Abstract
Despite antismoking campaigns, cigarette smoking remains a pervasive addiction with significant societal impact, accounting for one of every five deaths. Smoking cessation therapies to help smokers quit are ineffective with a high recidivism rate. With the knowledge that nicotine is the principal addictive compound of cigarettes, we have developed an antismoking vaccine based on the highly immunogenic properties of the hexon protein purified from the serotype 5 adenovirus (Ad) capsid. We hypothesized that an effective antinicotine vaccine could be based on coupling the nicotine hapten AM1 to purified Ad hexon protein. To assess this, AM1 was conjugated to hexon purified from serotype 5 Ad to produce the HexonAM1 vaccine. C57Bl/6 mice were sensitized by 10 daily nicotine administrations (0.5 mg/kg, subcutaneous) to render the mice addicted to nicotine. Control groups were sensitized to phosphate-buffered saline (PBS). The mice were then immunized with HexonAM1 (4 μg, intramuscular) at 0, 3, and 6 weeks. By 6 weeks, the HexonAM1-vaccinated mice had serum antinicotine antibody titers of 1.1×106±7.6×104. To demonstrate that these high antinicotine titers were sufficient to suppress the effects of nicotine, HexonAM1-vaccinated mice were evaluated for nicotine-induced hypoactive behavior with nicotine challenges (0.5 mg/kg wt) over 5 weeks. In all challenges, the HexonAM1-vaccinated mice behaved similar to PBS-challenged naive mice. These data demonstrate that a vaccine comprised of a nicotine analog coupled to Ad hexon can evoke a high level of antinicotine antibodies sufficient to inhibit nicotine-induced behavior. The HexonAM1 vaccine represents a platform paradigm for vaccines against small molecules.
Rosenberg and colleagues describe an antinicotine vaccine comprising a nicotine analog (AM1) coupled to hexon protein purified from the adenovirus serotype 5 capsid. They show that this vaccine can elicit high levels of antinicotine antibodies in vivo and that this leads to significant inhibition of nicotine-induced behaviors in C57BL/6 mice.
Introduction
Tobacco use is endemic in the United States and worldwide, with approximately 45 million or nearly 19% of all adults in the United States currently cigarette smokers (King et al., 2011). Smoking is the leading cause of preventable death worldwide, causing more than 5 million deaths per year, and current trends indicate that tobacco use will cause more than 8 million deaths annually by 2030 (World Health Organization [WHO], 2009). In the United States alone, tobacco usage is responsible for about one in five deaths annually (Adhikari et al., 2008). Compared to nonsmokers, cigarette smokers die 13 to 14 years earlier (Fellows et al., 2002).
Cigarette smoking is an addiction, primarily because of the psychoactive properties of nicotine in tobacco smoke (Stolerman and Jarvis, 1995; Office of the Surgeon General, 2010). One potential therapy to treat nicotine addiction is immunotherapy, in which high levels of high-affinity circulating antinicotine antibodies could sequester nicotine in the blood, thereby preventing nicotine from reaching the nicotinic receptors in the central nervous system (CNS) (Kosten and Owens, 2005; Maurer and Bachmann, 2007; Cerny and Cerny, 2009; Kinsey et al., 2009). Although nicotine itself is a small molecule and not immunogenic, the immune system can be induced to recognize nicotine as foreign by attaching nicotine or its analogs to a protein (LeSage et al., 2006; Cerny and Cerny, 2009; Moreno et al., 2010). Clinical trials of vaccines to elicit antinicotine antibodies have been shown to be effective in a subset of smokers, but to date, antinicotine vaccines have failed to generate sufficiently high antinicotine antibody titers to be broadly effective (Maurer and Bachmann, 2007; Cerny and Cerny, 2009).
Based on the knowledge that the capsid proteins of the serotype 5 adenovirus (Ad) are highly immunogenic in humans, we hypothesized that covalently linking a nicotine analog to serotype 5 Ad capsid proteins would elicit high titers of circulating antibodies against nicotine. Leveraging the knowledge that adenoviral hexon capsid protein is the most immunogenic component of Ad (Haase et al., 1972; Molinier-Frenkel et al., 2002), we have developed an antismoking vaccine based on the conjugation of the nicotine analog AM1 (Moreno et al., 2010) to Ad capsid hexons, to produce the HexonAM1 vaccine. The data demonstrates that Ad hexon can be used as a platform to conjugate small molecules like AM1 to evoke high titers of high-affinity antinicotine antibodies sufficient to shield the brain from nicotine.
Methods
Purification of hexon
A recombinant serotype 5 E1a−, partial E1b−, E3− Ad vector (Ad5) with β-galactosidase in the expression cassette in the E1− region was propagated and purified to produce Ad5LacZ (Rosenfeld et al., 1992). Disruption of Ad5 vector (Fig. 1A) was carried out by treating with lithium iodide (22% final concentration; Sigma-Aldrich, St. Louis, MO) in the presence of 0.17 mM sodium thiosulfate at 36°C for 30 min (Neurath et al., 1970). Disrupted Ad5 capsid proteins were diluted 25-fold in 50 mM phosphate buffer pH 7.0 and mixed by vortexing. The capsid proteins were loaded onto a 5-mL prepacked QHP anion exchange column (GE Healthcare, Piscataway, NJ) previously equilibrated with 50 mM phosphate buffer pH 7.0. The column was washed with 10 mL of phosphate buffer pH 7.0. The hexon was eluted from the column with 15 mL of 50 mM phosphate buffer pH 7.0 plus 0.4 M sodium chloride. Eluate was concentrated using Amicon 30K concentrator, diluted 25-fold with phosphate-buffered saline (PBS) and further concentrated. Protein concentration of the purified hexon was determined by the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL).
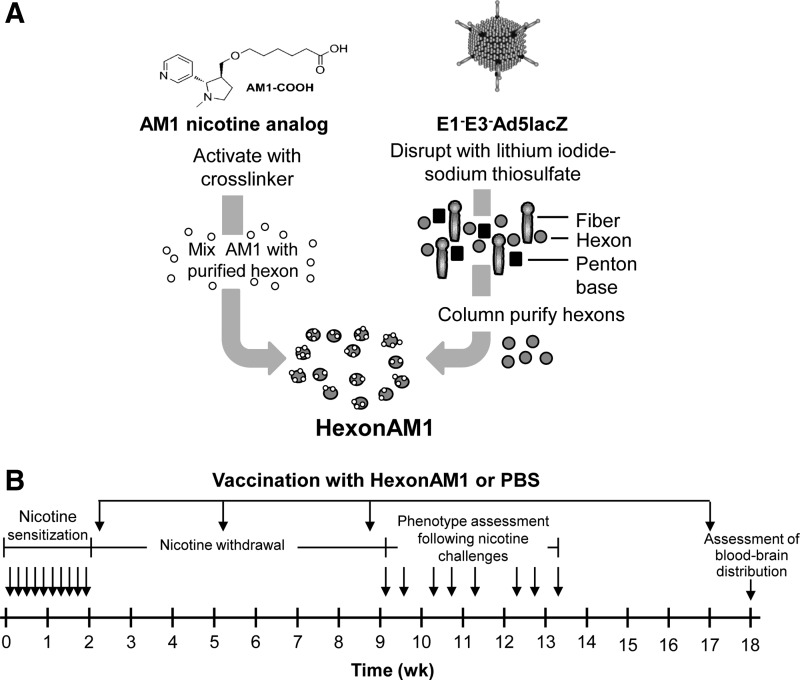
FIG. 1.
Production of HexonAM1 and experimental design. (A) Production of HexonAM1. Shown is a schematic of conjugation of a nicotine analog (AM1) to the purified hexon of disrupted E1−E3− Ad5. (B) Experimental design with timing of presensitization with nicotine, immunization with HexonAM1, phenotype assessment following nicotine challenges and assessment of nicotine blood–brain distribution. Following nicotine presensitization over 2 weeks, the mice were vaccinated with HexonAM1 at 2, 5, 8, and 17 weeks. The timing of phenotypic changes following nicotine challenges are shown by the arrows for each administration at 9 to 13 weeks. At the terminus of the study at 18 weeks, the mice were tested for [3H]nicotine biodistribution in the blood and brain.
HexonAM1 vaccine
The nicotine analog AM1 [rac 6-((trans-1-methyl-2-(pyridin-3-yl)pyrrolidin-3-yl)methoxy)hexanoic acid; Moreno et al., 2010] was activated overnight at 4°C after the addition of 7.2 μL charging solution, made by dissolving 2.4 mg of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride and 2 mg of N-hydroxysulfosuccinimide in 4 μL H2O and 40 μL dimethylformamide (Hicks et al., 2011). Conjugation of 200 μg of hexon with charged AM1 (1:4 hapten to hexon weight ratio) was carried out by incubating overnight at 4°C in 200 μL of PBS, pH 7.4. The conjugated hexon-based vaccine was purified from unreacted small molecules by dialysis against 100 mM Tris-HCl buffer (pH 7.8) containing 20% sucrose. The amount of hexon protein eluated was quantified by the bicinchoninic acid assay (Pierce Biotechnology).
Western analysis
Polyclonal antibody sera to nicotine were produced by conjugating AM1 to KLH at a ratio of 2:1 (Carrera et al., 1995) and 0.1 mg formulated in Freund's adjuvant intramuscularly administered to BALB/c mice (CFA; Sigma-Aldrich). Polyclonal sera derived from a 10-week bleed were used for Western analysis of the conjugated HexonAM1. HexonAM1 protein components were resolved by a 4% to 12% polyacrylamide SDS gel under reducing conditions and transferred to a polyvinylidene difluoride membrane, probed with the antinicotine polyclonal sera or, to assess for the adenovirus components, anti-adenovirus antibody (Abcam, Cambridge, MA). The membranes were developed with horseradish peroxidase–conjugated goat antimouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA), and ECL reagent (GE Healthcare).
Sensitization of mice and nicotine-induced locomotor behavior
All animal studies were carried out under protocols specifically approved for this study by the Weill Cornell Institutional Animal Care and Use Committee.
To determine the effect of the vaccine on mice sensitized to nicotine, nonvaccinated naive C57BL/6 male mice (n=16/group) were administered either saline (PBS) or nicotine [(−) nicotine hydrogen tartrate (Sigma), 200 μL, 0.5 mg/kg wt] subcutaneously in nape of neck, daily, 10 times over the course of 2 weeks (Fig. 1B). The mice were monitored for nicotine-induced changes in activity using infrared beam–equipped open field chambers (20×20 cm chamber, AccuScan Instruments, Columbus, OH). Mice were allowed to habituate to the testing room for >30 min prior to each test. Mice were placed in the chamber for 15 min to record baseline behavior, then removed, injected with PBS or nicotine (0.5 mg/kg wt) subcutaneously and returned to the chamber for 15 min. Locomotor activity was collected as ambulatory distance traveled (cm). Following the last immunization with HexonAM1 (at 8 weeks), mice were tested twice per week for changes in their behavioral activity over a 5-week period (9 to 13 weeks) for a total of eight nicotine challenges (0.5 mg/kg wt) or PBS (n=8/group).
Immunization with HexonAM1 vaccine
Three days following the end of nicotine sensitization, C57BL/6 mice were immunized (n=8) by intramuscular injection to the quadriceps with 4 μg of HexonAM1 vaccine in 50-μL volume, formulated in 20% Adjuplex® (Advanced BioAdjuvants, Omaha, NE) in PBS at 2 weeks, and boosted with the same vaccine mixture at 5, 8, and 17 weeks (Fig. 1B). Adjuplex is an adjuvant made with a purified lecithin and carbomer homopolymer. The quadriceps muscle was used for both the prime and boost vaccine administrations. Blood was collected from the transected tail vein, allowed to clot, centrifuged at 10,000×g for 20 min, and the resulting serum was stored at −20°C.
In vitro assessment of antinicotine antibody titers
Wells of flat bottomed 96-well EIA/RIA plates (Corning, New York, NY) were coated with 100 μL of 1 μg/mL AM1 bovine serum albumin (BSA), conjugated at a ratio of 1:2, in carbonate buffer, pH 9.4 overnight at 4°C (as described above but substituting BSA for KLH). Twofold serial dilutions of collected mouse serum from 0, 3, 6, 12, 14, and 18 weeks were added to each well and incubated for 90 min, 23°C. Serum from Ad5LacZ-immunized mice was used as a negative control for all time points. The enzyme-linked immunosorbent assay was developed as previously described (Hicks et al., 2012).
Nicotine pharmacokinetics
To assess the blood/brain distribution of nicotine in immunized versus nonimmunized mice, naive or HexonAM1 conjugate immunized mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) 2 min prior to tail vein administration of 0.4 μg of nicotine (equivalent to one cigarette) with 1.0 μCi [3H]nicotine (PerkinElmer, Waltham, MA; Fig. 1B). One minute later, the mice were sacrificed and the brain and trunk blood was collected separately. The blood samples were allowed to clot and serum was collected following a 10-min centrifugation at 10,000 rpm. Afterwards, 50 μL of serum were mixed with 20 μL equilibrated Sepharose G (GE Healthcare) in 200 μL phosphate buffer, incubated at room temperature for 90 min, and centrifuged at 2000 rpm for 10 min. Supernatant was separated from the Sepharose G pellet, and the pellet was resuspended in 200 μL of phosphate buffer. Brain tissue was homogenized in PBS, and 300 μL of brain homogenate and 50 μL of serum or Sepharose G isolates were added to separate 5 mL of liquid scintillant (Ultima Gold™, PerkinElmer), assayed in triplicate for tritium and normalized with a standard quenching curve. For the blood compartment, nicotine was normalized to serum volume and brain was normalized to brain wet weight. To correct the brain nicotine content for the nicotine in the brain's blood compartment, we used the estimate of cerebral blood volume of 4.4% from magnetic resonance imaging (MRI; Chugh et al., 2009) and calculated the amount of nicotine associated with that volume and corrected each brain nicotine value.
Statistics
All data are expressed as either geometric means (for serum titers) or group means (behavior parameter)±standard error. Comparisons for the blood–brain distribution experimental groups and mean behavior times were performed with unpaired two-tailed Student's t-test and ANOVA for multiple paired datasets. The t-test score is a measure of the significance of the difference between the means of the populations divided by the standard error of the difference. Behavioral data statistical comparisons between experimental and control groups were performed by two-way repeated-measures ANOVA, matching both row and column factors, with immunization as the between subjects variable and nicotine challenge day as the within subjects variable, and with Bonferroni ad hoc comparisons (Prism 6, GraphPad Software, La Jolla, CA).
Results
Characterization of HexonAM1
Western analysis of the HexonAM1 vaccine demonstrated efficient covalent coupling of the nicotine analog to the hexon protein (Supplementary Fig. S1A and S1B; Supplementary Data are available online at www.liebertpub.com/hum) and also showed that the vaccine is not a mixture of hexon protein and free AM1. Based on these data, the HexonAM1 vaccine created with the AM1 analog and purified hexon was used for subsequent experiments.
Nicotine sensitization and immunization of HexonAM1-vaccinated mice
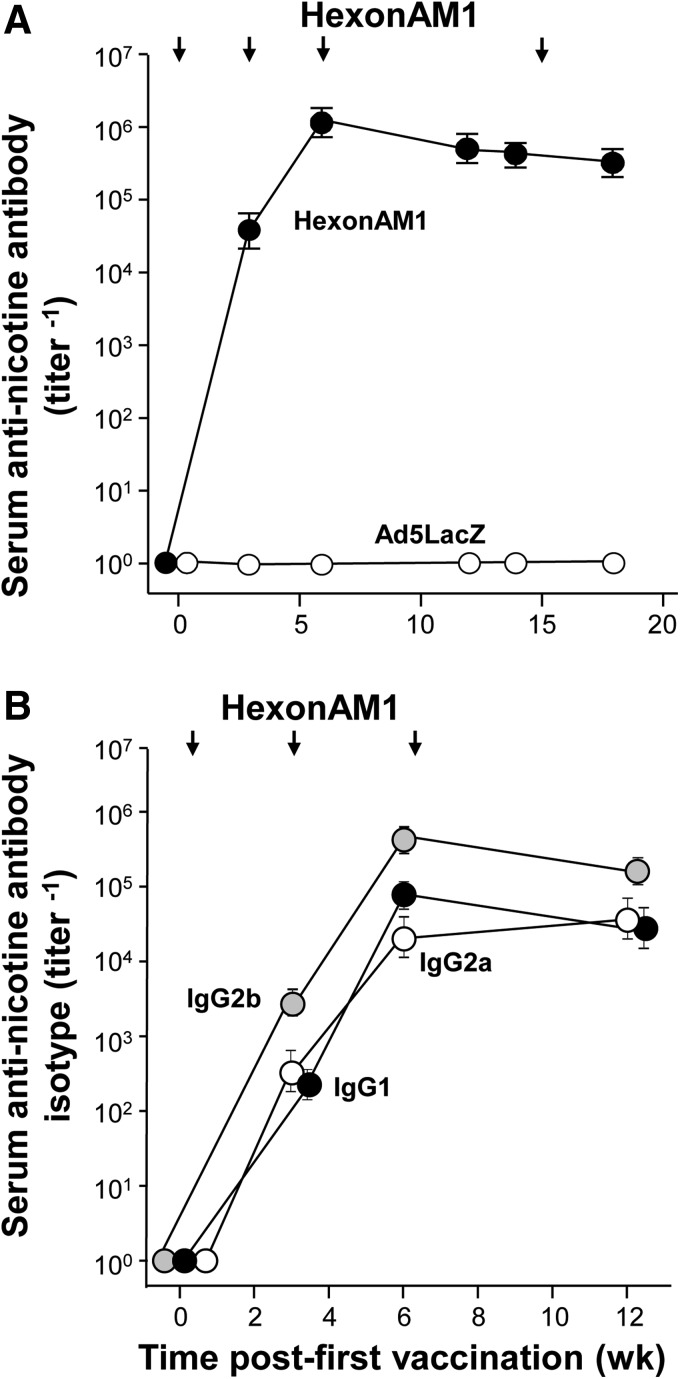
To establish efficacy of the hexon-conjugated vaccine in a mouse model analogous to that of a daily human smoker, we sensitized the mice by administering a subcutaneous bolus of nicotine, daily over a 2-week period prior to vaccination (Fig. 1B). To assess the ability of HexonAM1 to elicit and maintain high titers of antinicotine antibody in serum, HexonAM1 was administered intramuscularly to C57BL/6 mice at the 4-μg dosage at 2, 5, 8, and 17 weeks, and the mice were bled repeatedly to determine serum antinicotine antibody titers. The antinicotine vaccine evoked high levels of antibody at a mean titer of 1.1×106±7.6×104 and 3.1×105±4.2×104 at weeks 6 and 18, respectively (Fig. 2A). Quantification of the time course of isotype-specific titers revealed antinicotine IgG2b, IgG1, and IgG2a titers were detectable at week 3 and increased substantially at week 6, with IgG2b titers a log higher than other isotypes (Fig. 2B).
FIG. 2.
Antinicotine antibody titers in mice after HexonAM1 vaccine administration. C57Bl/6 mice (n=8/group) were sensitized to nicotine (0.5 mg/kg) or to phosphate-buffered saline (PBS), daily 10 times over 2 weeks, followed by immunization intramuscularly with 4 μg of HexonAM1 vaccine at 2, 5, 8, and 17 weeks. (A) Levels of antinicotine antibodies following HexonAM1 vaccination. (B) Antibody IgG isotype titers evoked by the HexonAM1 vaccine.
HexonAM1 vaccinated mice sequester nicotine to the blood compartment
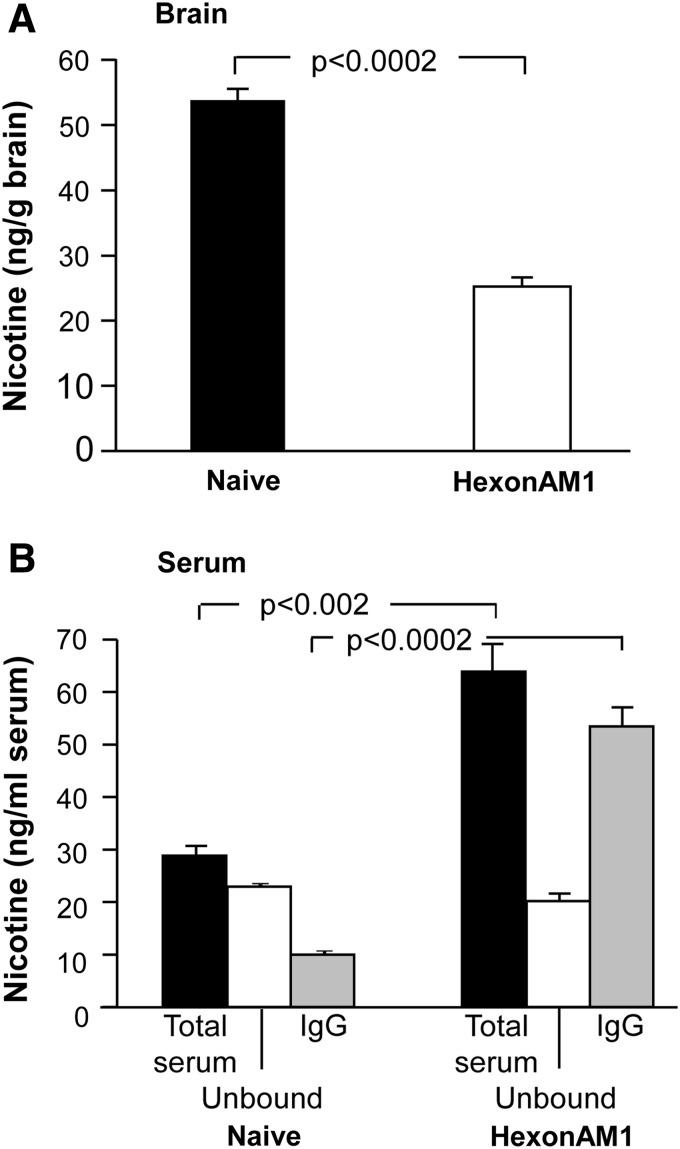
To test whether vaccination with HexonAM1 would express levels of antinicotine antibodies sufficient to shield the brain from systemically administered nicotine, we intravenously challenged HexonAM1 mice with [3H]nicotine in 0.4 μg of nicotine at week 18 postsensitization (Fig. 3). At 1 min after administration, 83% of the total serum nicotine (61.5±4.8 ng/mL) was IgG-bound serum nicotine (51.4±3.3 ng/mL) demonstrating a 5.2-fold increase in IgG-bound serum nicotine compared to naive control mice (9.6±0.4 ng/mL; p<0.0002; Fig. 3B). Conversely, nicotine levels in the brain of HexonAM1-vaccinated mice (25.1±1.4 ng/g brain) were reduced by 53% compared to naive control mice (53.7±1.7 ng/g brain; Fig. 3A), demonstrating a 3.4-fold reduction in the ratio of brain to blood nicotine levels in the HexonAM1-vaccinated mice (p<0.0002).
FIG. 3.
Levels of nicotine in serum and brain of HexonAM1-vaccinated C57Bl/6 mice challenged with nicotine. Shown are nicotine levels in the serum (ng/mL serum) and brain (ng/g brain) of naive or HexonAM1-immunized mice 18 weeks postsensitization. [3H]nicotine in 0.4 μg nicotine was administered intravenously to C57Bl/6 mice (n=3/group). Evaluations were at 1 min following [3H]nicotine challenge. (A) Brain nicotine levels. Shown is data for nonimmunized (naive) and HexonAM1 immunized mice (p<0.0002). (B) Serum nicotine levels. Serum was assessed for IgG-bound [3H]nicotine relative to the [3H]nicotine in the supernatant. Shown is data for nonimmunized (naive) and HexonAM1-immunized mice. For each group, the data includes: total serum nicotine, unbound nicotine, and IgG-bound nicotine (serum, p<0.002; IgG, p<0.0002). Comparisons between groups were conducted by unpaired two-tailed t-test.
Alleviation of nicotine-suppressed locomotor behavior in HexonAM1-vaccinated mice
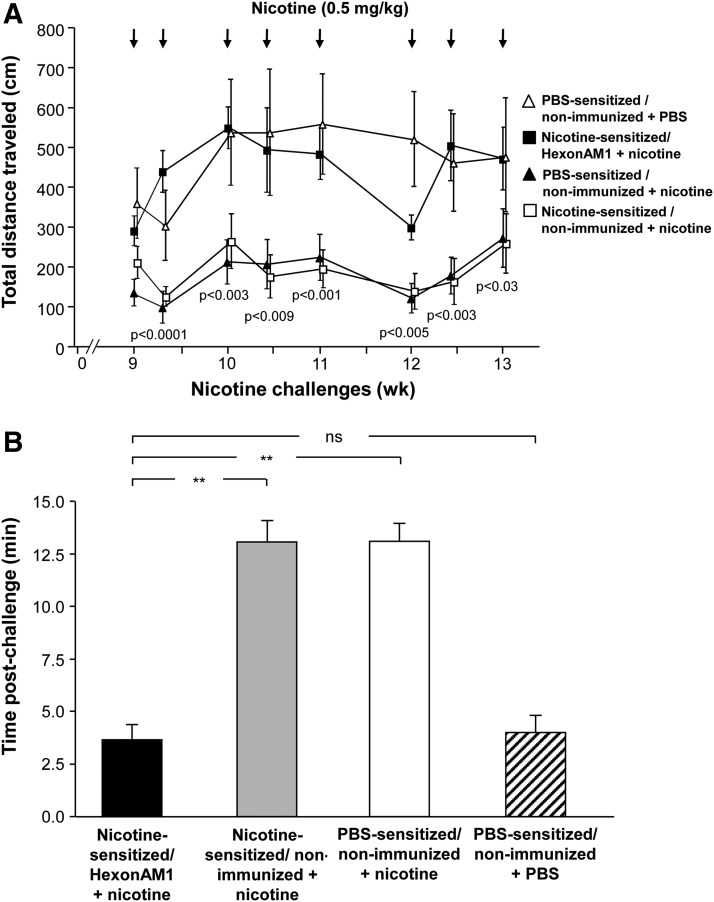
In nicotine-sensitized, HexonAM1-vaccinated mice, repeat challenge with nicotine no longer had the typical nicotine-induced suppression of locomotor activity (Figs. 4, 5). Both nicotine-sensitized and PBS-sensitized mice were repeatedly challenged over a 5-week period (9 to 13 weeks postsensitization) with nicotine bolus subcutaneously (0.5 mg/kg), and the behavior activity assessed for 15 min after each nicotine administration.
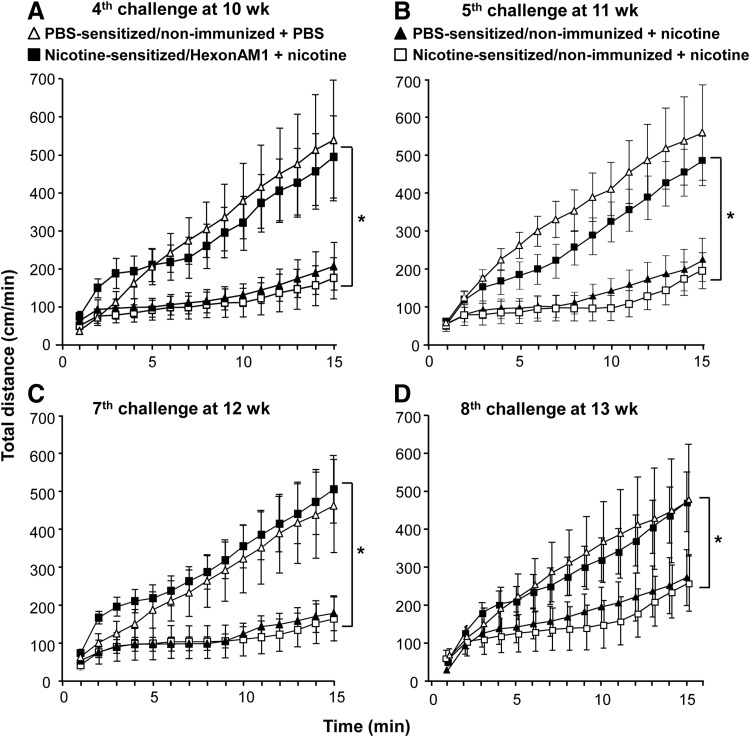
FIG. 4.
Examples of HexonAM1-mediated prevention of nicotine-induced hypolocomotor activity. Assessments of distance traveled over 15 min in nicotine-sensitized and PBS-sensitized mice were compared for nonimmunized and HexonAM1-immunized mice over 5 weeks with frequent challenges of nicotine or PBS. (A–D) Examples of cumulative distance traveled assessed as a function of time post-administration of PBS or nicotine. (A) Fourth nicotine challenge (day 74, 10 weeks postsensitization); (B) fifth nicotine challenge (day 78, 11 weeks postsensitization); (C) seventh nicotine challenge (day 88, 12 weeks postsensitization); and (D) eighth nicotine challenge (day 92, 13 weeks postsensitization).
FIG. 5.
Summary of all nicotine challenges of HexonAM1 vaccination abrogation of nicotine-induced hypoactivity over multiple weeks. Total distances traveled in nicotine-sensitized and PBS-sensitized mice were compared for nonimmunized and HexonAM1-immunized mice over 5 weeks with frequent challenges of nicotine or PBS. The mice were vaccinated 3 days prior to the beginning of the nicotine challenges at 9 weeks. (A) Locomotor activity in HexonAM1-vaccinated mice compared to controls following repeated nicotine challenges. Shown is data for nicotine-sensitized HexonAM1-immunized mice+nicotine (■); nicotine-sensitized nonimmunized mice+nicotine (□); PBS-sensitized nonimmunized mice+nicotine (▲); and PBS-sensitized nonimmunized mice+PBS (Δ); p values are listed between the nicotine-sensitized mice only: HexonAM1-vaccinated+nicotine versus nonvaccinated+nicotine controls. (B) Summary of HexonAM1-vaccination prevention of nicotine-induced hypolocomotor activity. Assessments of distance traveled by the mice postnicotine/PBS challenge were determined in nicotine-sensitized and PBS-sensitized mice to compare the rapid response of the antinicotine antibodies in vivo. The time necessary for each nicotine-challenged mouse to achieve a cumulative distance of 150 cm postnicotine challenges was averaged for each treatment group. Mean time (in min) required by the mice to obtain 150 cm during the eight separate challenges [see (A)] is shown,±SEM. Comparisons between groups were conducted by unpaired two-tailed t-test; **p<0.0001; ns, not significant.
As examples of the ability of vaccination with HexonAM1 to prevent nicotine-induced hypolocomotor activity, nonvaccinated mice administered nicotine at weeks 10, 11, 12, and 13 demonstrated suppression of locomotor activity whether the mice had been sensitized with nicotine or not (Figs. 4A–4D). In contrast, in all four examples, the cumulative total distance over 15 min in the HexonAM1-vaccinated mice was the same as in the PBS-sensitized, nonimmunized mice challenged with PBS, instead of nicotine. Assessment of each of the eight nicotine challenges without and with HexonAM1 vaccination demonstrated significant HexonAM1-mediated alleviation of nicotine-induced hypolocomotor activity (70%–80%) at each challenge over the 5-week test period (Fig. 5; group interaction p values: sensitized HexonAM1+nicotine versus sensitized nonvaccinated+nicotine, p<0.008, F1,7=13.73; versus PBS-sensitized nonvaccinated+nicotine, p<0.002, F1,7=22.1; versus PBS-sensitized nonvaccinated+PBS, p>0.84, F1,7=0.04; two-way ANOVA with repeated measures with Bonferroni ad hoc comparisons). The interaction between group and day was not significant for all groups (p>0.13, p>0.19, p>0.29, respectively). Post hoc analysis using the Bonferroni's multiple comparisons test showed no significance.
As a way to summarize the data into a single parameter for all eight nicotine challenges, we assessed the time it took for the nonvaccinated and vaccinated mice to reach a total ambulatory activity of 150 cm (Fig. 5B). While it took an average of >13 min for the nonvaccinated+nicotine mice to reach a total ambulatory activity of 150 cm, it took an average of <4 min for nonvaccinated+PBS mice (i.e., no nicotine challenge) to reach 150 cm (p<0.0001 and p>0.36, respectively, compared to AM1-vaccinated mice, Fig. 5B). The cumulative distance traveled by the nonvaccinated naive controls (206±54 cm over 15 min) exhibited a noticeable nicotine-induced suppression of ambulatory activity compared to that of HexonAM1+nicotine mice (494±107 cm), which showed no nicotine-induced reduction in locomotor activity similar to nonvaccinated+PBS mice (538±157 cm; Fig. 4D). Interactions were significant between the vaccinated and nonvaccinated groups (Figs. 4A–4D, weeks 10, 11, 12, and 13, respectively; nicotine-sensitized HexonAM1+nicotine versus nicotine-sensitized nonvaccinated+nicotine, p<0.0001 for all four challenges, F14,196=20.5, 16.7, 10.2, 4.9 respectively, two-way ANOVA with repeated measures of time comparisons).
Discussion
Cigarette smoking by adults remains endemic across the globe, despite years of antismoking campaigns and high taxation (Fiore et al., 2008; King et al., 2011; Polosa and Benowitz, 2011). Tobacco addiction is the second-leading cause of death in the world, accounting for approximately 5 million deaths each year or 1 in 10 adult deaths, with an enormous toll on global health care and loss of productivity from death and illness (Adhikari et al., 2008; WHO, 2009; Office of the Surgeon General, 2010). Although some smokers can quit on their own, relapses are frequent, causing smokers to try multiple cessation therapies (Hughes, 2003; Nides, 2008). Pharmaceutical treatments, such as various nicotine delivery strategies, bupropion (Zyban), varenicline (Chantix), nortriptyline, and clonidine have been marketed to alleviate the symptoms of nicotine withdrawal (Nides, 2008; Polosa and Benowitz, 2011). However, the highly variable results of smoking cessation therapies continue to be ineffective in helping smokers quit, with a recidivism rate >70% to 80% by 6 months (Hughes, 2003; Fiore et al., 2008).
Nicotine is the major addictive component of cigarettes (Stolerman and Jarvis, 1995). It is a potent psychoactive drug that acts both as a stimulant and as a depressant (Benowitz, 1996; Picciotto, 2003). Previous research has demonstrated the feasibility of developing a therapeutic antinicotine vaccine, demonstrating that antinicotine antibodies can be evoked by active vaccination with nicotine–protein conjugates, including pseudomonas, tetanus, and diphtheria antigens (Pentel et al., 2000; Carrera et al., 2004; Maurer et al., 2005; Moreno et al., 2010; Polosa and Benowitz, 2011). Phase I and II clinical trials with three different nicotine–protein conjugate vaccines, TA-Nic (cholera toxin B+nicotine analog), NicVax (pseudomonas exoprotein A+3′AmNic analog), and NicQb (Qb bacteriophage+nicotine analog) have all generated antinicotine antibody titers in humans (Cerny, 2005; Hatsukami et al., 2005, 2011; Maurer et al., 2005; Cornuz et al., 2008; Wagena et al., 2008; Polosa and Benowitz, 2011). However, in the NicVAX phase II trial <30% of subjects had significantly high titers necessary to cure smokers of their nicotine addiction and to allow for continued abstinence from smoking (Cornuz et al., 2008; Hatsukami et al., 2011). Despite the improvements in eliciting specific antinicotine in humans, the failure to maintain abstinence among vaccinated smokers reveals a need for an improved antinicotine vaccine with greater immunogenicity.
We have developed a new antinicotine vaccine based on the highly immunogenic properties of the hexon protein of the serotype 5 Ad capsid (Youil et al., 2002; Sumida et al., 2005). Since hexons are the most immunogenic of the Ad capsid proteins (Haase et al., 1972; Molinier-Frenkel et al., 2002; Sumida et al., 2005), we hypothesized that an effective antinicotine vaccine created based on the conjugation of the nicotine AM1 hapten to Ad hexon protein would generate high titers of antinicotine antibodies sufficient to attenuate nicotine-induced behavior in a rodent model.
Hexon-conjugate vaccines
It has been known since the early 1970s that adenoviral hexons are highly immunogenic (Kasel et al., 1971; Haase et al., 1972). In subsequent years, investigators have used modified hexons (insertions and conjugations) to deliver a more immunogenic Ad virus for vaccination against bacteria, protozoa, and other viruses (Worgall et al., 2005; McConnell et al., 2006; Matthews et al., 2008, 2010; Palma et al., 2011). The hexon proteins in the Ad capsid may be responsible for the “adjuvant effect” of the adenovirus in vaccine preparations (Molinier-Frenkel et al., 2002). Through conjugation to free lysines in the Ad capsid proteins, molecules such as polymers, lipids, biotin, fluorophores, and metal nanoparticles have been covalently linked to Ad-hexons (Kramp et al., 1979; Singh and Kostarelos, 2009). Trials have used Ad viral capsid proteins to deliver macromolecules in therapeutic applications, such as MRI contrast agents, radiation sensitizers, and antigenic peptides (HIV-Tat) for vaccine development (Liepold et al., 2007; Vasalatiy et al., 2008; Yoshioka et al., 2008; Singh and Kostarelos, 2009). The high seropositivity to several strains of Ad in the human population, including Ad 2 and Ad5, has limited the success of Ad-based vaccine trials, such as HIV STEP clinical trial (Matthews et al., 2010). Based on this concept, investigators have focused on methods to circumvent pre-existing anti-Ad immunity. Ad5-hexons have been used to make chimeric hexons using other Ad-hexons (Ad1, 2, 6, and 12) that successfully evade anti-Ad immunity (Youil et al., 2002), and Suzuki-Kouyama et al. (2011) incorporated biotin-binding peptides into the hypervariability region (HVR5) on Ad-hexons to allow for immune evasion of PEG-Ad complexes. The use of hexons as a conjugate vaccine appears to be unaffected by anti-Ad neutralizing antibodies, allowing repeat vaccination of HexonAM1 in human clinical trials.
Taking advantage of the immunogenicity of Ad-hexon, administration of the HexonAM1 conjugates to mice succeeded in evoking high titers of antinicotine antibodies that persisted for at least 18 weeks, the last date tested. Importantly, the ability of the HexonAM1-generated antibodies to rapidly sequester circulating nicotine from binding to CNS receptors was demonstrated by the prevention of intravenously administrated nicotine molecules from reaching the brain. These antibodies remained bound to nicotine in the blood, suggestive of high-affinity binding to circulating nicotine. The rapid reduction in [3H]nicotine after 1 min in HexonAM1-vaccinated mice brains (53%) is significant reduction for a “proof-of-principal study”. As summarized in the recent review of (Raupach et al., 2012), preclinical nicotine conjugate vaccine studies showed a wide range of reduction in brain nicotine levels (30%–60%) following acute nicotine administration in vaccinated mice and rats. While the NicQβ conjugate vaccine has been reported to achieve up to a 90% reduction in nicotine brain levels in female Balb/c mice, vaccine dosage used in their trial was eight times the amount used in our current mouse trial (Cerny et al., 2002). Further testing with increasing doses of HexonAM1 vaccine may resolve this difference. In fact, Maurer et al. (2005) demonstrated that different doses and boosting schedules of the NicQβ vaccine resulted in wide range of serum titers and reduction of brain nicotine, of which those mice with the highest titers (>106) had reductions ranging from 40% to 90% (average of 60% reduction).
When mice sensitized to nicotine were vaccinated with HexonAM1 and subsequently challenged with nicotine, there was a dramatic alleviation of nicotine-induced hypoactivity behavior in the mouse locomotor activity in multiple nicotine challenges. While it has been shown that large doses of nicotine are required for alterations in mouse behavior and physiology, it is not known what the threshold of nicotinic receptor-binding is required to effect these nicotine-induced changes (Marks et al., 1985; Collins et al., 1988; Collins and Marks, 1989). In this study, we compared both acute effects of nicotine binding in vaccinated mouse brains and chronic effects of daily challenges with boluses of nicotine solutions on vaccinated mouse behavior.
To date there have been nine different nicotine-conjugate vaccines reported in the literature (LeSage et al., 2006; Hartmann-Boyce et al., 2012; Raupach et al., 2012), with only four successfully attaining clinical trials. Of these, the initial mice trials results were only reported for two vaccines (TA-NIC and Nic-QβNic002). Since each of these antinicotine vaccines used different nicotine haptens and different protein conjugates to present the nicotine haptens, it is difficult to compare their evoking potential of immunity in mice based solely upon the serum titer levels generated in rodents. However, as this study demonstrates, HexonAM1 vaccine evokes higher serum antinicotine antibody titers than any of the previous described nicotine-conjugate vaccines (Raupach et al., 2012), including those reported with NicQβ (Maurer et al., 2005), with a dosage a log less than the previous trials. Importantly, as the clinical trial results have shown for antinicotine vaccines, serum titer levels are not the sole predictor of “success” of the patients remaining nicotine/smoke-free. There appears to be a minimal titer that will allow the majority of the nicotine to be bound, but it does not change the psychological effect of smoking. The second component in preventing smoking recidivism is to block or greatly diminish the effects associated with nicotine administration with smoking. Similar to other antinicotine vaccine studies in rodents, our vaccine prevents the accumulation of nicotine in the brain and strongly abrogates the downstream effects following activation of the nicotinic receptor system, alleviating behavior suppression in mice.
It is reasoned that antidrug vaccines are therapeutic for chronic addictions like nicotine and would help with the addiction relapse and recidivism. In summary, HexonAM1 represents a novel platform active vaccine strategy for addictive drugs.
Supplementary Material
Acknowledgments
We thank Advanced BioAdjuvants, LLC (Omaha, NE) for supplying Adjuplex; and N. Mohamed and D.N. McCarthy for help in preparing this manuscript. These studies were supported, in part, by NIH R01 DA025305 and RC2 DA028847. MJH is supported in part by T32 HL094284. KDJ is supported in part by TRDRP (20XT-0156).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Adhikari B. Kahende J. Malarcher A., et al. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb. Mortal. Wkly. Rep. 2008;57:1226–1228. [PubMed] [Google Scholar]
- Benowitz N.L. Pharmacology of nicotine: addiction and therapeutics. Annu. Rev. Pharmacol. Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- Carrera M.R. Ashley J.A. Parsons L.H., et al. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Carrera M.R. Ashley J.A. Hoffman T.Z., et al. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg. Med Chem. 2004;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Cerny E.H. Cerny T. Vaccines against nicotine. Hum. Vaccin. 2009;5:200–205. doi: 10.4161/hv.5.4.7310. [DOI] [PubMed] [Google Scholar]
- Cerny E.H. Levy R. Mauel J., et al. Preclinical development of a vaccine ‘against smoking’. Onkologie. 2002;25:406–411. doi: 10.1159/000067433. [DOI] [PubMed] [Google Scholar]
- Cerny T. Anti-nicotine vaccination: where are we? Recent Results Cancer Res. 2005;166:167–175. doi: 10.1007/3-540-26980-0_12. [DOI] [PubMed] [Google Scholar]
- Chugh B.P. Lerch J.P. Yu L.X., et al. Measurement of cerebral blood volume in mouse brain regions using micro-computed tomography. Neuroimage. 2009;47:1312–1318. doi: 10.1016/j.neuroimage.2009.03.083. [DOI] [PubMed] [Google Scholar]
- Collins A.C. Marks M.J. Chronic nicotine exposure and brain nicotinic receptors—influence of genetic factors. Prog. Brain Res. 1989;79:137–146. [PubMed] [Google Scholar]
- Collins A.C. Miner L.L. Marks M.J. Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol. Biochem. Behav. 1988;30:269–278. doi: 10.1016/0091-3057(88)90455-8. [DOI] [PubMed] [Google Scholar]
- Cornuz J. Zwahlen S. Jungi W.F., et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows J.L. Trosclair A. Adams E.K., et al. Annual smoking-attributable mortality, years of potential life lost, and economic costs—United States, 1995–1999. MMWR Morb. Mortal. Wkly. Rep. 2002;51:300–303. [PubMed] [Google Scholar]
- Fiore MC. Jaen C.R. Baker T.B., et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. Overview and Methods; pp. 11–34. [Google Scholar]
- Haase A.T. Mautner V. Pereira H.G. The immunogenicity of adenovirus type 5 structural proteins. J. Immunol. 1972;108:483–485. [PubMed] [Google Scholar]
- Hartmann-Boyce J. Cahill K. Hatsukami D., et al. Nicotine vaccines for smoking cessation. Cochrane Database Syst. Rev. 2012;8:CD007072. doi: 10.1002/14651858.CD007072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami D.K. Rennard S. Jorenby D., et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin. Pharmacol. Ther. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Hatsukami D.K. Jorenby D.E. Gonzales D., et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 2011;89:392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M.J. De B.P. Rosenberg J.B., et al. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Mol. Ther. 2011;19:612–619. doi: 10.1038/mt.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M.J. Rosenberg J.B. De B.P., et al. AAV-directed persistent expression of a gene encoding anti-nicotine antibody for smoking cessation. Sci. Transl. Med. 2012;4:140ra87. doi: 10.1126/scitranslmed.3003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.R. Motivating and helping smokers to stop smoking. J Gen. Intern. Med. 2003;18:1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasel J.A. Couch R.B. Douglas R.G., Jr Antigenicity of alum and aqueous adenovirus hexon antigen vaccines in man. J. Immunol. 1971;107:916–919. [PubMed] [Google Scholar]
- King B. Dube S. Kaufmann R., et al. Vital signs: current cigarette smoking among adults aged>/=18 years–United States, 2005–2010. MMWR Morb. Mortal. Wkly. Rep. 2011;60:1207–1212. [PubMed] [Google Scholar]
- Kinsey B.M. Jackson D.C. Orson F.M. Anti-drug vaccines to treat substance abuse. Immunol. Cell Biol. 2009;87:309–314. doi: 10.1038/icb.2009.17. [DOI] [PubMed] [Google Scholar]
- Kosten T. Owens S.M. Immunotherapy for the treatment of drug abuse. Pharmacol. Ther. 2005;108:76–85. doi: 10.1016/j.pharmthera.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Kramp W.J. Six H.R. Drake S., et al. Liposomal enhancement of the immunogenicity of adenovirus type 5 hexon and fiber vaccines. Infect. Immun. 1979;25:771–773. doi: 10.1128/iai.25.2.771-773.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage M.G. Keyler D.E. Pentel P.R. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J. 2006;8:E65–E75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepold L. Anderson S. Willits D., et al. Viral capsids as MRI contrast agents. Magn. Reson. Med. 2007;58:871–879. doi: 10.1002/mrm.21307. [DOI] [PubMed] [Google Scholar]
- Marks M.J. Romm E. Bealer S.M., et al. A test battery for measuring nicotine effects in mice. Pharmacol. Biochem. Behav. 1985;23:325–330. doi: 10.1016/0091-3057(85)90577-5. [DOI] [PubMed] [Google Scholar]
- Matthews Q.L. Yang P. Wu Q., et al. Optimization of capsid-incorporated antigens for a novel adenovirus vaccine approach. Virol. J. 2008;5:98. doi: 10.1186/1743-422X-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews Q.L. Fatima A. Tang Y., et al. HIV antigen incorporation within adenovirus hexon hypervariable 2 for a novel HIV vaccine approach. PLoS One. 2010;5:e11815. doi: 10.1371/journal.pone.0011815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P. Bachmann M.F. Vaccination against nicotine: an emerging therapy for tobacco dependence. Expert Opin. Investig. Drugs. 2007;16:1775–1783. doi: 10.1517/13543784.16.11.1775. [DOI] [PubMed] [Google Scholar]
- Maurer P. Jennings G.T. Willers J., et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur. J. Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- McConnell M.J. Danthinne X. Imperiale M.J. Characterization of a permissive epitope insertion site in adenovirus hexon. J. Virol. 2006;80:5361–5370. doi: 10.1128/JVI.00256-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinier-Frenkel V. Lengagne R. Gaden F., et al. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 2002;76:127–135. doi: 10.1128/JVI.76.1.127-135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A.Y. Azar M.R. Warren N.A., et al. A critical evaluation of a nicotine vaccine within a self-administration behavioral model. Mol. Pharm. 2010;7:431–441. doi: 10.1021/mp900213u. [DOI] [PubMed] [Google Scholar]
- Neurath A.R. Stasny J.T. Rubin B.A. Disruption of adenovirus type 7 by lithium iodide resulting in the release of viral deoxyribonucleic acid. J. Virol. 1970;5:173–178. doi: 10.1128/jvi.5.2.173-178.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. Am. J. Med. 2008;121:S20–S31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Office of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. [PubMed] [Google Scholar]
- Palma C. Overstreet M.G. Guedon J.M., et al. Adenovirus particles that display the Plasmodium falciparum circumsporozoite protein NANP repeat induce sporozoite-neutralizing antibodies in mice. Vaccine. 2011;29:1683–1689. doi: 10.1016/j.vaccine.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentel P.R. Malin D.H. Ennifar S., et al. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol. Biochem. Behav. 2000;65:191–198. doi: 10.1016/s0091-3057(99)00206-3. [DOI] [PubMed] [Google Scholar]
- Picciotto M.R. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol. Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- Polosa R. Benowitz N.L. Treatment of nicotine addiction: present therapeutic options and pipeline developments. Trends Pharmacol. Sci. 2011;32:281–289. doi: 10.1016/j.tips.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach T. Hoogsteder P.H. Onno van Schayck C.P. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72:e1–16. doi: 10.2165/11599900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M.A. Yoshimura K. Trapnell B.C., et al. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Singh R. Kostarelos K. Designer adenoviruses for nanomedicine and nanodiagnostics. Trends Biotechnol. 2009;27:220–229. doi: 10.1016/j.tibtech.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Stolerman I.P. Jarvis M.J. The scientific case that nicotine is addictive. Psychopharmacology (Berl.) 1995;117:2–10. doi: 10.1007/BF02245088. [DOI] [PubMed] [Google Scholar]
- Sumida S.M. Truitt D.M. Lemckert A.A., et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005;174:7179–7185. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- Suzuki-Kouyama E. Katayama K. Sakurai F., et al. Hexon-specific PEGylated adenovirus vectors utilizing avidin-biotin interaction. Biomaterials. 2011;32:1724–1730. doi: 10.1016/j.biomaterials.2010.10.060. [DOI] [PubMed] [Google Scholar]
- Vasalatiy O. Gerard R.D. Zhao P., et al. Labeling of adenovirus particles with PARACEST agents. Bioconjug. Chem. 2008;19:598–606. doi: 10.1021/bc7002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagena E.J. de V.A. Horwith G., et al. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob. Res. 2008;10:213–218. doi: 10.1080/14622200701704921. [DOI] [PubMed] [Google Scholar]
- World Health Organization. France: WHO Library Cataloguing-in-Publication Data; 2009. WHO Report on the Global Tobacco Epidemic, 2009: Implementing smoke-free environments. [Google Scholar]
- Worgall S. Krause A. Rivara M., et al. Protection against P. aeruginosa with an adenovirus vector containing an OprF epitope in the capsid. J. Clin. Invest. 2005;115:1281–1289. doi: 10.1172/JCI23135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Y. Asavatanabodee R. Eto Y., et al. Tat conjugation of adenovirus vector broadens tropism and enhances transduction efficiency. Life Sci. 2008;83:747–755. doi: 10.1016/j.lfs.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Youil R. Toner T.J. Su Q., et al. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 2002;13:311–320. doi: 10.1089/10430340252769824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.