One-STrEP tag of eGFP-Nrf2 protein can bind tightly to Strep-Tactin bead, and, subsequently, the eGFP-Nrf2-One-Strep complex can then be eluted with desthiobiotin in a sequential manner. The eluted protein complexes can be separated on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel, and the gel will be stained with silver (Fig. 1A). One-STrEP tag with eGFP marker (eGFP-Os) alone was performed in parallel as a control. Representative bands found in the pull down of eGFP-Nrf2-Os but not eGFP-Os were excised as the potential Nrf2-interacting partner proteins. The identity of these peptides was analyzed by mass spectrometry using LTQ Orbitrap LC/MS/MS equipment as described in Material and Methods. As shown in Figure 1A, six representative bands (F1 to F6) from the eGFP-Nrf2-Os silver-stained gel were excised and subsequently subjected to mass spectrometry analysis. To eliminate nonspecific binding proteins, the bands in the respective positions in the eGFP-O silver stained gel as the F1 to F6 bands in the eGFP-Nrf2-O gel were cut out for analysis as well. As a result, several major Nrf2 binding proteins were identified (five of these are provided in Table 1). Four known Nrf2-interacting proteins (Keap, MafG, MafF, and MafK) were identified as expected. Among the other newly identified Nrf2-interacting proteins, IQGAP1 showed the strongest signal and the best score from mass spectrometry analysis. To check and verify the identified proteins, elution samples were subjected to Western blot analysis using anti-IQGAP1, anti-Keap1, and anti-Maf F/G/K antibodies. The results showed that all proteins were clearly detected as eGFP-Nrf2-One-strep partners (Fig. 1B). Based on these results, we further investigated the interaction and functional significance of IQGAP1 in Nrf2 signaling.
FIG. 1.
Purification and identification of novel Nrf2 partners. (A) Eluted samples from the One-strep pull-down assay were concentrated using Microcon YM-50 (Millipore) and subjected to polyacrylamide gel electrophoresis gel (6%–15% gradient) electrophoresis for silver staining. The representative protein bands in the lane of eGFP-Nrf2-Os (G-N-Os) were excised for LC/MS/MS analysis. To minimize the nonspecific background between eGFP-One-strep (G-Os; control) and eGFP-Nrf2-One-strep (G-N-Os), the same region of G-Os was also cut as a counterpart of G-N-Os. All excised samples were measured by LTQ_Orbitrap LC/MS/MS equipment (http://cabm-ms.cabm.rutgers.edu), and all mass data were analyzed using The Global Proteome Machine Organization Proteomics Database (Open Source Software, www.thegpm.org). (B) Based on the mass identification, same samples from (A) were subjected to Western blot analysis to check and verify the identified proteins. Newly identified IQGAP1 was blotted using anti-IQGAP1antibody, and four known Nrf2 partners, Keap1 and Maf F/G/K, were also blotted using anti-Keap1and anti-Maf F/G/K antibodies, respectively. (C) To verify the binding between IQGAP1 and Nrf2, GST-IQGAP1 protein was incubated with purified His-Nrf2 protein in vitro and followed by GST pull-down assay. Protein-bead complexes were then subjected to Western blot analysis against His-Nrf2 using anti-Nrf2 (C-20). (D) Schematic diagram shows the structure of different segments of IQGAP1 utilized for in vitro transcription/translation-binding assay. (E) IQGAP proteins (full-length or N-terminal fragment) were synthesized by in vitro transcription/translation, as described in Materials and Methods, and were incubated with GST-Nrf2. The protein complex was pulled down by glutathione-sepharose beads and subjected to Western blot analysis using an antibody against IQGAP1. (F) Whole-protein extract from MEF cells were subjected to co-IP to examine the binding affinity between the endogenous mouse Iqgap1 and Nrf2. (G) Western blot analysis showed that suppressed mouse Nrf2 in the Iqgap1-deficient MEF cells was rescued by transfection of the different amounts (2, 3, 5 μg) of Dsred-IQGAP1. CHD, calponin homology domain; WW, WW domain; IQ, IQ motif; GRD, GAP-related domain; RasGAP_C, RasGAP binding C-terminal. S, standard mouse Iqgap1 protein; WT, wild type; KO, knock-out; Dsred-IQGAP1, Dsredmono-IQGAP1; GST, glutathione S-transferase; IQGAP1, IQ motif containing GTPase activating protein 1; Keap1, kelch-like ECH-associated protein 1; MEF, mouse embryonic fibroblast; co-IP, coimmunoprecipitation;.
Table 1.
Identification of Nrf2 Partners from LC/MS/MS Silver-Stained Gel Were Excised and Subjected to LTQ_Orbitrap LC/MS/MS for Protein Identification
| F | Identifier | log(I) | rl | log(e) | Mr (kDa) | Description | Source |
|---|---|---|---|---|---|---|---|
| 1 | ENSP00000268182 | 6.13 | 59 | −424.6 | 189.1 | Ras GTPase-activating-like protein IQGAP1 (p195) | Uniprot/SWISSPROT P46940 |
| 3 | ENSP00000171111 | 5.27 | 11 | −74.5 | 69.6 | Kelch-like ECH-associated protein 1 (Cytosolic inhibitor of Nrf2) | Uniprot/SWISSPROT Q14145 |
| 6 | ENSP00000350369 | 6.05 | 13 | −69.5 | 17.8 | Transcription factor MafG | Uniprot/SWISSPROT O15525 |
| 6 | ENSP00000345393 | 5.62 | 8 | −41.0 | 17.7 | Transcription factor MafF | Uniprot/SWISSPROT Q9ULX9 |
| 6 | ENSP00000344903 | 6.25 | 17 | −80.1 | 17.5 | Transcription factor MafK | Uniprot/SWISSPROT O60675 |
IQGAP1 protein and four other known Nrf2 partner proteins were identified. Newly identified proteins are indicated with bold letters.
F, excised gel fraction number; log(I), base-10 log of the sum of the Intensities of the fragment ion spectra; log(e), based-10 of the expectation that this assignment in stochastic; rl, number of peptides found from this sequence only; Mr, protein molecular mass; Nrf2, nuclear factor erythroid 2-related factor 2.
To examine whether IQGAP1 interacts with Nrf2 directly, we performed an in vitro protein binding assay. GST-tagged, full-length IQGAP1 protein was incubated with purified His-tagged, full-length Nrf2 protein. The complex was isolated with glutathione-sepharose beads and analyzed by Western blotting using the antibodies against Nrf2 or IQGAP1. As shown in Figure 1C, Nrf2 binding to GST-IQGAP1 was specific, as no Nrf2 protein was pulled down by GST beads alone. We confirmed the direct interaction using an in vitro transcription/translation assay (Fig. 1E). Since the calmodulin-binding domain is located in the N-terminal of IQGAP1 (17, 20), we next examined whether the N-terminal domain of IQGAP1 can interact with Nrf2 directly. The N-terminal segment (amino acid residues 2-863) of IQGAP1 (N-IQGAP1) was synthesized by in vitro transcription/translation and incubated with GST-Nrf2 protein, and the complex was isolated by glutathione-sepharose beads and analyzed by Western blotting. As shown in Figure 1D and 1E, specific binding of N-IQGAP1 to Nrf2 was confirmed, as no N-IQGAP1 was pulled down when incubated with GST beads alone. This suggests that the IQGAP1's function in the Nrf2 signaling may be through this N-terminal domain.
In addition, we performed coimmunoprecipitation (co-IP) using an antibody against Nrf2 to detect Iqgap1 in mouse embryonic fibroblast (MEF) cells. The results showed that the mouse Nrf2 was bound to Iqgap1 in Iqgap1+/+ MEF (Fig.1F). Furthermore, Nrf2 protein level was significantly decreased in Iqgap1-deficient MEF cells and rescued by Dsredmono-IQGAP1 transfection (Fig.1G). This might be the result from the instability of Nrf2, because its mRNA was not altered in the qPCR analysis (data not shown). This suggests that Iqgap1 may play a critical role in the stability of Nrf2.
IQGAP1 enhances Nrf2 stability and HO-1 expression
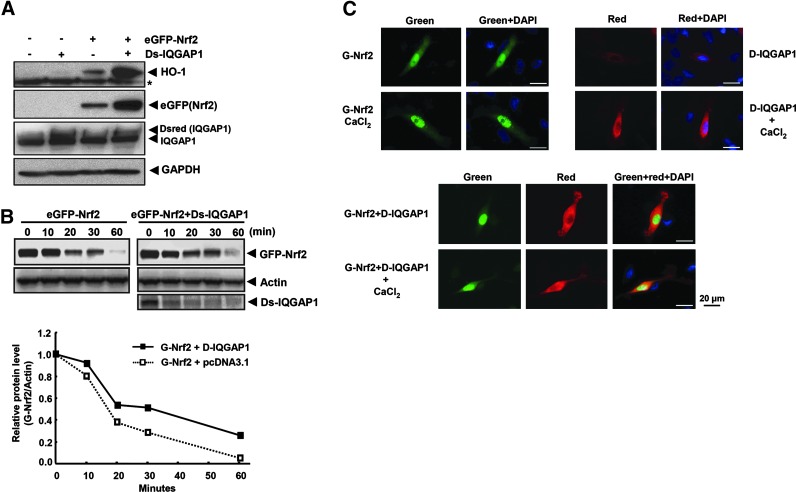
To investigate the role of IQGAP1 in Nrf2 signaling, HeLa cells were transfected with eGFP-Nrf2 and Dsredmono-IQGAP1 constructs for 24 h. Immuoblotting showed that Dsredmono-IQGAP1 stabilized the eGFP-Nrf2, resulting in a strong induction of Nrf2 downstream target gene HO-1 (Fig. 2A). In a separate experiment, likewise, eGFP-Nrf2 was also stabilized by Dsredmono-IQGAP1 (Fig. 2B). To further support the interaction between Nrf2 and IQGAP1, HeLa cells were transfected with eGFP-Nrf2 and/or Dsredmono-IQGAP1 for 24 h on cover-glass-bottomed dishes. The expressed proteins in the cells were then analyzed for their subcellular localization and the intensity of protein expressed using fluorescent microscopy. Epifluorescence results showed that the expression of eGFP-Nrf2 was strongly induced in the nucleus with cotransfection of eGFP-Nrf2 and Dsredmono-IQGAP1 constructs, as compared with the transfection of eGFP-Nrf2 alone. Furthermore, the localization of IQGAP1 protein from the cytosol to the nucleus was enhanced after calcium treatment for 1 h, especially in the eGFP-Nrf2/Dsredmono-IQGAP1 cotransfected cells (Fig. 2C).
FIG. 2.
IQGAP1 enhances the expression of Nrf2 and induction of HO-1 expression as well as the stability of Nrf2. (A) Based on mass data shown in Table 1, the newly identified IQGAP1 was then tested to see whether it could regulate the expression of HO-1, which is also regulated by Nrf2. HeLa cells were cotransfected with pDsredmono-IQGAP1 and pEGFP-Nrf2 plasmids for 24 h using jetPEI reagent. The cells were then lysed with RIPA lysis buffer, and 20 μg of proteins were subjected to Western blot analysis against HO-1, eGFP-Nrf2, and Dsred-IQGAP1 using anti-HO-1, anti-GFP, and anti-IQGAP1 antibodies, respectively. GAPDH was used for the equal loading control. pCDNA3.1 vector was used for the equal amount of transfection. GFP-Nrf2, pEGFP-Nrf2; Dsred-IQGAP1, pDsredmono-IQGAP1. (B) To measure the stability of Nrf2 by IQGAP1, HeLa cells were transfected with pEGFP-Nrf2 (500 ng) and either empty vector (pcDNA3.1) or pDsredmono-IQGAP1 (500 ng) constructs using jetPEI reagent for 24 h. The cells were then treated with cycloheximide (5 μg/ml) for different time intervals as just indicated. Cell lysates (20 μg) were subsequently subjected to immunoblotting against eGFP-Nrf2 or Dsred-IQGAP1 using anti-Nrf2 or anti-Dsred antibodies, respectively. Actin was used for the equal loading control. GFP-Nrf2, pEGFP-Nrf2; Dsred-IQGAP1, pDsredmono-IQGAP1. (C) HeLa cells were plated in glass-bottom culture dishes and transfected with pEGFP-Nrf2 and pDsredmono-IQGAP1 using jetPEI transfection reagent. After 24 h of transfection, the cells were fixed with 4% paraformaldehyde, and the images were taken by fluorescent microscopy. Green, Red, and DAPI filters were used for GFP, Dsred, and nucleus, respectively. GFP-Nrf2, pEGFP-Nrf2; Dsred-IQGAP1, pDsredmono-IQGAP1. magnification (34×). HO-1, heme oxygenase-1.
Silencing of IQGAP1 increases the instability of Nrf2 and results in the inhibition of Nrf2-mediated target genes
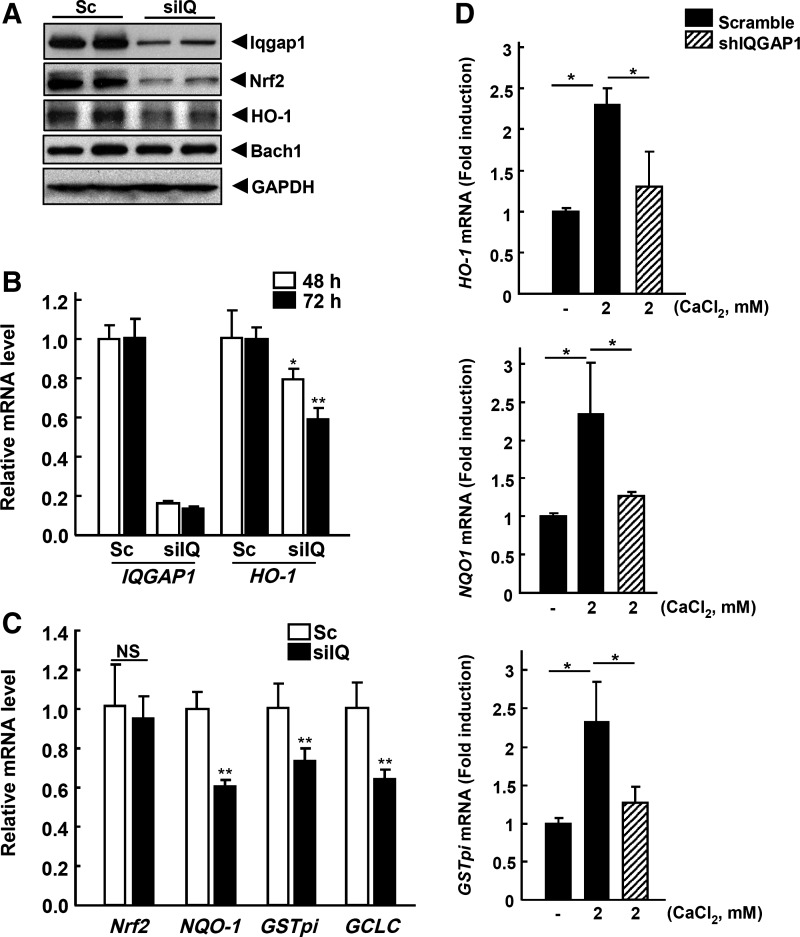
To confirm the function of IQGAP1 in Nrf2 signaling, HeLa cells were first transfected with siIQGAP (Santa Cruz Biotechnology) or shIQGAP1 (pGFP-V-RS-IQGAP1-77; Origene) for different time intervals. The results showed that siIQGAP1, treated for 72 h, knocked down the endogenous IQGAP1 and resulted in the inhibition of endogenous protein levels of both Nrf2 and HO-1. However, Bach1 protein (14), a negative regulator of Nrf2, was not changed (Fig. 3A). Likewise, IQGAP1 and HO-1 mRNAs were measured after treatment of the cells with siIQGAP1 for 48 and 72 h. siIQGAP1 decreased the mRNA expression of IQGAP1 and HO-1 in a time-dependent manner (Fig. 3B). In addition, Nrf2 and Nrf2-target genes NQO-1, GSTpi and GCLC mRNAs were also measured after siIQGAP1 treatment for 72 h. The results showed that Nrf2 mRNA expression level was not changed; however, Nrf2 target genes NQO-1, GSTpi, and GCLC were decreased by siIQGAP1 (Fig. 3C). These results suggested that IQGAP1 could play an important role in the stability of Nrf2 protein and that interaction between Nrf2 and IQGAP1 may be important for Nrf2's regulation of its downstream target genes. To examine the effect of calcium on the regulation of Nrf2 and Nrf2 target genes, such as HO-1, NQO-1, and GSTpi, mRNA were measured by real-time PCR after the treatment of HeLa cells with calcium. The results showed that the mRNA expression of HO-1, NQO-1, and GSTpi was increased by calcium and attenuated by shIQGAP1-77 (Fig. 3D).
FIG. 3.
siIQGAP1 decreases the stability of Nrf2 and attenuates HO-1 expression and ARE-luciferase activity. (A) HeLa cells were transfected with siIQGAP1 for 72 h using Lipofectamin 2000 reagent to silence or knockdown the endogenous IQGAP1 gene expression. The expression levels of endogenous IQGAP1, Nrf2, HO-1 and Bach1 were measured by Western blot analysis. (B) IQGAP1 and HO-1 mRNAs were measured by qPCR after transfection with siIQGAP1 for different time periods (48 and 72 h). (C) Nrf2, NQO-1, GSTpi, and GCLC mRNAs were also measured from the siIQGAP1-72 h-transfected samples. (D) HeLa cells were transfected with pGFP-V-RS-Sc plasmid or pGFP-V-RS-IQGAP1-77 for 3 days and treated with 2 mM CaCl2 for 6 h. Isolated mRNA was subjected to real-time qPCR for HO-1, NQO-1, and GSTpi. Beta-Actin was used for the normalization. *p<0.05 **p<0.01. Sc, scramble; siIQ, siIQGAP1; ARE, antioxidant responsive element; NQO-1, NAD(P)H: quinone oxidoreductase 1; NS, not significant.
Ca2+ activates Nrf2/ARE-luciferase activity and induces HO-1 expression
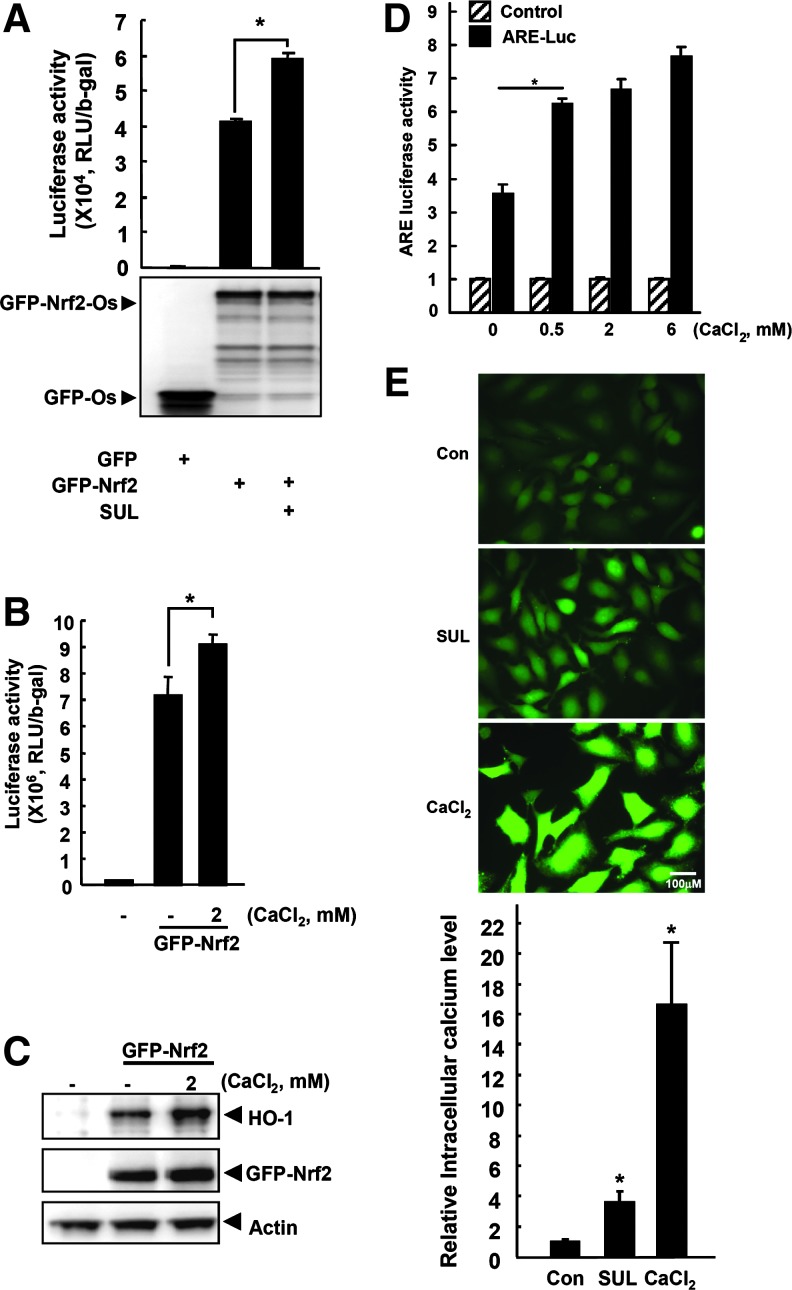
Before the initiation of our current study, the activity of eGFP-Nrf2-One-Strep was verified using reporter assay by treatment with sulforaphane (SUL), a naturally occurring Nrf2-ARE activator (Fig. 4A). Since IQGAP1 has been reported to be involved in calcium/calmodulin signaling, we examined whether Ca2+ could activate Nrf2/ARE-luciferase activity and induce endogenous HO-1 protein. The results showed that Nrf2/ARE-luciferase activity (Fig. 4B) and HO-1 protein (Fig. 4C) were significantly induced after the addition of CaCl2 to eGFP-Nrf2 transfected cells. Furthermore, the ARE activity was also activated by CaCl2 in a concentration-dependent manner (Fig. 4D). To provide further evidence between Nrf2 activation and the increased intracellular calcium level, HeLa cells were treated with SUL and CaCl2, and the level of intracellular calcium level was measured. As shown in Figure 4E, intracellular calcium levels were enhanced 16-fold by CaCl2 (2 mM), while SUL (20 μM) increased by 4-fold.
FIG. 4.
Calcium induces the activity of ARE-luciferase and the expression of Nrf2 target proteins. (A) To verify the Nrf2-One-strep tag construct for this system, HeLa cells were transfected with pEGFP-Nrf2-Os and pTI-ARE-Luc plasmids for 24 h, and the Nrf2 transactivation activity was examined by reporter assay after 6 h of treatment with 25 μM SUL, as an Nrf2 inducer. pEGFP-Os was also transfected as a control. (B) To check the involvement of the calcium ion in Nrf2 activity or Nrf2 partner complex, after cotransfection with pEGFP-Nrf2 and pTI-ARE-Luc constructs, HeLa cells were treated for 6 h with various concentrations of CaCl2. ARE-luciferase activity was then measured by reporter assay. (C) The level of endogenous HO-1 was also measured by Western blotting using the same samples from (B). (D) HeLa cells were transfected with pGL3-luc or pTI-ARE constructs for 24 h and treated with different concentrations of CaCl2 for 3 h, and ARE luciferase activity was measured. (E) To measure the changes in the intracellular calcium level, HeLa cells were treated with SUL, an ARE activator and CaCl2. The cells were seeded in 12-well plates and cultured overnight. Intracellular calcium level was measured using FluoForte® Calcium Assay Kit (ENZO Life Sciences) according to the manufacturer's instructions. Briefly, the cultured medium was removed, and the cells were incubated with 1 ml of FluoForte™ Dye-Loading Solution for 40 min at room temperature. Then, 20 μM SUL or 2 mM CaCl2 was added to the incubation solution. After 1 min of incubation, the cells were immediately imaged with a fluorescence microscope (Carl Weiss, Axiovert S100) using the FITC channel (left panel). Right panel shows a densitometry analysis of the pictures, which represent the relative intracellular calcium levels. Con, Control (0.1% DMSO); SUL, sulforaphane; Magnitude,×32. *p<0.001 (vs. control).
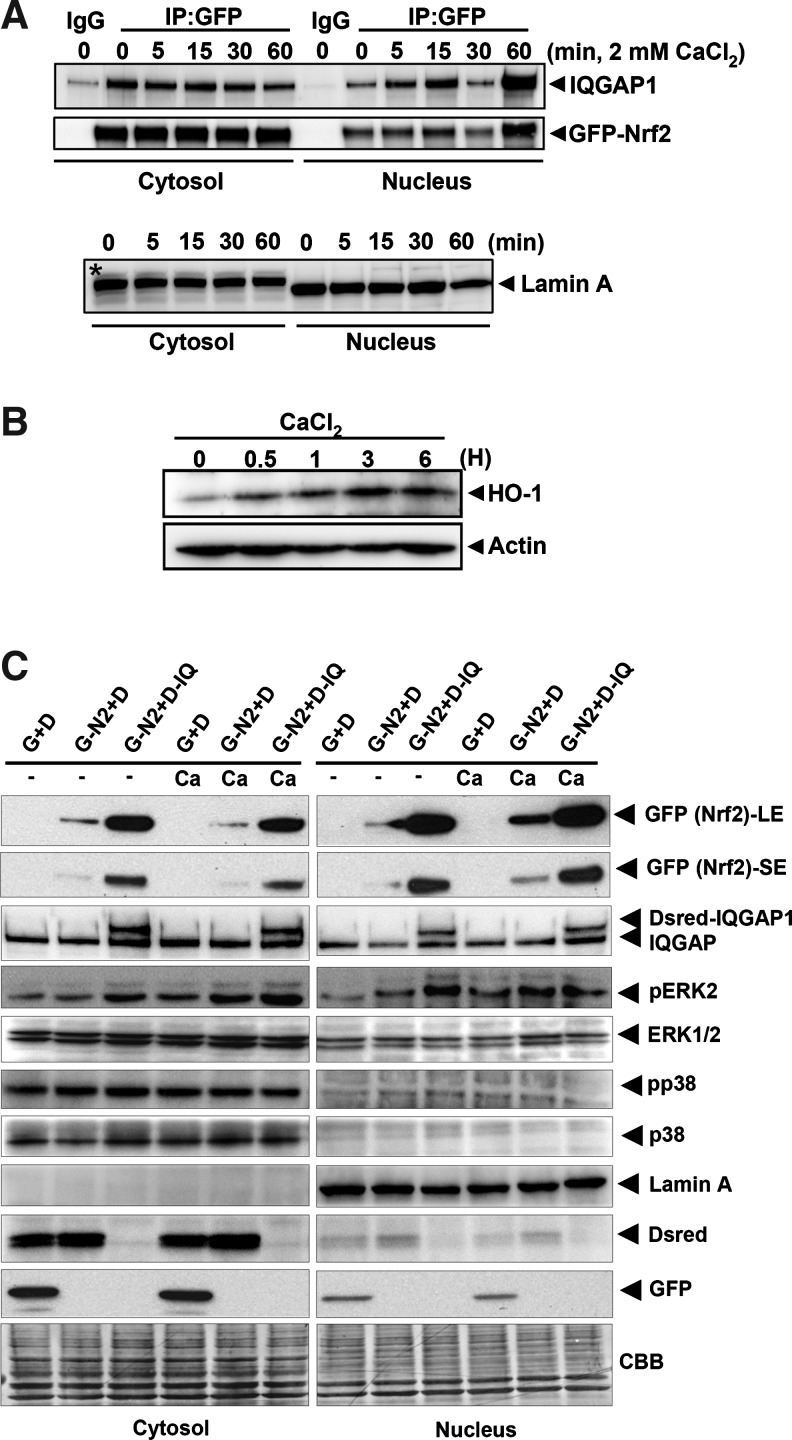
Nrf2-IQGAP1 complex is translocated into the nucleus by calcium treatment, resulting in the induction of HO-1 protein
As just discussed, the direct interaction between IQGAP1 and Nrf2 was verified using GST pull-down assay. To further investigate the functional importance of partnership between IQGAP1 and Nrf2, HeLa cells were transfected with pEGFP-Nrf2 and treated with CaCl2 for different time periods. Cytosolic and nuclear extracts were subjected to IP assay against EGFP using anti-GFP antibody and immunoblotted against IQGAP1. The results showed that the translocation of cytosolic IQGAP1 into the nucleus began at 5 min, peaked at 60 min after CaCl2 and eGFP-Nrf2 protein also showed a similar pattern of expression as IQGAP1 (Fig. 5A); Lamin A was used as a positive control for nuclear protein loading. In addition, HeLa cells were treated with 2 mM CaCl2 from 0 to 6 h, and the cytosolic extracts were then subjected to Western blotting using an antibody against anti-HO-1 (C-20) (Fig. 5B). The results showed that HO-1 protein was induced by single treatment with CaCl2 (Fig. 5B). The induction of HO-1 protein was seen at 30 min and peaked at 3 h to 6 h. These results suggest that calcium triggered the translocation of the Nrf2/IQGAP complex into the nucleus very quickly (<30 min), resulting in the Nrf2-mediated induction of HO-1.
FIG. 5.
Translocation of Nrf2-IQGAP1 complex into the nucleus by calcium treatment and induction of HO-1 protein. (A) To verify the involvement of calcium in endogenous Nrf2-IQGAP1 complexes, HeLa cells were transfected with pEGFP-Nrf2 (8 μg/100 mm dish) for 24 h and treated with CaCl2 (2 mM) for different times. Then, cytosolic and nuclear proteins were isolated using the M-PER kit (Thermo Scientific). Equal volumes of cytosolic and nuclear fractions were subjected to IP with the GFP antibody using Dynabead G (Invitrogen) beads. The procedures of this experiment are described in Materials and Methods. The IP samples were then subjected to Western blotting against endogenous IQGAP1 and eGFP-Nrf2 using anti-IQGAP1 or GFP antibody. Lamin A was used for nuclear fraction marker protein. *, represents nonspecific protein. (B) To examine the time course of induction of HO-1 by CaCl2, HeLa cells were treated with CaCl2 for different times. Then, cell lysates were subjected to Western blotting against HO-1 using anti-HO-1 (C-20) antibody. Actin was used for the control of the equal loading of protein. (C) To test whether calcium treatment facilitates the Nrf2 translocation into the nucleus, HeLa cells were transfected with pEGFP-Nrf2 (4 μg) or/and pDsredmomo-IQGAP1 (4 μg) constructs on 100 mm dishes for 24 h and treated with 2 mM CaCl2 for 30 min. Cytosolic and nuclear fractions were isolated using the NE-PER kit (Thermo Scientific). Then, fractionated lysates (15 μg) were subjected to Western blot analysis. Lamin A was used as an indicator of a nuclear fraction. Both eGFP and Dsredmono control vectors were used for equal transfection. CBB staining was used as an equal loading control. G, EGFP; D, Dsredmono; G-N2, eGFP-Nrf2; D-IQ, Dsredmono-IQGAP1; CBB, Coomassie brilliant blue staining; LE, longer exposure; SE, shorter exposure; Ca, CaCl2.
To further examine IQGAP1-Nrf2-Calcium signaling pathway, HeLa cells were transfected with eGFP-Nrf2 (G-N2) or/and Dsredmono-IQGAP1 (D-IQ) constructs and treated with 2 mM CaCl2 for 30 min. Figure 5C shows that the cotransfection of eGFP-Nrf2 and Dsredmono-IQGAP1 significantly increased the protein expression of eGFP-Nrf2 in both cytosolic and nuclear extracts as compared with the transfection of eGFP-Nrf2 alone. Furthermore, calcium treatment enhanced the translocation of eGFP-Nrf2 protein into the nucleus by either transfection of eGFP-Nrf2 alone or cotransfection of eGFP-Nrf2 and Dsredmono-IQGAP1 (Fig. 5C). Interestingly, calcium also induced ERK2 phosphorylation, and this was intensified in the presence of Dsredmono-IQGAP1 (Fig. 5C), suggesting that ERK2 activation by calcium may result in enhancing the translocation of Nrf2 into the nucleus and increase Nrf2-mediated gene expression.
Discussion
Nrf2 is a key transcriptional factor in the regulation of ARE-mediated gene expression in response to oxidative and electrophilic stresses. Although several functional proteins, such as Keap1 (31, 41), MAPKs (44), PKC (23), PI3K (43), CBP (29), and small Mafs (3), have been implicated in the Nrf2 transactivation activity, the precise molecular mechanism in controlling Nrf2 signaling under various cellular environments remains unclear and would probably depend on the cellular and tissue context.
The dogma of Nrf2-Keap1 signaling which states that Nrf2 is regulated by Keap1 anchoring to the perinuclear region of cytoskeletal proteins, such as F-actin (27), is evolving. For instance, this hypothesis may have been modified by Velichkova and Hasson (52), where they proposed that Keap1 does not anchor Nrf2 to the cytoskeleton. In response to oxidative stress, Nrf2 may not be released from the Keap1 anchored to actin but instead facilitate Keap1-Nrf2's translocation from the cytoplasm to the nucleus. This notion appears to be supported by Watai et al. (57), suggesting that the complex between Keap1 and actin cytoskeleton or cytoplasmic scaffold may not be that simple and straightforward, as they show no clear-cut colocalization between endogenous Keap1 and actin filaments (F-actin). In this context, the molecular mechanism based on the perinuclear localization of Keap1 may still be evolving.
To investigate the potential new interacting protein partners of Nrf2, the One-strep tag purification system coupled with the LCTQ_Orbitrap LC/MS/MS was utilized. This system is designed to purify intact protein complexes under physiological conditions inside the cells. One-strep tag consists of two strep tag II sequences (SAWSHPQFEK) along the edges, which would facilitate the binding affinity between One-strep tag and streptavidin bead (Strep-Tactin bead).
Before the purification of the Nrf2 complex, we have first performed a preliminary study on the potential role of calcium in Nrf2 activation utilizing Nrf2/ARE-luciferase reporter gene and Western blotting against HO-1 protein, an Nrf2-target gene. From these purification processes, we were able to isolate many possible Nrf2-partner proteins and identified them using LTQ_Orbitrap LC/MS/MS. Table 1 shows some of the representative proteins of the newly identified proteins (some of the newly identified proteins, other than IQGAP1, were not shown, as we are in the process of performing further investigation). In addition, some of the proteins that have low rl (a number of peptides were found from the sequencing) values were also not shown.
The involvement of calcium in Nrf2/ARE signaling was hypothesized and published by our group (9). Tertiary butylhydroquinone-induced Nrf2/ARE-luciferase activity and HO-1 expression were dramatically attenuated by treatment with EGTA, a chelating agent of Ca2+, in both HT-29 cells and HepG2 cells (data not shown). In contrast, Nrf2/ARE-luciferase activity and HO-1 expression were significantly increased by calcium in HeLa cells (Fig. 4B–D). It has been reported that diallyltrisulfide, a garlic organosulfur compound that can activate the Nrf2-ARE pathway, could also increase the intracellular level of calcium in the early hours of treatment (48). It has been reported that an increased level of intracellular calcium is associated with reduced glutathione level (26), and that calcium-dependent calcium/calmodulin-dependent protein kinase is implicated in a stress-mediated cellular defense response (21). Furthermore, microarray data reported by Lee et al. (37) showed that Nrf2+/+ mouse primary neuronal cultures have higher expression levels of genes encoding calcium homeostasis protein, such as visinin-like 1, calbindin-28K, synaptotagmin-1, hippocalcin, and calmodulin III, as compared with cells obtained from Nrf2−/− mice, suggesting that calcium may play an important role in Nrf2 signaling. Based on all these reports, it is possible that increased intracellular level of calcium by Nrf2 activators could play an important role in the overall Nrf2 signaling.
IQGAP1, as a scaffolding protein, can assemble with many proteins, such as calmodulin, actin, Cdc42, Rac1, E-cadherin, β-catenin, and CLIP-170, for coordinating different signaling pathways (5). As such, IQGAP1 complexation with other protein partners can be modulated by calmodulin association with the calcium ion [reviewed in ref. (4)]. Calcium ion, as an intracellular messenger, is ubiquitously distributed in the cells and plays an important role in controlling numerous cellular events (2). The major mediator of Ca2+ signaling is calmodulin, which modulates the function of various downstream targets (10). A rise in intracellular Ca2+ concentration induces conformational changes in calmodulin followed by binding to specific domains on target proteins. Taken together, our present results suggest that the intracellular calcium ion concentration [Ca2+]i may enhance the binding of calcium-binding proteins to IQGAP1, resulting in the translocation of the IQGAP1-Nrf2 complex to the nucleus and subsequently enhancing the expression of Nrf2-target genes (Fig. 5). The question remains as to how the IQGAP1-Nrf2 complex would activate Nrf2 transcription activation, and further studies will be needed.
In summary, we have isolated and identified IQGAP1 as a new Nrf2 signaling-partner protein using One-strep tag purification system. IQGAP1, a scaffold, actin, and calmodulin binding protein, was subsequently studied for physical interactions, signaling, and functions with Nrf2. IQGAP1 directly interacts with Nrf2 and enhances the stability of Nrf2, resulting in increased induction of Nrf2-target genes, including HO-1. In addition, since calcium mediates IQGAP1 signaling, treating the cells with calcium facilitates the translocation of the IQGAP1-Nrf2 complex into the nucleus, resulting in the enhanced induction of Nrf2-target genes such as HO-1 through the Nrf2/ARE pathway. Taken together, our present study suggests that IQGAP1 may play an important role in Nrf2 signaling and the regulation of Nrf2-mediated antioxidant enzymes such as HO-1.
Materials and Methods
Materials
Minimum essential medium (MEM), fetal bovine serum (FBS), penicillin/streptomycin antibiotics mixture, lipofectamin 2000 reagent, and Dynabead G were obtained from Invitrogen. The anti-IQGAP (H-109), anti-GFP (B-2), anti-Dsred (C-20), and anti-Maf F/G/K (H-100), anti-HO-1 (C-20), anti-Bach1 (C-20), anti-actin, anti-Lamin A primary antibodies, and secondary antibodies were purchased from Santa Cruz Biotechnology. The anti-pp38, anti-p38, anti-pERK1/2, and anti-ERK were purchased from Cell Signaling. ECL femto signal substrate and silver SNAP staining kit were obtained from Thermo Scientific. Transfection reagent jetPEI was purchased from Polyplus-Transfection (Bioparc). Strep tactin cartridge was obtained from EMD chemicals. Polypropylene column was purchased from Qiagen. Cover-glass-bottomed culture dishes were purchased from MatTek Corporation. Polyvinylidene difluoride (PVDF) was obtained from Millipore. SUL was purchased from Alexis. Protease inhibitor cocktail was from Roche Molecular Biochemicals. Mammalian expression vectors, pEGFP-C3, and pDsredmono-C1 were obtained from Clontech. All other chemicals used were of an analytical grade or the highest grade available.
Cell culture
HeLa cells from ATCC were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air in MEM supplemented with 10% heat-inactivated FBS and 50 U/ml of penicillin/streptomycin mixture (Gibco BRL). Cells were grown to 60%–80% confluence and trypsinized with 0.05% trypsin containing 2 mM EDTA.
Iqgap1(+/+) and Iqgap1(−/−) MEF cells (25) were maintained at 37°C in a humidified atmosphere of 5% CO2/95% air in DMEM supplemented with 10% heat-inactivated FBS and 50 U/ml of penicillin/streptomycin mixture (Gibco BRL).
Construction of plasmids
To identify and purify the unknown proteins in the Nrf2-partner complexes using Strep-tactin bead, a pEGFP-Nrf2-One-strep plasmid was constructed. Nrf2 gene (NM 006164, 605 aa) was first amplified with specifically designed primers (forward: 5′-AAA CTC GAG ATG ATG GAC TTG GAG CTG CCG CCG CCG GGA C-3′ and reverse: 5′-AAA GGA TCC TTA TTT TTC GAA CTG CGG GTG GCT CCA CGA TCC ACC TCC CGA TCC ACC TCC GGA ACC TCC ACC TTT CTC GAA CTG CGG GTG GCT CCA GTT TTT CTT AAC ATC TGG CTT CTT AC-3′) for tagging One-strep tag (SAWSHPQFEK(GGGS)2GGSAWSHPQFEK) in the C-terminal of Nrf2. Then, the Nrf2-One-strep gene was amplified using the Platinum pfx DNA polymerase kit (Invitrogen) following the manufacturer's instructions. Briefly, cDNA (0.5 μg) was denatured at 95°C for 2 min, cycled 24 times at 94°C for 15 s, 55°C for 15 s, and 68°C per min/per kb. The PCR reaction ended with an additional 10 min incubation at 68°C. The amplified PCR product was cut with BamHI and XhoI restriction enzymes and subcloned into pEGFPC3 vector. For the control, the One-strep tag was also linked to the C-terminal of eGFP gene. Based on the results of LC/MS/MS, IQGAP1 cDNA was subcloned into the pDsredmono-C1 vector (Clontech). The IQGAP1 cDNA was purchased from the I.M.A.G.E clone consortium (Invitrogen) and amplified by the PCR method with specifically designed primers containing appropriate restriction enzyme sites. Amplified IQGAP1 gene was cut by XhoI/ApaI restriction enzymes, and then subcloned into the pDsredmono-C1 vector. All PCR reaction conditions were the same as those in the previous experiment. We used the commercially available siIQGAP1 and shIQGAP plasmids (pGFP-V-RS-shIQGAP1-77) from Santa cruz biotechnology (Santa cruz, CA, USA) and Origene, respectively.
Nrf2 partner pull-down with One-strep tactin system
HeLa cells from 20 plates (15 cm) were scrapped and lysed in 4 ml of lysis buffer (25 mM NaCl,5 0 mM Tris-HCl containing protein inhibitor cocktail [Roche] in 0.2% Triton-X100, and 7.5% glycerol, W/O ETDA, pH 7.4). Cell lysis was performed by four freezing/thawing cycles in liquid nitrogen. After every single thawing cycle, pippetting was performed to disrupt the cells. After lysis, all procedures were followed by One-STrEP purification system (IBA GmbH). Briefly, lysates were then centrifuged at 15,000 rpm for 20 min at 4°C. The supernatant was applied to the One-strep tactin bead column packed with 1 ml bead in polypropylene column with gravity flow rate. Columns were then washed 5 times with 1 ml washing buffer (150 mM NaCl, 50 mM Tris-HCl, 0.2%, Triton X-100, and pH 8.0) and eluted with 500 ml elution buffer (150 mM NaCl, 50 mM Tris-HCl, 2.5 mM Desthiobiotin, and pH 8.0). This was repeated four times. Each small fraction was subjected to gel electrophoresis and silver staining to verify the elution of the Nrf2 complex. After silver staining of the gel, the eluted complexes were concentrated by centrifugation for 20 min at 4°C in Microcon YM50 (Millipore). Concentrated samples were subjected to PAGE gel electrophoresis and silver stained. Candidate bands were excised and analyzed for protein identification using LTQ_Orbitrap LC/MS/MS (Thermo Fisher Scientific) in the Rutgers/UmDNJ proteomic core facility (http://cabm-ms.cabm.rutgers.edu/index.html). Mass data were analyzed using The Global Proteome Machine Organization Proteomics Database (Open Source Software; www.thegpm.org).
Transient transfection and reporter gene activity assays
For the Nrf2 partner pull-down study using One-strep tactin system, HeLa cells (50% confluence) were plated in 20 of 15 cm culture dishes per group, and cells were cultured overnight. The cells were then transfected with either pEGFP-Nrf2-One-strep or pEGFPC3-One-strep plasmid for 24 h. Twelve micrograms of plasmid were mixed with jetPEI reagent (Polyplus-Trasfection™; Illkirch) in a ratio of 1 μg DNA to 4 μl jetPEI. Before harvesting, eGFP expression was checked for transfection efficiency using FITC filtered fluorescence microscopy. The cells were immediately washed once with ice cold phosphate-buffered saline (PBS, pH 7.4, w/o EDTA), scraped, and subjected to the Nrf2 partners pull-down experiment as described next. For the reporter gene activity assay, HeLa cells were plated in six-well plates at ∼4.0×105 cells/well. Sixteen hours after plating, cells were transfected with given plasmids using the jetPEI reagent according to the manufacturer's instructions. To each well, either 500 ng of pEGFP-Nrf2 and/or 200 ng of ARE-Luciferase construct (56) were added into 100 μl of NaCl (150 mM). Then, jetPEI reagent was added into a separate tube of 100 μl of NaCl (150 mM). The two different tubes were vigorously mixed and incubated at room temperature for 25 min. After that, the cells were incubated with transfection complexes for 20–24 h in a 37°C incubator. The cells were then washed once with PBS, scraped, and lysed in 200 μl of reporter lysis buffer (Promega) on ice for 10 min. After centrifugation at 12,000 rpm for 5 min at 4°C, 10 μl of lysate was mixed with luciferase substrate (Promega), and the ARE-luciferase activity was measured using a Sirius luminometer (Berthold Detection System). Luciferase activity was normalized by measuring the conventional β-gal activity by the transfection with pCDNA3.1-lacZ plasmid coding galactosidase.
Expression and purification of recombinant IQGAP1 and Nrf2 proteins
The constructs that encode human proteins GST-IQGAP1 (in pGEX-4T), GST-Nrf2 (in pGEX-4T), and His-Nrf2 (in pET28) were expressed in Escherichia coli BL21 cells. GST-Nrf2 or His-Nrf2 proteins were induced using 1 mM isopropyl β-D-thiogalactopyranoside (IPTG) for 4 h at 30°C. Expression of GST-IQGAP1 protein was induced by 0.5 mM IPTG for 8–10 h at room temperature. The recombinant proteins were purified by affinity chromatography using Glutathione Sepharose 4B beads (GE Healthcare) for GST-IQGAP1 or GST-Nrf2; or nickel-nitrilotriacetic acid agarose beads (Qiagen) for His-Nrf2. The concentration and purity of the recombinant proteins were evaluated by Coomassie brilliant blue staining.
In vitro binding assays
Eluted His-Nrf2 protein was incubated for at least 2 h at 4°C with GST-IQGAP1 protein in 0.5 ml incubation buffer (25 mM Tris-phosphate [pH 7.8], 2 mM DTT, 2 mM 1, 2- diaminocyclohexane-N,N,N′,N,-tetraacetic acid, 10% glycerol, 1% Triton X-100, and protease inhibitors). GST was used as a negative control for the in vitro binding assay. The complexes were isolated with glutathione-sepharose beads, resolved by SDS-PAGE gel, and processed by Western blotting using Nrf2 and IQGAP1 antibodies.
In vitro transcription/translation of IQGAP1 proteins
pcDNA3.1-IQGAP1 and pcDNA3.1-IQGAP1-N plasmid DNA was isolated using the Qiagen plasmid purification kit. In vitro transcription and translation (TNT) products were generated with the TNT Quick Coupled Transcription/Translation system (Promega) according to the manufacturer's instructions. Briefly, the plasmids (0.5 μg) were incubated with 40 μl of TNT Quick Master mix at 30°C for 1.5 h. The products were confirmed by resolving on SDS-PAGE gel and Western blotting. Ten micro liter of the TNT coupled IQGAP1 proteins were used for the in vitro binding assay with the purified GST-Nrf2 protein.
Western blot analysis
HeLa cells were plated in 6-well culture dishes at ∼4.0×105 cells/well for 16 h prior to plasmid transfection. After transfection, cells were scraped and lysed with RIPA lysis buffer (150 mM NaCl, 0.5% Triton X-100, 50 mM Tris–HCl, pH 7.4, 25 mM NaF, 20 mM EGTA, 1 mM DTT, 1 mM Na3VO4, and protease inhibitor cocktail tablet) for 30 min on ice followed by centrifugation at 14,800 g for 15 min. The protein concentration of the supernatant was measured by using the BCA reagent. Twenty microgram of protein was loaded onto a 4%–15% criterion Tris-HCl gel (Biorad) and electrotransferred onto PVDF membrane in Tris–glycine buffer (pH 8.4) containing 20% methanol. The membrane was then blocked in 5% fat-free dry milk in PBS with 0.1% Tween-20 for 1 h. Then, the membranes were probed with primary antibodies and horseradish peroxidase-conjugated secondary antibody by standard Western blotting procedures. The proteins were visualized with the femto signal chemiluminescent substrate (Pierce) under the image analyzer (Bio-Rad).
Real-time PCR
RNA was isolated by using TRIzol reagent (Invitrogen), and cDNA was mixed with SYBR green master mix and subjected to real-time PCR on a 7900HT sequence Detector (Applied Biosystems). The primer sequences of target mRNA were followed as Nrf2 (5′-TCT TGC CTC CAA AGT ATG TCA A-3′ and 5′-ACA CGG TCC ACA GCT CAT C-3′), IQGAP1 (5′-CGTCAGAACGTGGCTTATGA-3′ and 5′-CTC CTC CAG TTC TGT GGT GG-3′), HO-1 (5′-GAG TGT AAG GAC CCA TCG GA-3′ and 5′-GCC AGC AAC AAA GTG CAA G-3′), NQO-1 (5′-TCC TTT CTT CTT CAA AGC CG-3′ and 5′-GGA CTG CAC CAG AGC CAT-3′), GSTpi (5′-CTC AAA AGG CTT CAG TTG CC-3′ and 5′-ACC TCC GCT GCA AAT ACA TC-3′) and GCLC (5′-CTT TCT CCC CAG ACA GGA CC-3′ and 5′-CAA GGA CGT TCT CAA GTG GG-3′). The results were normalized to beta-actin (5′-GCA CAG AGC CTC GCC TT-3′ and 5′-GTT GTC GAC GAC GAG CG-3′).
Epifluorescence
HeLa cells grown on cover glasses were transfected with various plasmids and then fixed in 4% paraformaldehyde in PBS (pH 7.4) for 10 min. After briefly washing the cells in PBS solution, slides were mounted in a medium (VECTASHIELD; Vector Laboratories, Inc.) containing an antifade agent and DAPI. Sample images were taken with a fluorescence microscope (Nikon; ECLIPSE E 600 system), and the images were processed using Photoshop 8.0 (Adobe Systems).
Immunoprecipitation
HeLa or MEF cells were plated on 100 mm dishes and cultured until more than 95% confluence was achieved. Then, the cells were treated with either CaCl2 (2 mM), EGTA (5 mM) or BAPTA-AM (10 μM) for different times. Next, the cells were harvested and lysed with M-PER (Pierce) buffer to obtain the cytosolic extract. Four hundred micrograms of each cytosolic sample was subjected to IP assay using anti-GFP (B-2) or anti-Nrf2 (C-20) antibodies. For this experiment, Dynabead G beads (Invitrogen) were used for IP according to the manufacturer's protocol. Briefly, protein extracts were incubated overnight with primary antibodies in a cold room, and Dynabead protein G was added and incubated for 1 h. After washing the IP complexes, the samples were suspended in 200 μl of 2×SDS sample buffer. Twenty microliters of aliquots were then subjected to Western blotting using IQGAP1 (H-109) antibody.
Intracellular calcium measurements
HeLa cells were plated overnight on 12-well plates. Relative intracellular calcium levels were measured using FluoForte Calcium Assay Kit from Enzo Life Sciences. Fluorescenced calcium images were taken with an FITC filtered fluorescence microscope.
Statistical analysis
All experiments in this study were performed at least twice with similar results. The values are presented as mean±SD of three separate samples. Statistical analysis was performed by the two-tailed Student's t-test for unpaired data, with p<0.05 considered statistically significant.
Abbreviations Used
- γGCS
γ-glutamylcysteine synthetase
- ARE/EpRE
antioxidant responsive element/electrophile response element
- CBP
CREB binding protein
- GST
glutathione S-transferase
- HO-1
heme oxygenase-1
- IP
immunoprecipitation
- IPTG
isopropyl β-D-thiogalactopyranoside
- IQGAP1
IQ motif containing GTPase activating protein 1
- Keap1
kelch-like ECH-associated protein 1
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- MEM
minimum essential medium
- MRP1
multidrug-resistance-associated protein 1
- NQO-1
NAD(P)H: quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- PBS
phosphate-buffered saline
- PERK
PRKR-like endoplasmic reticulum kinase
- PI3K
phosphoinositide-3-kinase
- PKC
protein kinase C
- PVDF
polyvinylidene difluoride
- SUL
sulforaphane
- UGT
UDP-glucuronosyltransferase
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA94828 and 2R01CA118947 to A.N.K. and by the Intramural Research Program of the National Institutes of Health to D.B.S.
Author Disclosure Statement
There are no conflicts of interest.
References
- 1.Alam J. Stewart D. Touchard C. Boinapally S. Choi AM. Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Lipp P. Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Blank V. Small Maf proteins in mammalian gene control: mere dimerization partners or dynamic transcriptional regulators? J Mol Biol. 2008;376:913–925. doi: 10.1016/j.jmb.2007.11.074. [DOI] [PubMed] [Google Scholar]
- 4.Briggs MW. Sacks DB. IQGAP1 as signal integrator: Ca2+, calmodulin, Cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 5.Brown MD. Sacks DB. IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol. 2006;16:242–249. doi: 10.1016/j.tcb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Chan K. Han XD. Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–4616. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan K. Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci U S A. 1999;96:12731–12736. doi: 10.1073/pnas.96.22.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W. Sun Z. Wang XJ. Jiang T. Huang Z. Fang D. Zhang DD. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung KL. Yu S. Pan Z. Ma J. Wu TY. Kong AN. tBHQ-induced HO-1 expression is mediated by calcium through regulation of Nrf2 binding to enhancer and polymerase II to promoter region of HO-1. Chem Res Toxicol. 2011;24:670–676. doi: 10.1021/tx1004369. [DOI] [PubMed] [Google Scholar]
- 10.Chin D. Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 11.Clements CM. McNally RS. Conti BJ. Mak TW. Ting JP. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correa F. Mallard C. Nilsson M. Sandberg M. Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3beta. Neurobiol Dis. 2011;44:142–151. doi: 10.1016/j.nbd.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cullinan SB. Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 14.Dhakshinamoorthy S. Jain AK. Bloom DA. Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280:16891–16900. doi: 10.1074/jbc.M500166200. [DOI] [PubMed] [Google Scholar]
- 15.Enomoto A. Itoh K. Nagayoshi E. Haruta J. Kimura T. O'Connor T. Harada T. Yamamoto M. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59:169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 16.Greco T. Fiskum G. Neuroprotection through stimulation of mitochondrial antioxidant protein expression. J Alzheimers Dis. 2010;20(Suppl 2):S427–S437. doi: 10.3233/JAD-2010-100519. [DOI] [PubMed] [Google Scholar]
- 17.Hart MJ. Callow MG. Souza B. Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi A. Suzuki H. Itoh K. Yamamoto M. Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 19.Hayes JD. McMahon M. Chowdhry S. Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–1748. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 20.Ho YD. Joyal JL. Li Z. Sacks DB. IQGAP1 integrates Ca2+/calmodulin and Cdc42 signaling. J Biol Chem. 1999;274:464–470. doi: 10.1074/jbc.274.1.464. [DOI] [PubMed] [Google Scholar]
- 21.Howe CJ. LaHair MM. Maxwell JA. Lee JT. Robinson PJ. Rodriguez-Mora O. McCubrey JA. Franklin RA. Participation of the calcium/calmodulin-dependent kinases in hydrogen peroxide-induced Ikappa B phosphorylation in human T lymphocytes. J Biol Chem. 2002;277:30469–30476. doi: 10.1074/jbc.M205036200. [DOI] [PubMed] [Google Scholar]
- 22.Hu R. Saw CL. Yu R. Kong AN. Regulation of NF-E2-related factor 2 signaling for cancer chemoprevention: antioxidant coupled with antiinflammatory. Antioxid Redox Signal. 2010;13:1679–1698. doi: 10.1089/ars.2010.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang HC. Nguyen T. Pickett CB. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 24.Jain AK. Jaiswal AK. GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- 25.Jeong HW. Li Z. Brown MD. Sacks DB. IQGAP1 binds Rap1 and modulates its activity. J Biol Chem. 2007;282:20752–20762. doi: 10.1074/jbc.M700487200. [DOI] [PubMed] [Google Scholar]
- 26.Jurma OP. Hom DG. Andersen JK. Decreased glutathione results in calcium-mediated cell death in PC12. Free Radic Biol Med. 1997;23:1055–1066. doi: 10.1016/s0891-5849(97)00134-2. [DOI] [PubMed] [Google Scholar]
- 27.Kang MI. Kobayashi A. Wakabayashi N. Kim SG. Yamamoto M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc Natl Acad Sci U S A. 2004;101:2046–2051. doi: 10.1073/pnas.0308347100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaspar JW. Niture SK. Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katoh Y. Itoh K. Yoshida E. Miyagishi M. Fukamizu A. Yamamoto M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells. 2001;6:857–868. doi: 10.1046/j.1365-2443.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH. Yu S. Chen JD. Kong AN. The nuclear cofactor RAC3/AIB1/SRC-3 enhances Nrf2 signaling by interacting with transactivation domains. Oncogene. 2012 doi: 10.1038/onc.2012.59. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi M. Itoh K. Suzuki T. Osanai H. Nishikawa K. Katoh Y. Takagi Y. Yamamoto M. Identification of the interactive interface and phylogenic conservation of the Nrf2-Keap1 system. Genes Cells. 2002;7:807–820. doi: 10.1046/j.1365-2443.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi M. Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 33.Komatsu M. Kurokawa H. Waguri S. Taguchi K. Kobayashi A. Ichimura Y. Sou YS. Ueno I. Sakamoto A. Tong KI. Kim M. Nishito Y. Iemura S. Natsume T. Ueno T. Kominami E. Motohashi H. Tanaka K. Yamamoto M. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 34.Kong AN. Owuor E. Yu R. Hebbar V. Chen C. Hu R. Mandlekar S. Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE) Drug Metab Rev. 2001;33:255–271. doi: 10.1081/dmr-120000652. [DOI] [PubMed] [Google Scholar]
- 35.Lau A. Wang XJ. Zhao F. Villeneuve NF. Wu T. Jiang T. Sun Z. White E. Zhang DD. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JM. Chan K. Kan YW. Johnson JA. Targeted disruption of Nrf2 causes regenerative immune-mediated hemolytic anemia. Proc Natl Acad Sci U S A. 2004;101:9751–9756. doi: 10.1073/pnas.0403620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JM. Shih AY. Murphy TH. Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278:37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 38.Li Y. Jaiswal AK. Regulation of human NAD(P)H:quinone oxidoreductase gene. Role of AP1 binding site contained within human antioxidant response element. J Biol Chem. 1993;268:21454. [PubMed] [Google Scholar]
- 39.McMahon M. Itoh K. Yamamoto M. Chanas SA. Henderson CJ. McLellan LI. Wolf CR. Cavin C. Hayes JD. The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001;61:3299–3307. [PubMed] [Google Scholar]
- 40.Morse MA. Stoner GD. Cancer chemoprevention: principles and prospects. Carcinogenesis. 1993;14:1737–1746. doi: 10.1093/carcin/14.9.1737. [DOI] [PubMed] [Google Scholar]
- 41.Motohashi H. Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T. Lipton SA. Preventing Ca2+-mediated nitrosative stress in neurodegenerative diseases: possible pharmacological strategies. Cell Calcium. 2010;47:190–197. doi: 10.1016/j.ceca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakaso K. Yano H. Fukuhara Y. Takeshima T. Wada-Isoe K. Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen T. Sherratt PJ. Huang HC. Yang CS. Pickett CB. Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem. 2003;278:4536–4541. doi: 10.1074/jbc.M207293200. [DOI] [PubMed] [Google Scholar]
- 45.Rada P. Rojo AI. Chowdhry S. McMahon M. Hayes JD. Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rojo AI. Rada P. Egea J. Rosa AO. Lopez MG. Cuadrado A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol Cell Neurosci. 2008;39:125–132. doi: 10.1016/j.mcn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Rushmore TH. Pickett CB. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 48.Sakamoto K. Lawson LD. Milner JA. Allyl sulfides from garlic suppress the in vitro proliferation of human A549 lung tumor cells. Nutr Cancer. 1997;29:152–156. doi: 10.1080/01635589709514617. [DOI] [PubMed] [Google Scholar]
- 49.Salazar M. Rojo AI. Velasco D. de Sagarra RM. Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 50.Sun Z. Huang Z. Zhang DD. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Z. Wu T. Zhao F. Lau A. Birch CM. Zhang DD. KPNA6 (Importin {alpha}7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol Cell Biol. 2011;31:1800–1811. doi: 10.1128/MCB.05036-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velichkova M. Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol Cell Biol. 2005;25:4501–4513. doi: 10.1128/MCB.25.11.4501-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villeneuve NF. Lau A. Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vollrath V. Wielandt AM. Iruretagoyena M. Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakabayashi N. Slocum SL. Skoko JJ. Shin S. Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal. 2010;13:1649–1663. doi: 10.1089/ars.2010.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasserman WW. Fahl WE. Functional antioxidant responsive elements. Proc Natl Acad Sci U S A. 1997;94:5361–5366. doi: 10.1073/pnas.94.10.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watai Y. Kobayashi A. Nagase H. Mizukami M. McEvoy J. Singer JD. Itoh K. Yamamoto M. Subcellular localization and cytoplasmic complex status of endogenous Keap1. Genes Cells. 2007;12:1163–1178. doi: 10.1111/j.1365-2443.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y. Talalay P. Cho CG. Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]