Abstract
Objective
Magnetic resonance imaging (MRI) studies have demonstrated functional prefrontal cortical (PFC) abnormalities in pathological gambling (PG) and other psychiatric disorders characterized by impaired impulse control; e.g. cocaine dependence and bipolar disorder. These abnormalities are accompanied by impairments in white matter microstructures in the anterior (genual) corpus callosum (CC) in cocaine dependence and bipolar disorder. Prior studies have not examined white matter integrity in PG.
Methods
19 participants with PG and 19 matched control participants underwent diffusion tensor imaging (DTI) to compare white matter integrity in the CC, as assessed using fractional anisotropy (FA).
Results
In PG subjects as compared to control subjects, reduced FA values in the left and right genu of the CC were observed. Multiple regression analyses confirmed that PG status - in addition to age and past alcohol abuse/dependence (AA/AD) – was a significant predictor of genual FA values.
Conclusion
Findings of decreased FA values in the genu of the CC in PG subjects suggest that, like with other disorders of behavioral dyscontrol, white matter microstructural abnormalities contribute to the pathophysiology of PG. These differences appear particularly relevant to individuals with remitted AA/AD, highlighting the importance of considering co-occurring substance use disorders when investigating PG.
Keywords: corpus callosum, diffusion tensor imaging, fractional anisotropy, impulse control disorder, pathological gambling, substance use
Introduction
Pathological gambling (PG) is characterized in the Diagnostic and Statistical Manual 4th Edition (DSM-IV; American Psychiatric Association, 1995) as an impulse control disorder (ICD). Consistently, individuals with PG have scored high on measures of impulsivity (Blaszczynski et al., 1997; Potenza et al., 2003b). Functional magnetic resonance imaging (fMRI) studies have reported abnormalities in prefrontal cortex (PFC - particularly in its ventromedial component), particularly as related to function underlying impulse control (e.g., in risk/reward decision-making and cognitive control) in PG (Tanabe et al., 2007; Potenza et al., 2003a), and in other disorders characterized by impulsivity; e.g. cocaine dependence (Volkow and Fowler, 2000) and bipolar disorder (Blumberg et al., 2003). Studies using diffusion tensor imaging (DTI) - a technique allowing for the in vivo assessment of white matter integrity based on the directionality of diffusion of water molecules in the brain (Mori and Zhang, 2006) - have demonstrated microstructural abnormalities in the inferior frontal cortex and anterior corpus callosum (CC) in cocaine dependence (Lim et al., 2002; Lim et al., 2008; Moeller et al., 2005; Moeller et al., 2007), bipolar disorder (Yurgelun-Todd et al., 2007; Wang et al., 2008; Chaddock et al., 2009; Barnea-Goraly et al., 2009; Kafantaris et al., 2009), alcohol dependence (AD; Pfefferbaum et al., 2000; Pfefferbaum et al., 2002; Pfefferbaum et al.,2004) and ICDs, including kleptomania (Grant et al., 2006) and trichotillomania (Chamberlain et al., 2010). The extent to which white matter abnormalities exist in PG has not been previously assessed.
Clinical and population-based studies report elevated rates of drug and alcohol use disorders in PG (Desai and Potenza, 2008; Petry et al., 2005; Ladd and Petry, 2002). Clinical similarities between PG and substance use disorders have also been noted (Tavares et al., 2005), with the DSM-IV diagnostic criteria for PG sharing features with those for substance dependence (American Psychiatric Association, 1995). Based on these similarities, it has been proposed that PG might be best characterized as a behavioral addiction (Grant et al., 2006; Yip and Potenza, 2009) and is currently being considered for re-categorization as an addictive disorder in DSM-V (Holden, 2010). Given white matter deficits associated with AD (Pfefferbaum et al., 2000; Pfefferbaum et al., 2002; Pfefferbaum et al., 2004), elevated prevalence rates of AD in PG (Desai and Potenza, 2008; Petry et al., 2005), and the similarities between PG and substance addictions, research into the relationship between alcohol use disorders and CC white matter integrity in PG may inform the understanding of the pathophysiology of PG, and studies of white matter integrity in PG should consider alcohol use disorders.
Previous DTI studies suggest a complex relationship between white matter integrity and impulsivity measures across psychiatric disorders. For example in cocaine dependence, reduced anterior CC FA has been associated with Barratt Impulsiveness Scale (BIS-11) scores in some (Lim et al., 2008) but not other studies (Moeller et al., 2005). In contrast, more consistent findings have been observed between white matter integrity measures and BIS-11 scores in other populations; e.g., individuals with schizophrenia (Hoptman et al., 2002; Hoptman et al., 2004). These findings suggest that associations between FA and impulsivity measures may vary in groups of individuals with different psychiatric disorders, and, given the relevance of impulsivity to ICDs, further research is needed to better determine the relationship between anterior CC white matter integrity (e.g., genual FA) and impulsivity in individuals with PG.
As impulsivity is a complex entity (Moeller et al., 2001), the use of measures covering multiple domains of impulsivity and theoretically related constructs may help link aspects of impulsivity with specific anatomical features. For example, the Behavioral Inhibition System and Behavioral Activation System (BIS/BAS; Carver and White, 1994) scale – particularly the BAS subscales – may be helpful in identifying different aspects of behavioral activation (e.g., reward responsiveness versus fun-seeking), whereas the BIS-11, a widely used measure of impulsivity, may help in distinguishing between different aspects of impulsivity (e.g., cognitive versus motor impulsiveness; Patton et al., 1995).
This study used DTI to assess CC white matter integrity in PG subjects in comparison to healthy control subjects and to explore relations with impulsivity and consider potential contributions of substance addictions. We hypothesized that: 1) in comparison to controls, PG subjects would have reduced FA in the genu of the CC, a region linked to the functional abnormalities identified in PG in ventromedial PFC and implicated in other disorders characterized by impaired impulse control; 2) in comparison to controls, PG subjects would score higher on measures of impulsivity and behavioral activation (e.g., BIS-11, BIS/BAS subscales; 3) across all subjects, FA values would correlate inversely with measures of impulsivity and behavioral activation, as assessed by the BIS-11 and BIS/BAS subscales.
Methods
Participants and recruitment procedures
Participants were 19 individuals who met DSM-IV-TR diagnostic criteria for PG (12 men and 7 women) and 19 control participants (11 men and 8 women). Participants were screened using the Structured Clinical Interview for DSM-IV (SCID; First et al., 1995). PG diagnoses were confirmed using the Structured Clinical Interview for Pathological Gambling (SCI-PG; Grant et al., 2004). For the PG subjects, the mean (standard deviation) South Oaks Gambling Screen (SOGS; Lesieur and Blume, 1987) scores ranged between 6–18 (median score = 13) and the mean (standard deviation) score was 12.47 (3.16). Control subjects’ SOGS scores ranged between 0–2, and the mean (standard deviation) score was 1.06 (0.24). Exclusion criteria for controls included pregnancy, a current Axis I disorder apart from nicotine dependence or unstable medical condition.
Participants were recruited by media ads seeking adults for participation in a university-based research study. All participants provided written informed consent and the study protocols were approved by the Yale Human Investigations Committee.
Demographic measures
Gender, race/ethnicity, age and education information were obtained via self-report during intake.
Substance use history
One of the PG subjects and two of the controls reported current recreational marijuana use. Twelve of the PG subjects and four of the controls reported current daily cigarette smoking. Two of the PG subjects had a history of substance abuse/dependence: one subject reported past lifetime cocaine and sedative dependence, one subject reported past lifetime opioid dependence. Three of the PG subjects had a history of alcohol abuse (AA) and two had a history of AD. Two of the PG subjects with a history of AA also had a history of substance abuse/dependence: one subject reported past lifetime opiate prescription drug dependence, one subject reported past lifetime cocaine abuse. Alcohol and substance use history were not available for one PG subject, and this subject was excluded from analyses related to past substance and alcohol use.
Non-substance psychiatric comorbidity
Two of the PG subjects had current Axis I disorders: one of the PG subjects had current comorbid major depressive disorder, panic disorder, social phobia, specific phobia, obsessive-compulsive disorder and post-traumatic stress disorder. One of the PG subjects had comorbid anxiety disorder NOS. Complete Axis I histories were not available for three of the PG participants.
Impulsivity, behavioral inhibition and activation measures
The Barratt Impulsiveness Scale (BIS-11)
The Barratt Impulsiveness Scale (BIS-11; Patton et al., 1995) is a self-report measure designed to assess impulsivity. The 30-item scale factors into three subscales: Motor Impulsiveness (BIS-11-MI), Cognitive Impulsiveness (BIS-11-CI), Non-planning Impulsiveness (BIS-11-NPI).
The Behavioral Inhibition System and Behavioral Activation System (BIS/BAS) scales
Based on Gray’s theory of personality, the Behavioral Inhibition System and Behavioral Activation System (BIS/BAS; Carver and White, 1994) scale is self-report measure that factors into one BIS subscale and three BAS subscales: Fun-Seeking (BAS-FS), Drive (BAS-D), Reward Responsiveness (BAS-RR). Participants rate each of the 20 items on a four point Likert-type scale ranging from ‘strongly disagree’ to ‘strongly agree.’
Image acquisition
DTI data were acquired with a 3T Siemens Trio scanner at Yale’s Magnetic Resonance Research Center, and each participant underwent two separate image acquisitions. Diffusion sensitizing gradients were applied along 32 non-collinear directions using b values of 0 (b0 image = 1) and 1000 s/mm2 (TR = 7400, TE = 115, bandwidth = 1396, matrix = 128 × 128, FOV = 256 × 256 mm2), and 40 contiguous slices parallel to AC-PC line were acquired, and each slice was 3.0 mm thick.
Image processing
Diffusion-weighted images were visually inspected to ensure sufficient quality. Images were motion corrected using SPM5 (www.fil.ion/ucl.ac.uk/spm/software/spm5). All other image processing was conducted using the Yale BioImage Suite (www.bioimagesuite.org), following previously described techniques (Constable et al., 2008). For each subject, two separate image acquisitions were combined and averaged and used to compute the diffusion tensor. Intensity thresholds of the T2-weighted b0 image were matched on background value. Individual subject tensors were masked outside the brain. FA was calculated from the tensor data and individual subject FA maps were nonlinearly registered to a single control subject’s FA map. Parallel diffusivity values were defined as λ1, and perpendicular diffusivity values were defined as the mean of λ2 and λ3.
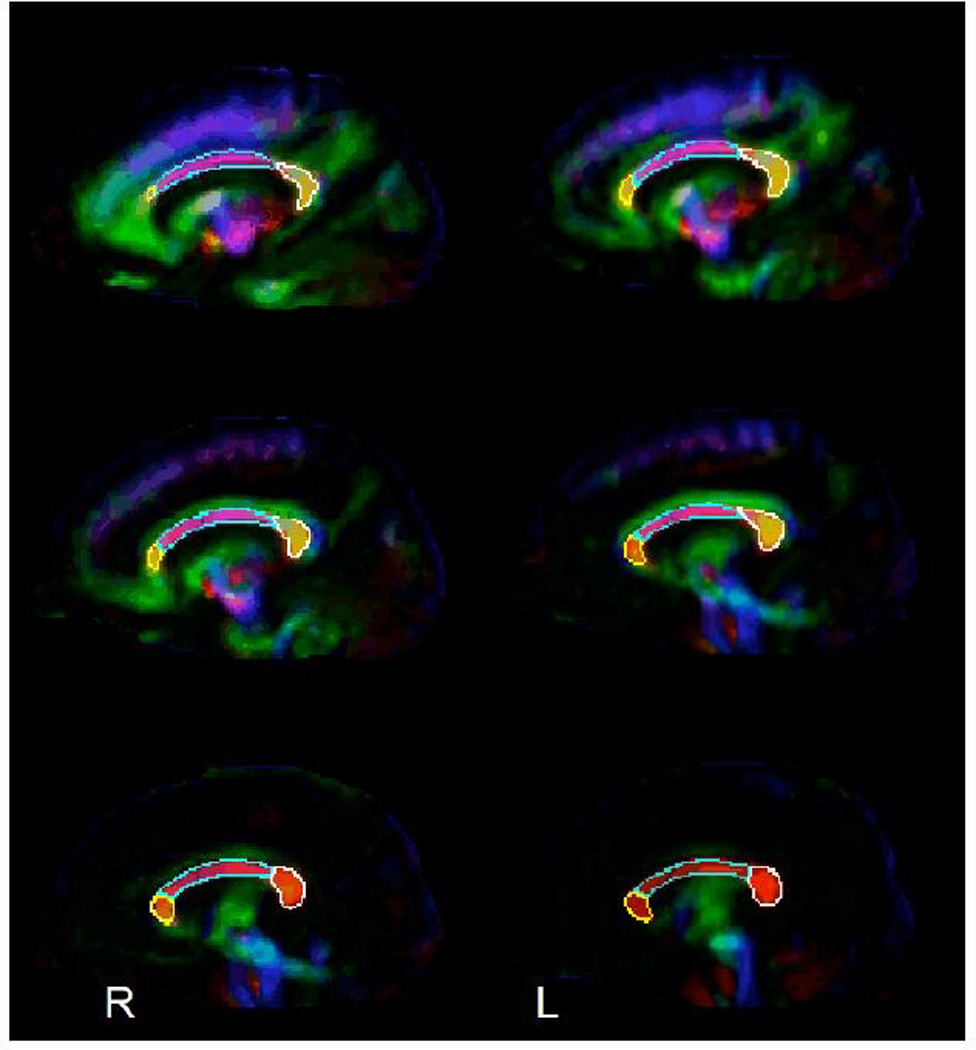
A composite tricolor directionality map was generated using the mean tensor of the control participants, and this map was used to manually parcellate the CC into six distinct regions of interest (ROIs; Fig. 1): the left and right genu, splenium and body. The genu was defined as the anterior curved aspect of the CC. The fiber tracts in the genu curved anterior to form, in part, the forceps minor. The posterior boundary of the genu (anterior aspect of body) was defined as where the fiber tracts became transverse in orientation with minimal or no curvature. The splenium was defined as the posterior curved aspect of the corpus callosum. The fiber tracts of the splenium curved posterior to form, in part, the forceps major. The anterior border of the splenium (posterior border of the body) was where fiber tracts became transverse in orientation, with minimal or no curvature. The body of the CC was defined by where the fiber tracts had a transverse orientation. ROIs were then applied directly to single-subject FA maps and used to generate individual FA values for each ROI on an individual subject basis as in previous DTI studies using the Yale BioImage Suite software package (Constable et al., 2008).
Figure 1.
Statistical comparisons
Demographic comparisons were conducted using chi-square and student’s t-tests. Individual subject FA values and parallel and perpendicular diffusivity values for each of the six ROIs were entered into SPSS 16.0. Normality was assessed using Kolmogorov Smirnov tests. BIS-11, BIS/BAS scores, FA and diffusivity values were compared using one-way analyses of variance analyses (ANOVAs). Age, gender, presence or absence of PG, substance use history, alcohol use history and tobacco-smoking status were entered into a multiple regression analyses to explore further the relationship between PG and diffusion parameters. Correlational analyses were conducted using Pearson’s r.
Results
Demographic measures
The results of between-group comparisons of demographic characteristics are shown in Table 1. Controls and PGs did not differ with respect to gender (x2 = 0.11, p = 0.74), ethnicity (x2 = 1.05, p = 0.31), age (t = 0.09, p = 0.93) or years of education (t = 1.50, p = 0.14).
Table 1.
Results of demographic and clinical comparisons between HC and PG subjects
| HCs (n = 19) | PGs (n = 19) | X2 | p | df | |
| Variables | N, % |
||||
| Female | 8, 42.1% | 7, 36.8% | 0.11 | 0.74 | 1 |
| Non-Caucasian | 8, 42.1% | 5, 26.3% | 1.05 | 0.31 | 1 |
| Mean Values ± St. Dev. |
t | p | df | ||
| Age, years | 36.53 ± 11.21 | 36.84 ± 11.76 | 0.09 | 0.93 | 1 |
| Education, years | 13.68 ± 1.97 | 12.74 ± 1.91 | 1.50 | 0.14 | 1 |
| Measures | Mean Values ± St. Dev. |
F | p | df | |
| SOGS | 1.06 ± 0.24 | 12.47 ± 3.12 | 240.08 | <0.001 | 1 |
| BIS-11 | 56.44 ± 10.93 | 69.47 ± 13.26 | 10.57 | 0.003 | 1 |
| BIS-NPI | 22.56 ± 5.68 | 27.68 ± 5.71 | 7.50 | 0.010 | 1 |
| BIS-MI | 20.94 ± 4.07 | 24.79 ± 5.20 | 6.23 | 0.017 | 1 |
| BIS-CI | 12.94 ± 3.51 | 17.00 ± 4.73 | 8.71 | 0.006 | 1 |
| BIS | 18.42 ± 3.06 | 19.32 ± 3.27 | 0.76 | 0.389 | 1 |
| BAS | 36.37 ± 5.64 | 41.89 ± 5.73 | 8.97 | 0.005 | 1 |
| BAS-FS | 10.21 ± 2.32 | 12.32 ± 1.97 | 9.06 | 0.005 | 1 |
| BAS-D | 9.58 ± 2.67 | 12.16 ± 2.71 | 8.71 | 0.006 | 1 |
| BAS-RR | 17.58 ± 1.71 | 17.42 ± 2.19 | 1.74 | 0.195 | 1 |
HC = Healthy control
PG = Pathological gambling
SOGS = South Oaks Gambling Screen
BIS-11 = Barratt Impulsiveness Scale total score
BIS-NPI = Barratt Impulsiveness Scale Non-planning Impulsiveness
BIS-MI = Barratt Impulsiveness Scale Motor Impulsiveness
BIS-C = Barratt Impulsiveness Scale Cognitive Impulsiveness
BIS = Behavioral Inhibition System
BAS = Behavioral Activation System total score
BAS-FS = Behavioral Activation System Fun-Seeking (BAS-FS),
BAS-D = Behavioral Activation System Drive
BAS-RR = Behavioral Activation System Reward Responsiveness
Impulsivity, behavioral inhibition and activation
The results of between-group comparisons of impulsivity-related measures are shown in Table 1. In comparison to controls, PGs had higher scores on the BIS-11-CI (F = 8.71, p = 0.006), BIS-11-MI (F = 6.23, p = 0.02), BIS-11-NPI (F = 7.50, p = 0.01), BAS-FS (F = 9.06, p = 0.005) and BAS-D (F = 8.71, p = 0.006).
FA and diffusivity values
Mean values and between-group comparisons of FA within the six sub-regions of the CC are shown in Table 2. In comparison to controls, PG subjects had reduced FA values in the left body (F = 4.33, p = 0.045) and left (F = 9.45, p = 0.004) and right genu (F = 8.59, p = 0.006). In comparison to controls, PG subjects also had significantly increased perpendicular diffusivity values in the left (F = 6.19, p = 0.018) and right (F = 5.48, p = 0.025) genu. No significant between group differences were observed for parallel diffusivity values.
Table 2.
Results of between group comparisons of CC diffusion parameters
| PGs versus matched HCs | |||||
| HCs (n = 19) | PGs (n = 19) | ||||
| ROIs | Mean FA values ± St. Dev. | F | p | df | |
| L Genu | 0.587 ± 0.060 | 0.520 ± 0.073 | 9.446 | 0.004 | 1 |
| R Genu | 0.587 ± 0.063 | 0.519 ± 0.078 | 8.585 | 0.006 | 1 |
| L Body | 0.557 ± 0.050 | 0.521 ± 0.057 | 4.326 | 0.045 | 1 |
| R Body | 0.536 ± 0.047 | 0.506 ± 0.058 | 3.098 | 0.087 | 1 |
| L Splenium | 0.620 ± 0.047 | 0.610 ± 0.060 | 0.328 | 0.570 | 1 |
| R Splenium | 0.609 ± 0.050 | 0.506 ± 0.058 | 0.242 | 0.626 | 1 |
| Mean Perpendicular ± St. Dev. | |||||
| L Genu | 0.512 ± 0.075 | 0.589 ± 0.113 | 6.186 | 0.018 | 1 |
| R Genu | 0.508 ± 0.077 | 0.580 ± 0.110 | 5.483 | 0.025 | 1 |
| L Body | 0.534 ± 0.063 | 0.575 ± 0.080 | 3.080 | 0.088 | 1 |
| R Body | 0.561 ± 0.064 | 0.591 ± 0.086 | 1.498 | 0.229 | 1 |
| L Splenium | 0.510 ± 0.086 | 0.523 ± 0.120 | 0.140 | 0.710 | 1 |
| R Splenium | 0.542 ± 0.105 | 0.531 ± 0.089 | 0.124 | 0.727 | 1 |
| Mean Parallel ± St. Dev. | |||||
| L Genu | 1.410 ± 0.162 | 1.338 ± 0.122 | 2.376 | 0.132 | 1 |
| R Genu | 1.416 ± 0.162 | 1.339 ± 0.120 | 2.825 | 0.101 | 1 |
| L Body | 1.385 ± 0.082 | 1.351 ± 0.111 | 1.172 | 0.286 | 1 |
| R Body | 1.372 ± 0.082 | 1.329 ± 0.095 | 2.253 | 0.142 | 1 |
| L Splenium | 1.533 ± 0.122 | 1.498 ± 0.145 | 0.655 | 0.424 | 1 |
| R Splenium | 1.553 ± 0.123 | 1.482 ± 0.126 | 3.106 | 0.086 | 1 |
St. Dev. = Standard deviation
FA = Fractional anisotropy
HC = Healthy control
PG = Pathological gambling
ROI = Regions of interest
L = Left
R = Right
Results of multiple regression analyses are displayed in Table 3. In these models, presence or absence of PG was a significant predictor of left and right genual FA (L: β = −0.38, p = 0.02; R: β = −.36, p = 0.03), and of left and right genual perpendicular diffusion values (β = 0.40, p = 0.03; β = 0.39, p = 0.04). Age was also a significant predictor of both left and right genual FA values (β = −0.37, p = 0.01; β = −0.39, p = 0.009), but did not significantly predict perpendicular genual diffusion values. Presence or absence of past AA/AD was not a significant predictor of left or right genual FA or perpendicular diffusion values, although a trend toward significance was observed for FA values (β = −0.30, p = 0.065; β = −0.30, p = 0.063). Gender, current tobacco smoking status and presence or absence of past SUDs did not significantly contribute to genual FA or perpendicular diffusion values. The only significant predictor of left body FA values was the presence or absence of past AA/AD (β = −0.37, p = 0.044).
Table 3.
Results of multiple regression analyses exploring the effects gender, age, current cigarette smoking, past SUDs, past AA/AD and current PG on CC diffusion parameters
| ROIs | Gender | Age | Current Cigarette Smoking |
Past SUDs | Past AA/AD | PG |
|---|---|---|---|---|---|---|
| B (p) | B (p) | B (p) | B (p) | B (p) | B (p) | |
| Fractional anisotropy | ||||||
| L Genu | 0.092 (0.526) | −0.365 (0.012) | 0.188 (0.238) | 0.119 (0.464) | −0.296 (0.065) | −0.379 (0.021) |
| R Genu | 0.073 (0.616) | −0.390 (0.009) | 0.160 (0.319) | 0.082 (0.618) | −0.300 (0.063) | −0.362 (0.029) |
| L Body | −0.048 (0.771) | −0.225 (0.157) | 0.256 (0.157) | 0.092 (0.615) | −0.365 (0.044) | −0.274 (0.130) |
| Perpendicular diffusion | ||||||
| L Genu | −0.081 (0.626) | 0.250 (0.120) | −0.188 (0.298) | 0.078 (0.676) | 0.266 (0.141) | 0.398 (0.033) |
| R Genu | −0.019 (0.908) | 0.289 (0.072) | −0.201 (0.263) | 0.083 (0.651) | 0.270 (0.131) | 0.390 (0.035) |
L = Left
R = Right
SUDs = Substance use disorders
AA/AD = Alcohol abuse or dependence
Correlations between clinical characteristics and FA
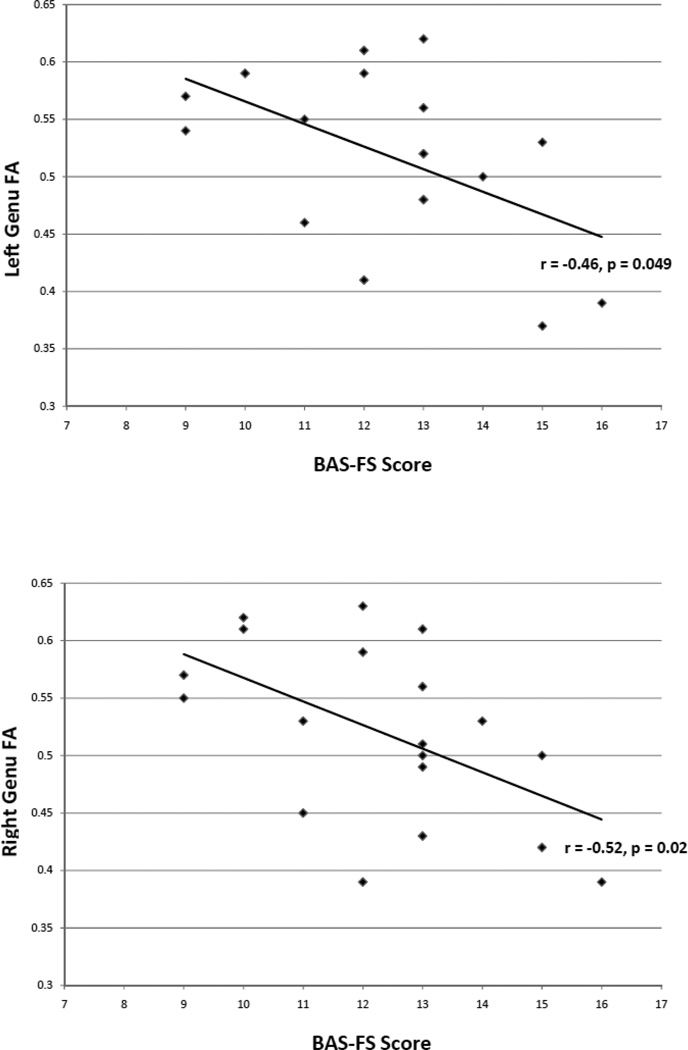
Among controls, BIS scores were positively correlated with right corpus body FA values (r = 0.47, p = 0.046). Among PG participants, BAS-FS scores were negatively correlated with FA values in the left and right genu (r = −0.46, p = 0.049; r = −0.52, p = 0.02; Fig. 2).
Figure 2.
No significant correlations between BIS-11 scores and CC FA values were observed.
Discussion
This study used DTI to compare CC white matter integrity in individuals with PG versus healthy controls. Our first hypothesis was largely supported. Consistent with this hypothesis, reduced FA values were observed in the genu of the CC among PG subjects, suggesting impairments in connectivity between frontal regions in PG. We additionally compared parallel versus perpendicular diffusivity values, and found increased perpendicular – but not parallel - diffusivity in the genu among PG subjects, suggesting decreased mylenation in this region (Song et al., 2002). Findings of reduced FA values in the genu persisted after controlling for age, gender, current tobacco smoking status, past AA/AD and SUDs using multiple regression. These findings are consistent with the other published DTI studies of ICDs (Grant et al., 2006; Chamberlain et al., 2010), and together suggest that deficits in frontal white matter microstructures might contribute to the pathophysiology of a range of impulsive disorders.
A significant effect of past AA/AD status was also observed on left body FA values, as well as a trend toward significance for genual FA values. These findings are consistent with prior DTI studies in chronic AD (Pfefferbaum et al., 2000; Pfefferbaum et al., 2002; Pfefferbaum et al.,2004), which found a significant main effect of past AA/AD on CC FA. Together, these results suggest that impairments in white matter integrity in PG could be exacerbated by the presence of prior AA/AD. However, future studies using a comparison group of controls with a history of AA/AD is needed to further substantiate this theory. The present findings complement neuropsychological research demonstrating ventral PFC deficits in both PG and AD - which have been hypothesized to reflect a shared pathophysiology - and AD-specific impairments in dorsal PFC functioning - hypothesized to reflect structural damage resultant from AD (Lawrence et al., 2009). Given the elevated rates of AA/AD in PG (Desai and Potenza, 2008; Petry et al., 2005), further research is needed to better characterize the interaction between PG and AA/AD with respect to white matter integrity. Additionally, the extent to which reduced white matter integrity in the genu in PG may be related to heavy alcohol exposure or represents an a priori vulnerability factor for PG and other disorders characterized by behavioral dyscontrol warrants additional investigation (e.g., in longitudinal studies).
Consistent with our second hypothesis, PG subjects scored significantly higher than controls on multiple measures related to impulsivity and behavioral activation. Consistent with previous studies, PG subjects scored significantly higher than controls on all three BIS-11 subscales (Motor, Cognitive, and Non-planning Impulsiveness; Fuentes et al., 2006; Ledgerwood et al., 2009). In contrast to a previous report of elevated BIS and BAS subscale (Reward-Responsiveness) scores among treatment-seeking individuals with PG (Goudriaan et al., 2006), we observed significant between-group differences for two of the BAS subscales (Fun-Seeking, Drive), but not for the third BAS subscale (Reward Responsiveness) or for the BIS subscale. Additional research is needed to better understand specific individual difference factors that may underlie differences amongst PG subjects with respect to impulsivity and behavioral inhibition and activation.
Partially consistent with our third hypothesis, reduced FA values were associated with higher scores on some but not all measures related to impulsivity and behavioral activation. Analogous to findings of some (Moeller et al, 2005) but not other studies of cocaine dependence (Lim et al., 2008), no significant correlations between FA values and BIS-11 scores were observed. These findings suggest that aspects of impulsivity assessed by the BIS-11 might be more closely related to specific functional brain activation differences (e.g., related to gray matter structure and function), consistent with findings in individuals with alcoholism (Beck, et al., 2009). A significant negative correlation between left and right genu FA values and BAS-FS subscale scores was observed among PG subjects. Elevated BAS-FS and BAS-D scores have been reported previously in other impulsive disorders with documented white matter deficits; e.g. substance use disorders (Franken et al., 2006) and bipolar disorder (Alloy et al., 2009). To our knowledge, this is the first study to explore the relationship between BIS/BAS subscale scores and white matter integrity as assessed via DTI.
In conjunction with prior reports in cocaine dependence (Moeller et al., 2005), findings in PG subjects of reduced FA values in the genu of the CC which negatively correlate with impulsivity-related measures suggest that deficits in genual CC white matter integrity might contribute to multiple disorders characterized by poor impulse control. Findings of an association between BIS scores and CC body FA values among control subjects are consistent with one previous report of a negative correlation between CC FA and cognitive control among methamphetamine users (Salo et al., 2009) and suggest that associations between white matter integrity and impulsivity-related measures extend to non-psychiatric populations.
Previous studies using fMRI have demonstrated functional impairments in frontal gray matter structures (e.g. ventromedial PFC) in PG (Tanabe et al., 2007; Potenza et al., 2003a). Findings of reduced FA in the genu of the CC among PG subjects suggest that these impairments may be partially accounted for by impairments in connectivity between frontal regions; however, further research is needed to examine directly relationships between white matter microstructural integrity and gray matter functional impairments in PG. Additionally, research is needed to determine whether the more severe white matter impairments observed among PG subjects with a history of comorbid AA/AD are best construed as vulnerability factors for multiple impulsive disorders, outcomes of engagement in multiple risk behaviors, or a direct effect of extensive alcohol exposure, or whether alternate explanations might account for the findings.
Clinically, these findings may inform data from treatment trials in which drugs (e.g., naltrexone and nalmefene) with efficacy in the treatment of AD appear beneficial in the treatment of PG (Grant et al., 2006; Grant et al., 2008), particularly among individuals with a familial history of alcoholism (Grant et al., 2008). Future studies are warranted to investigate directly how white matter microstructural integrity relates to the treatment of individuals with PG, particularly with respect to treatments with drugs like naltrexone that may target neural pathways underlying motivated behaviors in addictions. Additionally, white matter integrity may be altered by pharmacological or behavioral means (Harsan et al, 2008; Schlaug et al, 2009). For example, mindfulness based therapies have been associated with improved white matter integrity in the corona radiata, white tracts connecting the anterior cingulate cortex (implicated in self-control) to other brain regions (Tang et al., 2010). As mindfulness-based therapies have shown early promise in the treatment of substance addictions (Brewer et al, 2009), future studies of PG should evaluate the efficacy of mindfulness-based interventions as well as the behavioral factors (including impulsivity) and biological factors (including white matter integrity) that may mediate and/or moderate its effects.
Strengths and Limitations
This study has several limitations, including its reliance on self-report measures of impulsivity and related constructs, the relatively small sample size of PG subjects with past drug abuse/dependence and AA/AD and the absence of control subjects with a history of AA/AD, which prevented us from exploring directly for PG-by-AA/AD interaction effects on FA values. Our DTI analyses also include limitations, as we did not control for possible partial volume effects, or for the effects of individual variability in the fitting of the ROIs. These limitations are nonetheless similar to those in some other previously published DTI papers (e.g., Constable et al., 2008). It is additionally possible that our finding of no significant effect of past AA/AD on genual FA values might be due to Type II error, and future research including a larger sample of individuals with PG and remitted AA/AD is needed to further explore interactions between FA values and alcohol use disorders in PG. This study additionally included one PG subject and two controls with current recreational marijuana use. Although previous studies do not suggest decreased CC FA in marijuana use (reviewed in Arnone et al., 2006), there has been one previous report of increased mean diffusivity (MD) in the anterior CC among heavy marijuana users (Arnone et al., 2006). As such, future studies are needed to investigate the possible influence of marijuana use on white matter integrity in PG. However, the number of PG participants with a history of drug abuse/dependence and AA/AD is comparable to that reported in previously published fMRI studies of PG (e.g., Potenza et al., 2003b) and DTI studies of CD (Moeller et al., 2005). Additionally, there is heterogeneity amongst individuals with PG, with some groups proposed as being less impulsive than others (Blasczcynski and Nower, 2002; Ledgerwood and Petry, 2006). As the PG subjects in our group scored relatively highly on measures of impulsivity, the extent to which the findings are relevant to groups with lower levels of impulsivity warrants additional investigation. Future studies of larger samples could also investigate further the nature of the relationship between impulsivity and white matter integrity using larger sample, alternate analytical approaches, and a wider range of impulsivity measures (including behavioral assessments). A final limitation of this study is that it did not assess for a complete range of psychiatric disorders and some disorders characterized by impaired impulse control (e.g., attention-deficit/hyperactivity disorder; personality disorders) were not formally assessed.
This study also has several strengths, including a larger sample size comparable to several previous DTI studies (e.g. Moeller et al., 2005; Grant et al., 2006; Paul et al., 2008), the use of multiple impulsivity-related measures. To our knowledge, this is also the first study of white matter integrity in PG. Future research should explore further the interaction between PG, AA/AD and white matter integrity, identify the extent to which white matter differences are relevant to sub-groups of individuals with PG and the extent to which they relate to other clinically relevant measures (e.g. treatment outcome, neuropsychological task performance).
Acknowledgments
The authors would like to acknowledge Robert D Rogers for helpful discussions. The authors would like to acknowledge Katherine VanBuskirk for help with data management and collection. This study was supported in part by NIH grants R01 DA019039, RC1 DA028279, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, the Veterans Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center (MIRECC), and the State of Connecticut, Department of Mental Health and Addiction Services. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza consults for and is an advisor to Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Footnotes
Authors contribution:
Marc Potenza was responsible for the study concept and design. Sarah Yip and Cheryl Lacadie conducted the diffusion tensor analyses. Sarah Yip conducted the statistical comparisons of FA values and impulsivity measures, wrote the first draft of the manuscript and worked with Marc Potenza on subsequent drafts. Robert Fulbright advised on ROI identification. Patrick Worhunsky, Jiansong Xu and Todd Constable advised on imaging acquisition and analysis. All authors have reviewed and revised the manuscript during preparation and approved the content of this submission.
Financial Disclosures: The authors report that they have no financial conflicts of interest with respect to the content of this manuscript. The other authors reported no biomedical financial interests or other conflicts of interest.
References
- Alloy L, Bender R, Wagner C, et al. Bipolar spectrum-substance use co-occurrence: Behavioral approach system (BAS) sensitivity and impulsiveness as shared personality vulnerabilities. J Pers Soc Psychol. 2009;97:549–565. doi: 10.1037/a0016061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders 4th Edition. Washington (DC): American Psychiatric Publishing; 1995. [Google Scholar]
- Arnone D, Abou-Saleh MT, Barrick TR. Diffusion Tensor Imaging of the Corpus Callosum in Addiction. Neuropsychobiology. 2006;54:107–113. doi: 10.1159/000096992. [DOI] [PubMed] [Google Scholar]
- Arnone D, Barrick T, Chengappa S, et al. Corpus callosum damage in heavy marijuana use: Preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. NeuroImage. 2008;41:1067–1074. doi: 10.1016/j.neuroimage.2008.02.064. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Chang K, Karchemskiy A, et al. Limbic and corpus callosum aberrations in adolescents with bipolar disorder: a tract-based spatial statistics analysis. Biol Psychiatry. 2009;66:238–244. doi: 10.1016/j.biopsych.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, et al. Ventral Striatal Activation During Reward Anticipation Correlates with Impulsivity in Alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Nower L. A pathways model of problem and pathological gambling. Addiction. 2002;97:487–499. doi: 10.1046/j.1360-0443.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- Blaszczynski A, Steel Z, McConaghy N. Impulsivity in pathological gambling: The antisocial impulsivist. Addiction. 1997;92:75–87. [PubMed] [Google Scholar]
- Blumberg HP, Leung H, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:599–607. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Sinha R, Chen JA, et al. Mindfulness Training Reduces Stress Reactivity and Relapse in Substance Abuse: Results from A Randomized, Controlled Pilot Study. Substance Abuse. 2009;30:306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Person Soc Psychol. 1994;67:319–333. [Google Scholar]
- Chaddock C, Barker G, Marshall N, et al. White matter microstructural impairments and genetic liability to familial bipolar I disorder. Br J Psychiatry. 2009;194:527–534. doi: 10.1192/bjp.bp.107.047498. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Menzies LA, et al. Reduced brain white matter integrity in trichotillomania: a diffusion tensor imaging study. Arch Gen Psychiatry. 2010;67(9):965–971. doi: 10.1001/archgenpsychiatry.2010.109. [DOI] [PubMed] [Google Scholar]
- Constable RT, Vohr Ment L, et al. Prematurely Born Children Demonstrate White Matter Microstructural Differences in 12 Years of Age, Relative to Term Control Subjects: An Investigation of Group and Gender Effects. Pediatrics. 2008;121:306–316. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Patient Edition. Washington (DC): American Psychiatric Press Inc; 1995. [Google Scholar]
- Franken IHA, Muris P, Georgieva I. Gray's model of personality and addiction. Addict Beh. 2006;31:399–403. doi: 10.1016/j.addbeh.2005.05.022. 2006. [DOI] [PubMed] [Google Scholar]
- Fuentes D, Tavares H, Artes D, et al. Self-reported and neuropsychological measures of impulsivity in pathological gambling. J Int Neuropsychol Soc. 2006;12:907–912. doi: 10.1017/S1355617706061091. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W, et al. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug Alcohol Depend. 2006;84:231–239. doi: 10.1016/j.drugalcdep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Grant JE, Correia S, Brennan-Krohn T. White matter integrity in kleptomania: a pilot study. Psychiatry Res. 2006;147:233–237. doi: 10.1016/j.pscychresns.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Judson BA, Potenza MN. The neurobiology of substance and behavioral addictions. CNS Spectrums. 2006;11(12):924–930. doi: 10.1017/s109285290001511x. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Hartman B. A double-blind, placebo-controlled study of the opioid antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69:783–789. doi: 10.4088/jcp.v69n0511. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Hollander E, et al. Predicting treatment response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology. 2008;200:521–527. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Potenza MN, Hollander E, et al. Multicenter Investigation of the Opioid Antagonist Nalmefene in the Treatment of Pathological Gambling. Am J Psychiatry. 2006;163:303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- Grant JE, Steinberg MA, Kim SW, et al. Preliminary validity and reliability testing of a structured clinical interview for pathological gambling (SCI-PG) Psychiatry Res. 2004;128:79–88. doi: 10.1016/j.psychres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Steibel J, Zaremba A, et al. Recovery from chronic demyelination by thyroid hormone therapy: myelinogenesis induction and assessment by diffusion tensor magnetic resonance imaging. J Neurosci. 2008;28:14189–14201. doi: 10.1523/JNEUROSCI.4453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden C. Behavioral Addictions Debut in Proposed DSM-V. Science. 2010;327(5968):935. doi: 10.1126/science.327.5968.935. [DOI] [PubMed] [Google Scholar]
- Hoptman M, Ardekani B, Butler P, et al. DTI and impulsivity in schizophrenia: a first voxelwise correlational analysis. Neuroreport. 2004;15:2467–2470. doi: 10.1097/00001756-200411150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M, Volavka J, Johnson G, et al. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Kafantaris V, Kingsley P, Ardekani B, et al. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd G, Petry NM. Gender differences among pathological gamblers seeking treatment. Exp Clin Psychopharmacol. 2002;10:302–309. doi: 10.1037//1064-1297.10.3.302. [DOI] [PubMed] [Google Scholar]
- Lawrence A, Luty J, Bogdan N, et al. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104:1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Alessi SM, Phoenix N, et al. Behavioral assessment of impulsivity in pathological gamblers with and without substance use disorder histories versus healthy controls. Drug and Alcohol Depend. 2009;105:89–96. doi: 10.1016/j.drugalcdep.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood DM, Petry NM. Psychological experience of gambling and subtypes of pathological gamblers. Psychiatry Res. 2005;144:17–27. doi: 10.1016/j.psychres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Lesieur H, Blume S. The South Oaks Gambling Screen (SGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- Lim K, Choi SJ, Pomara N, et al. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim K, Wozniak J, Mueller B, et al. Brain macrostructural and miscrostructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller F, Barratt ES, Dougherty DM, et al. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, et al. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Pyschiatry Res Neuroimaging. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: Diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Patton J, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paul R, Grieve S, Niaura R, et al. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob Res. 2008;10:137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Stinson FS, Grant BF. Co-morbidity of DSM-IV pathological gambling and other psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan E. Disruption of Brain White Matter Microstructure by Excessive Intracellular and Extracellular Fluid in Alcoholism: Evidence from Diffusion Tensor Imaging. Neuropsychopharmacology. 2004;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan E. Microstructural But Not Macrostructural Disruption of White Matter in Women with Chronic Alcoholism. Neuroimage. 2002;15:708–718. doi: 10.1006/nimg.2001.1018. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, et al. In Vivo Detection and Functional Correlates of White Matter Microstructural Disruption in Chronic Alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003a;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. (2003) [DOI] [PubMed] [Google Scholar]
- Potenza MN, Steinberg MA, Skudlarski P, et al. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2003b;60:828–836. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl T, Buonocore M, et al. Cognitive Control and White Matter Callosal Microstructure in Methamphetamine-Dependent Subjects: A Diffusion Tensor Imaging Study. Biol Pschychiatry. 2009;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G, Marchina S, Norton A. Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci. 2009;1169:385–394. doi: 10.1111/j.1749-6632.2009.04587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, et al. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Lu Q, Geng X, et al. Short-term meditation induces white matter changes in the anterior cingulated. PNAS. doi: 10.1073/pnas.1011043107. 2010l Epub; 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wang F, Kalmar J, Edmiston E, et al. Abnormal corpus callosum integrity in bipolar disorder: a diffusion tensor imaging study. Biol Psychiatry. 2008;64:730–733. doi: 10.1016/j.biopsych.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, et al. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Potenza MN. Understanding behavioral addictions: Insights from research. In: Ries RK, Fiellin DA, Miller SC, et al., editors. Principles of Addiction Medicine: 4th Edition. Philedelphia: Lippincott Williams & Wilkins; 2009. pp. 45–63. [Google Scholar]
- Yurgelun-Todd D, Silveri M, Gruber S, et al. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9:504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]