Abstract
Diverse receptors on two types of cell mediate adaptive immunity in jawed vertebrates. In the lamprey, a jawless vertebrate, immunity is likewise compartmentalized but the molecular mechanics are very different.
About 500 million years ago, vertebrates divided into two forms marked by the possession or lack of jaws. Today, the jawed forms are by far the more common and include organisms as diverse as humans and sharks. Immunology research has understandably centred on the jawed vertebrates, in which it has long been known that adaptive (inducible) immunity is mainly accounted for by two distinct lineages of lymphocyte: T cells and B cells. But any understanding of vertebrate immunity has to consider our distant jawless cousins, which today are represented by only the lamprey and hagfish. On page 796 of this issue, Gou and colleagues1 do just that, and extend our concepts of the two-component nature of immune function2 through their investigation of the cellular basis of immunity in the lamprey.
The business ends of T and B cells are the components that recognize foreign antigen — respectively, the T-cell antigen-binding receptor (TCR), and the B-cell receptor, which is a membrane-anchored immunoglobulin that is subsequently released as a circulating antibody (Fig. 1a). Extraordinary diversity can be generated in both TCRs and immuno globulins outside the germline through somatic recombination, and also through hypervariation, in the case of immunoglobulins. Consequently, T and B cells can produce an immune response to all manner of invaders. One major (cellular) type of T-cell response involves killing host cells infected by viruses or bacteria. By contrast, the (humoral) B-cell-derived response consists of the production of antibodies that largely recognize and ultimately help to destroy aliens in the bloodstream.
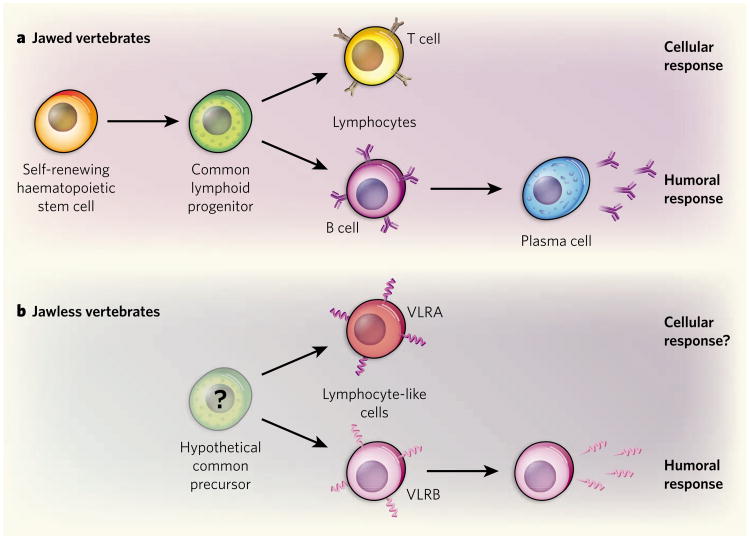
Figure 1. Compartmentalization of adaptive immunity in vertebrates.
a, In jawed vertebrates, a haematopoietic stem cell differentiates into a common lymphoid progenitor, and then into three lineages: T cells, which are responsible for the cellular immune response; B cells, responsible for the humoral response; and natural killer cells (not shown). On T cells, the receptor is the T-cell antigen-binding receptor and on B cells it is membrane-bound immunoglobulin. Alternative RNA processing changes membrane-bound immunoglobulin to a secreted antibody of the same specificity in a plasma cell. b, In jawless vertebrates, an as-yet-undefined precursor is thought to give rise to two lineages of lymphocyte-like cells defined by their expression of variable lymphocyte receptor (VLR) A or B. As Guo et al.1 show in the lamprey, VLRA is expressed only on the cell surface; VLRB is expressed initially on the cell surface and is also secreted. The molecular mechanisms by which adaptive immunity is compartmentalized into cellular and humoral responses in jawed and jawless vertebrates are fundamentally different. But in both cases there was evidently evolutionary pressure to create specific lineage-restricted immune systems.
But jawless vertebrates have much to offer immunologists. Lampreys have been shown to mount an adaptive form of specific humoral immunity to Gram-negative bacteria, as well as to a determinant unique to human type O red blood cells3. Despite the antigenic complexity of the red-blood-cell surface, induced molecules that agglutinated type O blood cells did not agglutinate type A or B cells. Furthermore, the molecular size of the reactive molecules was inconsistent with that of the immunoglobulins3. Experimental strategies, based on knowledge of the genes encoding immunoglobulins and TCRs, failed to detect the evolutionary equivalents (orthologues) of these molecules in jawless vertebrates. These negative results became understandable with the recognition that adaptive immune specificity in the lamprey and hagfish is mediated by variable lymphocyte receptors (VLRs) that are unrelated to immunoglobulin-type molecules4.
Much is now known about the structure and genetic diversification of VLRs, which consist of variable and invariant portions. On the basis of this, they are classified as VLRA and VLRB4,5, and the complex immune repertoires of both types are somatically derived through a gene-conversion-like mechanism that recombines relatively short DNA sequences encoding leucine-rich repeats6,7. A very different form of somatic recombination gives rise to diversified immunoglobulins and TCRs in the lymphocytes of jawed vertebrates.
But VLRs are produced by cells in the lamprey that morphologically resemble the lympho cytes seen in jawed vertebrates8,9. Gou et al.1 illustrate further parallels between VLR-bearing cells and conventional T and B cells. First, transfection of complementary DNAs encoding VLRA and VLRB into mammalian cell lines revealed that VLRB is both expressed at the cell surface and secreted, whereas VLRA is only surface expressed (Fig. 1b). The tissue distribution of VLRA is more anatomically restricted than that of VLRB. A known stimulant of T-cell proliferation in jawed vertebrates acts on VLRA-expressing cells to a greater degree than on VLRB-expressing cells. Putative orthologues of transcription products expressed in conventional T and B cells are associated with cell populations that are respectively restricted to VLRA and VLRB expression. Finally, two types of AID/APOBEC deaminase enzyme, both of which could potentially be involved in the generation of somatic variability7, are expressed selectively in the two lineages.
However, the relationship of the VLRA-and VLRB-expressing cell populations to the lymphocyte lineages in jawed vertebrates is far from clear. Confounding factors are the absence of conventional T- and B-cell receptors, which are the defining lineage-specific markers, and of their respective co-receptors. And there are no equivalents of those members of the major histocompatibility complex (MHC) family that interact specifically with T cells in jawed vertebrates.
The results of Gou et al.1 constitute a convincing case for the compartmental differentiation of immune functions in jawless as well as in jawed vertebrates. But the possible relationships between the VLR lymphocytelike cells and the mediators of adaptive immunity in jawed vertebrates have probably been obscured by the passage of evolutionary time. Given the extent of the evolutionary separation between jawed and jawless vertebrates, and what we know of the extraordinary levels of variation in genomic organization and the unique mechanisms associated with the rearranging antigen-binding receptors in the jawed vertebrates10, the use of a different molecule to carry out similar functions is not surprising. Whether or not the VLR system can create repertoires as complex as those seen with antibodies and TCRs remains an open question.
Ultimately, VLR diversity may well not match the extraordinarily fine specificity effected through immunoglobulins. But allelic exclusion, which restricts the rearrangement of TCRs and immunoglobulins to a single chromosome in jawed vertebrates, thus ensuring a single specific immune receptor on a lymphocyte, has also been demonstrated in lamprey VLRs4,6. Other defining characteristics of jawed vertebrate lymphocytes, including clonal selection of somatic cells, lymphocyte memory, affinity maturation and intercompartmental cooperativity, are potentially common to adaptive immunity in all vertebrates.
Overall, the new findings1 cause us to rethink the nature of immune function outside what now seems to be the ever-more-specialized case of T- and B-cell immunity in jawed vertebrates. The emerging theme reinforced here is that there was a compelling need for the evolution of effective host mechanisms to combat the elaborate immune-evasion strategies of pathogens. Adaptability in response to these strategies is achieved through integration of basic physiological processes, such as selective mobilization of DNA-repair mechanisms and differential processing of RNA10, with surprisingly few unique innovations. If the differences in the molecular basis of immune-receptor function between a lamprey and a human (or jawed fish) are as great as they seem, then there is no predicting what other adaptations may have arisen in the animal and plant kingdoms. Studies of immunity in different organisms will continue to provide examples of some of the most complex patterns of system integration and specialization in biology.
50 YEARS AGO
A general investigation has been in progress for some time in this Department into certain aspects of the chemistry of Ulex europaeus. This common furze has a local reputation as a supplementary animal foodstuff … No previous results are available on the carotene content of the green matter (spines) of furze and its seasonal variation, which are now reported … From October to February or March — when furze is fed to stock — the average carotene content is 126 mgm./kgm., indicating that it is a very good, and freely available, source of carotene. The relatively high values during the summer months point to the possibility of using dried furze meal as a supplementary source of carotene in animal feeding. The problem of retention of carotene in dried furze is being investigated.
100 YEARS AGO
The interesting problem of the dew-pond still awaits a definite solution. That these ponds are mainly fed by mist, and not dew, can hardly be doubted by anyone who has visited them at night, situated as they are on the topmost ridges of the Downs. In the driest summer the prevailing south-west wind, as it comes up from the sea, forms on these heights after dark thick clouds of mist, which soak everything that comes into contact with them … The source of the water in these ponds, therefore, seems evident, but the mechanism by which the mist is precipitated into the ponds is not so apparent … It appears to me that the only possible explanation is that the particles of mist must bear charges of electricity differing in potential from that of the earth. The charge on the earth would, of course, be most dense at the summits of the hills. Hence the tendency for the mist to deposit on the top of the ridge.
References
- 1.Gou P, et al. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper MD, Peterson RDA, Good RA. Nature. 1965;205:143–146. doi: 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- 3.Pollara B, Litman GW, Finstad J, Howell J, Good RA. J Immul. 1970;105:738–745. [PubMed] [Google Scholar]
- 4.Pancer Z, et al. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 5.Alder MN, et al. Science. 2005;310:1970–1973. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 6.Nagawa F, et al. Nature Immul. 2006;8:206–213. [Google Scholar]
- 7.Rogozin IB, et al. Nature Immul. 2007;8:647–656. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 8.Finstad J, Good RA. J Exp Med. 1964;120:1151–1168. doi: 10.1084/jem.120.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer WE, et al. Proc Natl Acad Sci USA. 2002;99:14350–14355. doi: 10.1073/pnas.212527499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litman GW, Cannon JP, Dishaw LJ. Nature Rev Immul. 2005;5:866–879. doi: 10.1038/nri1712. [DOI] [PMC free article] [PubMed] [Google Scholar]