Abstract
OBJECTIVE
Prior research indicates CT colonography (CTC) would be a cost-effective colorectal cancer (CRC) screening test if widespread availability were to increase overall CRC screening adherence rates. The primary aims of this multicenter study were to evaluate patient experience and satisfaction with CTC screening and compare preference against screening colonoscopy.
MATERIALS AND METHODS
A 12-question survey instrument measuring pretest choice, experience, and satisfaction was given to a consecutive cohort of adults undergoing CTC screening in three disparate screening settings: university academic center, military medical center, and community practice. The study cohort was composed of individuals voluntarily participating in clinical CTC screening programs.
RESULTS
A total of 1417 patients responded to the survey. The top reasons for choosing CTC for screening included “noninvasiveness” (68.0%), “avoidance of sedation/anesthesia” (63.1%), “ability to drive after the test” (49.2%), “avoidance of optical colonoscopy risks” (46.9%), and “identifying abnormalities outside the colon” (43.3%). Only 7.2% of patients reported pain during the CTC examination and only 2.5% reported greater than moderate discomfort. Of 441 patients who had experienced both CTC and optical colonoscopy, 77.1% preferred CTC and 13.8% preferred optical colonoscopy. Of all patients, 29.6% indicated that they may not have undergone optical colonoscopy screening if CTC were not available. Of all patients, 92.9% labeled their overall experience with CTC as “excellent” or “good,” and 93.0% indicated they would choose CTC for their next screening.
CONCLUSION
Respondents reported a very high satisfaction level with CTC, and those who had experienced both modalities indicated a preference for CTC over optical colonoscopy. These results suggest that CTC has the potential to increase adherence to CRC screening guidelines if widely available.
Keywords: colorectal cancer, CT colonography, optical colonoscopy, virtual colonoscopy
Colorectal cancer (CRC) is currently the second leading cause of cancer death in the United States [1]. However, it is well accepted that CRC screening with early detection and removal of adenomatous polyps would substantially reduce the mortality associated with CRC [2, 3]. Despite the obvious benefits of screening, adherence with current screening options including optical colonoscopy, flexible sigmoidoscopy, and fecal occult blood tests remains poor. In the case of optical colonoscopy, prior research suggests the invasiveness of the procedure as well as the risks and side effects of the accompanying sedation are likely contributors to suboptimal compliance with CRC screening recommendations [4]. The most recent National Institutes of Health (NIH) consensus statement (2010) indicates that only 55% of adults in the CRC screening target population (age 50 years and older) are compliant with current screening guidelines [5].
CT colonography (CTC) is a minimally invasive sedation-free imaging modality that has been shown to be comparable to optical colonoscopy in the detection of clinically significant colon polyps and CRC [6–9] and has been endorsed by some national guidelines as an acceptable option for CRC screening [2, 10, 11]. In addition to the excellent performance measures, the risks and complications related to this examination are very low [12]. Potentially, the addition of CTC to CRC screening options could markedly impact current low adherence rates, likely in a cost-effective manner [13]. A recent cost-effectiveness analysis indicated that modest increases in patient adherence to CRC screening guidelines with greater availability of CTC would place CTC among the cost-effective CRC screening options [14].
Central to the ultimate impact on screening rates are patients’ perceptions and attitudes toward CTC as a screening modality. Patient experience and response to CTC as a CRC screening test outside of clinical trials are not yet well established. A recent study from a single center suggested a significant patient preference for CTC over optical colonoscopy and gave evidence of patients’ willingness to receive screening only after CTC was offered, but this study was limited by a small sample size (n = 300) and limited survey instrument [15]. The current study represents the first large multicenter effort to assess patient response to CTC screening across diverse clinical settings where this test has become uniquely available, including a university hospital, military hospital, and community-based gastroenterology practice. The primary aims of this survey-based study were to assess the level of patient experience and satisfaction with respect to CTC in a screening population and to compare preference against optical colonoscopy for those who have undergone both tests.
Materials and Methods
This survey-based study was compliant with HIPAA, and institutional review board approval was obtained at all participating centers. A standardized survey instrument generated by the authors regarding the screening experience and attitudes toward CTC was administered to a series of consecutive patients over a 14-month period for each of the three disparate institutions. Responses were tabulated and analyzed.
Study Settings
Institution A
Institution A is an integrated CTC center (offering same-day optical colonoscopy if a polyp is detected at CTC), featuring an eight-physician community gastrointestinal practice. Institution A has had a CTC screening program in place since 2008 and has screened more than 2000 individuals.
Institution B
Institution B is a tertiary-care military treatment facility that serves a population of active duty military personnel, retirees, and their dependents. Institution B has had a CTC screening program in place since 2004 and has performed more than 10,000 CTC examinations, the vast majority representing average-risk CRC screening examinations.
Institution C
Institution C is a tertiary referral academic medical center. Institution C has had a CTC screening program in place since 2004 and has performed more than 8000 screening CTC examinations.
Data Collection
A patient questionnaire consisting of 12 questions in five dimensions was standardized across all three participating institutions (Table 1). The questionnaire used the more popular terminology, “virtual colonoscopy,” in place of the more technically correct “CT colonography.” The surveys were administered to a series of consecutively enrolled patients after screening CTC. Surveys were provided at the screening institution immediately after the examination or were mailed to patients within the 6 months after the examination. All survey responses were kept anonymous. There were no incentives offered for participation in the study, and there was no attempt to contact or follow up with survey recipients beyond initial disbursement of the survey. The overall survey response rate was 55.6% (1417/2548).
TABLE 1.
Study Questionnaire Summary
| Dimensions | Questions |
|---|---|
| Demographics and colorectal cancer screening history | What is your sex? |
| What was your age in years at the time of virtual colonoscopy screening? | |
| Which category best represents your racial or ethnic identity? | |
| Was this the first time you have received colon cancer screening? | |
| Reasons for selecting virtual colonoscopy | Which of the following advantages of virtual colonoscopy represent your main reason(s) for selecting this method of colon cancer screening? |
| If virtual colonoscopy had NOT been available to you, would you have chosen to be screened with traditional invasive colonoscopy instead? | |
| Level of comfort with virtual colonoscopy | What was your level of comfort during the virtual colonoscopy test? |
| If you experienced discomfort, please characterize the type of discomfort. | |
| Overall satisfaction with virtual colonoscopy | Please rate your overall experience with virtual colonoscopy. |
| After experiencing virtual colonoscopy, which method will you likely select for your next colon screening? | |
| Posttest preferences | If you have experienced both virtual colonoscopy and traditional colonoscopy, which test did you prefer? |
| Would you recommend virtual colonoscopy for friends or family undergoing colon cancer screening? |
CT Colonography Technique
The CTC protocol was largely standardized among the three participating centers—with only minor specific differences—and has been described elsewhere [16, 17]. Briefly, patients consume a standard low-volume bowel preparation on the day before the procedure, consisting of either magnesium citrate and bisacodyl (institutions A and C) or a 2-L polyethylene glycol bowel preparation alone (institution B). Single doses of 2% barium sulfate and diatrizoate were given for colonic stool and fluid tagging, respectively.
Before imaging, a small flexible rectal catheter was placed and the colon was insufflated using an automated carbon dioxide delivery system that is both pressure and volume regulated. The three centers used slightly different protocols with respect to pressure and timing of insufflation, enabling an assessment of the impact of these methods on patient comfort during the examination.
Patients underwent MDCT in the supine and prone positions with a low-dose CTC protocol. Additional decubitus views were obtained in cases with areas of persistent luminal collapse. No sedation or IV medications were administered as part of the CTC examination. The DICOM image data were networked to a dedicated CTC workstation for immediate interpretation by experienced radiologists at all centers.
Statistical Analysis
Questionnaire data were collected and collated in Microsoft Excel and analyzed using Stata Version 11 (StataCorp) and R 2.12.1 (R Development Core Team). Univariate and bivariate analyses were performed to evaluate differences across sites and within sites. Assessment of differences in dimensional variables was done using parametric tests (if assumptions were met). Categoric variables were analyzed by Pearson chi-square or Fisher exact test. A p value of less than 0.05 was considered statistically significant.
Subanalysis was performed for the responses gathered from institution C (n = 837), the only site in this study for which itemized survey responses were available. Association between survey responses and the demographic characteristics of sex, age (grouped into quartiles), and race or ethnicity (grouped into “white” and “other” because of the small number of nonwhite respondents) were examined, and a linear regression model was fit to examine the particular association between these demographic variables and preference for CTC over optical colonoscopy in patients who expressed a preference. When analyzing the association between race or ethnicity, sex, and age, p values were adjusted using the Bonferroni-Holm procedure because of the large number of hypothesis tests and the higher risk of spurious significance (type 1 error). A multivariable logistic regression model was fit to examine whether preference for CTC was associated with race or ethnicity, sex, and age.
Results
A total of 1417 patients were surveyed after receiving CTC studies at the three centers (Table 2). Institution C contributed the most patients with 837 (59.1%), and institutions A and B contributed 280 (19.8%) and 300 (21.2%) patients, respectively. The mean age [± SD] of the patients was 56.7 ± 7.8 years and was similar at all three centers (p = 0.15). Overall, 50.2% (712/1417) of patients were women. Whites represented the majority of patients in the survey sample, constituting 87.3% (1237/1417) of patients overall, with institution B contributing a higher percentage of nonwhites than the other two centers (18.7% African-American, 4.7% Hispanic, and 5.0% Asian).
TABLE 2.
Study Demographics and Characteristics at the Three Institutions
| Parameter | Institution A | Institution B | Institution C | Totals |
|---|---|---|---|---|
| No. of patients | 280 | 300 | 837 | 1417 |
| Average age (y) (SD) | 55.9 (6.6) | 56.9 (9.4) | 56.9 (7.4) | 56.7 (7.8) |
| Female (%)a | 119 (42.5) | 131 (43.7) | 462 (55.2) | 712 (50.2) |
| Racea | ||||
| White (%) | 241 (86.1) | 210 (70.0) | 786 (93.9) | 1237 (87.3) |
| Black or African American (%) | 26 (9.3) | 56 (18.7) | 17 (2.0) | 99 (7.0) |
| Hispanic (%) | 3 (1.1) | 14 (4.7) | 11 (1.3) | 28 (2.0) |
| Asian (%) | 7 (2.5) | 15 (5.0) | 12 (1.4) | 34 (2.4) |
| Other (%) | 2 (0.7) | 2 (0.7) | 5 (0.6) | 9 (0.6) |
| No response given (%) | 1 (0.4) | 3 (1.0) | 6 (0.7) | 10 (0.7) |
Chi-square test, p < 0.0001.
In total, 64.4% (913/1417) of the patients reported their CTC examination was their first CRC screening, ranging from 55.0% to 77.1% among the three institutions; 17.2% (244/1417) of respondents reported they had undergone optical colonoscopy in the past, ranging from 12.9% at institution C to 27.0% at institution B. Overall, 69.9% of patients reported they would have undergone optical colonoscopy if CTC had not been available; 8.3% indicated they would not have undergone optical colonoscopy, and 21.3% indicated they were not sure.
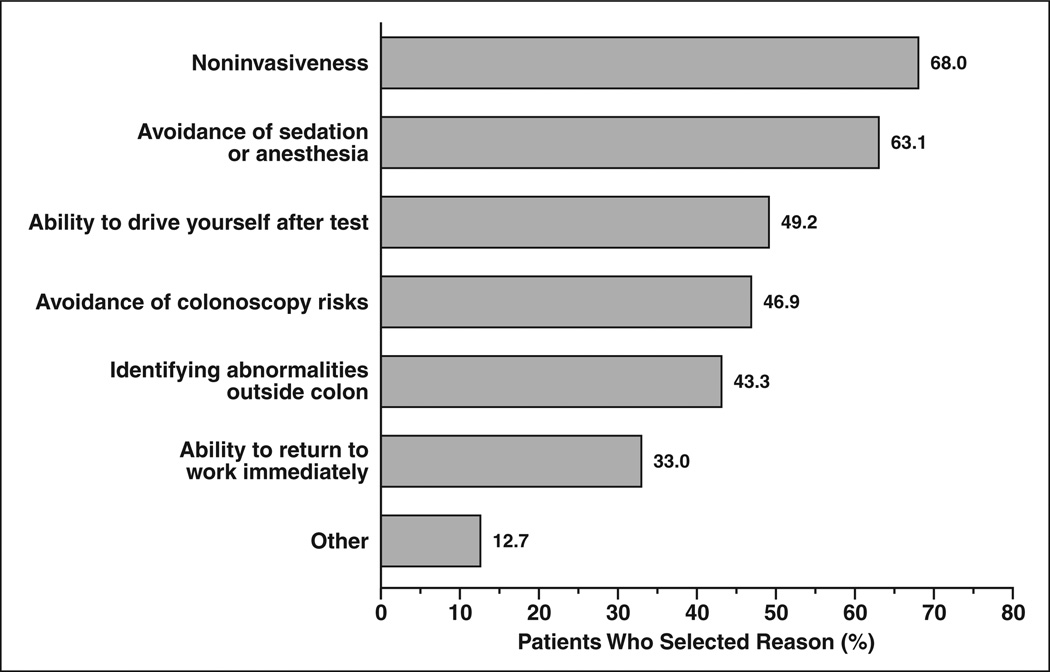
Figure 1 depicts the most frequently cited reasons for choosing CTC. Patients were allowed to select more than one reason and primarily centered on the relatively noninvasive nature of CTC and the perceived increased level of invasiveness and risks associated with colonoscopy. “Noninvasiveness” (68.0%), “avoidance of sedation/anesthesia” (63.1%), “ability to drive yourself after test” (49.2%), and “avoidance of colonoscopy risks” (46.9%) were the top four cited reasons for choosing CTC screening. Additionally, other advantages of CTC including “possibility of identifying abnormalities outside the colon” (43.3%) and “ability to return to work immediately” (33.0%) were common reasons for CTC choice.
Fig. 1.
Chart shows main reasons given by patients for selecting CT colonography.
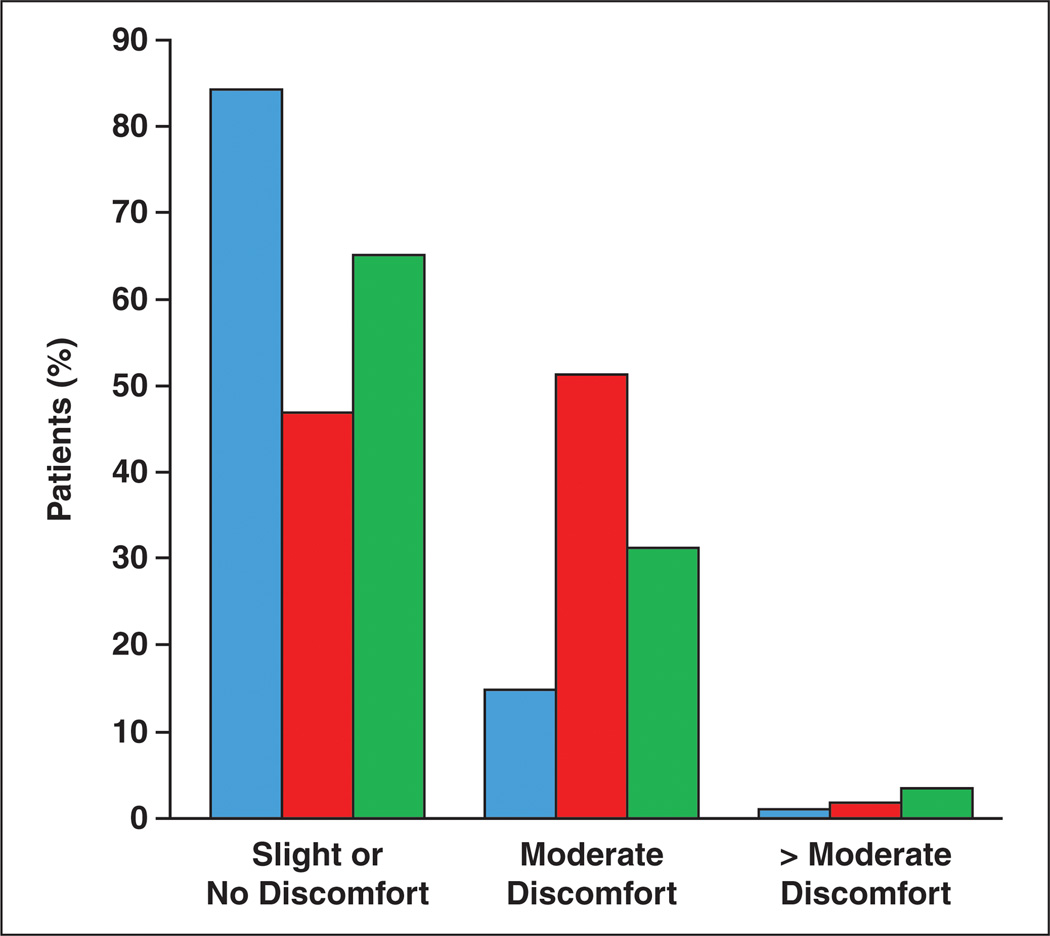
When asked about discomfort during CTC, only 7.2% of patients reported any pain, with only 10/1417 patients (0.7%) reporting pain that lasted beyond the duration of the examination. Additionally, 14.6% of patients reported no discomfort at all during the examination, with 41.5% reporting a “mild bloating” and 35.1% reporting “moderate cramping,” both of which were short-lived and limited to the duration of the examination. A total of 2.5% of patients reported greater than moderate discomfort. There were significant differences reported in the level of discomfort experienced across the different insufflation protocols of the three centers (Fig. 2). The percentage of patients reporting “no discomfort” or “slight discomfort” was 84.0% for institution A (initial insufflation pressure 17 mm Hg), 65.0% for institution C (initial insufflation pressure 20 mm Hg), and 46.7% for institution B (initial insufflation pressure 25 mm Hg) (p < 0.001 (Table 3). However, among all centers, the percentage of patients reporting “intolerable discomfort” was low and did not differ significantly, with values of 1.1%, 1.7%, and 3.3% at institutions A, B, and C, respectively (p = 0.061).
Fig. 2.
Chart shows level of discomfort by institution. Blue bars indicate institution A, red bars indicate institution B, and green bars indicate institution C.
TABLE 3.
Insufflation Protocol by Institution
| Institution | Insufflation Protocol | No or Slight Discomfort (%) |
|---|---|---|
| A | Initiate insufflation pressure at 17mm Hg; increase pressure as needed to achieve full colonic distention; final pressure usually between 21–25 mm Hg | 84.0 |
| C | Initiate insufflation pressure at 20 mm Hg; increase to 25 mm Hg after initial rectal distention | 65.0 |
| B | Initiate and maintain insufflation pressure at 25 mm Hg | 46.7 |
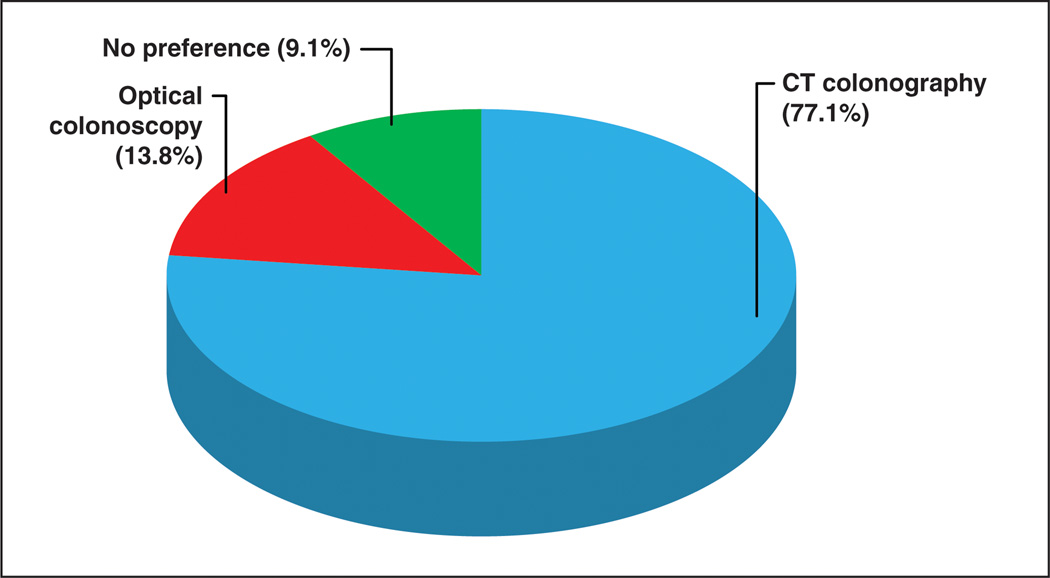
Overall, 92.4% of all patients rated their overall experience with CTC as either “excellent” (60.1%) or “good” (32.3%). Only 4.9% rated their experience as “fair,” and 2.2% rated their overall experience as “poor” (Fig. 3). Among those patients who had experienced both CTC and optical colonoscopy (n = 441), 77.1% preferred CTC, 13.8% preferred optical colonoscopy, and 9.1% had no preference (Fig. 4). Among patients who indicated they would be screened again in the future (n = 1319), 88.5% indicated they would likely select CTC for screening and 11.5% indicated they would likely opt for optical colonoscopy.
Fig. 3.
Chart shows overall individual experience with CT colonography.
Fig. 4.
Chart shows preference of screening test among patients who have experienced both CT colonography and optical colonoscopy.
Overall, 92.4% of patients indicated they would recommend CTC to friends or family members undergoing colon cancer screening, with institutions A (96.1%) and B (97.3%) reporting significantly higher rates than institution C (89.4%) (p < 0.0001). Subanalysis of itemized survey responses from institution C (n = 837) revealed no significant differences between whites and nonwhites in response to any survey question. Women and men differed significantly in response to only one question: Women were more likely to select “avoidance of sedation or anesthesia” as a reason for choosing CTC over optical colonoscopy than were men (69.3% vs 58.3%, p = 0.015).
For analysis of association between responses and age, the cohort was grouped into quartiles (30–51, 51–56, 56–61, and 61–92 years). As patient age increased, significantly fewer cited “ability to drive yourself after test” as a reason for choosing CTC over optical colonoscopy (61.2%, 53.6%, 47.2%, and 45.4%, respectively; p = 0.035). A similar trend was seen for “ability to return to work immediately” (43.9%, 40.2%, 32.0%, and 24.2%, respectively; p = 0.008). Otherwise, there were no significant differences in response seen among age quartiles.
Linear regression modeling revealed no significant association between race or ethnicity, age, sex, and preference for CTC over optical colonoscopy in patients who had undergone both tests. In comparison of individuals within this cohort who had experienced optical colonoscopy with those who had not, there was a significant difference in response to only one question: Patients who had experienced optical colonoscopy were less likely to select “noninvasiveness” as a reason for choosing CTC over optical colonoscopy (53.2% vs 70.6%, p < 0.001).
Discussion
CRC is a largely preventable cancer, but still causes approximately 50,000 deaths each year in the United States [1]. The most recent NIH consensus statement on CRC screening approximates the national screening rate among average-risk adults at 55%, lagging behind other malignancies for which screening is routinely performed, including cancers of the breast (68%) and cervix (82%) [18]. The comparatively low rate of compliance with national programmatic screening recommendations represents a major contributor to this public health challenge.
Although the cause is multifactorial, one reason that likely contributes to the relatively low rates of CRC screening is the nature of optical colonoscopy. Although it is an effective means for polyp and CRC detection, optical colonoscopy is an invasive procedure that in the United States typically involves some degree of sedation and analgesia, frequently with a combination of an opioid analgesic-benzodiazepine amnestic and occasionally a more potent hypnotic, such as propofol [19].
This study confirms that concerns about the invasive nature of optical colonoscopy and accompanying sedation are commonplace among patients who have opted for CTC screening. Indeed, approximately two thirds of patients in this study directly cite “noninvasiveness” and “avoidance of sedation/anesthesia” as primary reasons for selecting CTC. Related to concerns about invasiveness and sedation, nearly one half of patients further cited “ability to drive yourself after test” and “avoidance of colonoscopy risks” (including bleeding, perforation, and infection) as reasons for choosing CTC. A full third of patients also cited “ability to return to work immediately” as a reason for choosing CTC, reflecting a desire to avoid lost productivity or usage of medical leave to undergo a screening examination.
Offering an alternative CRC screening examination that is less invasive and performed without the need for anesthesia may help alleviate patient concerns about the risks of CRC screening with optical colonoscopy and could potentially help increase screening rates. CTC has been validated as a sensitive CRC screening option that has been included among the preferred screening options in recent national guidelines [2]. Previous studies have suggested that the availability of CTC as a convenient, minimally invasive, and sedation-free screening option may increase adherence with screening guidelines. In a review of data on patient preferences between CTC and optical colonoscopy from smaller single-center studies, six of seven reviewed studies showed a preference for CTC, usually by a wide margin [15].
Our study adds considerable evidence to previous research and was possible only because of the relatively unique availability of CTC screening without the need for out-of-pocket payment at the three participating centers. To date, this represents the largest survey of patient attitudes toward CTC in actual clinical practice (i.e., outside of any prospective trial), with more than 1400 patients responding. Furthermore, this study is the first multicenter study of its kind, allowing analysis of CTC screening across three distinct patient population settings: a university academic center, a military academic center, and a community-based private practice center. Finally, the patients included in this study represent an average-risk screening population and were not participating in other clinical trials.
Our data show that patients in this study had an overwhelmingly positive experience with CTC and that such experiences could be obtained in the varied clinical settings. Greater than 90% of all patients in this study rated their overall experience with CTC as either “excellent” or “good.” More than 10% of patients reported absolutely no discomfort with the examination, and less than 3% reported discomfort that was more than “moderate.” Among patients who did report discomfort, only 8% described the discomfort as “pain,” and less than 1% of all patients described the discomfort as persisting beyond the duration of the examination. This relative lack of postprocedural discomfort with CO2 is generally not the case with room air distention, as seen in colonoscopy and previously with CTC [20]. Additionally, differences in patient comfort from the three participating centers indicate that lower CO2 insufflation pressures may reduce patient discomfort even further during the examination, raising the possibility of even higher patient comfort in the future, especially because colonic distention remained adequate to achieve a diagnostic examination at lower pressures. Further highlighting the overall positive attitude toward CTC, more than 90% of all respondents indicated that they would recommend CTC to friends and family undergoing CRC screening.
Our data further suggest that the availability of CTC as a screening option has the potential to increase CRC screening rates. When asked if they would have undergone optical colonoscopy were CTC not available as a screening test, nearly 30% of the respondents in this study answered “no” (8.3%) or “not sure” (21.3%), suggesting that CTC could impact this sizeable group. Although nearly 70% indicated on the survey that they would have undergone optical colonoscopy, it is conceivable that fewer than this would actually follow-through with the considerably greater commitment of scheduling, prepping, and actually completing the optical colonoscopy examination; thus, this number is possibly an overestimation and could serve as a topic for future investigation.
Additionally, among the 441 patients in this study who had experienced both CTC and optical colonoscopy, CTC was preferred by a margin of 5.5:1. Finally, when asked about plans for future CRC screening, patients indicated they would choose CTC over optical colonoscopy at a rate of nearly 8:1. Among patients in this study, these data show a clear preference for CTC over optical colonoscopy and raise the possibility that adding the option of CTC as an alternative to optical colonoscopy holds the potential to persuade patients to participate in CRC screening when they otherwise may not. Future investigation into the ability of CTC to improve adherence to CRC screening guidelines is clearly warranted. Of note, a recently published Dutch randomized controlled trial found that participation in CTC screening was 55% higher than the optical colonoscopy arm [21], which provides important new evidence that further strengthens our own findings.
Subanalysis of itemized data from our largest center revealed no significant differences in response to survey questions with regard to race or ethnicity. Women and men differed significantly only in that men were less likely to select “avoidance of sedation or anesthesia” as a reason for choosing CTC. Differences among age groups were significant only in that, as they aged, respondents became less concerned about ability to drive and return to work immediately after the test; neither association is surprising. These data indicate a uniform acceptance of CTC as a screening modality across multiple demographics, strengthening the argument that widespread implementation of the modality as a CRC screening test would be successful.
Although the data from this survey study are promising, some methodologic issues limit the interpretation and generalization of the results. The individuals included in this survey were self-selected for CTC preference; they had already selected CTC as an acceptable screening method and may have been more likely than the general population to be biased toward CTC as a screening method. Because patients in this study were likely to volunteer for CRC screening and did not incur out-of-pocket expenses related to CRC screening, they may not be entirely representative of the target demographic of the CRC screening guidelines. Additionally, any survey study carries the risk of reporting bias from its participants, taking the form of a desire to “please” the provider by providing favorable responses or by misjudging the predictions of their future behavior, actions, or preferences. We attempted to address reporting bias by informing patients that the survey responses would remain confidential and anonymous; in addition, we offered no incentive for completion of the survey.
To maintain a consecutive patient cohort in the face of real-world logistical and workflow constraints at the CTC screening centers, not all patients were able to complete and return the survey the day of the CTC examination. These patients may have responded differently than the patients who did complete the survey on the same day. Furthermore, in the comparison of CTC and optical colonoscopy, recall bias could be present because some patients underwent optical colonoscopy multiple years before undergoing CTC. Finally, given the nature of the survey questions, we were unable to provide a control group that did not undergo CTC for comparison. Despite these limitations, the multicenter design and large number of respondents make this study valuable in understanding patient attitudes toward CTC.
It has been said that the best CRC screening test is the one that a patient is willing to undergo [22]. Given the need for ongoing screening in older adults, patient experience and satisfaction are very important components of any programmatic screening regimen. This large multicenter study both augments findings of previous studies showing a preference for CTC over optical colonoscopy and suggests a potential positive impact of CTC on overall CRC screening rates; if CTC were more widely available and this potential were realized, there could be profound implications for the cost-effectiveness of CRC screening [14]. In conclusion, our data show that patient experience with CTC as a primary CRC screening test is both overwhelmingly positive and has the potential to increase adherence to national CRC screening guidelines.
Acknowledgments
Supported by National Institutes of Health grant 1R01CA144835-01 (National Cancer Institute).
Footnotes
The opinions and assertions contained herein are the sole views of the authors and should not be construed as official or as representing the views of the U.S. Navy, Department of Defense, or Department of Veteran Affairs.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 4.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–141. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 5.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 6.Graser A, Stieber P, Nagel D, et al. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241–248. doi: 10.1136/gut.2008.156448. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CD, Chen M-H, Toledano AY, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207–1217. doi: 10.1056/NEJMoa0800996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 9.Regge D, Laudi C, Galatola G, et al. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA. 2009;301:2453–2461. doi: 10.1001/jama.2009.832. [DOI] [PubMed] [Google Scholar]
- 10.Summaries for patients: screening for colorectal cancer—U.S. Preventative Services Task Force recommendation. Ann Intern Med. 2008;149:1–44. doi: 10.7326/0003-4819-149-9-200811040-00246. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009. (corrected) Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313–316. doi: 10.1148/radiol.2392052002. [DOI] [PubMed] [Google Scholar]
- 13.Pickhardt PJ, Hassan C, Laghi A, Zullo A, Kim DH, Morini S. Cost-effectiveness of colorectal cancer screening with computed tomography colonography: the impact of not reporting diminutive lesions. Cancer. 2007;109:2213–2221. doi: 10.1002/cncr.22668. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the Medicare population. J Natl Cancer Inst. 2010;102:1238–1252. doi: 10.1093/jnci/djq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moawad FJ, Maydonovitch CL, Cullen PA, Barlow DS, Jenson DW, Cash BD. CT colonography may improve colorectal cancer screening compliance. AJR. 2010;195:1118–1123. doi: 10.2214/AJR.10.4921. [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ. Screening CT colonography: how I do it. AJR. 2007;189:290–298. doi: 10.2214/AJR.07.2136. [DOI] [PubMed] [Google Scholar]
- 17.Yee J. CT colonography: techniques and applications. Radiol Clin North Am. 2009;47:133–145. doi: 10.1016/j.rcl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Website. [Accessed February 13, 2012];National Center for Health Statistics. 2009: with special feature on medical terminology. www.cdc.gov/nchs/data/hus/hus09.pdf. Published 2010.
- 19.Waring JP, Baron TH, Hirota WK, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317–322. doi: 10.1067/s0016-5107(03)00001-4. [DOI] [PubMed] [Google Scholar]
- 20.Shinners TJ, Pickhardt PJ, Taylor AJ, Jones DA, Olsen CH. Patient-controlled room air insufflation versus automated carbon dioxide delivery for CT colonography. AJR. 2006;186:1491–1496. doi: 10.2214/AJR.05.0416. [DOI] [PubMed] [Google Scholar]
- 21.Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in populationbased screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55–64. doi: 10.1016/S1470-2045(11)70283-2. [DOI] [PubMed] [Google Scholar]
- 22.Woolf SH. The best screening test for colorectal cancer: a personal choice. N Engl J Med. 2000;343:1641–1643. doi: 10.1056/NEJM200011303432211. [DOI] [PubMed] [Google Scholar]