Adequate relief of perioperative pain has been deemed a fundamental right of patients, and obligation of practitioners.1 Inadequately relieved postoperative pain has numerous physiologic complications, attendant risk of increased morbidity, and causes needless suffering. More than 40% of postoperative patients report inadequate pain relief, or pain of moderate or greater intensity, despite treatment.1-3 Unrelieved acute postoperative pain is a risk factor for the development of chronic postsurgical pain.4,5 It seems axiomatic that the duration of analgesia should match the duration of pain.
But how best to do so? The trend in anesthesia care over the past decades has been the use of opioids with successively faster elimination and successively shorter duration of effect (fentanyl, sufentanil, alfentanil, remifentanil). Use of short-duration intraoperative opioids, awakening patients in discomfort, and then attempting to play “catch-up” in the recovery room, often with the standard postoperative opioid morphine - the opioid with the longest (and long - 1 hr to peak effect) time to onset, appears to some to be suboptimal. The transition, from intraoperative anesthesia based on ultrashort opioids to postoperative analgesia, can be challenging - particularly for painful operations.6
Focusing on perioperative opioids, intravenous patient-controlled analgesia (PCA) has become the mainstay for providing postoperative pain relief over the past 2-3 decades. It is generally accepted that PCA provides better analgesia and patient satisfaction compared with conventional or nurse-administered opioids.3,5,7 However there is also awareness of the limitations of PCA. PCA is better suited to maintaining analgesia, after administration of appropriate opioid loading doses, than to achieving analgesia de novo. While PCA may be considered advantageous because it consumes less nursing time,3 the cost of pumps, disposables, and adverse events clearly also factors into assessing the overall cost-effectiveness of PCA. Adverse events may relate in part to pump programming errors, equipment malfunctions, PCA-by-proxy, and respiratory depression. The incidence of significant respiratory depression with PCA ranges from 0.1-1%, depending in part on the definition.8-11 While this incidence has been variably referred to by some as low and by others as high, events can be catastrophic. It would seem axiomatic that decreasing postoperative opioid use, and the number of doses, would diminish the risk of untoward effects.
This issue of Anesthesia & Analgesia reports an investigation which reminds us of a longavailable, effective, and infrequently- (and perhaps under-) utilized alternative to the more conventional opioid paradigms described above.12 That alternative is intraoperative methadone. For patients undergoing complex thoracolumbar spine surgery, Gottschalk et al provided total intravenous anesthesia with propofol (50-150 μg/kg/min titrated to a target bispectral index) and either (by randomization) a sufentanil infusion (0.25 μg/kg/h after a loading dose of 0.75 μg/kg) or a single bolus dose of methadone (0.2 mg/kg), both supplemented with 0.1 μg/kg sufentanil for inadequate anesthesia. Postoperative analgesia was provided by PCA (fentanyl, morphine, or hydromorphone). Patients receiving methadone had significantly lower postoperative opioid requirements (median 98 vs 219 mg morphine equivalents 0- 72 hr postoperatively). Moreover, despite PCA, they also reported less postoperative pain. There was no difference in the incidence of side effects with methadone vs sufentanil. The authors conclude that a single intraoperative bolus of methadone improves postoperative pain control for patients undergoing complex spine surgery. Their results are unquestionably related to the slow rate of methadone elimination, and also attributed by the authors potentially to N-methyl-D-aspartate (NMDA) receptor antagonism by methadone.
Methadone is the opioid with the longest elimination half-life (Table 1). It is an efficacious and cost-effective analgesic for acute, chronic, neuropathic, and cancer pain, in adults, children and even neonates, and can be administered via oral, intravenous, and other parenteral routes.13-15 For cancer and neuropathic pain, it is an often-used alternative to morphine, and growing rapidly in first-line use.16,17 Nevertheless, it remains relatively invisible in the operating room and postoperatively.
Table 1.
Onset of effect and elimination of opioids
| t½ke0* | Elimination t½ | |
|---|---|---|
| remifentanil | 1 min | 0.5 hr |
| alfentanil | 1 min | 1 hr |
| sufentanil | 6 min | 8 hr |
| fentanyl | 5 min | 8-10 hr |
| morphine | 2-4 hr | 2-3 hr |
| methadone | 8 min | 24-36 hr |
Based on published data.45
The seminal investigations which introduced the use of methadone in the perioperative period were reported by Gourlay et al.18-20 They studied orthopedic (typically anterior spinal fusion) and general surgery (typically open cholecystectomy) patients administered 20 mg methadone as an IV bolus following induction of anesthesia. Postoperatively, approximately one-third were entirely pain free and did not request any analgesics during the 72 hr postoperative observation period, approximately one-third used only oral non-opioid analgesics (aspirin, paracetamol), with a median duration of methadone analgesia (time to first oral analgesic request) of 26 hr, and only one-third of patients requested postoperative opioid analgesics, with a median duration of methadone analgesia (time to first opioid analgesic request) of 20 hr. Importantly, no patient had postoperative respiratory depression (rate < 10 breaths/min), and postoperative nausea/vomiting was not different than that conventionally encountered. In a follow-up investigation, with a greater percentage of (presumably more painful) upper abdominal incisions, after 20 mg intraoperative methadone, patients needed a median of two 5 mg methadone doses in the recovery room (which thereafter provided postoperative analgesia for 21 hr), and an additional 5 mg dose on the ward at that time, which often provided analgesia until hospital discharge.19 A third investigation was a double-blind comparison of perioperative methadone and morphine for upper abdominal surgery.20 Patients received 20 mg opioid intraoperatively, and 5 mg doses in the recovery and surgical wards. In patients receiving methadone compared with morphine, the average time from initial pain control to first supplemental opioid dose in the surgical ward was significantly longer (21 vs 6 hr), and the average cumulative postoperative opioid use (12 vs 41 mg) and number of doses (2 vs 8) were smaller. Importantly, in these three investigations, there were no serious side effects, most notably respiratory depression, which is of particular concern with an opioid with prolonged effects. Together, this trio of investigations established methadone as providing excellent and prolonged intraoperative and postoperative analgesia, together with a fully acceptable safety profile.18-20 Other investigations subsequently confirmed the effectiveness and utility of perioperative methadone, including the advantages of longer analgesia, fewer postoperative opioid doses, lower cumulative postoperative opioid use, and lower pain intensity compared with morphine.21-23 The investigation by Gottschalk et al, again reminds us of these pharmacologic advantages.12
Nevertheless, methadone is one of the most thoroughly studied yet persistently misunderstood drugs in medicine! There are several misperceptions.
One misperception is that the onset of methadone analgesia is slow, and therefore it is unsuitable for perioperative use. In fact, central nervous system effect site methadone concentrations rapidly equilibrate with plasma concentrations, evidenced by the short lag time between plasma concentrations and effects (t1/2ke0 of 4 min).24 This is comparable to the rapid onset and effect compartment equilibration of fentanyl and sufentanil (5-6 min), which is exceeded only by that of remifentanil and alfentanil (t1/2ke0 of 1 min for both). In contrast, there is a well-known lag time for the apparent central nervous system effect compartment penetration and onset time of morphine, where t1/2ke0 has been reported to exceed 4 hr!25,26 Typical opioid t1/2ke0 values are compared in Table 1. The practical significance of these differences in central nervous system penetration is shown by comparing the time course of opioid plasma concentrations with the time course of effect, using miosis as a metric for the latter (Figure 1). Under similar conditions for all the opioids, the time course of miosis was quite similar to that of plasma concentrations, for alfentanil, fentanyl, and methadone. In contrast, for morphine, peak effects occurred hours after the peak of plasma concentrations. Onset of methadone analgesia is similarly rapid.24 These considerations suggest that methadone is a rapid-onset drug, with an onset time similar to that of fentanyl and sufentanil. For a patient in pain, intraoperatively or postoperatively, needing an opioid where rapid analgesia is desired, methadone certainly appears preferable to morphine.
Figure 1.
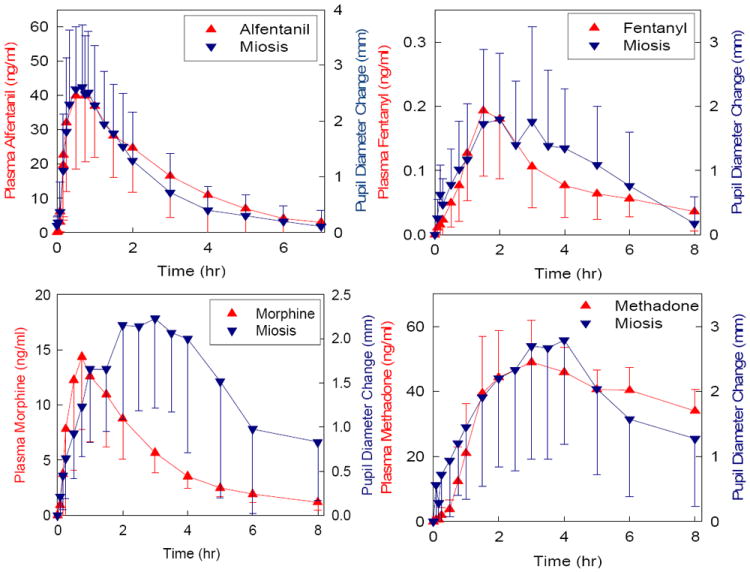
Relationship between opioid plasma concentration and onset of effect. Opioid effects was determined by pupil diameter change (miosis). Results are shown for alfentanil, fentanyl, methadone and morphine. Redrawn with permission.41-44
A second misperception is that the duration of methadone analgesia is shorter than its elimination half life (http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm142841.htm).18,27,28 This is only partially true, and certainly not specific to methadone, because the relationship between elimination half-life and duration of effect depends also on the dose administered (for methadone, or any other opioid, or drugs in general). The clinical effect of small doses will be terminated by redistribution (where the elimination half-life is irrelevant), while the effect of larger doses (achieving biophase concentrations well in excess of the minimal effective concentration) will be terminated by systemic elimination (where the elimination half-life is relevant) (Figure 2). Thus, targeting doses and concentrations as high as possible above the minimal analgesic concentration, but below the threshold for respiratory depression, will achieve the longest-lasting analgesia. At concentrations approximately ≥20 mg, the duration of methadone analgesia approximates its elimination half life.
Figure 2.
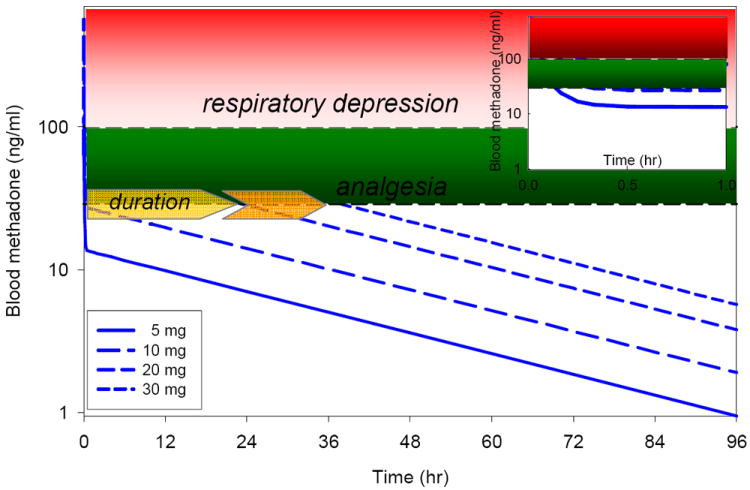
Relationship between methadone dose and duration of effect. Simulated methadone blood concentrations vs time are based on the pharmacokinetic parameters of Gourlay et al,18 as are the minimal effective analgesic methadone concentration (approximately 30 ng/ml), and the threshold for significant (5-6 breaths/min) respiratory depression (approximately 100 ng/ml).19 Data are shown for intravenous bolus methadone doses of 5, 10, 20 and 30 mg. Estimated duration of analgesia for these doses is approximately <0.5, <0.5, 24 and 36 hr. Duration of analgesia is governed by redistribution (and the redistribution half-life of approximately 5 min) for the 5 and 10 mg doses, but by elimination (and the elimination half-life of approximately 30 hr) for the larger doses. The inset shows plasma concentrations for the first hr after dosing. Due to rapid redistribution, anticipated respiratory depression would be less than 30-45 min, even at the higher single bolus doses.
A third misperception attends to the metabolism of methadone, which inactivates the drug. Methadone is considered to have a highly variable clearance and significant susceptibility to metabolic drug interactions.29 During the previous decade, its clinical metabolism and clearance have been attributed at various times to different cytochrome P450s (CYP), including CYP2D6, CYP2C19, and most consistently CYP3A4. Numerous studies, reviews, dosing guides, and the methadone label (approved by the FDA November 2007) warn about the potential for CYP3A4-mediated methadone drug interactions and the need to adjust dosing accordingly.30-33 Nevertheless, methadone metabolism may be less susceptible to inhibitory drug interactions that previously thought, and accumulated evidence demonstrates clearly that it is not a clinical CYP3A4 substrate and suggests instead that CYP2B6 is responsible for methadone metabolism and clearance.34-37
Based on these investigations, this author has used methadone as the primary intraoperative opioid for adult inpatients for over 25 yr. A single 20 mg intravenous bolus dose is administered before induction. This is reduced to 15 mg in patients “physiologically” older than 60 yr, because of declining methadone elimination and increased risk of respiratory depression with age.18,38 Other than occasional additional fentanyl (100-200 μg) use for induction/intubation, no other intraoperative opioids are administered. For postoperative analgesia, intravenous methadone (2-3 mg doses at >10 min intervals) is administered in the post-anesthesia care unit as needed, if a patient complains of pain and has an unstimulated respiratory rate greater than 10.20
There are also unanswered questions about perioperative methadone use. There is little information available about the pharmacokinetics and pharmacodynamics of methadone in pediatrics.22,23,39 More information is needed about the effectiveness and safety in outpatients,40 those who are opioid-tolerant, and, perhaps, the cost-effectiveness and safety of methadone compared with conventional PCA. Nevertheless, as reminded by Gottschalk, et al,12 perhaps it is opportune to rediscover, reappraise, and reinvigorate the use of methadone in the perioperative period.
Acknowledgments
Supported by NIH grants R01-DA14211, R01-DA25931, K24-DA00417
Footnotes
Conflict of interest: None
References
- 1.Brennan F, Carr DB, Cousins M. Pain management: a fundamental human right. Anesth Analg. 2007;105:205–21. doi: 10.1213/01.ane.0000268145.52345.55. [DOI] [PubMed] [Google Scholar]
- 2.Moss E, Taverner T, Norton P, Lesser P, Cole P. A survey of postoperative pain management in fourteen hospitals in the UK. Acute Pain. 2005;7:13–20. [Google Scholar]
- 3.Taylor A, Stanbury L. A review of postoperative pain management and the challenges. Curr Anaesthesia Critical Care. 2009;20:188–94. [Google Scholar]
- 4.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 5.Rathmell JP, Wu CL, Sinatra RS, Ballantyne JC, Ginsberg B, Gordon DB, Liu SS, Perkins FM, Reuben SS, Rosenquist RW, Viscusi ER. Acute post-surgical pain management: a critical appraisal of current practice. Reg Anesth Pain Med. 2006;31(suppl 1):1–42. doi: 10.1016/j.rapm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Bowdle TA, Ready LB, Kharasch ED, Nichols WW, Cox K. Transition to post-operative epidural or patient-controlled intravenous analgesia following total intravenous anaesthesia with remifentanil and propofol for abdominal surgery. Eur J Anaesthesiol. 1997;14:374–9. doi: 10.1046/j.1365-2346.1997.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- 7.Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD003348.pub2. CD003348. [DOI] [PubMed] [Google Scholar]
- 8.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–23. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro A, Zohar E, Zaslansky R, Hoppenstein D, Shabat S, Fredman B. The frequency and timing of respiratory depression in 1524 postoperative patients treated with systemic or neuraxial morphine. J Clin Anesth. 2005;17:537–42. doi: 10.1016/j.jclinane.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Weinger MB. Dangers of postoperative opioids. APSF workshop and white paper address prevention of postoperative respiratory complications. APSF Newsletter. 2007;21:63–7. [Google Scholar]
- 11.Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112:226–38. doi: 10.1097/ALN.0b013e3181c38c25. [DOI] [PubMed] [Google Scholar]
- 12.Gottschalk A, Durieux ME, Nemergut EC. Intraoperative methadone improves postoperative pain control in patients undergoing complex spine surgery. Anesth Analg. doi: 10.1213/ANE.0b013e3181d8a095. in press. [DOI] [PubMed] [Google Scholar]
- 13.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348:1223–32. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 14.Bryson J, Tamber A, Seccareccia D, Zimmermann C. Methadone for treatment of cancer pain. Curr Oncol Rep. 2006;8:282–8. doi: 10.1007/s11912-006-0034-4. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson AB. Methadone for cancer pain. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD003971.pub3. CD003971. [DOI] [PubMed] [Google Scholar]
- 16.Bruera E, Palmer JL, Bosnjak S, Rico MA, Moyano J, Sweeney C, Strasser F, Willey J, Bertolino M, Mathias C, Spruyt O, Fisch MJ. Methadone versus morphine as a first-line strong opioid for cancer pain: a randomized, double-blind study. J Clin Oncol. 2004;22:185–92. doi: 10.1200/JCO.2004.03.172. [DOI] [PubMed] [Google Scholar]
- 17.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, Kalso EA, Loeser JD, Miaskowski C, Nurmikko TJ, Portenoy RK, Rice AS, Stacey BR, Treede RD, Turk DC, Wallace MS. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 18.Gourlay GK, Wilson PR, Glynn CJ. Pharmacodynamics and pharmacokinetics of methadone during the perioperative period. Anesthesiology. 1982;57:458–67. doi: 10.1097/00000542-198212000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Gourlay GK, Willis RJ, Wilson PR. Postoperative pain control with methadone: Influence of supplementary methadone doses and blood concentration-response relationships. Anesthesiology. 1984;61:19–26. [PubMed] [Google Scholar]
- 20.Gourlay GK, Willis RJ, Lamberty J. A double-blind comparison of the efficacy of methadone and morphine in postoperative pain control. Anesthesiology. 1986;64:322–7. doi: 10.1097/00000542-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Richlin DM, Reuben SS. Postoperative pain control with methadone following lower abdominal surgery. J Clin Anesth. 1991;3:112–6. doi: 10.1016/0952-8180(91)90007-a. [DOI] [PubMed] [Google Scholar]
- 22.Berde CB, Beyer JE, Bournaki M-C, Levin CR, Sethna NF. Comparison of morphine and methadone for prevention of postoperative pain in 3- to 7-year-old children. J Pediatr. 1991;119:136–41. doi: 10.1016/s0022-3476(05)81054-6. [DOI] [PubMed] [Google Scholar]
- 23.Hamunen K. Ventilatory effects of morphine, pethidine and methadone in children. Br J Anaesth. 1993;70:414–8. doi: 10.1093/bja/70.4.414. [DOI] [PubMed] [Google Scholar]
- 24.Inturrisi CE, Colburn WA, Kaiko RF, Houde RW, Foley KM. Pharmacokinetics and pharmacodynamics of methadone in patients with chronic pain. Clin Pharmacol Ther. 1987;41:392–401. doi: 10.1038/clpt.1987.47. [DOI] [PubMed] [Google Scholar]
- 25.Dershwitz M, Walsh JL, Morishige RJ, Connors PM, Rubsamen RM, Shafer SL, Rosow CE. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000;93:619–28. doi: 10.1097/00000542-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Dahan A, Romberg R, Teppema L, Sarton E, Bijl H, Olofsen E. Simultaneous measurement and integrated analysis of analgesia and respiration after an intravenous morphine infusion. Anesthesiology. 2004;101:1201–9. doi: 10.1097/00000542-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Grochow L, Sheidler V, Grossman S, Green L, Enterline J. Does intravenous methadone provide longer lasting analgesia than intravenous morphine? A randomized, double-blind study. Pain. 1989;38:151–7. doi: 10.1016/0304-3959(89)90233-9. [DOI] [PubMed] [Google Scholar]
- 28.Gourlay GK, Plummer JL, Cherry DA, Cousins MJ. Does intravenous methadone provide longer lasting analgesia than intravenous morphine? Pain. 1990;42:383–6. doi: 10.1016/0304-3959(90)91151-8. [DOI] [PubMed] [Google Scholar]
- 29.Plummer JL, Gourlay GK, Cherry DA, Cousins MJ. Estimation of methadone clearance: application in the management of cancer pain. Pain. 1988;33:313–22. doi: 10.1016/0304-3959(88)90290-4. [DOI] [PubMed] [Google Scholar]
- 30.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 31.Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–81. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther. 2006;80:682–90. doi: 10.1016/j.clpt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Bruce RD, Altice FL, Gourevitch MN, Friedland GH. Pharmacokinetic drug interactions between opioid agonist therapy and antiretroviral medications: implications and management for clinical practice. J Acquir Immune Defic Syndr. 2006;41:563–72. doi: 10.1097/01.qai.0000219769.89679.ec. [DOI] [PubMed] [Google Scholar]
- 34.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–68. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport. Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL. Side effects of opioids during short-term administration: effect of age, gender, and race. Clin Pharmacol Ther. 2003;74:102–12. doi: 10.1016/S0009-9236(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Tong X, McCarver DG, Hines RN, Beard DA. Population-based analysis of methadone distribution and metabolism using an age-dependent physiologically based pharmacokinetic model. J Pharmacokinet Pharmacodyn. 2006;33:485–518. doi: 10.1007/s10928-006-9018-0. [DOI] [PubMed] [Google Scholar]
- 40.Callesen T, Bech K, Andersen J, Nielsen R, Roikjaer O, Kehlet H. Pain after primary inguinal herniorrhaphy: influence of surgical technique. J Am Coll Surg. 1999;188:355–9. doi: 10.1016/s1072-7515(98)00316-0. [DOI] [PubMed] [Google Scholar]
- 41.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass CYP3A activity. Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Kharasch ED, Whittington D, Hoffer C. Influence of hepatic and intestinal cytochrome P4503A (CYP3A) activity on the acute disposition and effects of oral transmucosal fentanyl citrate. Anesthesiology. 2004 doi: 10.1097/00000542-200409000-00022. in press. [DOI] [PubMed] [Google Scholar]
- 43.Kharasch ED, Hoffer C, Whittington D. The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br J Clin Pharmacol. 2004;57:600–10. doi: 10.1111/j.1365-2125.2003.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of P-glycoprotein in the intestinal absorption and clinical effects of morphine. Clin Pharmacol Ther. 2003;74:543–54. doi: 10.1016/j.clpt.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 45.Lötsch J. Pharmacokinetic-pharmacodynamic modeling of opioids. J Pain Symptom Manage. 2005;29:S90–103. doi: 10.1016/j.jpainsymman.2005.01.012. [DOI] [PubMed] [Google Scholar]


