Abstract
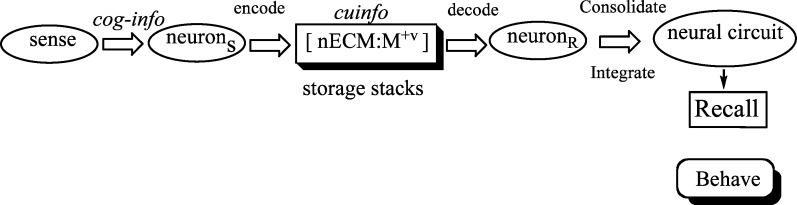
We propose a tripartite mechanism to describe the processing of cognitive information (cog-info), comprising the (1) neuron, (2) surrounding neural extracellular matrix (nECM), and (3) numerous “trace” metals distributed therein. The neuron is encased in a polyanionic nECM lattice doped with metals (>10), wherein it processes (computes) and stores cog-info. Each [nECM:metal] complex is the molecular correlate of a cognitive unit of information (cuinfo), similar to a computer “bit”. These are induced/sensed by the neuron via surface iontophoretic and electroelastic (piezoelectric) sensors. The generic cuinfo are used by neurons to biochemically encode and store cog-info in a rapid, energy efficient, but computationally expansive manner. Here, we describe chemical reactions involved in various processes that underline the tripartite mechanism. In addition, we present novel iconographic representations of various types of cuinfo resulting from“tagging” and cross-linking reactions, essential for the indexing cuinfo for organized retrieval and storage of memory.
Keywords: Memory, tripartite model, cognitive unit of information (cuinfo), neuron, extracellular matrix, trace metal
What is biologic memory? How can one describe the sensation of “memory” in molecular terms? What is the atomic correlate of memory? How is memory stored and lost? What formalism describes the encoding and storage of cognitive information (cog-info)?
One would like to formulate a molecular mechanism that is physiologically credible and biochemically based. It must operate rapidly (faster than neural firing at <100 ms) with available biological materials in an aqueous environment at 37 °C, using ∼400 cal/day, and offer huge computational capabilities. It should permit a chemical explanatory framework for describing “synaptic plasticity” and “long-term potentiation” (LTP)1−6 or forgetting.
In a previous article, we proposed a “tripartite” mechanism, wherein neurons, encased in a lattice of neural extracellular matrix (nECM), employ more than 10 trace metals (dopants) to encode, store, and decode cog-info.7 The neurons employ the nECM as an “information lattice”, comparable to the workings of a computer memory chip which encodes, stores, and retrieves binary “bits” (0/1) in an inorganic matrix. Neurons also release vesicles containing neurometals (Cu2+, Zn2+). We cited the literature which correlates the appropriate availability of trace elements as well as the functioning of nECM, with recall or memory. Due to limitations of space, we will not repeat the arguments and the cited references in our previous article.7 Rather, below we summarize and expand on the underlying chemistry8−27 and neuroelectric biology28−38 of the tripartite mechanism, as it relates to the nECM with trace metals.39−46
Definitions
The following terms are defined here for later discussions, as follows:
-
•
Tripartite System: Memory emerges from the dynamic interaction of three physiologic compartments:
-
1.
Neurons
-
2.
Neural extracellular matrix (nECM), encasing the neuron
-
3.
Trace metals, dispersed within the nECM (dopants)
-
1.
-
•
cog-info: Abbreviation of “cognitive information” referring to basic unit of information obtained from the senses, employed by the neuron to compute (mentate).
-
•
cuinfo: Cognitive unit of information, embodied as a [nECM:metal] complex (singular and plural); the molecular correlate of cog-info, equivalent to computer bit, which is used by the neuron as an information packet.
The computational components of the brain, the neuron and neural circuits, operate by electric and chemical signaling in an aqueous, but not empty, extracellular environment, comprising nECM with dispersed trace metal “dopants”.
Neuron
The neuron is intimately connected to the external nECM by numerous electrically active surface features, notably, integrins, gap junctions, nodes of Ranvier, and chemical and electrical synapses. These are sensitive to the nanoscale dielectric, piezoelectric, and elastic properties of the nECM. Some proteins (tenascin) can attach directly to the neural membrane, providing another channel for neural interdigitation with the nECM. Thus, the neuron can be described as a cell that is intimately connected to its environment by virtue of the many electroeffectors, sensors, and receptors on its surface membrane.
Comments on the Histology of Neurons
The structure of the neuron was initially elucidated from the histological works of Golgi and Cajal and many subsequent neurobiologists. They used a silver nitrate staining method, wherein atomic silver is deposited as nanoparticles, outlining cell structures.
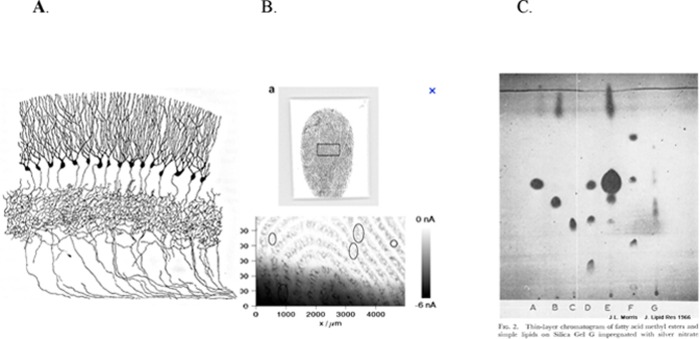
Analytic chemists know that soluble silver salts (i.e., AgNO3) in aqueous solutions are clear and uncolored. Experimental observations indicate that the cation Ag+ attaches particularly to lipids (especially double bonds (C=C).47,48 Exposure to a reducing agent generates elemental silver (Ag0), detected as a shiny surface precipitate (e.g., the Tollen’s test for reducing sugars). Shiny silver is easily oxidized, becoming visibly black (Ag2O). Thus, Golgi and Cajal used the Ag+ nitrate stain to selectively visualize the lipidic neural surface membranes as well as intraneural compartments (nucleus, vesicles) of the newly identified neurons with synaptic connectors (Figure 1A). The silver stain technique has been also used to identify lipids for forensic49 (Figure 1B), chromatographic applications47 (Figure 1C) and neurotoxicity testing.50
Figure 1.
Silver stain of (A) hippocampus neurons by Golgi (note the blank background). Reprinted from ref (98). (B) Forensic fingerprint (no cells). Reprinted with permission from ref (49). Copyright 2007 ASBMB. (C) Thin layer chromatogram of lipids. Reprinted with permission from ref (47). Copyright 1966 Elsevier.
As the nECM is mainly a three-dimensional (3D) mesh composed of polysaccharide with very few reducing sugars, it does not react with the Ag+. Subsequent histologists with other types of stains (Nissle, immunochemical) continued this tradition of exclusively imaging the neuron as the main player in brain function, ignoring the contribution of the nECM. The converse of the tale of the “Emperor’s New Clothes” comes to mind. Everyone, save one boy, perceived the Emperor as clothed, though he was naked. Here, the neuron is viewed as naked, in synaptic contact with other, similarly naked, neurons, though the neurons are “clothed” with nECM.
nECM Characterization
The nECM can be described as a block copolymer, comprising a number of glycosaminoglycans (GAGs), polysaccharides of varying dimensions, as well as various proteins (for more details, see the appropriate references in ref (7)).51−56 All these contribute many electron rich (Lewis base) moieties that cooperatively chelate individual metal cations. These anionic coordination groupings are termed “binding pocket”, “chelating node”, or “address”. The binding characteristics of each metal cation for the electron rich (i.e., amine, carboxyl, phosphate, sulfate, hydroxyl, ether) moieties of the nECM is reflected by quantitative chemical parameters such as
These parameters reflect the inherent affinity of metals (found in the brain) with the electron rich, moieties presented by the nECM. The entrapment of a metal cation “locked” at a specific configuration/shape, effectively is defined herein as cuinfo (see below), sensed by the neuron.
Metals
The elemental metals are each uniquely distributed among the brain’s anatomic compartments. While most of the metals are present within the neurons, a significant amount, about 10%, is present in the nECM. Table 1 summarizes the gross levels of a number of elements in the brain, arranged as sets of monovalent, divalent, and polyvalent elements.
Table 1. Groups of Brain Elements.
| metal | level | conc. unit |
|---|---|---|
| monovalent | ||
| K+ | 3.4 | M |
| Na+ | 2.7 | M |
| Rb+ | 3 | mM |
| Li+ | ∼1 | mM |
| Cs+ | ∼1 | mM |
| divalent | ||
| Mg+2 | 0.3 | M |
| Ca+2 | 60 | mM |
| Zn+2 | 6 | mM |
| polyvalent: redox active | ||
| Al+2/3 | 14–20 | uM |
| Co+1/2 | 6 | mM |
| Cu+1/2 | 3 | mM |
| Cr+2/3 | 153 | uM |
| Fe+2/3 | 40 | mM |
| Mn+2/3 | 211 | uM |
Their distribution is not homogeneous, differing among anatomic compartments. Today’s invasive techniques, such as neutron activation analysis, atomic spectroscopy, mass spectrometry, and fluorescent labeling,57 only permit such analysis on ex vivo slices of the brain.
The process by which neurons specifically accumulate and store each of the elements is not generally known. Vesicles containing Ca2+ are known to be released upon neural activation (firing). Little else has been described regarding the selectivity of neurons for one or another traces elemental cation or their accumulation of redox activators (ascorbate, glutathione). However, it is apparent that the trace metals and some oxidants within the blood permeate the nECM to reach the neurons via passage through the nECM surrounding the cell.52−56 Some metals may freely diffuse through the nECM; others may be actively transported by metallothioneins or other metal transporters.
Results and Discussion
Metal Binding Configurations
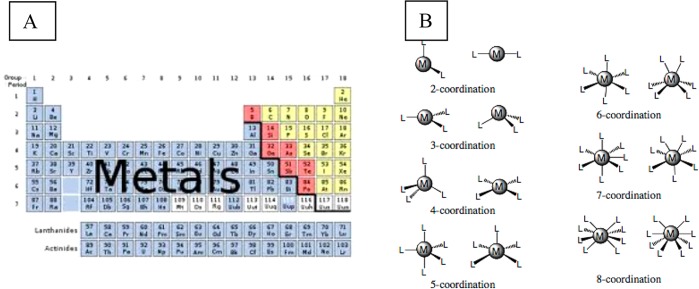
The elemental metal cations (Figure 2A) dispersed within the nECM each can achieve a number of bonding geometries consonant with their electron shell disposition. For example, a divalent metal complex wherein the central atom is combined with 6 different electron rich moieties could exist in 15 geometrically isomeric forms (Figure 2B). Most metal cations diffuse through the nECM as hydrated cations, combined with at lease 4 water molecules each.
Figure 2.
(A) Table of elements, emphasizing the physiologically relevant trace metals. (B) Configurational possibilities of a single metal cation binding to multiple anionic moieties (chelating node).
In aqueous environments, monovalent elemental cations (Li+, Na+, K+, Rb +, Cs+) are not capable of forming strong chelate bonds. They usually exist as tetrahedral water complexes, with coordination radii in the range 2.2–3.3 Å (0.2–0.3 nm). Chelate complexes with monovalent elements in water are not very stable and tend to disintegrate. Notwithstanding, these metals are present in the brain at millimolar levels with diffusion coefficients D ranging from 13 × 10–6 to 21 × 10–6 cm2/s. Thus, they are available to rapidly form short-lived complexes, possibly correlated to short-term memory (STM).
By contrast, divalent and polyvalent elemental cations can form a multitude of more stable chelate complexes, consonant with each element’s electron shell organization.8−19,58 For example, Ca2+ is tetra coordinate with a diffusion coefficient (in nECM) on the order of 2–3 × 10–6 cm2/s. Zn2+, which has a complex electron shell, is not redox active but can achieve a few bonding geometries, most frequently tetrahedral as well as square pyramidal and octahedral configurations (see Figure 2B). Similar descriptions can be made for all the di- and trivalent metal cations, each which can attach to selected coordination sites within the nECM, each achieving unique binding configurations. Assuming a chelation bond with two anionic sites, one could formulate the metal complexation reactions as (Figure 3):
Figure 3.
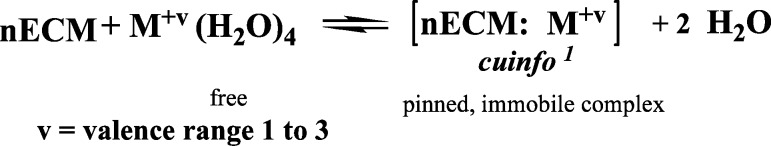
Reaction of hydrated metal cation with nECM.
The nECM is a highly hydrated gel with many associated water molecules attached thereto. The water allows electrical current to dissipate through the nECM and also serves to hydrate the M+v, discarded as leaving groups upon chelation. Common usage generally ignores the solvent when describing chemical reactions; so shall we.
The term “mechanism” is employed here, in a chemical manner. It describes a chemical reaction at molecular- or atomic-scale stages, where atomic elements interact with one another to make or break covalent or ionic chemical bonds, forming molecules and molecular complexes.
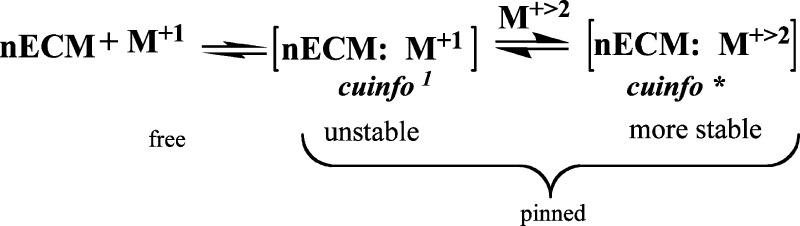
In that spirit, we propose that the formation of [nECM:metal] complexes with monovalent and polyvalent elemental cations represents the minimal cognitive unit of information (cuinfo). The initially formed, but unstable, monovalent complexes become transformed into polyvalent complexes, which are generally more stable, as follows (Figure 4):
Figure 4.
Scheme of the reaction of monovalent and divalent metal cation with nECM.
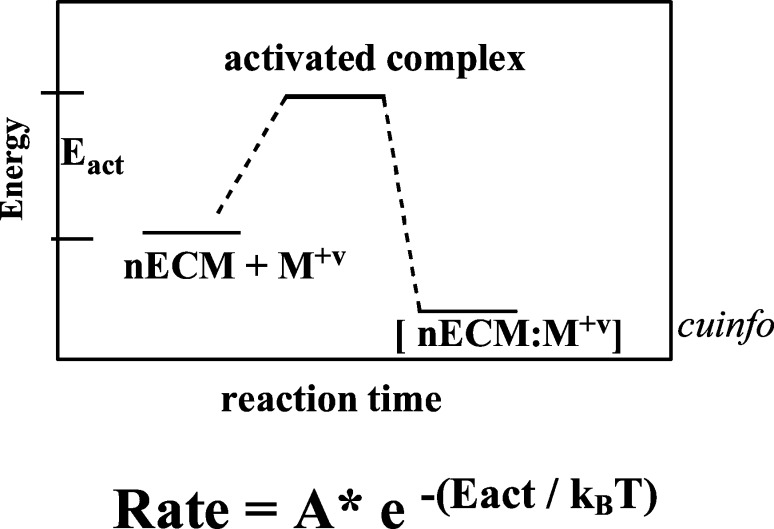
The energetic of metal complexation to the nECM to form cuinfo, is outlined in the energy diagram below (Figure 5):
Figure 5.
Free energy diagram for complexation of M+v to nECM, the kinetics of which are described by the Boltzmann rate equation. The energy of activation (Eact) is low, the rate is rapid (kB = Boltzmann constant), and the reaction is not very exothermic (v = valency state, usually from 0 to +3).
The illustration is meant to point out that the free energy (ΔG), enthalpy (ΔH), as well as the activation energy (Eact) for adsorption/desorption of metal cations to polyanionic substrates to form cuinfos are all low, characterized by rapid rates with little heat generation.
The energetic of metal complexation reactions (on the order of 5–10 kcal/mol) permit rapid, but not always reversible, dynamics. At physiologic levels (concentration of 10–6–10–9 M), the trace elements in the nECM bind with low energy requirements (5–10 kcal/mol, the brain expending 400 cal/day), generating little heat, but affording huge combinatorial coding options. By contrast, the Blue Gene IBM supercomputer operates at 100 petaflops/s and uses more that 6.6 MW/h.
Redox Reactions
Multivalent elements, such as copper, iron, and manganese, can achieve more than 2 oxidation states, each with a number of complexation geometries, as summarized in Table 2.
Table 2. Coordinating Configurations.
| oxid state | val config | coord no. | shape |
|---|---|---|---|
| Cu+1 | d10 | 4 | tetrahedral |
| Cu+2 | d9 | 4 | square planar |
| 5 | trigonal-bipyramid | ||
| 5 | square-pyramidal | ||
| 6 | octahedral | ||
| Zn+2 | d10 | 4 | tetrahedral |
| 5 | square-pyramidal | ||
| 6 | octahedral | ||
| Fe+2 | d6 | 4 | tetrahedral |
| 5 | trigonal bipyramid | ||
| 6 | octahedral | ||
| Fe+3 | d5 | 4 | tetrahedral |
| 6 | octahedral | ||
| 7 | pentagon-bipyramid |
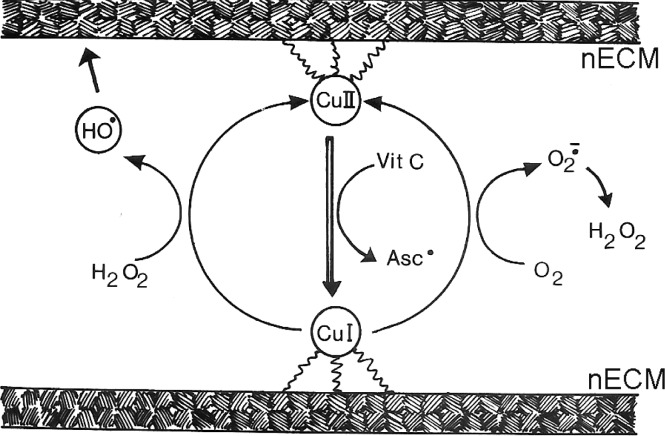
Small redox activators, notably oxygen, ascorbate (0.7 mM in nECM), and glutathione (1 mM),59−63 with diffusion coefficients of 2–4, 1.6, and 5.6 × 10–6 cm2/s, respectively, are also present in the nECM.64−66 Thus, the significant concentrations of redox ingredients for site-specific Fenton reactions with reactive metals are present in the neuron and the nECM. A Fenton reaction occur with cationic copper cycles between oxidation states of Cu(I) and Cu(II). Similarly, iron cycles between Fe(II) and Fe(III) (see Figure 6).
Figure 6.
Schematic representation of the site-specific Fenton reaction based on the interaction of nECM-bound Cu+1/2 with ascorbate, to generate reactive OH radicals, generating new keto C=O from C–OH groups.
Tagging Metal Complex via Redox Reactions
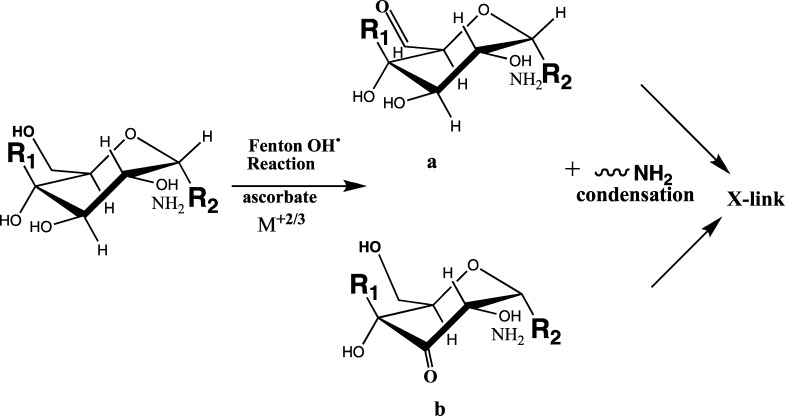
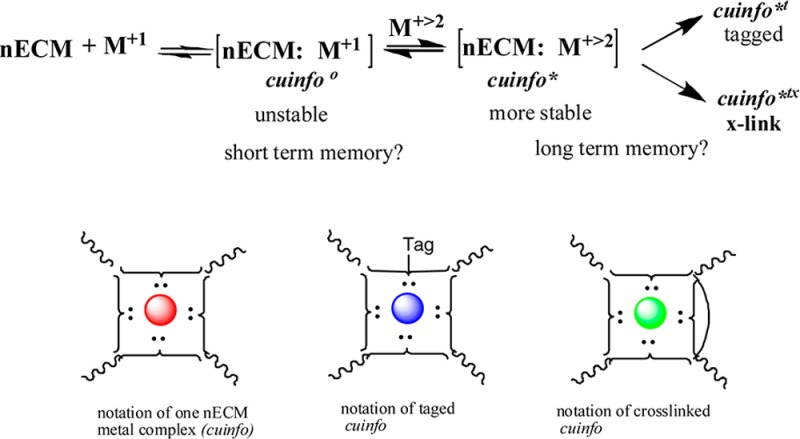
As a consequence of local redox reactions between oxidants (such as ascorbate and glutathione) and multivalent metal complexes, carbonyl groups could be formed on the many glycans comprising the nECM. The many sugars and proteins that make up the nECM are rich in oxidation prone groups (such as primary and secondary hydroxyl OH) that could be oxidized into carbonyl (C=O) groups. Thus, the OH radicals, locally generated by the Fenton reaction, can derivatize the nECM lattice, by introducing aldehyde or keto carbonyl groups at specific locations, or causing carbohydrate ring-opening, leading to the formation of flexible “hinges” (Figure 7). Thus, [nECM:M+2/3] complex, designated as cuinfo*, could be tagged with one or more unique carbonyl moieties, with concomitant unique nanostructural, viscoelastic, and dielectric alterations, referred to as cuinfo*t. This process would provide the cog-info complex with an identifier, a “tag” for sorting and easier recall, such as required to remember strong stimuli (pain, fear, hunger, pleasure, attention). Also, the tagged cuinfo*t could help index (classify) the stored cog-info, accelerating recall.
Figure 7.
Monomeric saccharide unit (glucose or glucosamine for example), within the nECM polymeric matrix (sites R and R2), showing multiple hydroxyl groups, capable of being oxidized to carbonyl groups by the Fenton reaction: aldehyde (a above) or keto group (only one isomer shown in b). A neighboring amino group from other glycans (e.g., amino sugars) or imbedded proteins can engage in condensation reactions causing cross-linking (see below).
There are multiple positions available in the saccharide subunits of the nECM (chondroitins, heparans, hyaluronates) for such carbonyl formation. Each saccharide (sugar) ring usually presents more than three oxidizable hydroxyl (OH) groups.
Ascorbate and glutathione are redox drivers of such reactions. For example, ascorbate is a very reactive molecule. In blood,59−63 it is sequestered within platelets, with high internal platelet ascorbate levels (mM range), compared to low blood plasma levels (μM range). An ascorbate level in neurons has been determined to be in the 1–10 mM range.55−57 Thus, the neuron has the resources (polyvalent metals and oxidants) to affect redox Fenton reactions within the nECM, whenever it is appropriately activated.
Stabilizing Metal Complexes within the nECM
a. Enzymatic Cross-linking
An additional mechanism that forms and affects long-term memory is cross-linking reactions affected by enzymes in the nECM, such as transglutaminases,12 which induce the formation of a covalent amide linkage between amine and primary amide moieties (such as Lys and Gln side chains), as follows (Figure 8):
Figure 8.
General reaction scheme of cross-linking by transamidation.
The resultant amide bond is not susceptible to peptidases. For example, a fibrin clot is rendered more stable by the cross-linking induced by factor XIII (a transglutaminase). Cross-linking renders the [nECM:metal] complex (a cuinfo) more stable, suitable for long-term storage, but available for recall.
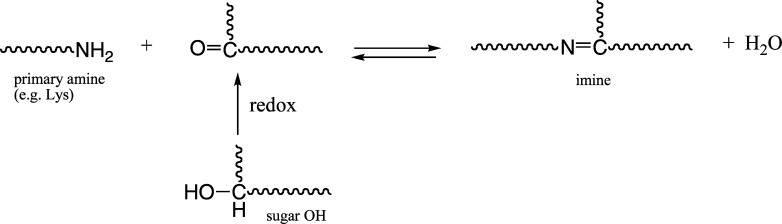
Nonenzymatic Cross-Linking via Free Radical (Fenton) Reactions
As a consequence of local redox reactions between oxidants (such as ascorbate and glutathione) and multivalent metal complexes, carbonyl groups (keto or aldehyde) could be formed on the many carbohydrates comprising the nECM (see above). The carbonyl groups can spontaneously condense with amine (−NH2) groups forming imines (Schiff bases) as follows (Figure 9):
Figure 9.
General reaction scheme of Schiff base cross-linking reaction.
The Schiff base reaction is reversible. It can be maintained by the hydrophobic conditions, such as found in hydrophobic pocket of proteins, which greatly increases its stability. Conversely, exposure to hydrating conditions with slight pH variations could reverse the direction of the reaction, breaking the immine linkage.
Thus, the redox reaction leading to aldo/keto group formation, could effectively derivatize the [nECM:metal] complex, and also lead to cross-linking reactions, stabilizing the ensemble, ensuring storage of encoded cog-info.
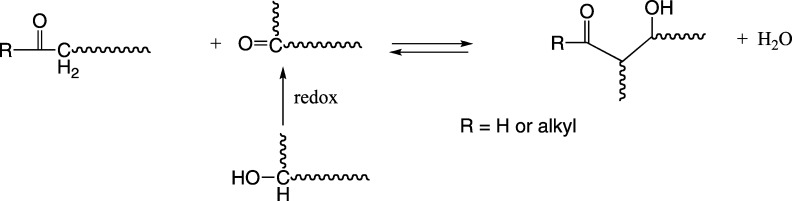
Other condensation reactions between carbonyl groups, giving rise to cross-links, are shown in Figure 10. Aldol condensation12 can occur in physiological media either by enzymatic catalysis (aldolases) or occur spontaneously by general base catalysis. The aldol condensation causes the formation of a covalent C–C bond. Such reactions have been shown to occur in the eye and the extracellular matrix of other tissues.
Figure 10.
General reaction scheme of aldol condensation after oxidation of hydroxyl group.
Other types of cross-links can be formed, such as those induced by lysyl oxidase-dependent and those originating from stochastic processes, such as the Maillard reaction, oxidation (for instance, dityrosine), and lipid peroxidation (such as malonyl dialdehyde-lysine cross-links).67−71
The essential point here is that a number of cross-linking reactions and locales are available for stabilizing the [nECM:metal] complexes, effectively ensuring long-term storage of cuinfo encoding cog-info, critical to memory. Alternatively, these processes can occur in an uncontrolled manner in pathological states in which memory is destroyed (e.g., dementia and Alzheimer’s disease).
Generations of cuinfo
The scheme whereby various generations of cuinfo are transformed by various reactions described above is outlined in Figure 11.
Figure 11.
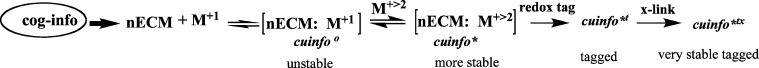
Reaction scheme for hierarchical formation of various types of cuinfo.
Iconographic representation of various classes of cuinfo is shown in Figure 12.
Figure 12.
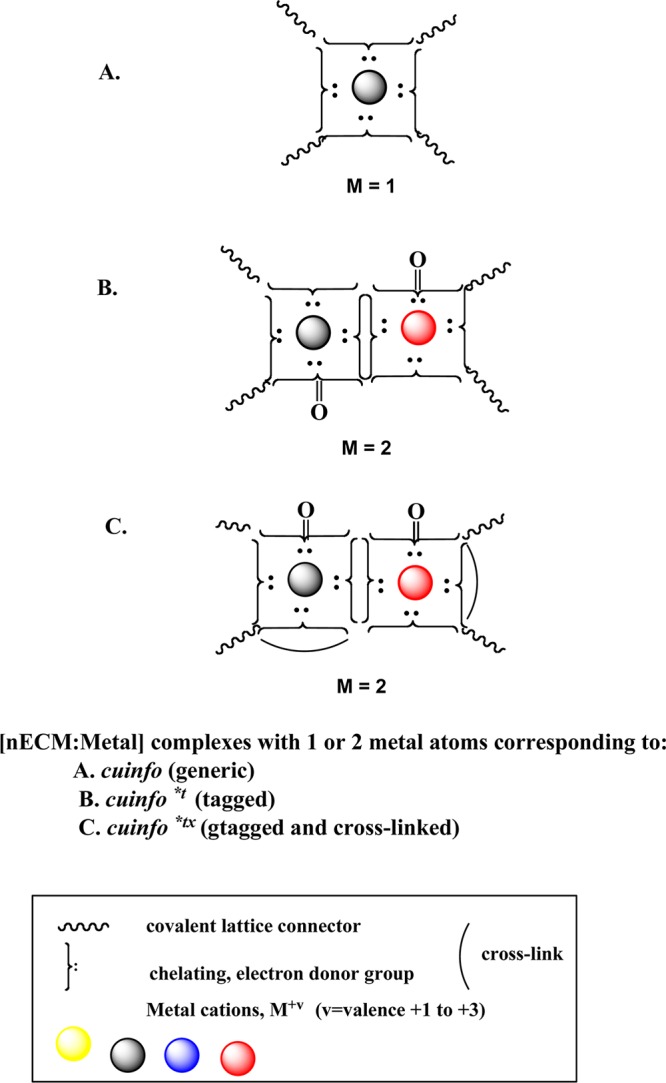
Iconographic icons of various types of cuinfo (singular/plural). (A) Metal complexes, the correlates of cuinfo*, formed with 1 or 2 metals per unit. (B) Tagged cuinfo*t, modified to express a keto (C=O) carbonyl, resulting from a Fenton-reaction generating OHradicals (tagged). (C) Cross-linked cuinfo*tx.
Pathways involving transglutaminase enzymes, or redox reactions (involving Schiff base condensation, etc), further stabilize the complex by introducing one or more cross-linkers, rendering the metal complex more enduring.
Tagging cuinfo
Aside from the above-discussed Fenton reaction, other modes of tagging cuinfo* could involve reactions such as acetylation, phosphorylation,72 sulfation, and methylation. These types of reactions are known to turn on/off certain metabolic pathways for proteins, DNA, and carbohydrates and are used in signal transductions.
Combinatorial Diversity of Tagging and Cross-Linking
We have described five types of tags (C=O, OAc, SO3–, PO3–, OMe) and three types of cross-links (tranglutaminates, Schiff base, and aldol condensates) (Figure 13). For every cuinfo complex, the nECM provides many potential moieties for affecting these reactions. Thus, in addition to the multitude of metal cations (n > 10) to form the cuinfo* complex, the Avogadro-scale combinatorial options17−19 for tagging and cross-linking each cuinfo* are also staggeringly huge (iconographic representation, Figure 13).
Figure 13.
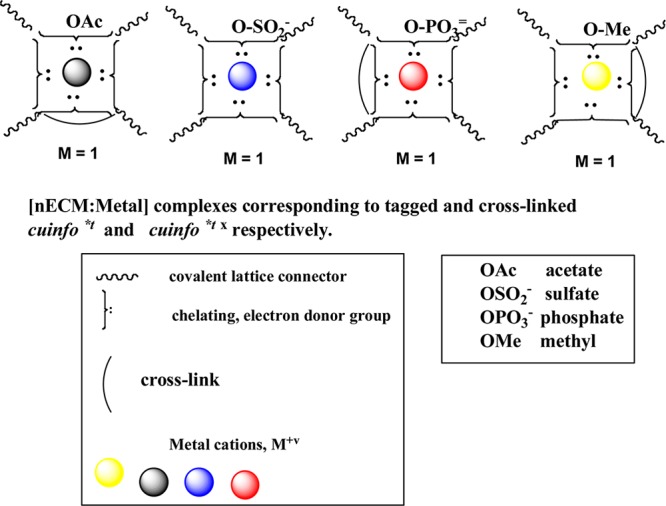
Iconographic representations of tagged and/or cross-linked cuinfo*t and cuinfo*tx, respectively, with different elemental cations, tagged with acetyl (Ac), sulfate (SO3–), phosphate (PO3–), or methyl (OMe) moieties. Such derivatized cuinfo serve to index the encoded cog-info for organized storage required for easier recall.
Hypothetical Model of cuinfo Formed with Tenascin and Zn2+
Tenascins73,74 are scattered throughout the nECM as monomers, trimers, or hexamers (star shapes).75−78 Knockout mice deficient in tensacins exhibited defective memory.79−82
The fibrinogen globe terminating the tenascins contains polypeptides that are homologous to the C-termini of the β and γ chains of fibrinogen. This region (D-domain) in fibrinogen has been shown to bind Zn2+ with an affinity on the order of KD 18 uM, affecting coagulation parameters, such as clotting time, clot turbidity, ultrastructure, and viscoelasticity.83−92 Also, the C-terminal epitope (Haptide) has been shown to be capable of attaching directly to and penetrating the lipid bilayer membrane of mesenchymal cells,93−97 as well as neurons (Marx unpublished).
Thus, we propose a hypothetical scheme whereby the tenascins attach directly to the neuron via their Haptide termini. The binding of Zn2+ alters the conformation/position of the epitope, effectively encoding cog-info as a cuinfo*, affected/sensed by the neurons with iontophoretic/piezoelectric actuators embedded within the membrane (Figure 14).
Figure 14.
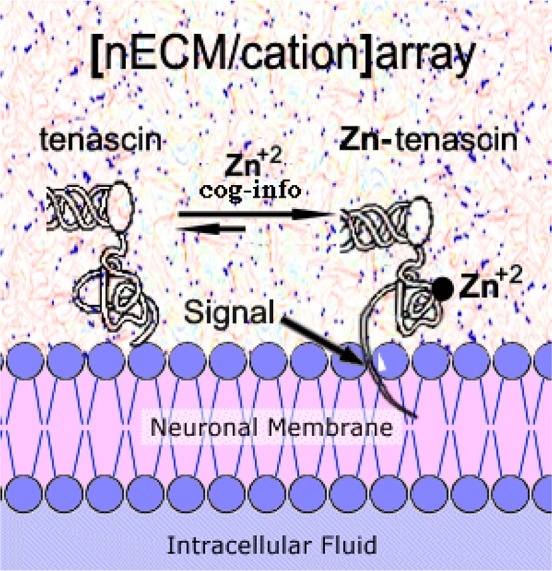
Hypothesized encoding event of tenascin (a component of the nECM) in direct contact with the neuronal membrane. Upon binding, a Zn2+ atom, a conformational/positional change in the Haptide epitope perturbs the membrane, serving as an encoding event, to form the cuinfo*.
The above Figure 14 presents a hypothesized scheme whereby Zn2+ binding to a fibrinogen-like region of tenascin induces a local conformational change in the protein, sensed at the neural surface as a “cuinfo event”. Other cations could also be entrapped by the nECM to form variant cuinfo*. Consonant with each element’s unique bonding traits (chelating bond lengths and angles), each type of cation binding event would impose its unique imprint (signal) on the conformation/position of the haptide epitope, induced or sensed by the neuron.
How can one describe a physiologically credible system wherein cog-info is transformed into memory? The proposed mechanism must conform to the limiting conditions of chemistry and physiology, in terms of low energy and rapid kinetics, but provide large encoding capacity. It should be couched in terms reflecting the biochemical underpinnings of all physiologic processes.
Golgi and Cajal developed the silver staining method, whose underlying chemistry we review here. Neurobiologists generally overlooked the nECM, imaging neurons “a la Cajal”, as if they were naked, suspended in space. Ironically, Cajal’s neural images with blank backgrounds blinded subsequent generations of neurobiologists to the importance of the nECM for neural function. By contrast, the tripartite mechanism focuses on the nECM as a key component in neural processing of cog-info (mentation), ultimately sensed as recall.
Neurons are remarkably like blood platelets. Both accumulate metabolites, cofactors, and reactants in cytoplasmic granules or vesicles, which are released upon activation. For example, platelets accumulate ascorbate, zinc, fibrinogen, coagulation factors, and so forth in various granules or special cytoplasmic compartments which are released upon activation, all aiding the formation of a stable blood clot. The neurons also accumulate NTs, ascorbate, and trace metals within vesicles, released by action potentials. But to what purpose? We suggest that the neurons manipulate or sense the surrounding ensembles of [nECM:metal] complexes (cuinfo), using piezoelectric, viscoelastic, and iontophoretic sensors/actuators to encode and decode cognitive information (cog-info), from which the neural circuit integrates and consolidates memory. The nECM around the neuron is a relatively static lattice, but the trace metals distributed therein are mobile (dopants), whose reversible binding or desorption from a particular address are sensed by the neuron.
However, such sensing is not binary (n = 2), but multinary (n > 10). We respectfully defer more detailed discussion of the computational aspects of the tripartite mechanism to a subsequent manuscript (MBM Pt4).
Each bound element imposes a unique geometry to the adsorption locale (address), which is reflected by conformational twisting/dielectric modification, sensed by the neuron. However, metal binding reactions are inherently reversible, with a lifetime depending on the particular element and the anionic moieties. For example, monovalent cations form relatively short-lived complexes, compared to di- and trivalent elemental metals.
Cross-linking imposes covalent constraints on the [nECM:metal] complexes (cuinfo), rendering them much more stable, as exemplified by the cross-linking of fibrin by factor XIII. Transglutaminase enzymes impose covalent cross-links, rendering the cuinfox much more resistant to degradative enzymes, effectively permanent. Some cross-linking reactions (Schiff base, aldol condensation, Maillard reaction) are themselves reversible, with stability dictated by local pH and/or enzymes. Cross-linking (i.e., stabilizing) the cuinfox is iconographically represented by the “bow” notation in the Figure 12.
We conceive a process whereby the input of sensorial cog-info is encoded within the nECM by metal “dopants”. The initially formed, but unstable, monovalent metal complex (template cuinfo1), is transposed and transformed into ever more stable sets of polyvalent metal complexes (derivative cuinfo) by various types of chemical transformations. We described Fenton reactions (redox) (Figure 6), condensation reactions between oxidized saccharide units (Figures 8, 9, and 12), as well as cross-linking by enzymatic pathways. Some of these occur in the ECM of various types of tissue, notably bone, skin, and eyes. Detailed evaluation of such reactions in brain tissue awaits further elaboration.
A hypothetical overview of the process of sensing an external event as cog-info involves its transformation into a storable form in the brain but outside the neuron (as cuinfo), available for recall by the neural circuitry. The tripartite mechanism permits one to chemically describe a sequence of processes, to rationalize the encoding and recall of cog-info as memory (Figure 15). It addresses the issue of how cog-info can be stored for long periods for recall (short- and long-term memory), and forgotten (by degradation or lack of critical components).
Figure 15.
Conceptualized process whereby cog-info is encoded as [nECM:metal] complexes (cuinfo) and decoded by the neuron and neural circuitry, resulting in recall (memory), driving behavior.
Conclusions
The chemical structures and processes described above are the basis for a molecular description of memory. It explains the highly efficient almost unlimited computational power of the human brain, using minimal amount of energy (400 cal/day equivalent to energy consumption of a standard laptop computer) with minimal heat generation, in aqueous media. The chemical interactions of the tripartite mechanism underlie “long term potentiation (LTP)” or synaptic plasticity, manifest as short- and long-term memory. Hopefully, the above discussion will stimulate efforts to characterize molecular correlates of declarative, episodic, procedural, and other types of memory.
Acknowledgments
Dedicated by G.M. to his late wife, the artist Georgette Batlle, for hearth, heart, and art. We thank our friends and family for their encouragement. Special thanks are due to Prof. Randy Gallistel (Rutgers University) and Prof. Tamar Zelniker (Tel Aviv University), whose critical comments guided our composition of these serialized expositions. We have no conflict of interest but are looking for academic and commercial collaborators.
Author Contributions
Both authors have equal contribution.
The authors declare no competing financial interest.
References
- Hopfield J. J. (1982) Neural networks and physical systems with emergent selective computational abilities. Proc. Natl. Acad. Sci. U.S.A. 79, 2554–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compte A.; Brunel N.; Goldman-Rakic P. S.; Wang X. J. (2000) Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb. Cortex 10, 910–923. [DOI] [PubMed] [Google Scholar]
- Craver C. F. (2003) The making of a memory mechanism. J. Hist. Biol. 36, 153–195. [DOI] [PubMed] [Google Scholar]
- Amit D. J. (2013) Hebb versus biochemistry: The fundamental viewpoint. In Dan Amit memorial volume. In print. [Google Scholar]
- Mongillo G.; Barak O.; Tsodyks M. (2008) Synaptic theory of working memory. Science 319, 1543–1546. [DOI] [PubMed] [Google Scholar]
- Gallistel C. R., and King A. P. (2009) Memory and the Computational Brain, Wiley Blackwell, New York. [Google Scholar]
- Marx G.; Gilon C. (2012) The molecular basis of memory. ACS Chem. Neurosci. 3, 633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. (1960) The Nature of the Chemical Bond, 3rd ed, Cornell University Press, Ithaca, NY. [Google Scholar]
- Angeleci R. J. (1973) Stability of coordination compounds. In Inorganic Biochemistry, Vol 4, Chapter 2, Elsevier Scientific Publishers, Amsterdam. [Google Scholar]
- Greenwood N. N., and Earnshaw A. (1997) Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK. [Google Scholar]
- Berg J. M., Tymoczko J. L., and Stryer L. (2002) Textbook of Biochemistry, Freeman & Company, New York. [Google Scholar]
- Lehninger A., Nelson D. L., and Cox M. M. (2009) Principles of Biochemistry, Freeman & Company, New York. [Google Scholar]
- Dwyer F. P., and Mellor D. P. (1964) Chelating Agents and Metal Chelates, Academic Press, New York. [Google Scholar]
- Martell A., and Smith P. M.(1977) Critical Stability Constants, Vols. 4–4, Plenum Press, New York. [Google Scholar]
- Kettle S. F. A. (1971) Coordination Compounds, Thomas Nelson & Sons, Edinburgh, UK. [Google Scholar]
- Clark R. W.; Bonicamp J. M. (1998) The Ksp–Solubility Conundrum. J. Chem. Educ. 75, 1182–1188. [Google Scholar]
- Tielrooij K. J.; Garcia-Araez N.; Bonn M.; Bakker H. J. (2010) Cooperativity in ion hydration. Science 328, 1006–1009. [DOI] [PubMed] [Google Scholar]
- Knobloch B.; Mucha A.; Operschall B. P.; Sigel H.; Jeżowska-Bojczuk M.; Kozłowski H.; Sigel R. K. O. (2011) Stability and Structure of Mixed-Ligand Metal Ion Complexes That Contain Ni2+, Cu2+, or Zn2+, and Histamine, as well as Adenosine 5′-Triphosphate (ATP4) or Uridine 5′-Triphosphate (UTP4): An intricate network of equilibria. Chem.—Eur. J. 17, 5393–5403. [DOI] [PubMed] [Google Scholar]
- Solov’ev V.; Marcou G.; Tsivadze A.; Varnek A. (2012) Complexation of Mn2+, Fe2+, Y3+, La3+, Pb2+, and UO22+ with organic ligands: QSPR Ensemble Modeling of Stability Constants. Ind. Eng. Chem. Res. 51, 13482–13489. [Google Scholar]
- Van Harreveld A.; Dafny N.; Khattab F. I. (1971) Effects of calcium on the electrical resistance and the extracellular space of cerebral cortex. Exp. Neurol. 31, 358–367. [DOI] [PubMed] [Google Scholar]
- Balt S.; de Bolster M. W.; Booij M.; van Herk A. M.; Visser-Luirink G. (1983) Binding of metal ions to polysaccharides. V. Potentiometric, spectroscopic, and viscosimetric studies of the binding of cations to chondroitin sulfate and chondroitin in neutral and acidic aqueous media. J. Inorg. Biochem. 19, 213–226. [DOI] [PubMed] [Google Scholar]
- Olsher U.; Izatt R. M.; Bradshaw J. S.; Kent N. (1991) Coordination chemistry of lithium ion: A crystal and molecular structure review. Chem. Rev. 91, 137–164. [Google Scholar]
- Guo X.; Zhu G.; Fang Q.; Xue M.; Tian G.; Sun J.; Li X.; Qiu S. (2005) Synthesis, structure and luminescent properties of rare earth coordination polymers constructed from paddle-wheel building blocks. Inorg. Chem. 44, 3850–3855. [DOI] [PubMed] [Google Scholar]
- Meighan S. E.; Meighan P. C.; Choudhury P.; Davis C. J.; Olson M. L.; Zornes P. A.; Wright J. W.; Harding J. W. (2006) Effects of extracellular matrix-degrading proteases matrix metalloproteinases 3 and 9 on spatial learning and synaptic plasticity. J. Neurochem. 96, 1227–1241. [DOI] [PubMed] [Google Scholar]
- Vorisek I.; Sykova E. (2009) Measuring diffusion parameters in the brain: comparing the real-time iontophoretic method and diffusion-weighted magnetic resonance. Acta Physiol. 195, 101–110. [DOI] [PubMed] [Google Scholar]
- Bates F. S.; Hillmyer M. A.; Lodge T. P.; Bates C. M.; Delaney K. T.; Fredrickson G. H. (2012) Multiblock polymers: Panacea or pandora’s box?. Science 336, 434–440. [DOI] [PubMed] [Google Scholar]
- Geddes L. A.; Baker L. E. (1967) The specific resistance of biological material—a compendium of data for the biomedical engineer and physiologist. Med. Biol. Eng. 5, 271–293. [DOI] [PubMed] [Google Scholar]
- Trubatch J.; Van Harreveld A. (1972) Spread of iontophoretically injected ions in a tissue. J. Theor. Biol. 36, 355–66. [DOI] [PubMed] [Google Scholar]
- Llinas R. R. (1988) The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science 242, 1654–1665. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Connors B. W. and Pradiso M. A. (2001) Neuroscience: Exploring the Brain, 2nd ed., p 97, Lippincott Williams and Wilkins, Baltimore. [Google Scholar]
- Jefferys J. G. R. (1995) Nonsynaptic modulation of neuronal activity in the brain: Electric currents and extracellular ions. Physiol. Rev. 75, 689–723. [DOI] [PubMed] [Google Scholar]
- Bokil H.; Laaris N.; Blinder K.; Ennis M.; Keller A. (2001) Ephaptic interactions in the mammalian olfactory system. J. Neurosci. 21, RC173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabìtová S., and Nicholson C. (2007) Biophysical properties of brain extracellular space explored with ion-selective microelectrodes, integrative optical imaging and related techniques. In Electrochemical Methods for Neuroscience (Michael A. C., and Borland L. M., Eds.), Chapter 10, CRC Press, Boca Raton, FL. [PubMed] [Google Scholar]
- Deitmer J. W.; Rose C. R. (2010) Ion changes and signaling in perisynaptic glia. Brain Res. Rev. 63, 113–129. [DOI] [PubMed] [Google Scholar]
- Tich J., Erhart J., Kittinger E., Privratska J., and Privatska J. (2010) Fundamentals of Piezoelectric Sensorics: Mechanical, Dielectric, and Thermodynamical Properties of Piezoelectric Materials/Edition 1, Springer-Verlag, New York. [Google Scholar]
- Tamano H.; Takeda A. (2011) Dynamic action of neurometals at the synapse. Metallomics 3, 656–661. [DOI] [PubMed] [Google Scholar]
- Gianni C., and Menciassi A. (Eds.) (2012) Piezoelectric nanomaterials for biomedical applications, Nanomedicine and Nanotoxicology Series, Springer-Verlag, New York. [Google Scholar]
- Liu Y.; Zhang Y.; Chow M. J.; Chen Q. N.; Li j. (2012) Biological ferro-electricity uncovered in aortic walls by piezoresponse force microscopy. Phys. Rev. Lett. 108, 078103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson R. E.; Frederickson C. J.; Danscher G. (1990) In situ binding of bouton zinc reversibly disrupts performance on a spatial memory task. Behav. Brain Res. 39, 25–33. [DOI] [PubMed] [Google Scholar]
- Takeda A. (2000) Movement of zinc and its functional significance in the brain. Brain Res. Rev. 34, 137–148. [DOI] [PubMed] [Google Scholar]
- Frederickson C. J.; Won Suh S.; Silva D.; Frederickson C. J.; Thompson R. B. (2000) Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. Suppl. 1471S–1483S. [DOI] [PubMed] [Google Scholar]
- Mathie A.; Sutton G. L.; Clarke C. E.; Veale E. L. (2006) Zinc and copper: Pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 111, 567–583. [DOI] [PubMed] [Google Scholar]
- Madsen E.; Gitlin J. D. (2007) Copper and iron disorders of the brain. Annu. Rev. Neurosci. 30, 317–337. [DOI] [PubMed] [Google Scholar]
- Paoletti P.; Pergnano A. M.; Barbour B.; Casado M. (2009) Zinc at glutamatergic synapses. Neuroscience 158, 126–136. [DOI] [PubMed] [Google Scholar]
- Lutsenko S.; Bhattacharjee A.; Hubbard A. L. (2010) Copper handling machinery of the brain. Metallomics 2, 596–608. [DOI] [PubMed] [Google Scholar]
- Tamano H.; Takeda A. (2011) Dynamic action of neurometals at the synapse. Metallomics 3, 656–661. [DOI] [PubMed] [Google Scholar]
- Morris L. J. (1966) Separation of lipids by silver ion (Ag+) chromatography. J. Lipid Res. 7, 717–732. [PubMed] [Google Scholar]
- Bothun G. D. (2008) Hydrophobic silver nanoparticles trapped in lipid bilayers: Size distribution, bilayer phase behavior, and optical properties. J. Nanobiotechnol. 6, 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.; Girault H. H. (2007) Fingerprint imaging by scanning electrochemical microscopy (and silver stain). Electrochem. Commun. 9, 1778–1782. [Google Scholar]
- Switzer R. C. (2000) Application of silver degeneration stains for neurotoxicity testing. Toxicol. Pathol. 28, 70–83. [DOI] [PubMed] [Google Scholar]
- Maroudas A.; Weinberg P. D.; Parker K. H.; Winlove C.P . (1988) The distributions and diffusivities of small ions in chondroitin sulphate, hyaluronate and some proteoglycan solutions. Biophys. Chem. 32, 257–270. [DOI] [PubMed] [Google Scholar]
- Scott J. E. (1995) Extracellular matrix, supramolecular organisation and shape. J. Anat. 187(Pt 2), 259–269. [PMC free article] [PubMed] [Google Scholar]
- Hrabetová S.; Masri D.; Tao L.; Xiao F.; Nicholson C. (2009) Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J. Physiol. 587(Pt 16), 4029–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargova L.; Homola A.; Cicanic M.; Kuncova K.; Krsek P.; Marusic P.; Sykova E.; Zamecnik J. (2011) The diffusion parameters of the extracellular space are altered in focal cortical dysplasias. Neurosci. Lett. 499, 19–23. [DOI] [PubMed] [Google Scholar]
- Androjna C.; Gatica J. E.; Belovich J. M.; Derwin K. A. (2008) Oxygen diffusion through natural extracellular matrices: Implications for estimating ″critical thickness″ values in tendon tissue engineering. Tissue Eng,. Part A. 14, 559–569. [DOI] [PubMed] [Google Scholar]
- Bath B. D.; White H. S.; Scott E. R. (2000) Electrically facilitated molecular transport. Analysis of the relative contributions of diffusion, migration, and electro-osmosis to solute transport in an ion-exchange membrane. Anal. Chem. 72, 433–42. [DOI] [PubMed] [Google Scholar]
- Taki M.; Iyoshi S.; Ojida A.; Hamachi H.; Yamamoto Y. (2010) Development of highly sensitive fluorescent probes for detection of intracellular copper(i) in living systems. J. Am. Chem. Soc. 132, 5938–5939. [DOI] [PubMed] [Google Scholar]
- Fabbrizzi L.; Poggi A. (2012) Anion recognition by coordinative interactions: Metal–amine complexes as receptors. Chem. Soc. Rev. 42, 1681–1699. [DOI] [PubMed] [Google Scholar]
- McCulloch R. K.; Vandongen R. (1992) Measurement of ascorbic acid in platelets and its relationship to polymorphonuclear leukocyte levels. Clin. Chim. Acta 213, 15–22. [DOI] [PubMed] [Google Scholar]
- Seghieri G.; Martinoli L.; di Felice M.; Anichini R.; Fazzini A.; Ciuti M.; Miceli M.; Gaspa L.; Franconi F. (1998) Plasma and platelet ascorbate pools and lipid peroxidation in insulin-dependent diabetes mellitus. Eur. J. Clin. Invest. 28, 659–63. [DOI] [PubMed] [Google Scholar]
- Sahud M. A.; Aggeler P. M. (1970) Utilization of ascorbic acid during platelet aggregation. Proc. Soc. Exp. Biol. Med. 134, 13–18. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V.; Davis P. S.; Emery H.; Lander H. (1972) Platelet ascorbic acid levels in normal subjects and in disease. J. Clin. Pathol. 25, 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S.; Fujisawa H.; Kametani F.; Sakurai H. (1992) Generation of active oxygen species in blood platelets: Spin trapping analysis. Free Radical Res. Commun. 15, 319–324. [DOI] [PubMed] [Google Scholar]
- Rice M. E. (1999) Ascorbate compartmentalization in the CNS. Neurotox. Res. 1, 81–90. [DOI] [PubMed] [Google Scholar]
- Qiu S.; Li L.; Weeber E. J.; May J. M. (2007) Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J. Neurosci. Res. 85, 1046–1056. [DOI] [PubMed] [Google Scholar]
- May J. M. (2012) Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 56, 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj R. H.; Shipanova I .N.; Faust F. M. (1996) Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J. Biol. Chem. 271, 19338–19345. [DOI] [PubMed] [Google Scholar]
- Monnier V. M.; Mustata G. T.; Biemel K. L.; Reihl O.; Lederer M. O.; Zhenyu D.; Sell D. R. (2005) Cross-linking of the extracellular matrix by the Maillard reaction in aging and diabetes: an update on ″a puzzle nearing resolution″. Ann. N.Y. Acad. Sci. 1043, 533–544. [DOI] [PubMed] [Google Scholar]
- Sell D. R.; Biemel K. M.; Reihl O.; Lederer M. O.; Strauch C. M.; Monnier V .M. (2005) Glucosepane is a major protein cross-link of the senescent human extracellular matrix. Relationship with diabetes. J. Biol. Chem. 280, 12310–12315. [DOI] [PubMed] [Google Scholar]
- Kishida K. T., Klann E. (2009) Reactive oxygen species, synaptic plasticity and memory. In Oxidative Neural Injury (Veasey S. C., Ed.), Ch 1, pp 1–28. Humana Press, New York. [Google Scholar]
- Fan X.; Sell D. R.; Zhang J.; Nemet I.; Theves M.; Lu J.; Strauch C.; Halushka M. K.; Monnier V. M. (2010) Anaerobic vs aerobic pathways of carbonyl and oxidant stress in human lens and skin during aging and in diabetes: A comparative analysis. Free Radical Biol. Med. 49, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalak G.; Vogel V. (2012) Extracellular phosphorylation and phosphorylated proteins: Not just curiosities but physiologically important. Sci. Signaling 5(255), re7. [DOI] [PubMed] [Google Scholar]
- Erickson H. P. (1994) Evolution of the tenascin family-implications for function of the C-terminal fibrinogen-like domain. Perspect. Dev. Neurobiol. 2, 9–19. [DOI] [PubMed] [Google Scholar]
- Lafleur D. W.; Chiang J.; Fagin J. A.; Schwartz S. M.; Shah P. K.; Wallner K.; Forrester J. S.; Sharifi B. G. (1997) Aortic smooth muscle cells interact with tenascin-C through its fibrinogen-like domain. J. Biol. Chem. 272, 32798–32803. [DOI] [PubMed] [Google Scholar]
- Srinivasan J.; Schachner M.; Catterall W. A. (1998) Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl. Acad. Sci. U.S.A. 95, 15753–15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P.; Fischer D.; Harig M.; Schulthess T.; Margolis R. K; Chicket Ehrismann R.; Margolis R. U. (1997) The fibrinogen like globe of tenascin-C mediates its interactions with neurocan and phophacan/protein-tyrosine phosphatase-β. J. Biol. Chem. 272, 15501–15509. [DOI] [PubMed] [Google Scholar]
- Schenk S.; Chiquet-Ehrismann R.; Battegy E. J. (1999) The fibrinogen-globe of tenascin-C promotes basic-FGF-induced endothelial cell elongation. Mol. Biol. Cell 10, 2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz M.; Jones F.; Jones P. L. (2000) The Tenascin family of ECM glycoproteins: Structure, function, and regulation during embryonic development and tissue remodeling. Dev. Dyn. 218, 235–259. [DOI] [PubMed] [Google Scholar]
- Brenneke F.; Bukalo O.; Dityatev A.; Lie A. A. (2004) Mice deficient for the extracellular matrix glycoprotein tenascin-r show physiological and structural hallmarks of increased hippocampal excitability, but no increased susceptibility to seizures in the pilocarpine model of epilepsy. Neuroscience 124, 841–855. [DOI] [PubMed] [Google Scholar]
- Syková E.; Vorísek I.; Mazel T.; Antonova T.; Schachner M. (2005) Reduced extracellular space in the brain of tenascin-R- and HNK-1-sulphotransferase deficient mice. Eur. J. Neurosci. 22, 1873–1880. [DOI] [PubMed] [Google Scholar]
- Pradel G.; Schmidt R.; Schachner M. (2000) Involvement of L1.1 in memory consolidation after active avoidance conditioning in zebrafish. J. Neurobiol. 43, 389–403. [PubMed] [Google Scholar]
- Kawakami K.; Matsumoto K. (2011) Behavioral alterations in mice lacking the gene for tenascin-X. Biol. Pharm. Bull. 34, 590. [DOI] [PubMed] [Google Scholar]
- Marx G.; Gurfel D.; Hopmeier P. (1987) Zinc alters fibrin ultrastructure. Thromb. Haemostasis 57, 73–76. [PubMed] [Google Scholar]
- Marx G. (1987) Protofibrin coagulation induced by calcium or zinc. Biopolymers 26, 911–920. [DOI] [PubMed] [Google Scholar]
- Marx G. (1988) Elasticity of fibrin and protofibrin gels is differentially modulated by calcium and zinc. Thromb. Haemostasis 59, 500–503. [PubMed] [Google Scholar]
- Marx G. (1988) Modelling cation-driven (proto)fibrin coagulation. Biopolymers 29, 1233–1241. [DOI] [PubMed] [Google Scholar]
- Marx G. (1988) Divalent cation induce protofibril gelation. Am. J. Hematol. 27, 104–109. [DOI] [PubMed] [Google Scholar]
- Marx G. (1988) Mechanism of fibrin coagulation based on selective, cation-driven protofibril association. Biopolymers 27, 763–774. [DOI] [PubMed] [Google Scholar]
- Marx G. (1988) Zinc binding to fibrinogen and fibrin. Arch. Biochem. Biophys 266, 285–288. [DOI] [PubMed] [Google Scholar]
- Hopmeier P.; Halbmayer M.; Fischer M.; Marx G. (1990) Zinc modulates thrombin adsorption to fibrin. Thrombos. Res. 58, 293–302. [DOI] [PubMed] [Google Scholar]
- Marx G.; Krugliak J.; Shaklai M. (1991) Nutritional zinc increases platelet sensitivity to agonists. Am. J. Hematol. 38, 161–165. [DOI] [PubMed] [Google Scholar]
- Marx G.; Korner G.; Mou X.; Gorodetsky R. (1993) Packaging zinc, fibrinogen and factor XIII in platelet α-granules. J. Cell. Physiol. 156, 437–442. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R.; Vexler A.; An J.; Mou X.; Marx G. (1998) Haptotactic and growth stimulatory effects of fibrin(ogen) and thrombin on cultured fibroblasts. J. Lab. Clin. Med. 131, 269–280. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R.; Vexler A.; Shamir M.; An J.; Levdansky L.; Shimeliovich I.; Marx G. (2003) New cell attachment peptide sequences from conserved epitopes in the carboxy termini of fibrinogen. Exp. Cell Res. 287, 116–129. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R.; Levdansky L.; Vexler A.; Shimeliovich I.; Kassis I.; Ben-Moshe M.; Magdassi S.; Marx G. (2004) Liposome transduction into cells enhanced by haptotactic peptides (Haptides) homologous to fibrinogen C-termini. J. Controlled Release 95, 477–488. [DOI] [PubMed] [Google Scholar]
- Marx G.; Ben-Moshe M.; Magdassi S.; Gorodetsky R. (2004) Fibrinogen C-terminal peptidic sequences (Haptides) modulate fibrin polymerization. Thromb. Haemostasis 91, 43–51. [DOI] [PubMed] [Google Scholar]
- Marx G., and Gorodetsky G. (2009) Haptotactic peptides. U.S. Patent #7,544,655.
- Golgi C. (1906) Nobel Lecture: The Neuron Doctrine – Theory and Facts. The Nobel Prize in Physiology or Medicine 1906, Nobel Foundation. [Google Scholar]