Abstract
Catalysis of Cope-type rearrangements of bishomoallylic hydroxylamines is demonstrated using chiral thiourea derivatives. This formal intramolecular hydroamination reaction provides access to highly enantioenriched α-substituted pyrrolidine products, and represents a complementary approach to metal-catalyzed methods.
Enantioselective intramolecular olefin hydroamination affords a direct and atom-economical synthetic approach to chiral nitrogen heterocycles with useful properties as biologically active compounds,1 small-molecule catalysts,2 and ligands.3 Significant advances in metal-catalyzed asymmetric intramolecular hydroaminations have relied mostly on highly air- and moisture-sensitive lanthanide, group 4 metal (Zr, Ti), or highly electropositive main group metal (Mg, Li) complexes.4 Late transition metal-catalyzed hydroamination4s-t and carboamination4a,5 reactions have been identified that hold promise for broader functional group tolerance and concomitant substrate generality.6,7
The retro-Cope elimination reaction (Figure 1) represents a mechanistically distinct alternative to metal-catalyzed processes for alkene hydroamination. In this type of transformation, a hydroxylamine undergoes reaction with an olefin in a pericyclic pathway involving concerted C–H and C–N bond formation (Figure 1).8,9 Protic solvents have been shown to accelerate the Cope-type hydroamination,10 and computational studies have identified specific stabilizing H-bonding interactions in the polar cyclization transition structure to account for this effect.11
Figure 1.
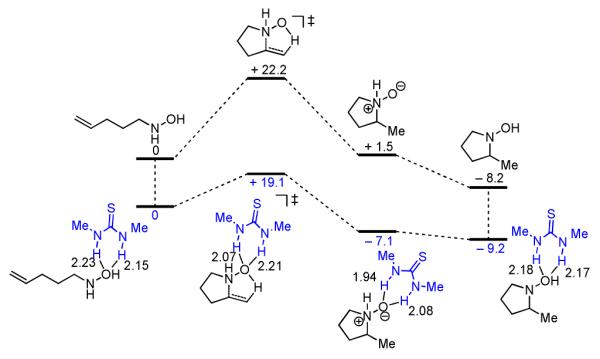
Calculated electronic energies for the optimized structures of the substrate, transition state, N-oxide intermediate, and product for both an uncatalyzed- (black, top) and a N,N'-dimethylthiourea-catalyzed (blue, bottom) Cope hydroamination reaction (B3LYP/6-331+G(d, p) level of DFT). Relative energies are in kcal/mol and key hydrogen bonding distances are shown in Angstroms.
We considered whether ureas and thioureas,12 which have demonstrated utility as enantioselective dual hydrogen-bond donor catalysts, could facilitate the Cope-type hydroamination in a similar manner.13 In order to evaluate this hypothesis, we analyzed the effect of a simple thiourea on the reaction coordinate for the Cope reaction using DFT methods (Figure 1). This analysis predicts that the thiourea lowers the activation barrier for the cyclization by 3.1 kcal/mol, thereby supporting the hypothesis that this family of H-bond donors could serve as viable catalysts for the hydroamination reaction. We report here the development of chiral thiourea derivatives bearing electron rich aromatic components that catalyze highly enantioselective Cope-type hydroaminations, providing access to enantioenriched α-substituted pyrrolidine products.
The principle we applied to the design of chiral catalysts for the Cope hydroamination is outlined in Scheme 1. In prior work on enantioselective catalytic Claisen rearrangements,14 the combined effect of hydrogen-bonding and secondary stabilization in the dipolar transition structure was shown to underlie the observed rate acceleration and asymmetric induction.15 We reasoned that the polar character of the Cope-type hydroamination transition structure might render it susceptible to analogous cooperative, attractive, non-covalent interactions with the appropriate polyfunctional catalyst framework.
Scheme 1.
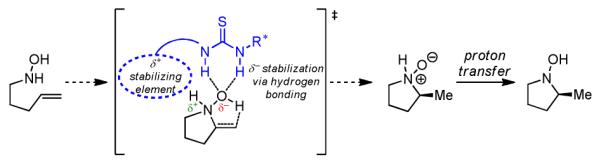
Design Strategy for Enantioselective Cope-Type Hydroamination Catalyst
The cyclization of hydroxylamine 1a was evaluated in the presence of a variety of thiourea catalysts bearing polarizable and conformationally constrained aromatic components (Table 1). While catalysts 3a and 3b (Table 1, entries 2–3) provided modest acceleration over the background, uncatalyzed reaction, catalysts containing electron rich π-systems, such as extended arenes or aryl-pyrroles, led to more significant rate enhancements. For catalysts 3b–3e (Table 1, entries 3–6), an increase in yield and enantioselectivity was observed as the expanse of the α-aryl group of the pyrrolidine was increased. Comparable correlations between arene expanse and rate and enantioselectivity have been observed previously for this class of catalysts (3c–3e) in the context of polyene cyclizations and episulfonium ion ring-openings.16 In these systems, stabilizing cation-π interactions between catalyst arene and the cationic transition state were identified.
Table 1.
Catalyst Evaluationa
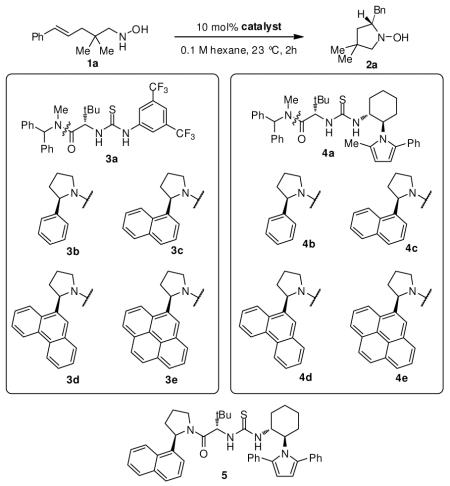
| entry | catalyst | yieldb | eec |
|---|---|---|---|
| 1 | none | 8 | -- |
| 2 | 3a | 43 | 9 |
| 3 | ent-3b | 45 | −14 |
| 4 | 3c | 58 | 40 |
| 5 | 3d | 67 | 85 |
| 6 | 3e | 74 | 87 |
| 7 | 4a | 77 | 76 |
| 8 | 4b | 85 | 86 |
| 9 | ent-4c | 82 | −89 |
| 10 | 4d | 60 | 81 |
| 11 | 4e | 64 | 85 |
| 12 | 5 | 83 | 91 |
Hydroxylamine 1a was generated in situ from the corresponding trifluoroacetate salt by addition of aqueous potassium carbonate. Reactions were performed on a 0.046–0.049 mmol scale, and were quenched by the addition of p-NO2 benzoyl chloride.
Yields of the O-benzoylated products were determined by 1H NMR analysis using mesitylene as in internal standard.
Determined by HPLC analysis using commercial chiral columns.
Catalysts bearing aryl-pyrrole components capable of similar interactions15 were also found to be effective and highly enantioselective catalysts for the model transformation (Table 1, compare entries 2 and 7). For catalysts containing both a pyrrole and an α-aryl pyrrolidine moiety, the level of enantioinduction was relatively insensitive to the size of the aryl group on the pyrrolidine portion of the catalyst (Table 1, entries 8–11). These results suggest that catalyst-transition state interactions with the aryl pyrrole are stronger than those with the aryl pyrrolidine for this class of catalysts. After extensive optimization of these structural motifs, thiourea 5 was identified as the optimal catalyst for the hydroamination.17
With the optimal catalyst identified, the substrate scope of the hydroamination reaction was evaluated. Several 4-alkenyl-1-hydroxylamines bearing electronically diverse (E)-styryl groups underwent cyclization in the presence of thiourea 5 in good yields and moderate to excellent enantioselectivities (Table 2, entries 1–10). Ortho, meta, and para substitution on the arene were all well tolerated (Table 2, entries 3, 6 and 7), as were halogen- and oxygen-containing functionality (Table 2, entries 3 and 5). Substrates with geminal disubstitution at the 2-position reacted more rapidly and with higher enantioselectivity than substrates lacking such groups (Table 2, entries 1, 8, and 9), presumably as a result of a favorable Thorpe-Ingold effect in the cyclization reaction.18 Terminal olefin substrate 1j also underwent cyclization to the desired pyrrolidine product, albeit with slightly lower enantioselectivity than the corresponding styrenyl analogues (Table 2, entries 11–12). The catalytic efficiency of the system is also notable, as the catalyst loading could be decreased from 10 to 2 mol % with only slight diminution of enantioselectivity (Table 2, entry 2).19
Table 2.
Scope of Enantioselective Hydroaminationa
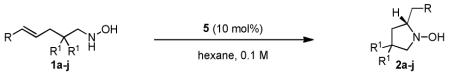
| entry | R | R1 | product | time (h) | temp (d°C) | yieldb | eec |
|---|---|---|---|---|---|---|---|
| 1 | C6H5 | Me | 2a | 12 | 3 | 83 | 94 |
| 2d | C6H5 | Me | 2a | 72 | 3 | 79 | 92 |
| 3 | p-ClC6H4 | Me | 2b | 5 | 3 | 87 | 95 |
| 4 | p-(Me)C6H4 | Me | 2c | 36 | 3 | 84 | 87 |
| 5 | p-(OMe)C6H4 | Me | 2d | 96 | 3 | 96 | 91 |
| 6 | o-ClC6H4 | Me | 2e | 5 | 3 | 91 | 92 |
| 7 | m-ClC6H4 | Me | 2f | 5 | 3 | 87 | 94 |
| 8 | C6H5 | -CH2(CH2)3CH2- | 2g | 5 | 3 | 82 | 96 |
| 9 | C6H5 | H | 2h | 72 | 30 | 68 | 81 |
| 10 | p-ClC6H4 | H | 2i | 36 | 30 | 93 | 83 |
| 11 | H | Me | 2j | 2 | 3 | 86 | 78 |
| 12e | H | Me | 2j | 2 | 3 | 91 | 85 |
Reactions were performed on a 0.18–0.25 mmol scale, and were quenched by the addition of p-NO2 benzoyl chloride. For entries 1–3, 6–8, and 11–12 the hydroxylamine was generated in situ from the corresponding trifluoroacetate salt by addition of aqueous potassium carbonate.
Isolated yields of O-benzoylated products after purification on silica gel.
Determined by HPLC analysis of O-benzoylated products using commercial chiral columns.
mol% of 5 was used.
10 mol% of 3e was used as the catalyst.
In conclusion, we have developed a highly enantioselective thiourea-catalyzed Cope-type hydroamination that provides access to a variety of pyrrolidine products under mild reaction conditions. Thiourea catalysts capable of stabilizing the dipolar transition structure in the pericyclic reaction via multiple non-covalent interactions were found to be most effective for catalysis and enantioenduction. Elucidation of the specific catalyst-substrate interactions at play is the focus of ongoing study, and will be applied to future catalyst-design strategies.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the NIH (GM-43214).
Footnotes
Supporting Information Complete experimental procedures and characterization data for products and all isolated intermediates. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).(a) Hensler ME, Bernstein G, Nizet V, Nefzi A. Biorg. Med. Chem. Lett. 2006;16:5073. doi: 10.1016/j.bmcl.2006.07.037. [DOI] [PubMed] [Google Scholar]; (b) Li X, Li J. Mini-Rev. Med. Chem. 2010;10:794. doi: 10.2174/138955710791608334. [DOI] [PubMed] [Google Scholar]; (c) Lexa KW. Proteins. 2011;79:2282. doi: 10.1002/prot.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Galliford CV, Scheidt KA. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]; (d) Cole DC, Lennox WJ, Lombardi S, Ellingboe JW, Bernotas RC, Tawa GJ, Masandarani H, Smith DL, Zhang G, Coupet J, Schechter LE. J. Med. Chem. 2005;48:353. doi: 10.1021/jm049243i. [DOI] [PubMed] [Google Scholar]
- (2).(a) Zhang S, Wang W. In: Privileged Chiral Ligands and Catalysts. Zhou Q-L, editor. Wiley-VCH; Weinheim, Germany: 2011. pp. 409–439. [Google Scholar]; (b) Watson AJB, MacMillan D. W. C. Enantioselective Organocatalysis Involving Iminium, Enamine SOMO and Photoredox Activation. In: Catalystic Asymmetric Synthesis. 3rd ed. Ojima I, editor. Wiley & Sons; Hoboken, NJ: 2010. pp. 39–57. [Google Scholar]
- (3).Caputo CA, Jones ND. Dalton Trans. 2007:4627. doi: 10.1039/b709283k. [DOI] [PubMed] [Google Scholar]; For a review of chiral amine ligands, see:
- (4).(a) Chemler SR. Org. Biomol. Chem. 2009;7:3009. doi: 10.1039/B907743J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Müller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795. doi: 10.1021/cr0306788. [DOI] [PubMed] [Google Scholar]; (c) Giardello MA, Conticello VP, Brard L, Gagne MR, Marks TJ. J. Am. Chem. Soc. 1994;116:10241. [Google Scholar]; (d) Hong S, Tian S, Metz MV, Marks TJ. J. Am. Chem. Soc. 2003;125:14768. doi: 10.1021/ja0364672. [DOI] [PubMed] [Google Scholar]; (e) Kim JY, Living-house T. Org. Lett. 2005;7:1737. doi: 10.1021/ol050294z. [DOI] [PubMed] [Google Scholar]; (f) Collin J, Daran J, Jacquet O, Schulz E, Trifonov A. Chem. Eur. J. 2005;11:3455. doi: 10.1002/chem.200401203. [DOI] [PubMed] [Google Scholar]; (g) Riegert D, Collin J, Meddour A, Schulz E, Trifonov A. J. Org. Chem. 2006;71:2514. doi: 10.1021/jo052322x. [DOI] [PubMed] [Google Scholar]; (h) Gribkov DV, Hultzsch KC, Hampel F. J. Am. Chem. Soc. 2006;128:3748. doi: 10.1021/ja058287t. [DOI] [PubMed] [Google Scholar]; (i) Chapurina Y, Ibrahim H, Guillot R, Kolodziej E, Collin J, Trifonov A, Schulz E, Hannedouche J. J. Org. Chem. 2011;76:10163. doi: 10.1021/jo202009q. [DOI] [PubMed] [Google Scholar]; (j) Knight PD, Munslow I, O'Shaughnessy PN, Scott P. Chem. Comm. 2004:894. doi: 10.1039/b401493f. [DOI] [PubMed] [Google Scholar]; (k) Watson DA, Chiu M, Bergman RG. Organometallics. 2006;25:4731. doi: 10.1021/om0606791. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Wood MC, Leitch DC, Yeung CS, Kozak JA, Schafer LL. Angew. Chem., Int. Ed. 2007;46:354. doi: 10.1002/anie.200603017. [DOI] [PubMed] [Google Scholar]; (m) Gott AL, Clarke AJ, Clarkson GJ, Scott P. Organometallics. 2007;26:1729. [Google Scholar]; (n) Zi G, Liu X, Xiang L, Song H. Organomettalics. 2009;28:1127. [Google Scholar]; (o) Manna K, Xu S, Sadow AD. Angew. Chem., Int. Ed. 2011;50:1865. doi: 10.1002/anie.201006163. [DOI] [PubMed] [Google Scholar]; (p) Martinez PH, Hultzsch KC, Hampel F. Chem. Comm. 2006:2221. doi: 10.1039/b518360j. [DOI] [PubMed] [Google Scholar]; (q) Ogata T, Ujihara A, Tsuchida S, Shimizu T, Kaneshige A, Tomioka K. Tetrahedron Lett. 2007;48:6648. [Google Scholar]; (r) Zhang X, Emge TJ, Hultzsch KC. Angew. Chem., Int. Ed. 2012;51:394. doi: 10.1002/anie.201105079. [DOI] [PubMed] [Google Scholar]; (s) Shen X, Buchwald SL. Angew. Chem., Int. Ed. 2010;49:564. doi: 10.1002/anie.200905402. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Turnpenny BW, Hyman KL, Chemler SR. Organometallics. 2012;31:7819. doi: 10.1021/om300744m. [DOI] [PMC free article] [PubMed] [Google Scholar]; For recent reviews on hydroamination see:; For asymmetric intramolecular hydroaminations with lanthanide complexes, see:; For asymmetric intramolecular hydroamination with group 4 complexes see:; For asymmetric intramolecular hydroamination with main group metals see:; For asymmetric intramolecular hydroamination with late transition metals see:
- (5).(a) Yip K-T, Yang M, Law K-L, Zhu N-Y, Yang D. J. Am. Chem. Soc. 2006;128:3130. doi: 10.1021/ja060291x. [DOI] [PubMed] [Google Scholar]; (b) Zeng W, Chemler SR. J. Am. Chem. Soc. 2007;129:12948. doi: 10.1021/ja0762240. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zeng W, Chemler SR. J. Org. Chem. 2008;73:6045. doi: 10.1021/jo801024h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Duy MN, Wolfe JP. J. Am. Chem. Soc. 2010;132:12157. doi: 10.1021/ja106989h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liwosz TW, Chemler SR. J. Am. Chem. Soc. 2012;134:2020. doi: 10.1021/ja211272v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hopkins BA, Wolfe JP. Angew. Chem., Int. Ed. 2012;51:9886. doi: 10.1002/anie.201205233. [DOI] [PMC free article] [PubMed] [Google Scholar]; For example:
- (6).(a) Hamilton GL, Kang EJ, Mba M, Toste FD. Science. 2007;317:496. doi: 10.1126/science.1145229. [DOI] [PubMed] [Google Scholar]; (b) LaLonde RL, Sherry BD, Kang EJ, Toste FD. J. Am. Chem. Soc. 2007;129:2453. doi: 10.1021/ja068819l. [DOI] [PubMed] [Google Scholar]; (c) Zhang Z, Bender CF, Widenhoefer RA. Org. Lett. 2007;9:2887. doi: 10.1021/ol071108n. [DOI] [PubMed] [Google Scholar]; (d) Zhang Z, Bender CF, Widenhoefer RA. J. Am. Chem. Soc. 2007;129:14148. doi: 10.1021/ja0760731. [DOI] [PubMed] [Google Scholar]; (e) Kanno O, Kuriyama W, Wang ZJ, Toste FD. Angew. Chem., Int. Ed. 2011;42:9919. doi: 10.1002/anie.201104076. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Shapiro ND, Rauniyar V, Hamilton GL, Wu J, Toste FD. Nature. 2011;470:245. doi: 10.1038/nature09723. [DOI] [PMC free article] [PubMed] [Google Scholar]; Asymmetric hydroaminations of allenes and dienes have also been identified that proceed under mild reaction conditions:
- (7).(a) Dorta R, Egli P, Zurcher F, Togni A. J. Am. Chem. Soc. 1997;119:10857. [Google Scholar]; (b) Zhou J, Hartwig JF. J. Am. Chem. Soc. 2008;130:12220. doi: 10.1021/ja803523z. [DOI] [PubMed] [Google Scholar]; (c) Zhang Z, Lee SD, Widenhoefer RA. J. Am. Chem. Soc. 2009;131:5372. doi: 10.1021/ja9001162. [DOI] [PMC free article] [PubMed] [Google Scholar]; For asymmetric intermolecular hydroaminations see:
- (8).(a) Copper NJ, Knight DW. Tetrahedron. 2004;60:243. [Google Scholar]; (b) House HO, Manning DT, Melillo DG, Lee LF, Haynes OR, Wilkes BE. J. Org. Chem. 1976;41:855. [Google Scholar]; (c) House HO, Lee LF. J. Org. Chem. 1976;41:863. [Google Scholar]; (d) St. Black DC, Doyle JE. Aust. J. Chem. 1978;31:2317. [Google Scholar]; (e) Oppolzer W, Siles S, Snowden RL, Bakker BH, Petrzilka M. Tetrahedron Lett. 1979;20:4391. [Google Scholar]; (f) Oppolzer W, Spivey AC, Bochet CG. J. Am. Chem. Soc. 1994;116:3139. doi: 10.1021/ja910184j. [DOI] [PubMed] [Google Scholar]; (g) Krenske EH, Davison EC, Forbes IT, Warner JA, Smith AL, Holmes AB, Houk KN. J. Am. Chem. Soc. 2012;134:2434. doi: 10.1021/ja211568k. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a review on the transformation see:; See also:
- (9).(a) MacDonald MJ, Schipper DJ, Ng PJ, Moran J, Beauchemin AM. J. Am. Chem. Soc. 2011;133:20100. doi: 10.1021/ja208867g. [DOI] [PubMed] [Google Scholar]; (b) Guimond N, MacDonald MJ, Lemieux V, Beauchemin AM. J. Am. Chem. Soc. 2012;134:16571. doi: 10.1021/ja303320x. [DOI] [PubMed] [Google Scholar]; For an elegant catalytic tethering approach to asymmetric intermolecular Cope-type hydroaminations, see:
- (10).(a) Beauchemin AM, Moran J, Lebrun M-E, Séguin C, Dimitrijevic E, Zhang L, Gorelsky SI. Angew. Chem., Int. Ed. 2008;47:1410. doi: 10.1002/anie.200703495. [DOI] [PubMed] [Google Scholar]; (b) Moran J, Gorelsky SI, Dimitrijevic E, Lebrun M-E, Bédard A-C, Séguin C, Beauchemin AM. J. Am. Chem. Soc. 2008;130:17893. doi: 10.1021/ja806300r. [DOI] [PubMed] [Google Scholar]
- (11).Acevedo O, Jorgensen WL. J. Am. Chem. Soc. 2006;128:6141. doi: 10.1021/ja057523x. [DOI] [PubMed] [Google Scholar]
- (12).(a) Taylor MS, Jacobsen EN. Angew. Chem., Int. Ed. 2006;45:1520. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; (b) Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]
- (13).(a) Curran DP, Kuo LH. Tetrahedron Lett. 1995;36:6647–6650. [Google Scholar]; (b) Kirsten M, Rehbein J, Hiersemann M, Strassner T. J. Org. Chem. 2007;72:4001–4011. doi: 10.1021/jo062455y. [DOI] [PubMed] [Google Scholar]; For acceleration of the Claisen rearrangement by a similar strategy see:
- (14).(a) Uyeda C, Jacobsen EN. J. Am. Chem. Soc. 2008;130:9228. doi: 10.1021/ja803370x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Uyeda C, Rötheli AR, Jacobsen EN. Angew. Chem., Int. Ed. 2010;49:9753. doi: 10.1002/anie.201005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Uyeda C, Jacobsen EN. J. Am. Chem. Soc. 2011;133:5062. doi: 10.1021/ja110842s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Knowles RR, Lin S, Jacobsen EN. J. Am. Chem. Soc. 2010;132:5030. doi: 10.1021/ja101256v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lin S, Jacobsen EN. Nat. Chem. 2012;4:817. doi: 10.1038/nchem.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).See supporting information for complete catalyst optimization.
- (18).Jung ME, Pizzi G. Chem. Rev. 2005;105:1735. doi: 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]; For a review on the Thorpe-Ingold effect see:
- (19).In order to prevent racemic cyclization of any unreacted hydroxylamine during workup, the catalytic reactions were quenched by the addition of p-nitro benzoyl chloride. The resultant O-benzoylated products can be reduced to the corresponding pyrrolidine in good yield and without erosion of enantiomeric excess. See supporting information for further details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


