Abstract
Significance: Diet-derived antioxidants are now being increasingly investigated for their health-promoting effects, including their role in the chemoprevention of cancer. In general, botanical antioxidants have received much attention, as they can be consumed for longer periods of time without any adverse effects. Flavonoids are a broadly distributed class of plant pigments that are regularly consumed in the human diet due to their abundance. One such flavonoid, fisetin (3,3′,4′,7-tetrahydroxyflavone), is found in various fruits and vegetables, such as strawberry, apple, persimmon, grape, onion, and cucumber. Recent Advances: Several studies have demonstrated the effects of fisetin against numerous diseases. It is reported to have neurotrophic, anticarcinogenic, anti-inflammatory, and other health beneficial effects. Critical Issues: Although fisetin has been reported as an anticarcinogenic agent, further in-depth in vitro and in vivo studies are required to delineate the mechanistic basis of its observed effects. In this review article, we describe the multiple effects of fisetin with special emphasis on its anticancer activity as investigated in cell culture and animal models. Future Directions: Additional research focused toward the identification of molecular targets could lead to the development of fisetin as a chemopreventive/chemotherapeutic agent against cancer and other diseases. Antioxid. Redox Signal. 19, 151–162.
Introduction
Dietary modifications can lead to extensive differences in the risks and incidences of several types of cancers. Recently, the use of natural dietary substances found in fruits, vegetables, and herbs has received considerable attention as chemopreventive and chemotherapeutic agents worldwide (14, 26, 28, 70). The approach of cancer prevention using nontoxic novel plant-derived agents has been encouraged by many researchers. Flavonoids are commonly found in most plants and exert a significant range of biological activities such as antioxidant, anticarcinogenic, anti-inflammatory, antibacterial, immune-stimulating, and antiviral (13, 24, 55). Flavonoids and their polymers comprise one of the largest groups of phytonutrients that afford beneficial health effects. These important polyphenolic compounds under the class of plant secondary metabolites exert significant effects in the biological system. It was reported that the average total intake of flavonoids in the United States was 1 g/day (34, 58); however, the intake differs extensively (7).
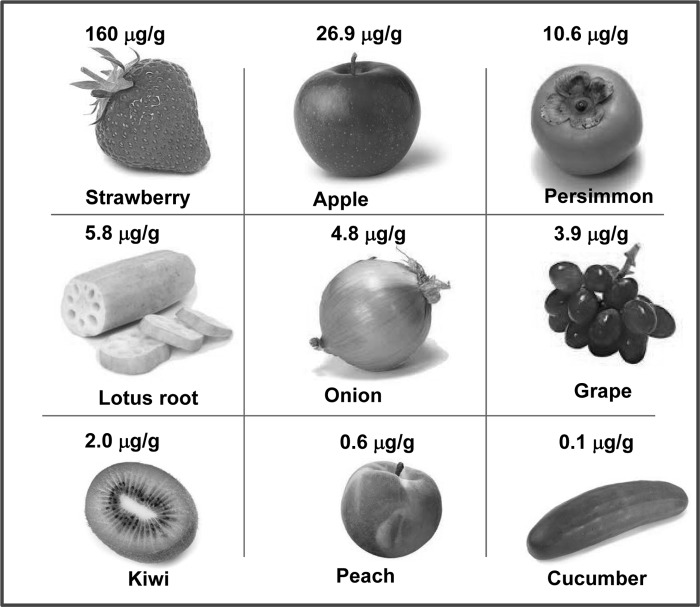
Fisetin (3,3′,4′,7-tetrahydroxyflavone, Figure 1) is a bioactive flavonol molecule found in fruits and vegetables such as strawberry, apple, persimmon, grape, onion, and cucumber at concentrations in the range of 2–160 μg/g (4). A preliminary database was built from the field study in Iwate Prefecture, Japan, to estimate the amount of flavonoids and isoflavonoids ingested by Japanese (33). The intake of flavonoids and isoflavonoids was estimated by calculations from the database and based upon a preliminary examination of 40 food items, covering at least 80% of total food consumption. Fisetin was measured in freeze-dried vegetables and fruits after acid hydrolysis of the parent glycosides. Average daily intake of fisetin per capita of flavonoids was computed as 0.4 mg. The highest concentration of fisetin was found in strawberries (160 μg/g) followed by apple (26.9 μg/g) and persimmon (10.5 μg/g) (33). Other dietary sources of fisetin and their concentrations are provided in Figure 2. In the past years, fisetin was a subject of research because of its presence in various human foods and its antiproliferative (20, 29, 30, 48, 61, 62, 65), apoptotic (10, 29, 61, 64), and antioxidant (22) activities. Fisetin has been reported as a chemopreventive/chemotherapeutic agent in several types of cancer and also as a neuroprotective agent. Several studies indicate that fisetin is a promising novel antioxidant. In this review article, we discuss the current information available in the literature on the multiple effects of fisetin, both in cell lines and in vivo models.
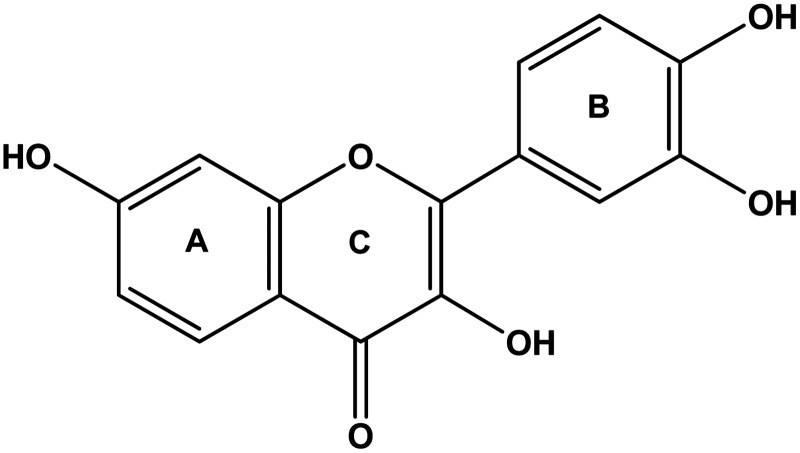
FIG. 1.
Structure of fisetin.
FIG. 2.
Dietary sources of fisetin. The concentration of fisetin was measured in freeze-dried vegetables and fruits after acid hydrolysis of the parent glycosides. Adapted from Kimira et al. (33).
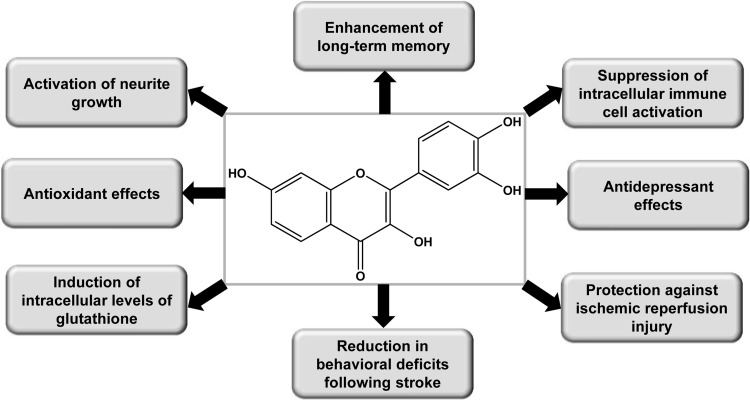
Fisetin as an Antioxidant
The ability of flavonoids to scavenge free radicals contributes to their marked antioxidant activity and significant biological effects. The oxygen radicals damage lipids, amino acids, carbohydrates, and nucleic acids. When an imbalance occurs between antioxidants and reactive oxygen species, it results in oxidative stress and is a consequence of a mismatch between the production of the reactive oxygen species and the ability to defend against them (63). It has been implicated in the development of many diseases, including diabetes mellitus, retinal degeneration, neurodegenerative diseases, mutagenesis, carcinogenesis, and ageing (6). Antioxidative properties of fisetin have been examined by both cyclic voltammetry- and quantum-chemical-based calculations (46). The trolox-equivalent activity concentration (TEAC) value of fisetin has been reported to be 2.80±0.06 (23). Fisetin was found to be a planar molecule exerting a cross-conjugation effect. The hydroxyl bond (OH) dissociation energy and dipole moment specified that fisetin had high antioxidant capacity (46). It has been reported that fisetin strongly binds between the polar head and hydrophobic tail of the phospholipids around the interfacial region of the egg phosphatidylcholine liposomes (59). This region is easily available to the free radicals (18) and serves as the reaction site for the antioxidant activity of the fisetin molecule and inhibits lipid peroxidation. The high antioxidant capacity was confirmed by semiempirical calculations for fisetin, with the effectiveness for extracting OH from the surrounding medium predicted to decrease in the order 3-OH>3′-OH>4′-OH>7-OH (59). Recently, Mohapatra and Mishra studied the photophysiological action of fisetin incorporated in liposomes using a dimyristoyl phosphatidylcholine (DMPC) bilayer membrane system (47). It was found that fisetin partitioned well into the membrane in solid gel and liquid crystalline phases, and the fisetin molecules were generally present near the head group region of the lipid bilayer membrane. There were variations of fluorescence intensity, lifetime, and anisotropy parameters in cholesterol-containing DMPC membranes, in mixed phospholipids, and as a function of temperature by membrane-bound fisetin, suggesting that fisetin can be an efficient fluorescent molecular probe for sensing lipid-bilayer membrane-related changes (47). Fisetin has been reported to inhibit human low-density lipoprotein (LDL) oxidation in vitro (49). It induced quinone oxidoreductase activity in murine hepatoma 1c1c7 cells in a time- and dose-dependent manner, and the induction of activity was associated with increase in mRNA expression. Fisetin also activated the antioxidant-/electrophile-response element as shown by transfection studies (22). Fisetin along with quercetin and myricetin had the lowest oxidation potential, more active than trolox, and seemed to be the most active compound in ferric-reducing antioxidant power assay, which determines the reducing capacity of a compound directly. It was suggested that the o-dihydroxy structure in the B ring and the 3-hydroxy group and 2,3-double bond in the C ring contribute to the antioxidant activity of fisetin (Fig. 1) (15). The effects of fisetin, morin, and myricetin were investigated on the susceptibility of LDL to oxidative modification and on oxLDL uptake in macrophages. Fisetin had stronger inhibitory activity than morin and myricetin in inhibiting Cu2+-mediated LDL oxidation measured by thiobarbituric acid-reactive substances assay, conjugated diene formation, and electrophoretic mobility. It was found that fisetin, morin, and myricetin prevented LDL from oxidation, in part, through reducing CD36 gene expression in macrophages, a possible effect in ameliorating atherosclerosis (37).
Fisetin has been shown to increase intracellular glutathione (GSH) levels in the mouse hippocampal HT-22 cells both in the presence and absence of glutamate. The increase in GSH metabolism provides protection from glutamate, as glutamate decreases the level of GSH by inhibiting the uptake of cystine necessary for the production of GSH (23). Treatment of primary rat neurons with fisetin afforded protection against the peroxynitrite donor, SIN-1-mediated alterations in inducing extracellular signal-regulated kinase (ERK1/2)/c-myc phosphorylation, nuclear NF-E2-related factor-2 (Nrf2) levels, glutamate cystine ligase levels, GSH concentration, and cell viability (8). Recently, the effect of fisetin on the upregulation of heme oxygenase-1 (HO-1) was investigated in human umbilical vein endothelial cells. The induction of HO-1 in fisetin-stimulated endothelial cells was diminished by small interfering RNA and pharmacological inhibitors of PKC-δ and p38 mitogen-activated protein kinase (MAPK). Treatment with fisetin caused increased Nrf2 nuclear translocation and activity. There was also reduction in hydrogen peroxide-induced cell death after fisetin treatment, which was reversed by ZnPP, an inhibitor of HO-1 (36).
Fisetin and Cancer
Emerging data from in vitro and in vivo studies indicate that fisetin possesses antiproliferative properties against several cancers (66). Its potential value in cancer prevention and treatment was further underscored from recent reports that show that fisetin may reduce angiogenesis and consequently suppress tumor growth through inhibition of urokinase plasminogen activator (uPA) (25). A screening study that evaluated the effect of 17 structure-related flavonoids found fisetin to be a potent inhibitor of the matrix metalloproteinase (MMP)-1 activity, a key enzyme in remodeling and degradation of the extracellular matrix, with an important role in cancer progression (40).
Fisetin is known to rapidly compromise microtubule drug-induced mitotic block in a proteasome-dependent manner in several human cell lines. It caused premature initiation of chromosome segregation and exit from mitosis without normal cytokinesis in unperturbed human cancer cells. The consequences of fisetin treatment on the localization and phosphorylation of several mitotic proteins were analyzed in cell culture studies, and it was found that Aurora B, Bub1, BubR1, and Cenp-F rapidly lost their kinetochore/centromere localization upon addition of fisetin to the culture medium. In addition, Aurora B kinase was identified as a novel direct target of fisetin, and its activity was significantly reduced by fisetin in vitro (57).
Fisetin was tested for genotoxicity in human lymphoblastoid cell lines, and the cells were analyzed for malsegregating chromosomes as well as for the induction of micronuclei. Olaharski et al. employed the CREST micronucleus assay to discriminate between micronuclei formed from chromosomal breakage (CREST-negative) and chromosomal loss (CREST-positive) in fisetin-treated cells. A statistically significant increase in CREST-positive micronuclei suggested that at low concentrations, fisetin exerted genotoxic effects primarily through chromosomal loss (52). Further studies showed that fisetin is an effective inhibitor of the nuclear enzyme human topoisomerase II-α, essential for DNA replication, and interfered with chromosome segregation during the anaphase and telophase periods of the cell cycle. It was thereby concluded that fisetin acted both as an aneugen (affecting cell division and mitotic spindle apparatus resulting in the loss or gain of whole chromosomes, thereby inducing aneuploidy) and a clastogen (causing breaks in chromosomes, leading to sections of the chromosome being deleted, added, or rearranged). At low doses, fisetin was capable of interfering with proper chromosomal segregation and acted as an aneugen, whereas at higher concentrations, fisetin through effective inhibition of topoisomerase II inhibitor exerted clastogenic effects causing double-stranded DNA breaks in the cells (52). Treatment with fisetin suppressed activation of HMC-1 mast cells by interfering with cell-to-cell interaction and inhibiting the activity of nuclear factor-kappa B (NF-κB) and MAPK, demonstrating its potential as an anti-inflammatory agent (50).
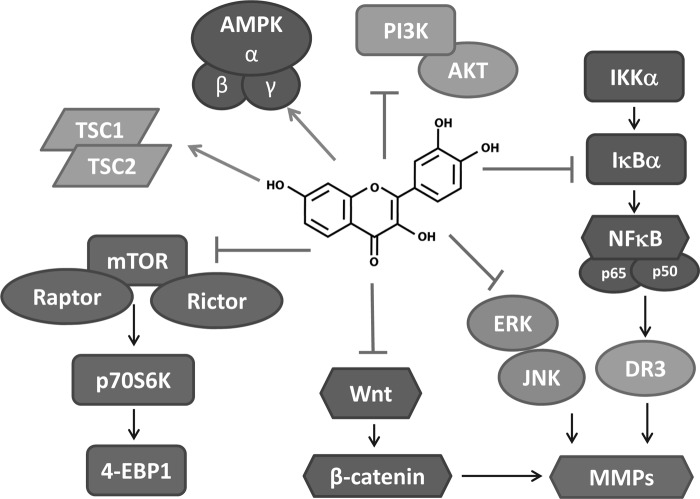
Fisetin induced apoptotic cell death in a variety of cancer cell lines. However, there is clear evidence that fisetin-mediated antiproliferative and proapoptotic effects were targeted specifically to cancer cells, and the normal cells were much less affected by fisetin treatment (27, 29, 30). It is possible that differential modulation of cell-signaling pathways in cancer cells versus their normal counterparts is responsible for this selective effect of fisetin. In the next section, we have reviewed the available literature on the effects of fisetin in various cancers, both in cell lines and in vivo models. The molecular targets of fisetin are shown in Figure 3. The anticarcinogenic effects of fisetin in in vitro and in vivo models are provided in Tables 1 and 2, respectively.
FIG. 3.
Molecular targets of fisetin.
Table 1.
Summary of the Anticarcinogenic Effects of Fisetin in In-Vitro Studies
| Target organ | In-vitro studies | References |
|---|---|---|
| Lung | Decreased cancer cell viability and clonogenecity, increased PTEN, decreased PI3-K and Akt phosphorylation, activated TSC and AMPK, decreased phosphorylation and activation of mTOR, decreased phosphorylation of p70S6K1, eIF-4E, and 4E-BP1, inhibited mTORC constituents such as Rictor, Raptor, GβL, and PRAS40 | 27 |
| Inhibited adhesion, migration, and invasion via inhibition of ERK1/2, downregulated MMP-2, uPA, NF-κB, and AP-1 | 38 | |
| Colon | Decreased cancer cell viability | 35, 61 |
| Induced cell cycle arrest, inhibited cdk-4 and 6 | 41 | |
| Induced cytotoxicity via securin degradation independent of p53 | 72 | |
| Induced apoptosis via extrinsic and intrinsic pathways, activated p53, increased TRAIL protein levels | 39, 61 | |
| Inhibited EGFR and NF-κB, decreased COX2 and PGE2, inhibited Wnt/β-catenin signaling, downregulated TCF-4, decreased cyclin D1 and MMP-7 | 61 | |
| Enhanced radiosensitivity of p53-mutant colon cancer cells, augmented radiation-induced G2/M arrest and apoptosis | 9 | |
| Prostate | Antiproliferative | 20 |
| Induces cell cycle arrest, induced cell death through apoptosis, and autophagy | 29, 62 | |
| Augmented TRAIL-mediated cytotoxicity, increased TRAIL protein expression, inhibited NF-κB | 67 | |
| Induced autophagy, activated AMPK, inhibited mTOR activity, mTOR complex formation, inhibited mTOR targets rpS6, eIF4B, and 4EBP1, suppressed Cap-dependent translation, Akt phosphorylation | 62 | |
| Inhibited adhesion, migration, and invasion, suppressed PI3K/Akt/JNK signaling downregulated MMPs | 11 | |
| Inhibited AR transactivation of target genes, including PSA, through interaction with AR | 30 | |
| Pancreas | Inhibited cell growth, induced apoptosis, inhibited invasion, suppressed DR3-mediated NF-κB activation, decreased MMP-9 | 48 |
| Melanoma | Decreased cell viability, induced G1-phase arrest, downregulated Wnt protein and its coreceptors, increased Wnt inhibitors Dickkopf and WIF-1, decreased nuclear β-catenin levels, downregulated β-catenin targets such as c-myc, Brn-2 and MITF | 65 |
AMPK, AMP-activated protein kinase; AR, androgen receptor; COX, cyclooxygenase; DR, death receptor; EGFR, epidermal growth factor receptor; eIF4B, eukaryotic initiation factor 4B; ERK1/2, extracellular signal-regulated kinase; MITF, microphthalmia-associated transcription factor; MMP, matrix metalloproteinase; NF-κB, nuclear factor-kappa B; PGE2, prostaglandin E2; PSA, prostate-specific antigen; PTEN, phosphatase and tensin homolog; TCF, transcription factor T-cell factor; TRAIL, TNF-related apoptosis-inducing ligand; uPA, urokinase plasminogen activator; WIF-1, Wnt inhibitory factor; XIAP, X-linked inhibitor of apoptosis.
Table 2.
Summary of the Anticarcinogenic Effects of Fisetin in In-Vivo Studies
| Target organ | In-vivo studies | References |
|---|---|---|
| Lung | Protects against B(a)P-induced lung carcinogenesis, reduced histological lesions, restored enzymic and nonenzymic anti-oxidants | 54 |
| Anti-angiogenic, reduced microvessel density, decreased tumor volume more effectively with cyclophsophamide | 69 | |
| Prostate | Slowed tumor growth, decreased serum PSA levels | 30 |
B(a)P, benzo(a)pyrene.
Fisetin and lung cancer
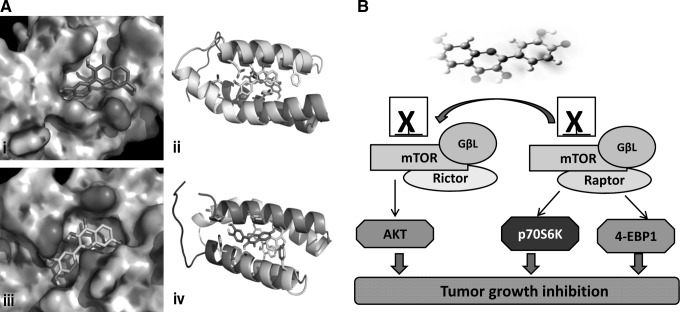
Lung cancer is reckoned to be one of the most common cancers in the world for several decades, and metastatic lung cancer is responsible for more than 90% of lung cancer-related deaths (12). Different molecular mechanisms enable tumor cells to infiltrate the surrounding tissue, invade blood vessels, and spread to distant sites (12). Efforts are being directed to discover novel agents that can modulate these pathways and prevent migration and invasion of tumor cells (1). Initial studies exploring the effect of fisetin on lung cancer showed that fisetin effectively inhibited adhesion, migration, and invasion of human A549 lung cancer cells (38). This was associated with decreased phosphorylation of ERK1/2 and downregulation of the expression of MMP-2 and uPA at both the protein and mRNA levels. Fisetin inhibited the nuclear translocation and activation of transcription factors NF-κB and AP-1, upstream regulators of MMP-2 and uPA (38). These studies were extended in our laboratory, where we showed that fisetin decreased the viability and clonogenicity of A549 lung cancer cells through modulation of AMP-activated protein kinase (AMPK)/Akt/mammalian target of rapamycin (mTOR) signaling (27). The study identified mTOR as a key target of fisetin and used in silico modeling to examine the physical interaction of fisetin with the mTOR molecule. It was shown that fisetin binds to two sites on the mTOR molecule. The binding energies were in the −7 to −8 kcal/mol range, and the binding site included hydrogen bonding to a glutamate by two OH groups (Fig. 4A). The proposed mechanism through which fisetin targets mTOR signaling in cancer cells is shown in Figure 4B. Cell culture studies showed that fisetin treatment decreased the protein expression of PI3K (p85/p110), and inhibited the phosphorylation of Akt, mTOR, p70S6K1, and eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1). Fisetin-treated cells exhibited inhibition of the constituents of mTOR-signaling complex such as Rictor, Raptor, GβL, and PRAS40, suggesting that the growth inhibitory effects of fisetin were mediated, in part, through inhibition of mTOR signaling (27). Further proof of its anticancer activity was obtained from an in vivo study where fisetin conferred protection against benzo(a)pyrene [B(a)P]-induced lung carcinogenesis. Treatment with fisetin reduced histological lesions, lipid peroxidation, and the levels of enzymatic and nonenzymatic antioxidants in the lungs of B(a)P-exposed mice (54). In another study, fisetin administered intraperitoneally to Lewis lung carcinoma-bearing mice resulted in a marked decrease in tumor volume when compared to the untreated mice. Fisetin treatment in combination with cyclophosphamide was found to induce greater tumor inhibition, when assessed by tumor growth curves. The decrease in tumor volume was linked to the antiangiogenic effect of fisetin, as treated tumors had significant reduction in the microvessel density (69). These studies demonstrate that fisetin, alone or as an adjuvant, could be effective in the treatment of solid tumors.
FIG. 4.
Fisetin and mTOR signaling. (A) Hypothetical model of the mammalian target of rapamycin (mTOR) molecule in complex with fisetin. (i, iii) Blind docking of fisetin to the mTOR target was performed with autodock 4 by setting grid sizes that included the entire mTOR molecule. The structure consists of four stacked alpha helices. The grid size for the docking site was expanded to include the entire mTOR molecule, and fisetin was docked. Using autodock 4, it was shown that fisetin bound to two sites on the mTOR target. The binding energies were in the −7 to −8 kcal/mol range for the binding constant. (ii, iv) The binding in the best site included hydrogen bonding to a glutamate by two hydroxyl groups. Adapted from Khan et al. (27). (B) Proposed mechanism through which fisetin targets mTOR signaling in cancer cells.
Fisetin and colon cancer
Colon cancer is one such type of cancer where dietary modification has been well documented to play an important role in reducing the risk of carcinogenesis. In this context, the effects of dietary flavonoids have been examined in colon cancer cells. Kuntz et al. screened more than 30 flavonoids for their effects on cell proliferation and potential cytotoxicity in the human colon cancer cell line Caco-2, which displays features of small-intestinal epithelial cells, and in the HT-29 cells, which resemble colonic crypt cells. Studies on the dose-dependent effects of flavonoids showed antiproliferative activity of all compounds with EC50 values ranging between 39.7±2.3 and 203.6±15.5 μM (35). The mechanism of growth inhibition of fisetin was further explored, and it was shown that fisetin-induced cell cycle arrest in HT-29 cells was at least in part mediated through inhibition of cyclin-dependent kinases (cdks) (41). Cell culture studies demonstrated that fisetin decreased the protein levels of cdk-2 and 4. These findings were validated in cell-free systems where fisetin was shown to inhibit cdk-4 activity directly (41). We examined the molecular signaling pathways upstream of cdks that were modulated by fisetin- mediated growth inhibition. Our data showed that fisetin induced apoptosis and targeted the cyclooxygenase 2 (COX2) and Wnt/epidermal growth factor receptor (EGFR)/NF-κB-signaling in HT29 colon cancer cells (61). Fisetin selectively inhibited COX2 expression and decreased prostaglandin E2 (PGE2) secretion without affecting COX1 protein expression. Furthermore, fisetin-mediated inhibition of Wnt/β-catenin signaling was associated with downregulation of the transcription factor T-cell factor (TCF)-4, and decreased expression of β-catenin target genes such as cyclin D1 and MMP-7 (61).
In HCT-116 colon cancer cells, fisetin reportedly induced apoptosis via activation of both death receptor (DR) and mitochondrial-dependent pathways and subsequent activation of the caspase cascade (39). Fisetin suppressed the protein levels of antiapoptotic Bcl-xL and Bcl-2 and increased proapoptotic Bak and Bim. Fisetin-mediated activation of p53 contributed to the mitochondrial translocation of Bax via a transcription-independent pathway, and was responsible, at least in part, for the apoptosis observed in HCT-116 cells. Additionally, fisetin caused an increase in the protein levels of cleaved caspase-8, Fas ligand, DR5, and tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (39). Securin, overexpressed in several types of cancer, is known to negatively regulate the transcription and subsequent apoptotic activity of the tumor suppressor p53. It was shown that depletion of securin enhanced fisetin-induced apoptosis in human colon cancer cells, while fisetin-induced apoptosis was blocked by overexpression of wild-type or nondegradable securin (72). Moreover, fisetin inhibited securin expression regardless of p53 status, and knockdown of securin by siRNA enhanced fisetin-induced apoptosis in p53-null HCT116 cells (72). Fisetin pretreatment enhanced the radiosensitivity of p53-mutant HT-29 cancer cells, prolonged radiation-induced G2/M phase cell cycle arrest, and augmented radiation-induced caspase-dependent apoptosis. At the molecular level, fisetin induced the phosphorylation of p38 MAPK, and suppressed the phosphorylation of Akt and ERK1/2 in these cells (9). In conclusion, fisetin inhibits growth and induces apoptosis in colon cancer cells through targeting multiple pathways.
Fisetin and prostate cancer
Studies conducted to identify novel flavonoids with antiproliferative activity have ascertained fisetin as a potent inhibitor of prostate cancer cell growth (20). The efficacy of the synthetic flavonoid 2,2′-dihydroxychalcone (DHC) was compared with that of the naturally occurring fisetin. Both compounds decreased cell viability and clonogenicity associated with a concomitant increase in apoptosis in prostate cancer cells. The synthetic compound DHC, however, showed cytotoxic activity at lower concentrations (19). Gene expression studies showed that the cell cycle regulatory genes were among the most highly represented functional category of genes altered. Of the hundred cell cycle genes altered by DHC and fisetin, 27 genes with key functions in the G2/M phase were downregulated by both compounds. The other functional categories that were modulated included chromosome organization, apoptosis, and stress-response genes (19).
Fisetin has been found to induce cell death in prostate cancer cells through the activation of the apoptotic and autophagic pathways (19, 29, 62, 67). We showed that fisetin treatment resulted in induction of apoptosis in LNCaP cells, with activation of the caspase cascade. This was accompanied by arrest of cells in the G0/G1 phase of the cell cycle (29). Fisetin decreased the viability of prostate cancer cells, but had minimal effect on normal prostate epithelial cells (29). As in colon cancer cells (39), fisetin augmented TRAIL-mediated cytotoxicity and apoptosis in prostate cancer cells by activating the extrinsic and intrinsic apoptotic pathways (67). Cotreatment with TRAIL and fisetin caused significant activation of caspase-8 and caspase-3 and disruption of mitochondrial potential in these cells. Fisetin increased the protein expression of the TRAIL receptor and decreased the activation and nuclear translocation of NF-κB (67).
Fisetin also acts as a dual inhibitor of PI3K/Akt and mTOR signaling in prostate cancer cells (2). A recent study from our laboratory showed that fisetin treatment of androgen-independent phosphatase and tensin homolog (PTEN)-negative human PC-3 prostate cancer cells leads to the induction of programmed autophagic cell death (62). The process of autophagy is promoted by AMPK, a key energy sensor that regulates cellular metabolism to maintain energy homeostasis (31). Treatment of PC-3 cells with fisetin induced the activation of AMPK and inhibited not only the basal expression of mTOR but also its phosphorylation at Ser2481 and Ser2448. The decrease in mTOR activity was associated with downregulation of Raptor, Rictor, PRAS40, and GβL with subsequent loss of formation of mTOR complexes. The dual inhibition of mTOR complexes (Raptor and Rictor) resulted in negative regulation of the feedback loop through which the mTOR–Rictor complex activates Akt with subsequent repression of Akt signaling (62). Furthermore, fisetin through inhibition of mTOR targets rpS6, eIF4B, and 4EBP1 suppressed cap-dependent translation in PC-3 cells. Interestingly, another study by Yang et al. in MCF-7 breast cancer cells showed that fisetin-induced apoptosis was accompanied by inhibition of autophagy (71). These diverse reports indicate that there are multiple mechanisms through which fisetin exerts its antitumor activity, which may vary with the cell type. Fisetin can inhibit the adhesion, migration, and invasive behavior of cancer cells. Chien et al. demonstrated that the antimetastatic potential of fisetin was related to suppression of the PI3K/Akt and JNK signaling with concomitant downregulation of MMPs in prostate cancer cells (11).
The androgen receptor (AR) signaling pathway, which is essential for prostate development and normal function, is central to prostate cancer's pathogenesis and progression. The key enzyme in AR signaling, 5α-reductase, catalyzes the conversion of testosterone to the more potent androgen dihydrotestosterone, a process known to amplify androgenic response and linked to the development of prostate cancer in humans (21). Interestingly, fisetin possesses higher affinity for the AR than dihydrotestosterone (30). We showed that fisetin interacts with the ligand-binding domain of the AR and interferes with its amino-/carboxyl-terminal interactions with subsequent reduction in receptor stability. This resulted in blunting of AR-mediated transactivation of target genes, including the prostate-specific antigen (PSA), an established diagnostic serum marker for monitoring the presence and progression of prostate cancer in human patients (30). Furthermore, treatment with fisetin significantly slowed the progression of tumor growth in athymic nude mice implanted with CWR22Rν1 prostate cancer cells. Importantly, the tumor growth inhibition followed a significant decrease in the serum levels of PSA, indicating an AR-specific effect (30). These findings further suggest that fisetin could be exploited to delay the progression of prostate cancer.
Fisetin and pancreatic cancer
Effective systemic therapies for pancreatic adenocarcinoma are lacking, in part because of the intrinsic drug resistance of this disease. Studies performed in our laboratory demonstrate that fisetin negatively regulates the growth of chemoresistant pancreatic cancer cells (48). DRs of the TNF receptor superfamily have been implicated in the constitutive activation of prosurvival NF-κB signaling in pancreatic cancer cells. We showed that fisetin induced apoptosis and inhibited invasion of pancreatic cancer cells through suppression of DR3-mediated NF-κB activation. Fisetin treatment resulted in dose-dependent inhibition of cancer cell growth and proliferation with concomitant induction of apoptosis (48). The effect of fisetin was analyzed on a subset of 113 genes with known relevance to cancer cell transformation and tumorigenesis. Compared to vehicle-treated cells, fisetin treatment caused significant modulation in the expression of many genes that were either unregulated or downregulated ranging between 1.5-fold and 7-fold. The highly upregulated genes were mostly related to cell cycle control, invasion, and metastasis (p21, p16, IκBα, NME4, and KISS1), whereas the downregulated genes included the antiapoptotic BCL2L1 and genes involved in cell invasion, proliferation, and metastasis such as NF-κB, MMP-9, EGFR, and HER-2. Interestingly, maximal decrease was observed in the DR3 gene expression and was accompanied with a parallel increase in the expression levels of IκBα, the NF-κB inhibitor. Cell culture studies further showed that fisetin treatment resulted in a decrease in the activity of NF-κB/p65, MMP-9, and X-linked inhibitor of apoptosis (XIAP) and signaling molecules associated with chemoresistance in pancreatic cancer cells. Importantly, we found that transient downregulation of DR3 by RNA interference or blocking with an extracellular-domain-blocking antibody significantly augmented fisetin-induced changes in cell proliferation, cell invasion, and apoptosis with concomitant decrease in MMP-9, XIAP, and NF-κB DNA-binding activity (48). These observations validated findings from other cancer cell models that point to NF-κB/MMP signaling as an important target of fisetin.
Pancreatic cancer cells are known to express the multidrug resistance-associated protein (MRP)-1 responsible for the efflux of drugs from the cell. Nguyen et al. screened the effect of dietary flavonoids on the transport of daunomycin and vinblastine in a pancreatic adenocarcinoma cell line. They found that fisetin significantly decreased the accumulation of both drugs in the plasma in contrast to other flavonoids (51). However, another study done in doxorubicin-resistant human sarcoma cells showed that fisetin significantly enhanced doxorubicin accumulation in these cells (3). These apparently contrasting data suggest that in-depth studies are needed to explore the efficacy of fisetin in pancreatic cancer.
Fisetin and melanoma
Malignant melanoma is a deadly human cancer with no effective cure for the metastatic disease. We recently showed that treatment of metastatic human melanoma cells with fisetin resulted in decreased cell viability with G1-phase arrest (65). Fisetin disrupted the Wnt/β-catenin signaling pathway in melanoma cells and downregulated Wnt protein and its coreceptors. In contrast, the protein expression of endogenous Wnt inhibitors Dickkopf and Wnt inhibitory factor (WIF)-1 was increased with fisetin treatment. Fisetin-mediated increase in the cytosolic levels of Axin and β-transducin repeat-containing protein (β-TrCP) and decrease in the phosphorylation of glycogen synthase kinase-3 (GSK3)-β corresponded to decreased nuclear β-catenin levels (65). Fisetin interfered with the functional cooperation between β-catenin and the transcription factor LEF-1/TCF-2, resulting in downregulation of known β-catenin targets such as c-myc, Brn-2, and microphthalmia-associated transcription factor (MITF) (65). We showed that fisetin was able to over-ride the proliferative effect of MITF, an oncogene amplified in ∼20% of metastatic melanomas, and induced growth repression in MITF-overexpressing melanoma cells (65). We validated our in vitro findings in a melanoma xenograft mouse model. Intraperitoneal administration of fisetin to athymic nude mice implanted with metastatic melanoma cells resulted in significant inhibition of melanoma tumor development and was associated with a decrease in endogenous MITF protein levels (65). This study published in the Journal of Investigative Dermatology was highlighted and chosen for commentary by Arbiser and Fisher, where they emphasized the need to explore the potential of dietary agents in the fight against cancer (5). The editorial described fisetin as a natural fist against melanoma and appropriately elucidated that a rejuvenated interest in natural products combined with modern methods of target identification might be an important tool in the fight against melanoma. Figure 5 depicts the proposed mechanisms through which fisetin targets β-catenin/MITF signaling in melanoma cells.
FIG. 5.
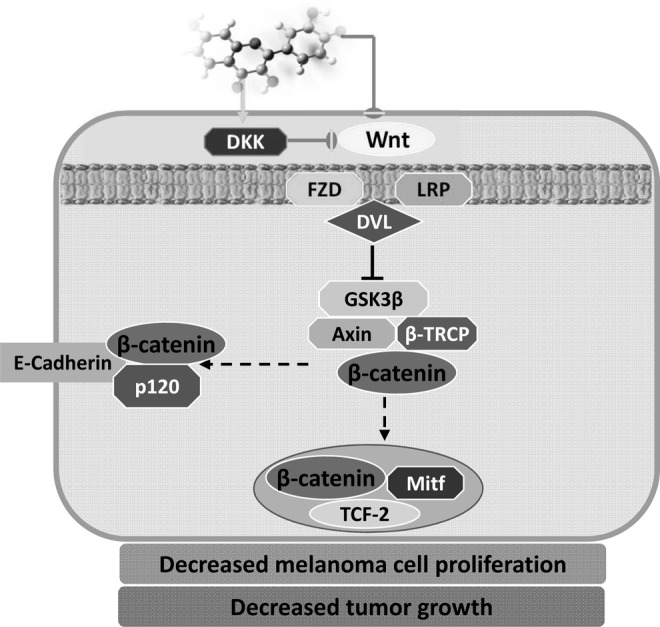
Proposed mechanism through which fisetin targets β-catenin/MITF signaling in melanoma cells.
Fisetin and Neuroprotection
It is well known that cerebrovascular and neurodegenerative diseases are a leading cause of death worldwide. Oxidative stress can lead to neuronal death in stroke and oxidative reactions have been recognized in arteriosclerosis, which is the main pathological condition leading to stroke. The neuroprotective effects of fisetin are shown in Figure 6. Several flavonoids were studied for their ability to induce neurite outgrowth in PC12 cells, which is a well-studied model system of neuronal differentiation. It was found that fisetin was the most effective flavonoid that induced neurite outgrowth by inducing ERK1/2 activation and PC12 cell differentiation (56). To investigate if ERK1/2 activation by fisetin is required for neurite outgrowth, three structurally unrelated inhibitors of the MAPK pathway were used, and fisetin-induced ERK1/2 activation was blocked by all of these inhibitors, suggesting the involvement of ERK1/2 in fisetin-mediated effects. Moreover, similar effects were observed on treatment with these inhibitors on fisetin-induced neurite outgrowth. Treatment of the PC12 cells with farnesyl transferase inhibitor FTI277 blocked fisetin-induced ERK1/2 activation and caused significant reduction in fisetin-induced differentiation, demonstrating that Ras activation is required for the observed effects of fisetin (56). Fisetin activated ERK1/2 and induced cAMP-response element-binding protein (CREB) phosphorylation in rat hippocampal slices and enhanced object recognition in mice. It was shown that fisetin enabled long-term memory potentiation and therefore may be useful for treating patients with memory disorders (43). This is consistent with recent studies showing that activation of ERK provides neuroprotection, while specific inhibition of ERK activation enhances neuronal cell death. Furthermore, reduced signaling by growth factors such as EGF-1 that activate the Ras-ERK cascade has been found in patients suffering from Huntington's disease (HD) as well as in disease models, signifying that ERK activation might provide a novel therapeutic approach to prevent neuronal dysfunction. In agreement with the aforementioned ERK-mediated neurotrophic effect of fisetin, most of the data available in literature indicate that fisetin does not exert an inhibitory effect on ERK1/2 signaling. Kim et al. showed that fisetin suppressed the activation of NF-κB and JNK MAPKs, but not ERK in lipopolysaccharide (LPS)-stimulated mouse macrophages (32). In another comparative study where apigenin, luteolin, and fisetin were investigated for their inhibitory effects on TNF-α-induced NF-κB transcriptional activation, it was shown that fisetin enhanced and sustained the activation of ERK and JNK in response to TNF-α (16). However, in contrast to the above reports, one study has shown that fisetin suppressed the invasion and migration of lung cancer cells through inhibition of the ERK activity (38). Given these conflicting results, further studies are required to understand the dual effects of fisetin on ERK signaling and to delineate the intricate mechanisms involved in its effect on normal versus cancer cells.
FIG. 6.
Neuroprotective effects of fisetin.
Maher et al. developed a cell culture-based assay as a screening tool to identify compounds that might be effective for the treatment of stroke. In mouse hippocampal cell line HT22 in combination with chemical ischemia, they did an initial screening for potential neuroprotective drugs among a group of flavonoids. The biochemical assays for ATP, GSH, and for the long-term induction of antioxidant proteins were performed to provide further screening. Fisetin was tested in the small-clot embolism model of cerebral ischemia in rabbits based upon the results of these screens. It was found that there was reduction in the behavioral deficits after stroke, upon treatment with fisetin (45). Additional studies investigated the effect on the infarct size and the inflammatory response in a mouse model of stroke. Treatment with fisetin afforded protection to brain tissue against ischemic reperfusion injury when given before ischemia and also when applied 3 h after ischemia. There was inhibition of the infiltration of macrophages and dendritic cells into the ischemic hemisphere and suppression of the intracerebral immune cell activation. Fisetin treatment caused suppression of NF-κB activation and JNK1/2/Jun phosphorylation, associated with inhibition of LPS-induced TNF-α production (17). In vitro studies showed that fisetin treatment suppressed the production of TNF-α, nitric oxide, and PGE2 in LPS-stimulated BV-2 microglial cells or primary microglial cultures. There was inhibition of the gene expression of TNF-α, interleukin (IL)-1β, COX-2, and inducible nitric oxide synthase at both mRNA and protein levels and suppression of IκB degradation, nuclear translocation of NF-κB, and phosphorylation of p38 MAPK in the LPS-stimulated BV-2 microglial cells. Treatment with fisetin reduced the cytotoxicity of LPS-stimulated microglia toward B35 neuroblastoma cells in a coculture system, suggesting that fisetin has a strong anti-inflammatory activity in brain microglia (74).
Fisetin has been shown to affect multiple pathways involved in the maintenance of neuronal function during aging. It acts as an antioxidant, increases GSH, maintains mitochondrial function in the presence of oxidative stress, has anti-inflammatory activity against microglial cells, and inhibits the activity of 5-lipoxygenase, signifying that fisetin causes reduction in the age-related decline in brain function (42). The ability of fisetin to prevent neuronal dysfunction was examined in three different models of HD: (a) in PC12 cells that expressed the mutant huntingtin (Httex1) protein under the control of an inducible promoter, (b) in Drosophila expressing mutant Httex1, and (c) in the R6/2 mouse model of HD. It was shown that fisetin reduced the impact of mutant huntingtin protein in all of these models and might be useful for the treatment of HD by inducing the activation of ERK1/2 (44).
The antidepressant potential of fisetin and its mechanisms were investigated in a recently published study. Data obtained from two mouse models employing despair tests suggested that fisetin dose-dependently inhibited the immobility time in both behavioral tests, while the doses that affected the immobile response did not affect locomotor activity. To discover the possible effect of fisetin in the noradrenergic and serotonergic systems, two behavioral models, reserpine-induced hypothermia and ptosis, in addition to p-chlorophenylalanine-induced depletion of serotonin, were used as indicators. The higher dose of fisetin antagonized the hypothermia induced by reserpine and produced an increase in serotonin and noradrenaline levels in the frontal cortex and hippocampus, showing that the antidepressant-like effect of fisetin was due to its regulatory effect on central serotonin and noradrenaline levels (73).
Pharmacokinetics and Bioavailability of Fisetin
Shia et al. have investigated the metabolism and pharmacokinetics of fisetin in rats (60). The activities of fisetin and its serum metabolites against 2,2′-azobis (2-amidinopropane hydrochloride) (AAPH)-induced hemolysis were evaluated and compared on an equimolar basis. After intravenous administration of fisetin (10 mg/kg body weight), the mean serum concentration–time profiles of metabolites showed that fisetin declined rapidly. The sulfates/glucuronides and glucuronides were largely at higher concentrations than the parent compounds at all time points, suggesting that fisetin was rapidly and extensively biotransformed by conjugation metabolism in the liver, mainly sulfation. Following oral dose (50 mg/kg body weight), fisetin levels were maintained after the first pass through the intestine and liver as shown by presence of the parent compound in serum. The (AUC)0-2880 of fisetin sulfates/glucuronides was 2.2-fold that of fisetin glucuronides, demonstrating that fisetin was rapidly and extensively metabolized to sulfates and glucuronides. Interestingly, it was observed that less sulfation occurred in enterocytes than in hepatocytes when serum levels of metabolites were compared after intravenous dose (60). It was shown that after treatment of 50 mg/kg body weight of fisetin, Cmax and AUC0-2880 values of fisetin sulfate/glucuronide were 27-fold and 59-fold greater than the Cmax and AUC0-4320 values of 5-OH-flavone sulfate/glucuronide, respectively, following 40 mg/kg body weight of 5-OH-flavone. After an equal dose, the AUC0-4320 of 7-OH-flavone sulfate/glucuronide was found to be even lower than that of 5-OH-flavone sulfate/glucuronide (60). There was significant inhibition of AAPH-induced hemolysis by both fisetin and its serum metabolites, signifying that fisetin sulfates/glucuronides retain free-radical scavenging activity due to the presence of residual phenolic groups after conjugation metabolism (60).
It has been reported that the maximum fisetin concentration reached 2.5 μg/ml at 15 min, and the plasma concentration declined biphasically with a rapid half-life of 0.09 h and a terminal half-life of 3.1 h after administration of fisetin at a dose of 223 mg/kg intraperitoneally in mice (68). These studies clearly demonstrate that the metabolism of fisetin has a significant role in its biological responses and anticancer activities. Ragelle et al. conducted a study to incorporate fisetin into a nanoemulsion to improve its pharmacokinetics and therapeutic efficacy (53). It has been shown that the fisetin nanoemulsion injected intravenously showed no significant difference in systemic exposure compared to free fisetin in mice, but when given intraperitoneally as compared to free fisetin, a 24-fold increase in the relative bioavailability of fisetin was found. The antitumor activity of the fisetin nanoemulsion in Lewis lung carcinoma-bearing mice occurred at lower doses compared to free fisetin (53).
Conclusion and Perspectives
Many botanical antioxidants are considered for health-promoting effects. One such antioxidant, fisetin, has recently received some attention for its beneficial effects against several diseases. Ischemic stroke is recognized as the multiphasic process composed of necrosis and apoptosis evolving into an immune-mediated process, leading to further lesion growth. Compounds capable of affecting these features could afford protection against the disease. Fisetin has been reported to reduce ischemia-induced brain damage. Additional studies are required to establish the neuroprotective properties of fisetin and to understand the precise molecular mechanisms involved in the animal models of neurodegenerative diseases. Fisetin has also been reported as a potential cancer preventive agent in several types of cancer. However, in vivo studies are still lacking with this natural compound to further establish its role and leading the way for future clinical trials. The investigation of the molecular mechanisms associated with the anticancer effects exhibited by fisetin is essential. Further research could lead to the development of fisetin as a chemopreventive/chemotherapeutic agent against cancer and other diseases. Single-agent-targeted therapies have rarely cured patients with cancer. Since fisetin exhibits several activities that are potentially beneficial in treating cancer, further studies directed toward target identification, and pathway analysis could pave the way for the addition of fisetin to the armamentarium of anticancer therapy.
To translate the chemopreventive potential of fisetin to clinical use, well-designed clinical trials along with the development of reliable analytical biomarkers are required. Pharmacokinetics studies will provide additional information to understand its effect on cancer in humans. To develop fisetin clinically, in-depth research studies involving suitable animal models, feasibility of use by humans, and optimization of the desired physiological responses in targeted patients or healthy populations are required.
Abbreviations Used
- AAPH
2-amidinopropane hydrochloride
- AMPK
AMP-activated protein kinase
- AR
androgen receptor
- AUC
area under the curve
- B(a)P
benzo(a)pyrene
- Cdks
cyclin-dependent kinases
- COX
cyclooxygenase
- CREB
cAMP-response element-binding protein
- DHC
2,2′-dihydroxychalcone
- DMPC
dimyristoylphosphatidylcholine
- DR
death receptor
- EGFR
epidermal growth factor receptor
- eIF4E
eukaryotic initiation factor 4E
- ERK1/2
extracellular signal-regulated kinase
- GSH
intracellular glutathione
- GSK3-β
glycogen synthase kinase-3β
- HD
Huntington's disease
- HO-1
heme oxygenase-1
- IL
interleukin
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MITF
microphthalmia-associated transcription factor
- MMP
matrix metalloproteinase
- MRP
multidrug resistance-associated protein
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor-kappa B
- OH
hydroxyl
- PGE 2
prostaglandin E2
- PSA
prostate-specific antigen
- PTEN
phosphatase and tensin homolog
- TCF
transcription factor T-cell factor
- TEAC
trolox-equivalent antioxidant capacities
- TNF-α
tumor necrosis factor-α
- TRAIL
TNF-related apoptosis-inducing ligand
- uPA
urokinase plasminogen activator
- WIF-1
Wnt inhibitory factor
- XIAP
X-linked inhibitor of apoptosis
- Β-TrCP
β-transducin repeat-containing protein
Acknowledgment
The authors are thankful for support from National Institutes of Health, National Cancer Institute Grants R01CA120451 (to H.M.) and R03CA153961 (to N.K.).
References
- 1.Adhami VM. Khan N. Mukhtar H. Cancer chemoprevention by pomegranate: laboratory and clinical evidence. Nutr Cancer. 2009;61:811–815. doi: 10.1080/01635580903285064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhami VM. Syed D. Khan N. Mukhtar H. Dietary flavonoid fisetin: a novel dual inhibitor of PI3K/Akt and mTOR for prostate cancer management. Biochem Pharmacol. 2012;84:1277–1281. doi: 10.1016/j.bcp.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelini A. Di Ilio C. Castellani ML. Conti P. Cuccurullo F. Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin- resistant sarcoma cells (MES-SA/DX-5): implications for natural sedatives as chemosensitizing agents in cancer therapy. J Biol Regul Homeostat Agents. 2010;24:197–205. [PubMed] [Google Scholar]
- 4.Arai Y. Watanabe S. Kimira M. Shimoi K. Mochizuki R. Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 5.Arbiser JL. Fisher DE. Fisetin: a natural fist against melanoma? J Invest Dermatol. 2011;131:1187–1189. doi: 10.1038/jid.2011.39. [DOI] [PubMed] [Google Scholar]
- 6.Aruoma OI. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res. 2003;523:9–20. doi: 10.1016/s0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 7.Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003;133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- 8.Burdo J. Schubert D. Maher P. Glutathione production is regulated via distinct pathways in stressed and non-stressed cortical neurons. Brain Res. 2008;1189:12–22. doi: 10.1016/j.brainres.2007.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen WS. Lee YJ. Yu YC. Hsaio CH. Yen JH. Yu SH. Tsai YJ. Chiu SJ. Enhancement of p53-mutant human colorectal cancer cells radiosensitivity by flavonoid fisetin. Int J Radiat Oncol Biol Phys. 2010;77:1527–1535. doi: 10.1016/j.ijrobp.2010.02.043. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC. Shen SC. Lee WR. Lin HY. Ko CH. Shih CM. Yang LL. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76:351–359. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 11.Chien CS. Shen KH. Huang JS. Ko SC. Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 12.Dela Cruz CS. Tanoue LT. Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duarte J. Perez-Vizcaino F. Zarzuelo A. Jimenez J. Tamargo J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1993;239:1–7. doi: 10.1016/0014-2999(93)90968-n. [DOI] [PubMed] [Google Scholar]
- 14.Fahey JW. Talalay P. Kensler TW. Notes from the field: “green” chemoprevention as frugal medicine. Cancer Prev Res (Phila) 2012;5:179–188. doi: 10.1158/1940-6207.CAPR-11-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Firuzi O. Lacanna A. Petrucci R. Marrosu G. Saso L. Evaluation of the antioxidant activity of flavonoids by “ferric reducing antioxidant power” assay and cyclic voltammetry. Biochim Biophys Acta. 2005;1721:174–184. doi: 10.1016/j.bbagen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Funakoshi-Tago M. Nakamura K. Tago K. Mashino T. Kasahara T. Anti-inflammatory activity of structurally related flavonoids, apigenin, luteolin and fisetin. Int Immunopharmacol. 2011;11:1150–1159. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Gelderblom M. Leypoldt F. Lewerenz J. Birkenmayer G. Orozco D. Ludewig P. Thundyil J. Arumugam TV. Gerloff C. Tolosa E. Maher P. Magnus T. The flavonoid fisetin attenuates postischemic immune cell infiltration, activation and infarct size after transient cerebral middle artery occlusion in mice. J Cereb Blood Flow Metab. 2012;32:835–843. doi: 10.1038/jcbfm.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon MH. Roedig-Penman A. Antioxidant activity of quercetin and myricetin in liposomes. Chem Phys Lipids. 1998;97:79–85. doi: 10.1016/s0009-3084(98)00098-x. [DOI] [PubMed] [Google Scholar]
- 19.Haddad AQ. Fleshner N. Nelson C. Saour B. Musquera M. Venkateswaran V. Klotz L. Antiproliferative mechanisms of the flavonoids 2,2′-dihydroxychalcone and fisetin in human prostate cancer cells. Nutr Cancer. 2010;62:668–681. doi: 10.1080/01635581003605524. [DOI] [PubMed] [Google Scholar]
- 20.Haddad AQ. Venkateswaran V. Viswanathan L. Teahan SJ. Fleshner NE. Klotz LH. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9:68–76. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 21.Hiipakka RA. Zhang HZ. Dai W. Dai Q. Liao S. Structure-activity relationships for inhibition of human 5alpha-reductases by polyphenols. Biochem Pharmacol. 2002;63:1165–1176. doi: 10.1016/s0006-2952(02)00848-1. [DOI] [PubMed] [Google Scholar]
- 22.Hou DX. Fukuda M. Johnson JA. Miyamori K. Ushikai M. Fujii M. Fisetin induces transcription of NADPH:quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int J Oncol. 2001;18:1175–1179. doi: 10.3892/ijo.18.6.1175. [DOI] [PubMed] [Google Scholar]
- 23.Ishige K. Schubert D. Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 24.Jang HS. Kook SH. Son YO. Kim JG. Jeon YM. Jang YS. Choi KC. Kim J. Han SK. Lee KY. Park BK. Cho NP. Lee JC. Flavonoids purified from Rhus verniciflua stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta. 2005;1726:309–316. doi: 10.1016/j.bbagen.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Jankun J. Selman SH. Aniola J. Skrzypczak-Jankun E. Nutraceutical inhibitors of urokinase: potential applications in prostate cancer prevention and treatment. Oncol Rep. 2006;16:341–346. [PubMed] [Google Scholar]
- 26.Khan N. Adhami VM. Mukhtar H. Apoptosis by dietary agents for prevention and treatment of prostate cancer. Endocr Relat Cancer. 2010;17:R39–R52. doi: 10.1677/ERC-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan N. Afaq F. Khusro FH. Mustafa Adhami V. Suh Y. Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130:1695–1705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan N. Afaq F. Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10:475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 29.Khan N. Afaq F. Syed DN. Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–1056. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan N. Asim M. Afaq F. Abu Zaid M. Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–8563. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J. Kundu M. Viollet B. Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SC. Kang SH. Jeong SJ. Kim SH. Ko HS. Inhibition of c-Jun N-terminal kinase and nuclear factor kappa B pathways mediates fisetin-exerted anti-inflammatory activity in lipopolysccharide-treated RAW264.7 cells. Immunopharmacol Immunotoxicol. 2012;34:645–650. doi: 10.3109/08923973.2011.648270. [DOI] [PubMed] [Google Scholar]
- 33.Kimira M. Arai Y. Shimoi K. Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol. 1998;8:168–175. doi: 10.2188/jea.8.168. [DOI] [PubMed] [Google Scholar]
- 34.Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- 35.Kuntz S. Wenzel U. Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- 36.Lee SE. Jeong SI. Yang H. Park CS. Jin YH. Park YS. Fisetin induces Nrf2-mediated HO-1 expression through PKC-delta and p38 in human umbilical vein endothelial cells. J Cell Biochem. 2011;112:2352–2360. doi: 10.1002/jcb.23158. [DOI] [PubMed] [Google Scholar]
- 37.Lian TW. Wang L. Lo YH. Huang IJ. Wu MJ. Fisetin, morin and myricetin attenuate CD36 expression and oxLDL uptake in U937-derived macrophages. Biochim Biophys Acta. 2008;1781:601–609. doi: 10.1016/j.bbalip.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Liao YC. Shih YW. Chao CH. Lee XY. Chiang TA. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J Agric Food Chem. 2009;57:8933–8941. doi: 10.1021/jf902630w. [DOI] [PubMed] [Google Scholar]
- 39.Lim do Y. Park JH. Induction of p53 contributes to apoptosis of HCT-116 human colon cancer cells induced by the dietary compound fisetin. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1060–G1068. doi: 10.1152/ajpgi.90490.2008. [DOI] [PubMed] [Google Scholar]
- 40.Lu W. Zhu J. Zou S. Li X. Huang J. The efficient expression of human fibroblast collagenase in Escherichia coli and the discovery of flavonoid inhibitors. J Enzyme Inhib Med Chem. 2012 doi: 10.3109/14756366.2012.681650. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Lu X. Jung J. Cho HJ. Lim DY. Lee HS. Chun HS. Kwon DY. Park JH. Fisetin inhibits the activities of cyclin-dependent kinases leading to cell cycle arrest in HT-29 human colon cancer cells. J Nutr. 2005;135:2884–2890. doi: 10.1093/jn/135.12.2884. [DOI] [PubMed] [Google Scholar]
- 42.Maher P. Modulation of multiple pathways involved in the maintenance of neuronal function during aging by fisetin. Genes Nutr. 2009;4:297–307. doi: 10.1007/s12263-009-0142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maher P. Akaishi T. Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A. 2006;103:16568–16573. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maher P. Dargusch R. Bodai L. Gerard PE. Purcell JM. Marsh JL. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington's disease. Hum Mol Genet. 2011;20:261–270. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher P. Salgado KF. Zivin JA. Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. doi: 10.1016/j.brainres.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markovic ZS. Mentus SV. Dimitric Markovic JM. Electrochemical and density functional theory study on the reactivity of fisetin and its radicals: implications on in vitro antioxidant activity. J Phys Chem A. 2009;113:14170–14179. doi: 10.1021/jp907071v. [DOI] [PubMed] [Google Scholar]
- 47.Mohapatra M. Mishra AK. Photophysical behavior of fisetin in dimyristoylphosphatidylcholine liposome membrane. J Phys Chem B. 2011;115:9962–9970. doi: 10.1021/jp1123212. [DOI] [PubMed] [Google Scholar]
- 48.Murtaza I. Adhami VM. Hafeez BB. Saleem M. Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappa B. Int J Cancer. 2009;125:2465–2473. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myara I. Pico I. Vedie B. Moatti N. A method to screen for the antioxidant effect of compounds on low-density lipoprotein (LDL): illustration with flavonoids. J Pharmacol Toxicol Methods. 1993;30:69–73. doi: 10.1016/1056-8719(93)90009-4. [DOI] [PubMed] [Google Scholar]
- 50.Nagai K. Takahashi Y. Mikami I. Fukusima T. Oike H. Kobori M. The hydroxyflavone, fisetin, suppresses mast cell activation induced by interaction with activated T cell membranes. Br J Pharmacol. 2009;158:907–919. doi: 10.1111/j.1476-5381.2009.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen H. Zhang S. Morris ME. Effect of flavonoids on MRP1-mediated transport in Panc-1 cells. J Pharmaceut Sci. 2003;92:250–257. doi: 10.1002/jps.10283. [DOI] [PubMed] [Google Scholar]
- 52.Olaharski AJ. Mondrala ST. Eastmond DA. Chromosomal malsegregation and micronucleus induction in vitro by the DNA topoisomerase II inhibitor fisetin. Mutat Res. 2005;582:79–86. doi: 10.1016/j.mrgentox.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 53.Ragelle H. Crauste-Manciet S. Seguin J. Brossard D. Scherman D. Arnaud P. Chabot GG. Nanoemulsion formulation of fisetin improves bioavailability and antitumour activity in mice. Int J Pharm. 2012;427:452–459. doi: 10.1016/j.ijpharm.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 54.Ravichandran N. Suresh G. Ramesh B. Siva GV. Fisetin, a novel flavonol attenuates benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Food Chem Toxicol. 2011;49:1141–1147. doi: 10.1016/j.fct.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Rice-Evans CA. Miller NJ. Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 56.Sagara Y. Vanhnasy J. Maher P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J Neurochem. 2004;90:1144–1155. doi: 10.1111/j.1471-4159.2004.02563.x. [DOI] [PubMed] [Google Scholar]
- 57.Salmela AL. Pouwels J. Varis A. Kukkonen AM. Toivonen P. Halonen PK. Perala M. Kallioniemi O. Gorbsky GJ. Kallio MJ. Dietary flavonoid fisetin induces a forced exit from mitosis by targeting the mitotic spindle checkpoint. Carcinogenesis. 2009;30:1032–1040. doi: 10.1093/carcin/bgp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scalbert A. Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 59.Sengupta B. Banerjee A. Sengupta PK. Investigations on the binding and antioxidant properties of the plant flavonoid fisetin in model biomembranes. FEBS Lett. 2004;570:77–81. doi: 10.1016/j.febslet.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 60.Shia CS. Tsai SY. Kuo SC. Hou YC. Chao PD. Metabolism and pharmacokinetics of 3,3′,4′,7-tetrahydroxyflavone (fisetin), 5-hydroxyflavone, and 7-hydroxyflavone and antihemolysis effects of fisetin and its serum metabolites. J Agric Food Chem. 2009;57:83–89. doi: 10.1021/jf802378q. [DOI] [PubMed] [Google Scholar]
- 61.Suh Y. Afaq F. Johnson JJ. Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappa B-signaling pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suh Y. Afaq F. Khan N. Johnson JJ. Khusro FH. Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–1433. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 64.Sung B. Pandey MK. Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappa B-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 65.Syed DN. Afaq F. Maddodi N. Johnson JJ. Sarfaraz S. Ahmad A. Setaluri V. Mukhtar H. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/beta-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291–1299. doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syed DN. Suh Y. Afaq F. Mukhtar H. Dietary agents for chemoprevention of prostate cancer. Cancer Lett. 2008;265:167–176. doi: 10.1016/j.canlet.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Szliszka E. Helewski KJ. Mizgala E. Krol W. The dietary flavonol fisetin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells. Int J Oncol. 2011;39:771–779. doi: 10.3892/ijo.2011.1116. [DOI] [PubMed] [Google Scholar]
- 68.Touil YS. Auzeil N. Boulinguez F. Saighi H. Regazzetti A. Scherman D. Chabot GG. Fisetin disposition and metabolism in mice: identification of geraldol as an active metabolite. Biochem Pharmacol. 2011;82:1731–1739. doi: 10.1016/j.bcp.2011.07.097. [DOI] [PubMed] [Google Scholar]
- 69.Touil YS. Seguin J. Scherman D. Chabot GG. Improved antiangiogenic and antitumour activity of the combination of the natural flavonoid fisetin and cyclophosphamide in Lewis lung carcinoma-bearing mice. Cancer Chemother Pharmacol. 2011;68:445–455. doi: 10.1007/s00280-010-1505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res. 2012;65:565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Yang PM. Tseng HH. Peng CW. Chen WS. Chiu SJ. Dietary flavonoid fisetin targets caspase-3-deficient human breast cancer MCF-7 cells by induction of caspase-7-associated apoptosis and inhibition of autophagy. Int J Oncol. 2012;40:469–478. doi: 10.3892/ijo.2011.1203. [DOI] [PubMed] [Google Scholar]
- 72.Yu SH. Yang PM. Peng CW. Yu YC. Chiu SJ. Securin depletion sensitizes human colon cancer cells to fisetin-induced apoptosis. Cancer Lett. 2011;300:96–104. doi: 10.1016/j.canlet.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 73.Zhen L. Zhu J. Zhao X. Huang W. An Y. Li S. Du X. Lin M. Wang Q. Xu Y. Pan J. The antidepressant-like effect of fisetin involves the serotonergic and noradrenergic system. Behavior Brain Res. 2012;228:359–366. doi: 10.1016/j.bbr.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 74.Zheng LT. Ock J. Kwon BM. Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int Immunopharmacol. 2008;8:484–494. doi: 10.1016/j.intimp.2007.12.012. [DOI] [PubMed] [Google Scholar]