Abstract
Imbalanced protein load within cells is a critical aspect for most diseases of aging. In particular, the accumulation of proteins into neurotoxic aggregates is a common thread for a host of neurodegenerative diseases. Recent work demonstrates that age-related changes to the cellular chaperone repertoire contributes to abnormal buildup of the microtubule-associated protein tau that accumulates in a group of diseases termed tauopathies, the most common being Alzheimer’s disease (AD). The Hsp90 co-chaperone repertoire has diverse effects on tau stability; some co-chaperones stabilize tau while others facilitate its clearance. We propose that each of these proteins may be novel therapeutic targets. While targeting Hsp90 directly may be deleterious at the organismal level, perhaps targeting individual co-chaperone activities will be more tolerable.
The identification of mutations in the MAPT gene itself in families suffering from certain types of early-onset dementia has demonstrated that defects in tau alone are capable of causing neurodegenerative disease independent of amyloid. These disorders are collectively termed “tauopathies”, and include progressive supranuclear palsy, Pick’s disease, frontal temporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) and argyrophilic grain’s disease [1]. Interestingly, while mice over-expressing the variant of tau identified from FTDP-17 fail to develop amyloid pathology, they do have profound tau pathology, neuron loss and cognitive deficits only a few months after birth [2]. Suppressing tau expression in this model prevented further neuron loss and rescued memory deficits [2]. Thus, the formation of soluble toxic tau species may underlie the development of tau-associated neurodegeneration [2], while insoluble aggregates may be the result of a protective function of last resort in terminally-differentiated neurons [3]. Enhancing the degradation of these soluble intermediates may be the most valid therapeutic strategy targeting tau.
The tau protein is intrinsically disordered in the absence of binding partners [4]. It normally promotes microtubule polymerization and stability. It has been thought to undergo folding and cleavage once aggregation has initiated [5–7]. Chaperone proteins, including Hsp27, Hsp90, HSP70 and the E3 ubiquitin ligase CHIP (carboxy-terminus of Hsc70-interacting protein), can recognize abnormal tau and reduce its concentration by facilitating its degradation and dephosphorylation [8–12]. These chaperones were also shown to protect against tau-induced cell toxicity. The need for these cytosolic chaperones becomes increasingly high when non-dividing cells, such as neurons, age. Indeed, several studies have suggested that aging decreases the activity of heat shock factor 1 (HSF1)-responsive gene expression, a critical system that allows the cell to deal with stress-induced dynamics [13, 14]. Thus the age-associated disruption of the ordered sequestration of pathologic proteins, triggering the aberrant accumulation of toxic intermediate species [15, 16], may be an essential pathological mechanism in the disease process.
Hsp90 is another molecular chaperone that is involved in the folding and stabilization of many “client” (i.e. mutant or mis-folded) proteins. Hsp90 exists as a homo-dimer and has 4 ATPase sites that are essential for its holdase activity. Compounds have been generated that inhibit Hsp90 ATPase activity, which prevents its re-folding activity [17]. The action of these drugs has two effects; 1) enhanced degradation of client proteins bound by Hsp90 and 2) activation of HSF1, since HSF1 activity is prevented by Hsp90 binding [18]. Our recent work suggests that it is the former activity of these Hsp90 inhibitors that reduces p-tau accumulation and selectively targets aberrant p-tau species that promote proteotoxicity [19]. This was the first therapeutic strategy designed to potentiate an endogenous transcription-independent chaperone response to remove abnormal proteins in tauopathies such as AD. Over the past decade, a vast number of interchangeable components that associate with the Hsp70/Hsp90 complex have been identified, and these interactions are tightly regulated based on the needs of the cell. This is in contrast to the long-held notion that protein degradation is simply mediated via a “garbage disposal” phenomenon [20, 21].
Since Hsp90 inhibition was able to reduce tau levels, we speculated that endogenous regulators of Hsp90 ATPase activity might also be therapeutic targets for tauopathies. Hsp90 co-chaperones are proteins that have distinct cellular functions apart from chaperoning and are dependent upon obligate chaperones (i.e. Hsp70 and Hsp90) to perform their chaperoning function. Some co-chaperones facilitate re-folding of an abnormal substrate whereas others promote degradation. Exploitation of this bifurcation in the Hsp90-client cycle may provide key insights into the processing of disease associated proteins, particularly tau. Therefore the repertoire of “co-chaperones” that optimally removes or re-folds specific pathologic proteins likely varies from disease to disease. Very recently, this notion was elegantly validated when mutant cystic fibrosis conductance regulator was rescued by the co-chaperone Aha-1 [22]. Thus, we speculated that identifying those select co-chaperones involved in the processing of abnormal tau and understanding this mechanism of re-folding versus degradation could allow for the discovery of novel and much more specific targets for future drug development in AD. To investigate this hypothesis and generate new therapeutic leads, we used siRNA studies to knockdown individual components of the known co-chaperone repertoire in a cell model of tau accumulation. The proteins chosen for knockdown experiments are described in Table 1.
Table 1.
Hsp90 co-chaperones that may be implicated in tau regulation.
| Cytosolic Co- chaperones |
Full Name | Aliases | MIM ID | Function |
|---|---|---|---|---|
| p23 | Prostaglandin E Synthase 3 | PTGES3; TEBP1 | 607061 | Hsp90 ATPase activation |
| Hop | Hsp70/Hsp90-Organizing Protein | STIP1; STI1 | 605063 | Hsp70/Hsp90 interaction |
| Cdc37 | Cell Division Cycle 37 | p50 | 605065 | kinase refolding |
| Aha-1 | Activator of Hsp90 ATPase 1 | AHSA1 | 608466 | CFTR folding |
| PP5 | Protein Phosphatase 5 | PPP5C | 600658 | Dephosphorylation |
| PPID | Peptidyl-Prolyl Isomerase D | Cyp40; CYPD | 601753 | Prolyl isomerase |
| FKBP52 | FK506-Binding Protein 52 | FKBP4; FKBP59 | 600611 | Prolyl isomerase |
| FKBP51 | FK506-Binding Protein 51 | FKBP5; FKBP54 | 602623 | Prolyl isomerase |
| S100A1 | S100 Calcium-Binding Protein A1 | S100α; S100A | 176940 | Neurite outgrowth |
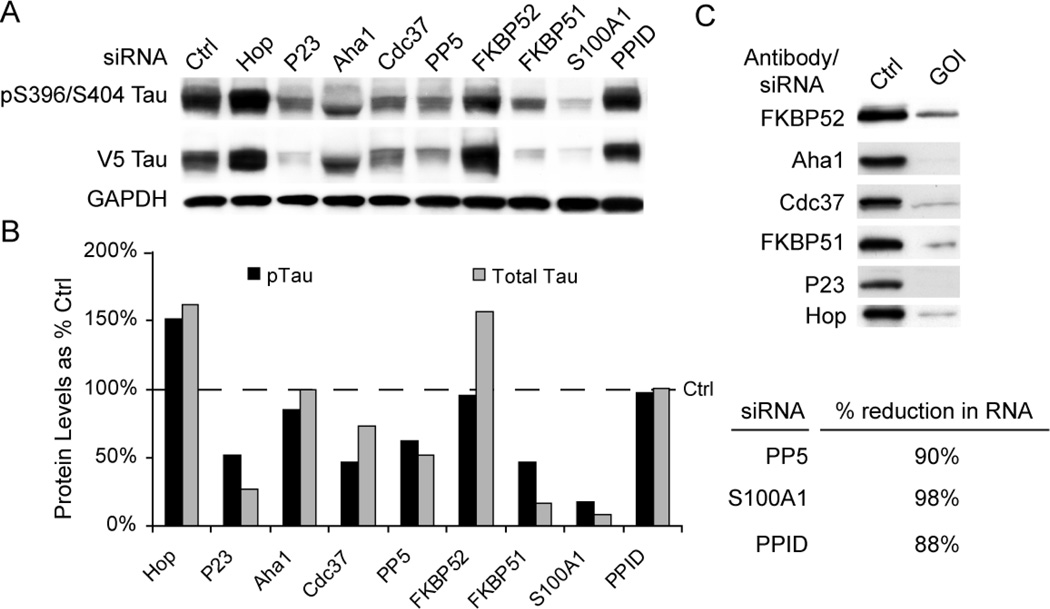
SiRNA targeting the Hsp90 co-chaperone P23 was previously shown to cause reductions in tau levels, presumably by inhibiting its ATPase activity [19]. With this in mind, we wanted to determine if other Hsp90 co-chaperones could elicit a similar response. Using a structure-based approach, we investigated how depletion of several proteins that were known to facilitate Hsp90 client folding affected tau biology. Several of these proteins contained tetratricopeptide (TPR) domains, which are known to facilitate interaction with Hsp90 and Hsp70. This included protein phosphatase 5 (PP5), FK506 binding protein 52 (FKBP52), FKBP51, S100A1 and cyclophilin 40 (Cyp40 or PPID). We also investigated Aha1 and Cdc37 which are well-documented Hsp90 co-chaperones, but lack TPR domains. HeLa cells stably transfected with tau were individually transfected siRNAs targeting each of these 7 genes as well as P23, Hop, and a non-silencing control. Cells were harvested 72 hours later and tau levels were analyzed by Western blot (Fig 1A). Quantitation of this mini-screen showed that the most pronounced reductions in both total and pS396/S404 tau levels compared to control siRNA were observed with P23, FKBP51 and S100A1 knockdown (Fig 1B). Cdc37 siRNA reduced phospho-tau to a greater extent than total tau. Aha1 knockdown changed the appearance of tau, but did not affect its levels. FKBP52 appeared to specifically increase total tau levels, while our control siRNA for Hop increased both phospho- and total tau. PPID had no effect on tau. Knockdown efficiency of these samples was analyzed by either Western blot where antibodies were readily available, or real-time PCR. All siRNAs used for this study demonstrated excellent knockdown efficiency (Fig 1C). Subsequent studies have confirmed roles for FKBP51 and 52 [23–25], Cdc37 [26], and PP5 [27–29].
Figure 1. Hsp90 chaperones differentially affect tau levels.
(A) Hela cells stably over-expressing V5-tagged wildtype human tau were transiently transfected with indicated siRNAs or scrambled negative control siRNA (Ctrl) for 72 hours and analyzed by Western. Hop siRNA was used as a positive enhancing control and p23 as a positive reducing control. (B) Western blot quantitation by densitometry. Levels of pTau (black bars) were calculated from pS396/S404 Tau in panel A after GAPDH normalization and are presented as a percentage of Ctrl (dashed line). Total Tau (gray bars) were calculated from V5 Tau in panel A after GAPDH normalization. (C) SiRNA knockdown was evaluated by Western or quantitative real time PCR (qPCR). GOI indicates gene of interest for the siRNA targeted. Ctrl indicates cells transfected with scrambled negative control. % reductions in RNA as measured by qPCR were calculated comparing Ctrl siRNA with GOI targeted siRNA.
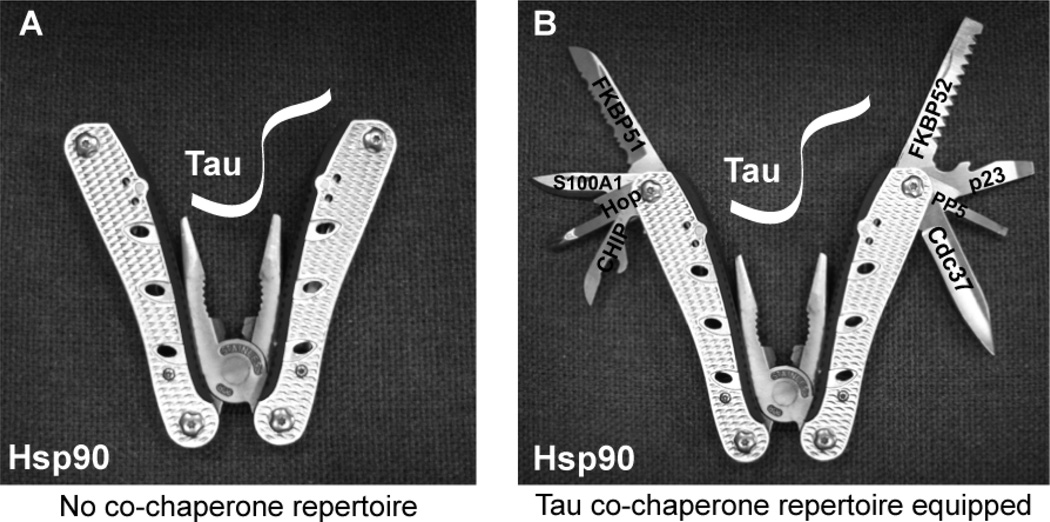
Based on these results, a picture of the Tau/Hsp90 machinery begins to emerge. We can imagine that Hsp90 is similar to a multi-tool or a pocket knife, where Hsp90 serves as the grasping tool for holding tau in place, while also acting as a scaffold for other tools (co-chaperones) to gain proximity to tau (Fig 2). These co-chaperones can then act on the bound tau without requiring absolute specificity for it. We can speculate about the mechanisms by which each protein regulates tau based on known functions. FKBP51 and FKBP52 are peptidyl prolyl isomerases that catalyze the conversion between cis and trans conformations around proline residues [30, 31]. The mid-domain of tau contains a proline rich region, and cis/trans conversion may have significant impact on the structure of tau, giving access to distinct protein phosphatases and kinases [23, 32–34]. The protein P23 is required for Hsp90 ATPase activity, and thus refolding activity [35]. Thus, suppressing the interaction of P23 with Hsp90 or suppressing its levels could turn Hsp90 into a pro-degradation machine, facilitating tau turnover. Hop is required for passing substrates like tau between Hsp70 and Hsp90 [36]. Since depleting Hop facilitates tau accumulation, it suggests that both Hsp70 and Hsp90 are involved in the chaperoning of tau; in fact, each may be dependent on the other to facilitate clearance. Cdc37 is important for recruiting kinases to the chaperone network, including CdK5 and Akt, which are both able to phosphorylate tau. Cdc37 also prevents nucleotide exchange at two of the four sites on the Hsp90 dimer: This locks Hsp90 into a “re-folding” complex. It is now known that Cdc37 not only facilitates tau stability, but also shifts the balance of tau kinases to favor CdK5 and Akt phosphorylation. Further evaluation of this protein could provide key insights about tau phosphorylation dynamics [26]. The roles of Aha1, S100A1 and PPID are less clear, although PPID seems to have a minimal impact on tau stability. Aha1 is similar to Cdc37, except it recruits nuclear hormone receptors to the Hsp90 complex and facilitates nuclear translocation and transcription of steroid induced genes. S100A1 is a calcium binding protein that interacts with Hsp90. It is induced in the CA1 region of the hippocampus following a tetanic burst that facilitates long term potentiation [37]. As Figure 1 indicates, knockdown of S100A1 most potently reduces tau levels. This suggests that S100A1 may have a vital role in controlling tau stability. Ultimately, each Hsp90 co-chaperone may be a unique drug target for chaperone clients, which includes tau and other proteins associated with neurodegenerative disease. Thus, further exploration of the mechanisms of these proteins, as well as understanding the cellular processes that these proteins control could be critical for therapeutic development using the chaperone system.
Figure 2. The Hsp90 complex depicted as a multi-tool.
(A) Hsp90 resembles a multi-tool with the pliers turned inward representing the “holdase” function; tau enters through the top of the enzyme and is grasped at the expense of ATP. (B) Co-chaperones use Hsp90 to identify the client (tau) and are attached to the outer scaffold of the dimer. Each co-chaperone has a unique role that can affect tau triage and function.
MATERIALS AND METHODS
Antibodies, siRNAs and chemicals
PHF1 (anti–S396/S404 p-tau) was provided by P. Davies, Albert Einstein College of Medicine, Yeshiva University, New York, New York, USA. Anti-FKBP51 and anti-FKBP52 were provided by Dr. David F. Smith and Dr. Marc Cox (Mayo Clinic) JJ3 (anti-p23) and F5 (anti-Hop) were provided by Dr. David O. Toft (Mayo Clinic). Anti-V5 was obtained from Invitrogen., Carlsbad, CA. Anti- Aha1 was from Rockland Immunochemicals (Gilbertsville, PA). Anti-Cdc37 was from Neomarkers (Fremont, CA). Anti-GAPDH was obtained from BIODESIGN International (Saco, ME). Secondary antibodies were obtained from Southern Biotech (Birmingham, AL). All antibodies were used at a 1:1,000 dilution with the exception of PHF1, which was used at a dilution of 1:200. All siRNAs were obtained from Qiagen; contact corresponding author for siRNA sequences. SiRNA efficiency for protein knockdown was validated either by Western blot (Fig. 1C) or by qPCR (PP5, S100A1 and PPID) as previously described ([38];Fig. 1D).
Cell culture and transfection
HeLa cells were grown in Opti-Mem plus 10% FBS (Invitrogen) and passaged every 3–5 days based on 90% confluence. SiRNA experiments were carried out as previously described [39]. Briefly, 20nM siRNAs in Opti-Mem, with 2.5 µL of siLentFect transfection reagent (Bio-Rad) used per well. This mixture was incubated for 20 minutes and then added to 50%–60% confluent HeLa cells. Cells were harvested in M-PER buffer (Pierce) containing 1X Protease inhibitor cocktail (Calbiochem), 1 mM Phenylmethylsulfonyl fluoride and 1X Phosphatase inhibitor I and II cocktails (Sigma).
REFERENCES
- 1.Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: a comparative study and review. Ann Neurol. 1996;40(2):139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- 2.Santacruz K, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickey CA, et al. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J Neurosci. 2006;26(26):6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukrasch MD, et al. Structural polymorphism of 441-residue tau at single residue resolution. PLoS Biol. 2009;7(2):e34. doi: 10.1371/journal.pbio.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver CL, et al. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neurobiol Aging. 2000;21(5):719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 6.Guillozet-Bongaarts AL, et al. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiol Aging. 2005;26(7):1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Pickering-Brown S, et al. Pick's disease is associated with mutations in the tau gene. Ann Neurol. 2000;48(6):859–867. [PubMed] [Google Scholar]
- 8.Abisambra JF, et al. Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J Neurosci. 2010;30(46):15374–15382. doi: 10.1523/JNEUROSCI.3155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou F, et al. Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A. 2003;100(2):721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279(17):17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- 11.Shimura H, et al. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J Biol Chem. 2004;279(6):4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 12.Petrucelli L, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet. 2004;13(7):703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 13.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15(2):657–664. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 15.Katsuno M, et al. Pharmacological induction of heat-shock proteins alleviates polyglutamine-mediated motor neuron disease. Proc Natl Acad Sci U S A. 2005;102(46):16801–16806. doi: 10.1073/pnas.0506249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waza M, et al. Modulation of Hsp90 function in neurodegenerative disorders: a molecular-targeted therapy against disease-causing protein. J Mol Med. 2006 doi: 10.1007/s00109-006-0066-0. [DOI] [PubMed] [Google Scholar]
- 17.Panaretou B, et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. Embo J. 1998;17(16):4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou J, et al. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94(4):471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 19.Dickey CA, et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest. 2007;117(3):648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg AL, Stein R, Adams J. New insights into proteasome function: from archaebacteria to drug development. Chem Biol. 1995;2(8):503–508. doi: 10.1016/1074-5521(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 21.Dalton WS. The proteasome. Semin Oncol. 2004;31(6 Suppl 16):3–9. doi: 10.1053/j.seminoncol.2004.10.012. discussion 33. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Hsp90 cochaperone Aha1 downregulation rescues misfolding of CFTR in cystic fibrosis. Cell. 2006;127(4):803–815. doi: 10.1016/j.cell.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Jinwal UK, et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J Neurosci. 2010;30(2):591–599. doi: 10.1523/JNEUROSCI.4815-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambraud B, et al. The immunophilin FKBP52 specifically binds to tubulin and prevents microtubule formation. FASEB J. 2007;21(11):2787–2797. doi: 10.1096/fj.06-7667com. [DOI] [PubMed] [Google Scholar]
- 25.Quinta HR, et al. Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth. J Neurochem. 2010;115(3):716–734. doi: 10.1111/j.1471-4159.2010.06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jinwal UK, et al. The Hsp90 kinase co-chaperone Cdc37 regulates tau stability and phosphorylation dynamics. J Biol Chem. 2011 doi: 10.1074/jbc.M110.182493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong CX, et al. Dephosphorylation of microtubule-associated protein tau by protein phosphatase 5. J Neurochem. 2004;88(2):298–310. doi: 10.1111/j.1471-4159.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu F, et al. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22(8):1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu F, et al. Dephosphorylation of tau by protein phosphatase 5: impairment in Alzheimer's disease. J Biol Chem. 2005;280(3):1790–1796. doi: 10.1074/jbc.M410775200. [DOI] [PubMed] [Google Scholar]
- 30.Baughman G, et al. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15(8):4395–4402. doi: 10.1128/mcb.15.8.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peattie DA, et al. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 1992;89(22):10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14(2):231–241. [PubMed] [Google Scholar]
- 33.McPherson PS. Regulatory role of SH3 domain-mediated protein-protein interactions in synaptic vesicle endocytosis. Cell Signal. 1999;11(4):229–238. doi: 10.1016/s0898-6568(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 34.Pawson T. Tyrosine kinase signalling pathways. Princess Takamatsu Symp. 1994;24:303–322. [PubMed] [Google Scholar]
- 35.McLaughlin SH, et al. The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins. J Mol Biol. 2006;356(3):746–758. doi: 10.1016/j.jmb.2005.11.085. [DOI] [PubMed] [Google Scholar]
- 36.Song Y, Masison DC. Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Sti1 (Hop1) J Biol Chem. 2005;280(40):34178–34185. doi: 10.1074/jbc.M505420200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lisachev PD, et al. A Comparison of the Dynamics of S100B, S100A1, and S100A6 mRNA Expression in Hippocampal CA1 Area of Rats during Long-Term Potentiation and after Low-Frequency Stimulation. Cardiovasc Psychiatry Neurol. 2010;2010 doi: 10.1155/2010/720958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dickey CA, et al. Selectively reduced expression of synaptic plasticity-related genes in APP+PS1 transgenic mice. J.Neurosci. 2003;23(12):5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koren J, et al. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J Biol Chem. 2009 doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]