Abstract
This review discusses selected classical works and contemporary research on recovery of contralesional fine hand motor function following lesions to motor areas of the cerebral cortex in non-human primates. Findings from both the classical literature and contemporary studies show that lesions of cortical motor areas induce paresis initially, but are followed by remarkable recovery of fine hand/digit motor function that depends on lesion size and post-lesion training. Indeed, in recent work where considerable quantification of fine digit function associated with grasping and manipulating small objects has been observed, very favorable recovery is possible with minimal forced use of the contralesional limb. Studies of the mechanisms underlying recovery have shown that following small lesions of the digit areas of primary motor cortex (M1), there is expansion of the digit motor representations into areas of M1 that did not produce digit movements prior to the lesion. However, after larger lesions involving the elbow, wrist and digit areas of M1, no such expansion of the motor representation was observed, suggesting that recovery was due to other cortical or subcortical areas taking over control of hand/digit movements. Recently, we showed that one possible mechanism of recovery after lesion to the arm areas of M1 and lateral premotor cortex is enhancement of corticospinal projections from the medially located supplementary motor area (M2) to spinal cord laminae containing neurons which have lost substantial input from the lateral motor areas and play a critical role in reaching and digit movements. Because human stroke and brain injury patients show variable, and usually poorer, recovery of hand motor function than that of nonhuman primates after motor cortex damage, we conclude with a discussion of implications of this work for further experimentation to improve recovery of hand function in human stroke patients.
Keywords: Motor cortex, lesion, hand, grasp, manipulation
1. Introduction
Our goal is to provide an overview of selected classical works that, largely through the use of surgical ablation techniques, have provided foundational support for our contemporary understanding of the neuroanatomical and functional characteristics of the motor cortex. These classical works, coupled with contemporary studies, provide an excellent forum to discuss their implications for the clinical features and expectations of stroke recovery. To accomplish our goal, we have limited the scope of this review primarily to the effects of long-term motor cortex lesions in nonhuman primates on contralateral upper limb function, in particular for reaching and grasping objects, including the use of precision grip of small objects. It is not surprising that the study of upper limb recovery has attracted so much attention over the years, considering the common neurological occurrence of paresis and the difficulty many patients have regaining dexterous movements. In accord with others, it is our contention that continued study of the behavioral, neuroanatomical, neuronal, and biochemical consequences of damage to motor cortex or its descending projections that affects upper limb reaching function will help us better translate between basic science advances and their clinical application [17, 110].
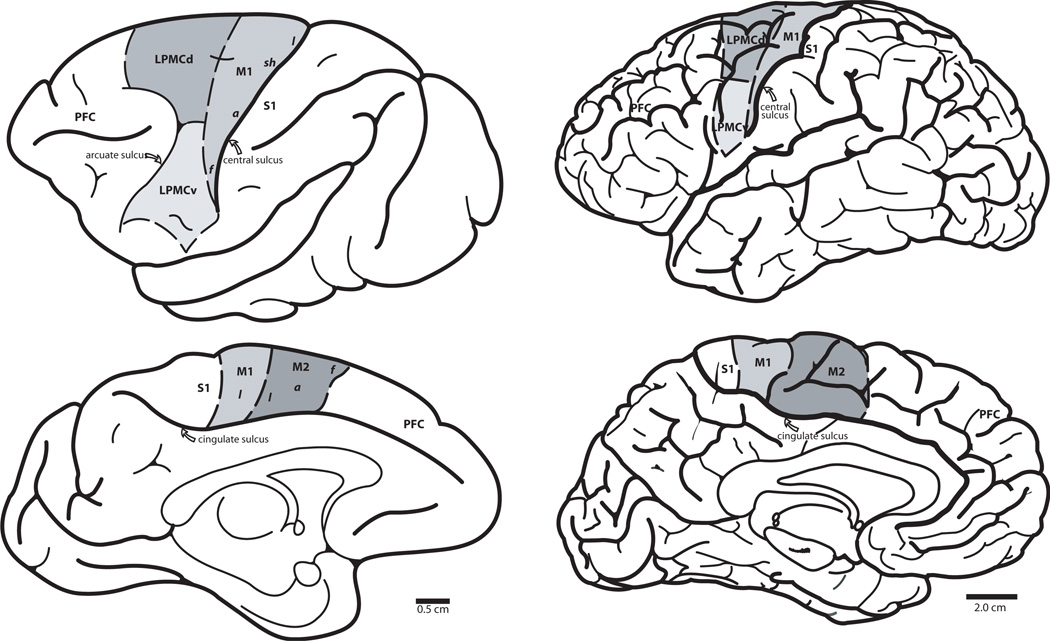
In this review, we will reference work on the motor cortex and the areas immediately adjacent. Our use of the term “motor cortex” will exclusively refer to the primary motor cortex (M1) located in the precentral gyrus of man and higher-order non-human primates. Other motor areas referred to throughout this discussion will include the lateral premotor cortex (LPMC), located just anterior to precentral sulcus in man and anterior to M1 in macaques (dorsal portion of LPMC being dorsal to the superior limb of the arcuate sulcus and ventral portion being caudal to the inferior limb), and the supplementary motor area (M2), located medially to LPMC in man and macaques (Fig. 1). Given that the reciprocal sensory information terminates immediately adjacent to the primary motor area in the postcentral gyrus, we will also discuss the basic implications of the primary sensory (S1) area (Fig. 1).
Fig. 1.
Drawings of lateral and medial (sagittal) views of monkey (Macaca mulatta) on the left side and human cerebral cortex on the right side showing major motor and sensory areas and prefrontal cortex. Abbreviations: M1: primary motor area, LPMCd: dorsal portion of lateral premotor cortex, LPMCv: ventral portion of lateral premotor cortex, M2: supplementary motor area, PFC: prefrontal cortex, SI: primary somatosensory area, f: face, a: arm, sh: shoulder, l: leg.
2. Localization and Mapping of Motor Cortex
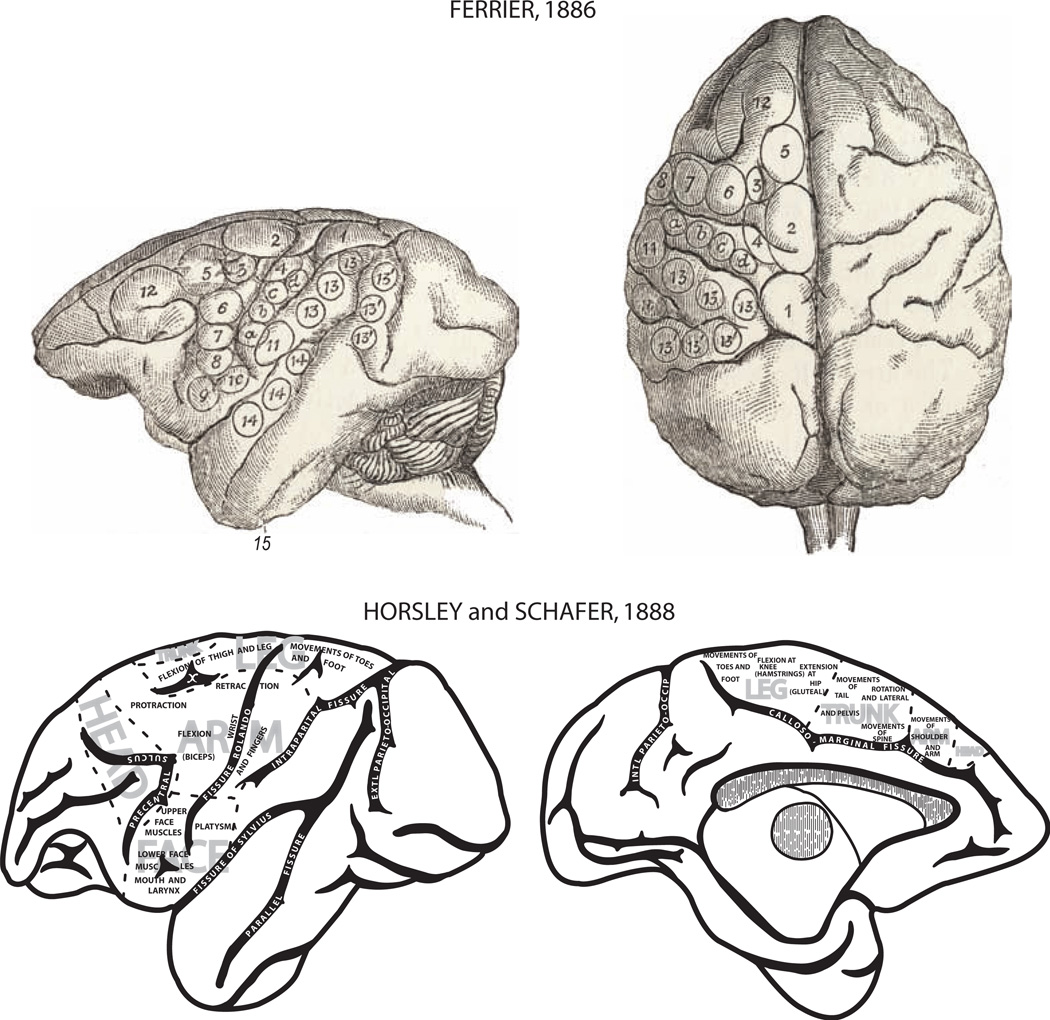
Early investigations into recovery after motor cortex lesions began with reports that damage to the precentral gyrus caused no sensory loss, and resultant movement problems varied in intensity and duration (e.g., [30]). This work originated in the classical era of functional localization, which was a highly controversial topic in the early to middle parts of the 19th century. During this time period, some scientists, led in part by the dominant influence of Pierre Flourens, held that the cerebral cortex was the seat of intelligence and sensation, while motor function was subserved by subcortical structures including the cerebellum ([34] as cited by [16]). Such views were based on experimental studies conducted by Flourens in “lower animals” such as dogs, frogs, and birds. In retrospect, it is thought that the lower functional capacity of these animals, and the probability that these experiments were conducted in young animals, may have contributed to his inability to localize motor function at the cortical level [33]. However, in 1870, Fritsch and Hitzig identified distinctive sites on the cortical surface of the dog brain, which, following the direct application of low levels of electrical current, elicited contralateral movements in isolated body parts including the regions of forepaw, hind paw and face ([35] as cited by [112]). This ground-breaking publication provided key support for not only the controversial idea of cortical localization, but also the long awaited experimental documentation for a cortical role in motor function. Subsequent studies in higher animals (e.g., monkeys), using more refined stimulation of the brain surface, in conjunction with ablation methods, demonstrated more detailed evidence of localization of motor function in the frontal lobes and major sensory functions in the parietal, occipital and temporal lobes (Fig. 2) [30, 45]. Indeed, when parts of the gyri spanning the Rolandic fissure (central sulcus) were lesioned by electrocautery, it was possible to observe in monkeys motor deficits to individual limbs on one side of the body (hemiplegia) (Fig. 3). These motor deficits were very similar to those observed in humans after stroke or traumatic brain injury that affected well-defined parts of the brain as confirmed post-mortem [30]. Recovery from such experimental lesions was typically poor, with at least weakness and paresis persisting for long periods. Similarly, surgical removal of portions of cortex that produced muscle contractions when stimulated led to long duration paralysis of the limbs on the opposite side of the body, although some recovery of trunk muscles was observed (Fig. 3) [45]. In many cases, it appears from the descriptions and published figures that these lesions spanned the central sulcus, thereby affecting both sensory and motor areas and extended into adjacent premotor cortex (Fig. 3). Moreover, it is also likely that the lesion may have included the underlying subcortical white matter of the corona radiata.
Fig. 2.
Montage depicting motor organization of the cerebral cortex determined by the application of electrophysiologic stimulation of the cortical surface in monkeys. Top: Lateral (left) and dorsal (right) views of the cortex with distinct movement representations outlined by irregular circles with numbers published by the British neurologist David Ferrier [29]. The sites that evoked movements in the upper limb are numbered 4 (“retraction with abduction of the opposite arm”), 5 (“extension forward of the opposite arm and hand”), a, b, c, d (“individual and combined movements of the fingers and wrist” for prehension of the opposite hand), 6 (supination and flexion of the forearm). Bottom: Lateral (left) and medial (right) views of the cortex with movement representations published by Horsley and Schafer [45]. This map provides one of the most comprehensive representations of the motor cortex published in the 1800s. Of notable significance was the early recognition of head, arm, trunk, and leg representations on the lateral surface of the hemisphere as well as the medial surface.
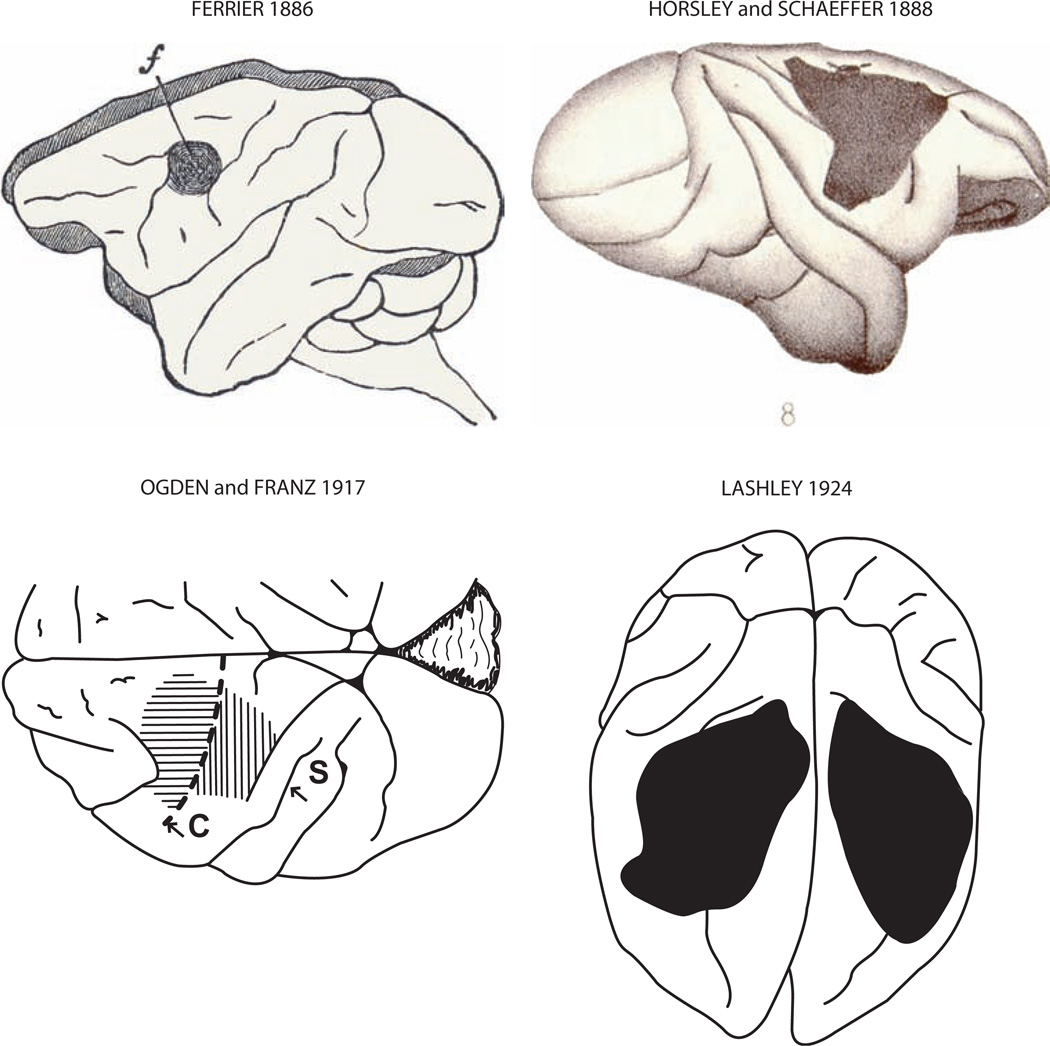
Fig. 3.
Montage depicting the precentral motor lesion site in monkeys in the classic studies of Ferrier [29], Horsley and Schaffer [45], Ogden and Franz [79] and Lashley [52] (Fig. 1, American Medical Association, Archives of Neurology and Psychiatry, reproduced with permission). In the Ogden and Franz map, the horizontal lines indicate the first surgical ablation which involved the excitable precentral motor cortex. The vertical hatching over S1 indicates an apparent abnormality of that area. In the other maps, the frontal motor lesion site is represented by the blackened region.
3. Injury to the Motor Cortex and Recovery
These very early investigations were followed by studies of motor recovery in the early 20th century following carefully performed surgical lesions to specific areas of M1 in nonhuman primates including lemurs, macaque monkeys and great apes [40, 59, 70] (see [112] for a review). In general, these studies confirmed previous work indicating initial flaccid paralysis of the contralesional limb(s); but in contrast to previous work, remarkable recovery of limb function was observed during the postlesion period of two through eight weeks. For example, it was reported that “full recovery” was possible after removing the arm area of M1 (identified using electrical stimulation with low currents) in great apes (e.g., [39, 59]). These lesions were typically quite large, with depths of 6–8mm and probably included some damage to the subcortical white matter of the corona radiata. In a series of experiments, Graham Brown and Sherrington [39] investigated the results of motor cortex damage in a chimpanzee that received a lesion of the arm area of left motor cortex on July 27, 1912 and had apparently recovered full function of the right arm by December, 1912. A second surgery was then performed where the arm area of right motor cortex was lesioned. This had no effect on right arm motor function and thus, was unlikely to have been responsible for its recovery. Also notable was that the left arm recovered function more quickly than the right arm had recovered after the first surgery. Next, in a third surgery, the arm area of the right postcentral gyrus was lesioned on February 5, 1913 and, “within 90 minutes of coming out of narcosis the ape gave the left hand at command” (presumably to shake the experimenter’s hand) and “None of the movements of the left arm were absolutely lost, but there was a considerable weakness in some of them.” Within a month (before March 15, 1913 when this note was published),
…the movements of the arm gradually improved and became stronger. He now sometimes feeds himself, for instance, with the left hand alone. He often transfers a banana from one hand to the other and it has been observed on several occasions that he can do this accurately without looking at either hand.
Years later, Leyton and Sherrington [59] described another chimpanzee that recovered from an isolated ablation lesion to the distal arm area (thumb, fingers, wrist, and elbow representation) of left motor cortex to the point of being able to use precision grip to pick up small food objects with the contralesional hand having no postlesion “therapy” or training. However, some loss of independent movement of the index and strength of thumb grip apparently remained. After this partial recovery, and during a second surgical exposure, stimulation of the left hemisphere yielded no response from the lesion site or the intact postcentral cortex. Collectively, the findings from these two classic reports led to the conclusion that recovery of hand movement in higher-order primates could not be attributed to taking over of function by the motor cortex in the opposite hemisphere or by the postcentral gyrus (S1) of the lesioned hemisphere.
Another important observation of Leyton and Sherrington [59], which demonstrated that they were unable to evoke distal upper limb movements by stimulating the undamaged portion of M1 from the first lesion, warrants further discussion. Specifically, during the second surgery, they stimulated noninvolved (spared) cortex surrounding the previous M1 lesion in an attempt to elicit distal upper limb movements. Stimulation of intact cortex dorsal to the lesion site evoked shoulder movements and stimulation of cortex ventral to the lesion-evoked face movements. However, no peripheral movements were observed in the hand and wrist and only questionably at the elbow. They concluded from this observation that portions of motor cortex that normally controlled face, trunk and lower limb movements of M1 did not have the capacity to take over the function of the damaged portion of the M1 wrist and hand representation. In a corollary component of their study, Leyton and Sherrington [59] also investigated the potential neuroanatomical consequences of the cortical lesions from histologically processed tissue sections through the medulla oblongata and spinal cord. Using the Marchi technique, microscopic observations revealed that the cortical lesion produced substantial myelin degeneration of the descending cortical projection in the pyramidal tract, both at the level of the medullary pyramids (on the side ipsilateral to the cortical lesion) as well as in the lateral and ventral corticospinal tracts (CSTs) in the cervical enlargement (contralateral to the lesion). In contrast, minimal tissue deterioration was noted in thoracic cord spinal levels and none at levels through the lumbar enlargement. These findings provided strong neuroanatomical evidence that the lesion was primarily restricted to the cortical neuron field projecting to spinal cord levels controlling the upper limb.
Complete recovery from large lesions that affected the entire “stimulable cortex” (M1 + dorsal part of LPMC) of one (left) hemisphere, were also demonstrated in early work (Fig. 3) [79]. Immediately after the lesion, there was flaccid paralysis of the contralesional limbs as reported in previous studies. However, constraint of the ipsilesional upper limb and daily movement therapy of the contralesional upper and lower limb (similar to constraint-induced movement therapy presently used for hemiplegic stroke patients — [104, 113]) produced what Ogden and Franz considered full recovery of upper and lower limb function, as well as body posture. Interestingly, much of this recovery occurred over the first two weeks following the lesion and appeared largely complete at three weeks postlesion as the monkey was able to “pick small objects from the floor and convey them to the mouth” [79]. Indeed, they described this monkey using the contralesional hand to catch a fly “that had alighted in the monkey’s cage” about three months after the lesion. As they eloquently stated: “The coordination and quickness for the performance of this act will readily be appreciated.” Unfortunately, they did not specifically state whether the animal recovered precision grasp between the thumb and index finger and it does not appear that they specifically tested for, or reliably measured this ability. Animals that did not receive constraint of the ipsilesional upper limb and therapy for the impaired limbs remained greatly impaired in movements of the contralesional hand and digits (and of the contralateral leg as well as postural impairments) for up to six months after the lesion (see experiments 2 and 3 of Ogden and Franz [79]). Based on their observations, and those of others, it was concluded that full recovery was possible even after extensive damage to the entire stimulable (motor) cortex.
Similarly, Lashley [52] observed in monkeys who learned a complex series of movements to open “problem boxes” containing food rewards, that after a surgically induced lesion of stimulable cortex followed by subsequent recovery from paresis, the animals could again perform the complex task two months after the lesion almost as well as before the lesion (Fig. 3). Interestingly, after training to learn the task exposure to the testing apparatus was prohibited two months before the lesion and two months after the lesion to address the issue of acquired motor skill retention. Although the lesions for this study were described as involving the “precentral gyrus”, the mapped lesions, as determined from the accompanying figures, appeared to also include what is currently considered the premotor cortex as well as the caudal region of the prefrontal cortex — see Figs. 1–3 of [52]. Furthermore, examination of the coronal sections in these figures also indicates some minimal involvement of the adjacent parietal somatosensory cortex. Even with this apparent larger cortical lesion, it was concluded that complex motor habits acquired prior to an experimental lesion of precentral gyrus were fully retained after the lesion, although some clumsiness may affect performance. These findings were later confirmed in monkeys with smaller lesions confined to the precentral motor areas (M1 and LPMC) [43, 46]. It is also important to note that Lashley [52] cited previous work conducted by Rothmann in 1907 in which he “observed learning in a rhesus monkey in which one precentral gyrus had been extirpated and the pyramidal tract of the other had been sectioned in the cervical region” [92]. This observation demonstrated that monkeys could learn a new motor task following a precentral gyrus lesion and cervical disconnection of the corticospinal projection from the opposite hemisphere. From a clinical standpoint, these results were very encouraging in terms of implications for rehabilitation of hand function in humans after stroke. In particular, acquired brain lesions affecting the lateral cortical motor areas, while preserving other cortical structures and their subcortical projections, including the medial areas along the interhemispheric fissure, resulted in the potential for recovery without extensive retraining. Furthermore, this body of work suggested that favorable recovery was possible even if the lesion extended into premotor areas with extensive training that included constraint of the ipsilesional limb. However, these findings seem to have been largely forgotten until constraint-induced movement therapy (CIMT) for hemiplegia was reintroduced by Taub et al. some 70 years later [103], but was based on results of experiments inducing deafferentation of the upper limb by sectioning dorsal roots in macaques [51, 102] rather than on stroke or motor cortex injury experiments.
After recovery of the right side was considered complete in experiment 1 of Ogden and Franz [79], a subsequent similar lesion was made in the stimulable cortex (M1 + dorsal LPMC) in the right hemisphere of the same monkey (experiment 2 of Ogden and Franz [79]). Following this lesion, the animal did not receive constraint of the recovered right upper limb or any therapy to the left limbs other than normal movements performed in its cage, and in a large exercise room. Walking and jumping showed some recovery but the animal tended to fall toward the left side and did not always reach the target of a jump, suggesting left lower limb weakness. However, the left upper limb showed very poor recovery such that during climbing:
…the right arm and hand are used for pulling and the left is apparently used only for support. When food is given, even though the food be close to the left hand, the animal always reaches for the food with the right. Unlike a normal monkey which grasps and holds food with both hands and feet, this animal uses only the right hand and right foot.”
Considering movement impairment resulting from brain injury, insights into our current understanding of the underlying corticospinal projections and transcallosal connections were evident in these early works. If the arm area of M1 was lesioned, resultant deficits appeared in the contralateral upper limb and recovery of function was possible. After recovery, when a subsequent lesion to the arm area of M1 of the other hemisphere was made, this new lesion did not reinstate deficits in the hand contralateral to the first lesion. Moreover, movement recovery was quicker in the second limb [59]. Similarly, Ogden and Franz [79] observed that lesion of the entire stimulable cortex of the other hemisphere did not affect the ipsilesional recovered limb, but produced contralesional hemiparesis that did not fully recover unless therapy was provided that included constraint of the recovered limb. The lack of effect of the second lesion on the hand contralateral to the first lesion provides strong evidence that the intact contralesional motor cortex did not take over control of the hand affected by the first lesion through ipsilateral pyramidal pathways. Moreover, the finding that recovery of the hand contralateral to the second lesion was quicker than expected is consistent with current theories that lesion to one hemisphere allows the other hemisphere to exert a form of dominance through transcallosal inhibition (TCI) of the injured hemisphere. Damage in both hemispheres after the second lesion may reinstate more balanced TCI between the hemispheres, thereby allowing better control of movement of both limbs.
As mentioned previously, an important finding from the work of Leyton and Sherrington [79] was that after recovery from the initial M1 lesion, they were unable to evoke contralesional distal upper limb movements when they stimulated the undamaged portions of M1 that were left intact from the first surgery. It appeared then, that the undamaged face, shoulder, trunk, and leg areas of M1 had not taken over the function of the damaged portion of M1. These results are consistent with the findings of Ogden and Franz [79], who showed that recovery of hand function (and leg/trunk function) was possible even after complete destruction of the entire M1 (and dorsal LPMC). Indeed, the observation that recovery was still possible after such large lesions, albeit when a form of what is currently called constraint-induced therapy [105] is applied to rehabilitate the monkeys, provides strong evidence that a simple reorganization of undamaged parts of M1 and/or adjacent LPMC cannot fully explain recovery of upper (or lower) limb function. That is, at least in the case of damage involving the portion of M1 and LPMC controlling an entire limb. Thus, in nonhuman primates and possibly stroke patients, other spared cortical or subcortical areas may be capable of taking over some of the functions of the lateral motor cortices. This would be consistent with our recent report that M2 generates new connections (synaptic boutons) onto contralateral ventral horn neurons of the cervical enlargement following removal of the arm representation of M1 and LPMCd, and this plasticity correlates with recovery of dexterous hand movements [64]. The issue of rehabilitation training and its contribution to recovery of hand function after motor cortex damage will be discussed in more detail later.
4. Fine Motor Control Deficits Following Motor Cortex Injury
Following the classical work of the late 19th and early 20th centuries, further experimentation in apes and macaque monkeys provided evidence that lesions restricted to M1 produced flaccid paresis initially followed by substantial recovery and lasting deficits primarily in fine control of digit movements for manipulating small objects, especially in chimpanzees but also in macaques (Fig. 4) [37]. Lesions of premotor areas in addition to M1 produced more substantial disturbances such as spasticity and forced grasping initially, but these resolved after several weeks [37], which is consistent with the report of Lashley [52]. In contrast, Denny-Brown and Botterell later reported that lesions of area 4 produced flaccidity initially but was followed by “a spastic type of paralytic weakness” with heightened tendon reflexes whereas lesions of premotor areas produced “a mild plastic rigidity without loss of power of contraction and without increase in tendon reflexes” (Fig. 4) [23]. However, it was clear that even after large motor cortical lesions, the loss of use of an extremity was incomplete because given sufficient provocation such as fright or anger, a lesioned animal will effectively use the impaired extremity in climbing to escape or fighting back, even though under normal circumstances the extremity appears nonfunctional [23]. Such findings further support the ideas from Ogden and Franz [79] that under certain emotionally motivated conditions, an apparently severely impaired extremity can be retrained for complex motor acts, although it was thought that retraining fine control of the digits was not possible. These observations provided additional behavioral evidence suggesting other brain areas were indeed capable of taking over some functions of the lateral motor cortex. Although multiple cortical and subcortical neural networks are likely to be involved in this surprising restoration of movement, a potential contribution of the cingulate motor areas warrants consideration for several reasons. First, the rostral (M3) and caudal (M4) cingulate motor areas are well protected from lateral cortical injury as they form the cortex lining the lower bank and fundus of the cingulate sulcus. Second, they both receive substantial limbic cortical inputs [67, 69] which provide the cingulate motor cortices with a rich source of motivational and emotional influence that are essential requisites for the initiation and execution of exploratory movement involving the trunk and limbs. Finally, the cingulate motor cortices have substantial connections with the primary, lateral premotor and supplementary motor cortices and both M3 and M4 give rise to descending projections to many subcortical motor targets including the facial nucleus and spinal cord (for review, see [68]).
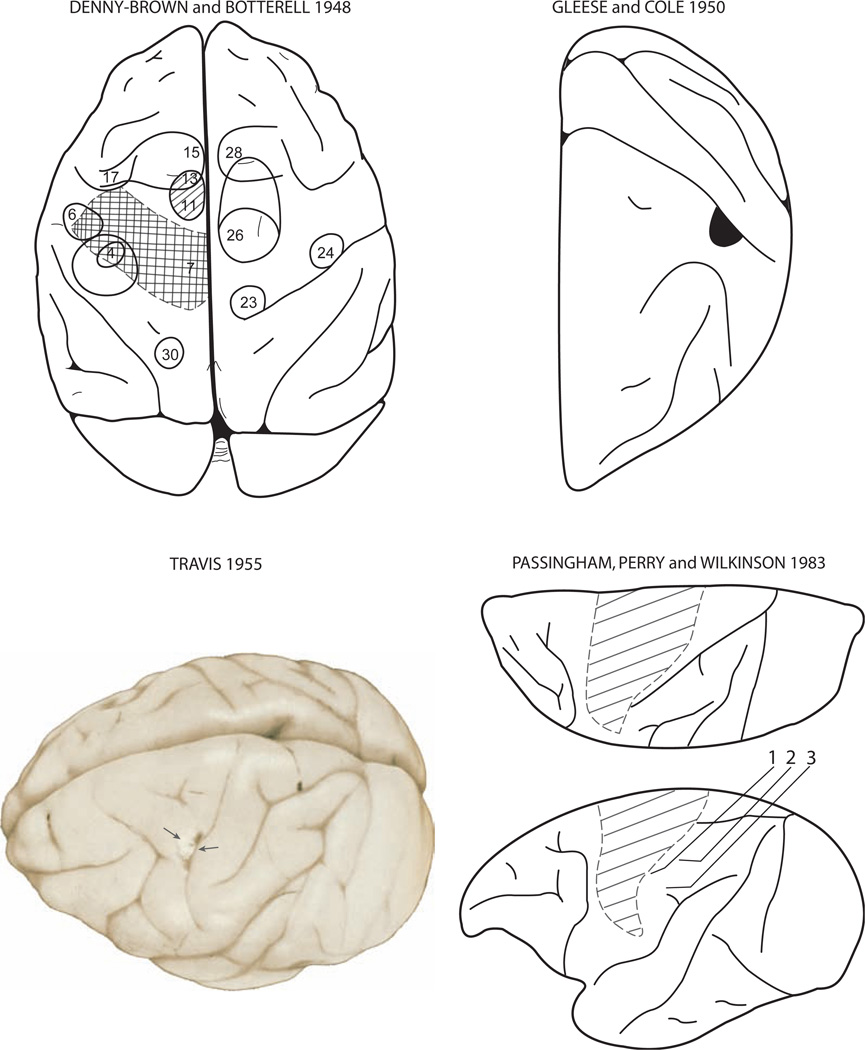
Fig. 4.
Montage depicting the precentral motor lesion site in monkeys in the classic studies of Denny-Brown and Botterell ([23]; Fig. 6), Glees and Cole ([38]; Fig. 8, Am Physiolog Soc, J Neurophysiol, used with permission), Travis ([107]; Fig. 6, Oxford University Press, Brain, used with permission) and Passingham et al. ([84]; Fig. 1, Oxford University Press, Brain, used with permission). In the Denny-Brown map, the crosshatching indicates the surgical ablation which involved the arm and leg representations of the precentral motor cortex. In contrast to the typical large precentral lesion induced in most studies, the M1 lesion created in the Glees and Cole work (blackened area abutting the central sulcus), as well as the Travis [107] work (pericentral region indicated by the arrows), was small and discretely limited to the distal forelimb region of the arm representation. The lesion site in the Passingham figure is depicted by the diagonal lines and involved the face, arm, shoulder and leg representations of the precentral motor cortex.
5. Neuroplasticity Following Motor Cortex Injury
Important experiments relevant to the effects of motor cortex lesions on development of reaching/grasping and differences in the effects of M1 and LPMC lesions as a function of age (infant vs. juvenile/adult monkeys and apes) were also carried out by Kennard in collaboration with Fulton during the 1930s and 1940s (Fig. 4) [47–50]. These classic experiments clearly showed that recovery was much more rapid in infant monkeys (7 days–3 months old) than in older animals (2–4 years). For example, complete lesions of M1 in very young infant macaques (7 days old) were associated with relatively little immediate effect and “complete recovery”, including grasping and finger movements, by two months of age [47]. Older infants (42 days) also showed remarkable recovery even after removal of an entire hemisphere. For example, some recovery was noted within 24 hours and after a week, the infant could walk and climb. After a month, the infant could reach and grasp, albeit awkwardly. In contrast, adults with such a lesion showed much poorer recovery over the first postlesion month. Further research in which M1 and LPMC were removed bilaterally in a single operation or serially (i.e., left hemisphere and then right hemisphere 1.5–8 months later) again showed much better short-term motor recovery in infants than adults [50], but recovery over the long-term (up to two years) was studied only in infants as adults were all euthanized within 10–48 days of the lesion. Overall, these experiments showed that the infant brain was able to reorganize more rapidly than the adult brain to allow better recovery of motor function quite soon after the lesion(s). However, as discussed by Passingham et al. (see below), these experiments did not establish poorer long-term recovery in adults than in infants because the adults were not given up to two years to recover [84].
Experiments carried out in the 1950s strongly suggested that recovery of precision grip and fine digit control were possible following lesions of the entire arm or hand/digit areas of M1 (Fig. 4) [38, 107]. In particular, Travis [107] stated in reference to a rhesus monkey with a large lesion to the left precentral forelimb area: “After two weeks he picked up small pieces of food by apposition of the right thumb and index finger.” Smaller lesions localized to the precentral hand/digit area (Fig. 4) were also made by Travis [108] and she reported that “after recovery from the anaesthetic the hand contralateral to the lesion was used almost as well as the normal hand.” Glees and Cole [38] also reported, in contrast to earlier observations [40, 59], that stimulation of spared perilesional areas of M1 elicited hand/digit movements where prior to lesion, these movements were not evident. Thus, it appeared that the intact perilesional areas had taken over digit function of the damaged tissue areas. However, it is important to note that in the work of Leyton and Sherrington [59] during the first operation the entire elbow, wrist and digit areas of M1 were excised whereas in the Glees and Cole work, only the thumb area was removed (Fig. 4).
More recent experimental work using intracortical microstimulation has complemented and expanded upon the findings of Glees and Cole. Specifically, Nudo et al. [74] elegantly demonstrated in squirrel monkeys that very small focal lesions affecting subsectors of M1 that elicit digit movements produces reorganization in spared subsectors to recover these M1 movement representations. Indeed, hand movement representations expanded into areas that formerly elicited shoulder/elbow movements, but only if rehabilitation in the form of training of skilled hand movements is provided after the lesion [77, 78]. Similarly, in macaque monkeys (Macaca fascicularis), it has also been shown that M1 hand area lesions in infant monkeys are associated with reorganization of perilesional cortex to innervate hand/digit muscles [93]. However, in the same species, a similar lesion induced in adult monkeys did not produce reorganization of motor cortex and, instead, was associated with reorganization of premotor cortex as short-term damage to this area reinstated the original deficit [62]. It is now well known based on observations from spike triggered averaging and single pulse intracortical microstimulation that single cortico-motoneuronal cells project to multiple muscles [10, 15, 31]. Furthermore, cortico-motoneuronal cells projecting to a given muscle controlling hand, wrist, elbow, and/or shoulder movements are distributed over large areas of M1 and overlap considerably in cat [1, 98] and monkey [25]. This expansive organization has also been postulated in humans based on transcranial magnetic stimulation observations [24]. Thus, muscle/movement map expansions in motor cortex may result after limited injury through altered connectivity within the cortex including the descending outputs ending directly in spinal motor areas, especially when use of the impaired limb is encouraged. However, others have reported in macaques that stimulation of the perilesional M1 after ibotenic acid lesions which damaged both M1 and S1 hand areas, did not produce visible movement of the “recovered” hand [62]. Notably, in this experiment, recovery was minimal, achieving only 30% of prelesion success rate on the task. It was also reported that reversible muscimol lesions to intact premotor areas reinstated impairment of the recovered hand, suggesting that these areas were responsible for the minimal recovery observed. Similarly, Nudo et al. have reported that following focal ischemic infarction affecting the distal forelimb (DFL) representation of M1 in squirrel monkeys, that initially produced severe deficits in reach/grasp motor abilities, was associated with enlargement of the DFL map in M2 [27]. Such findings are consistent with our recent report demonstrating that recovery of hand function following surgical removal of M1 and LPMC arm areas is associated with intraspinal sprouting and generation of new corticospinal connections from M2 into ventral horn neuron pools in C5-T1 segmental levels [64]. Thus, whether perilesional M1 or more distal sites in premotor cortex reorganize to assist in recovery may depend on lesion size, type (i.e., ischemic, chemical, surgical removal) and, possibly, location.
6. Measuring and Quantifying Movement and Skill
Also notable in the work of Glees and Cole [38] was that they developed a novel method to measure gripping strength between the thumb and index finger while pulling open a small “matchbox” drawer with a string to which they could attach different weights (see their Fig. 5). One rhesus monkey learned to perform the easiest version of the task (without weights) with both hands after the arm area of M1 in both hemispheres had both been lesioned by surgical removal (with no prelesion training on the task). Lesions were done serially, with the left hemisphere being lesioned first followed by lesion of the right hemisphere after recovery of the right hand. These observations demonstrated that a monkey could learn a difficult novel fine motor task after a large lesion of M1 of both hemispheres, although they commented that learning was slower than in the case of intact monkeys on this task. This finding also supported previous observations of learning a new fine motor task after lesion of M1 in one hemisphere and lesion of the pyramidal tract out of the other hemisphere ([92] as reported by Lashley [52]). Moreover, study in one of these monkeys was done with the weighted drawer device after two lesions to the arm areas of left M1. Here, an initial lesion of the entire excitable arm area was completed, which was followed 1½ months later by “undercutting of the newly excitable area of left motor cortex” in a second operation. After this lesion, the monkey learned to open the device only with the left hand as the right hand remained severely impaired for some time after the second lesion, but was eventually used for gross movements such as climbing in the cage. This is consistent with the work from many studies showing that M1 lesions, as well as lesions to the CST at the medullary level, are associated with recovery of gross motor function [44, 54, 55].
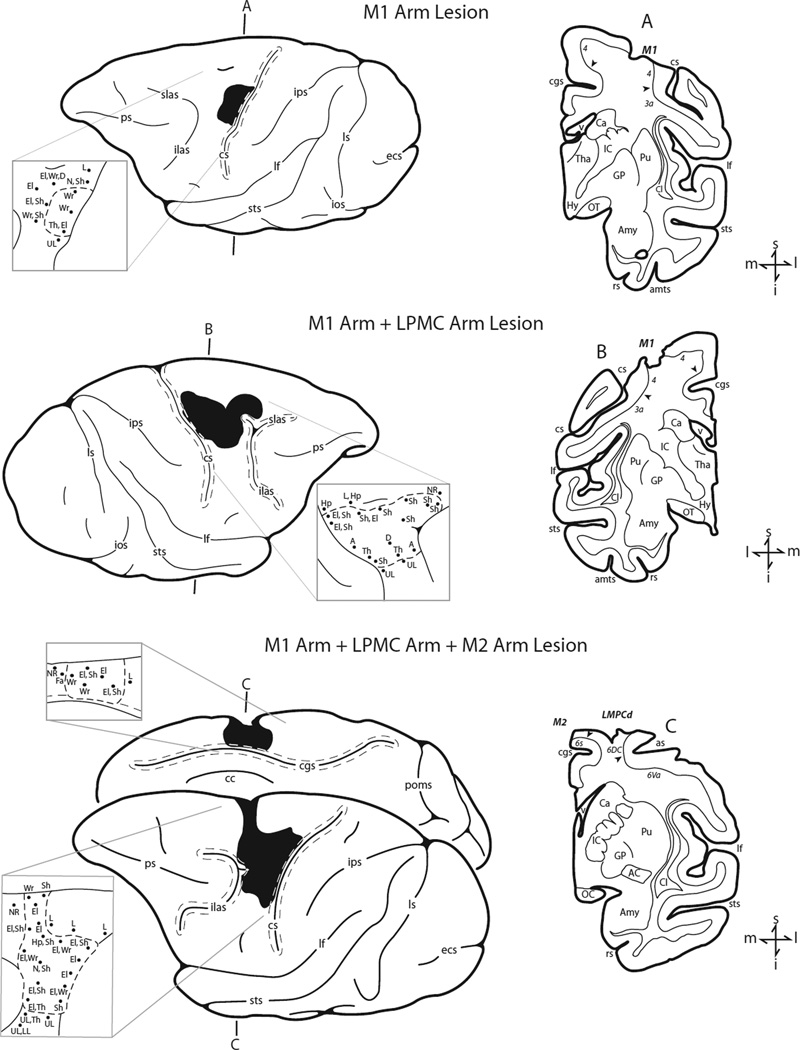
Fig. 5.
Lesions of M1 arm area, M1 + LPMC arm areas and M1 + LPMC + M2 arm areas are depicted as performed for studies of volumetric effects of frontal lobe motor area lesions [21]. Arm representations were identified using intracortical microstimulation.
Unilateral lesion of premotor areas alone (i.e., with M1 intact) in monkeys has been shown to have minimal effects on fine hand motor function. For example, lesions limited to M2 unilaterally have been reported to have little effect on posture or movement in macaque monkeys [108] or man [85]. However, bilateral lesions of M2 in macaques had much greater effects on posture, produced hypertonia and even clonus in the digits [108]. Although Travis [108] did not evaluate fine motor function in this work, it is likely that fine motor function was compromised. Other work showed minimal effects of a bilateral M2 lesion on hand fine motor function, although there were some effects on upper limb posture/movement due to hypertonia at shoulder and elbow [43]. Later work also demonstrated no deficits in unimanual fine motor tasks after M2 lesions but a deficit of bimanual control if the two hands must simultaneously perform different tasks, such as when mirror-type movements are involved [8, 9]. In contrast, Passingham et al. observed that monkeys with M2 lesions also performed poorly in a simple arbitrary task involving raising the arm to receive a food reward [106]. Interestingly, these monkeys performed the task better when performance was triggered by an external stimulus than when required to simply initiate the movement at their own pace. Monkeys with anterior cingulate lesions had similar impairments, but monkeys with LPMC lesions did not. Further study suggested that individuals/monkeys with M2 lesions perform better in response to external cues because they can use these cues as “instructions” [14]. Earlier work by Passingham et al. showed that individuals/monkeys with unilateral LPMC lesions without damage to M1 or M2 areas demonstrated deficits in responding to visual cues related to upper limb movements (e.g., pulling and/or squeezing a handle) under certain conditions, but did not have difficulty performing reach/grasp movements to pick up a peanut in a box [41, 42, 82, 83].
We did not find any major reports of investigations into effects of lesions to cortical motor areas on hand motor function in the 1960s [110]. However, there was one study that examined the effects of such lesions to different precentral motor areas on spinal cord distribution of outputs using the standard Marchi method to detect degenerating myelin, as well as the then newer Nauta method that permitted identification of degenerating axons in the spinal gray matter [61]. It was reported that motor deficits following the lesion of the precentral arm motor area were similar to those described previously [106] and that the observations with the Marchi method were also similar to previous findings (e.g., [2]). The novel findings with the Nauta method were that contralateral corticospinal projections from the precentral arm area were found in proximal and distal spinal motor neurons pools whereas ipsilateral corticospinal projections were limited to only proximal spinal motor neurons [61]. These findings suggested that M1 of the undamaged hemisphere may assist in recovery of proximal arm joint motions (shoulder and elbow) but not so for the wrist and digit joints.
7. Training, Rehabilitation, and Recovery
Subsequent work in the 1970s focused on the effects of postlesion training (rehabilitation) on recovery of upper limb strength. From an important and rarely cited series of papers, it was demonstrated that recovery of proximal flexor muscle strength (to 90% of prelesion performance levels) was much better than in distal muscles controlling grip strength (only to about 50% of prelesion performance levels) after unilateral precentral forelimb area ablation [4]. Secondly, similar recovery was possible after bilateral M1 forelimb area ablations, but required 5–6 months instead of three months. Ablation of the remainder of the precentral motor area reinstated the initial paresis for a short time, but recovery of distal strength was to similar levels as after ablation of the precentral forelimb area only [5]. These results suggest that although perilesional M1 and contralesional M1 may contribute to recovery of strength, they are not necessary since similar total recovery can occur without these areas. Surprisingly, however, ablation of the entire precentral motor area in a single surgery resulted in much poorer recovery of contralesional proximal and distal upper limb muscle strength than after serial ablations (i.e., M1 arm area, recovery, then remainder of M1 and/or contralesional M1). Black et al. also showed that daily training on the strength tasks with the contralesional arm led to better recovery of upper limb pulling and grip strength [6]. Moreover, starting rehabilitation training immediately after the lesion was found to produce much better recovery than starting four months after the lesion. It is important to note, however, that the monkeys were trained daily on the same task on which they were tested for recovery. Unfortunately, they did not assess whether training on the strength tasks influenced recovery of fine hand motor functions such as grasping and manipulating small objects, which are important skills in primates.
Following the work of Black et al. that focused on strength, there was a return to consideration of fine motor tasks, specifically precision grip and independent finger movements. Passingham et al., following up on the work of Kennard in the 1940s and 1950s, showed that there was no recovery of precision grip after complete unilateral removal of left M1 or M1 and S1 (Fig. 4) in infant rhesus monkeys (age 7 days–3 months) tested 1–2 years after the lesion, despite excellent recovery of locomotion and climbing abilities over 10 months postlesion [81]. Notably, they assessed use of precision grip by using an apparatus in which peanuts had to be removed from holes 2–6 cm in diameter or a cylindrical food pellet was used in a special device such that the food morsel could be “picked out only by inserting the fingers into two grooves (7-mm wide, 21-mm long, 12-mm deep) leading into the well from either side” (see Fig. 1 of Passingham et al. [81]). Although all monkeys with M1 lesions would use the right hand to acquire peanuts in the 2-cm hole when first tested, 3 of 4 monkeys with M1 + S1 lesions initially refused to use the right hand to reach for food and required some “training” (passively moving the right hand onto food) to use the right hand in these tasks. Moreover, only one monkey with M1 + S1 lesion (the one not requiring training) could retrieve a peanut from the 2-cm hole and the others were only successful on the 3-cm hole. Testing on the slot apparatus showed that all these animals could retrieve the food pellet with the left hand but only one animal (with a M1 lesion) could remove the pellet with the right hand with the slots in all four tested orientations (i.e., parallel, perpendicular and two oblique angles to the frontal plane of monkey).
These findings prompted an additional study to compare recovery of infants and adults to test the “Kennard Principle” suggesting that cortical damage in infant primates had little, if any, lasting effects on motor function whereas the same lesion in juveniles and adults led to lasting deficits on fine hand and foot motor function [84]. As mentioned above and discussed in the work of Passingham et al. [84], although Kennard conclusively demonstrated that infant monkeys show much faster initial recovery than adult animals from a variety of neocortical lesions, the postlesion survival durations were much longer for infants than for adult monkeys [37, 47–50]. Thus, the question of persistent deficits was not adequately assessed over a similar postlesion survival period in Kennard’s work. The same tests applied in previous work [81] were used by Passingham et al. to fully assess capability for precision grasp, as well as additional “problem box tests” in adults. All animals were allowed 19–26 months postlesion recovery with no special training (note that the same infants studied in Passingham et al., [81] were included in the 1983 report). Importantly, there were no obvious differences in the performance of monkeys with complete lesions as infants versus older monkeys on any tests and it was clear that the hand was used crudely when grasping by closing all fingers at once rather than with precision grip. However, all animals showed excellent recovery of locomotion, climbing and jumping (including safe landings). Thus, the results convincingly showed that adults could recover similarly to infants if given sufficient time. Moreover, they concluded that this study confirmed the suggestion that control of fine finger movements requires direct anatomical pathways from the cortex to motor neurons, which exist in the upper limb and foot areas of motor cortex in rhesus monkeys [55]. Indeed, anatomical study of the CST output pathways of sensorimotor areas of the non-lesioned hemisphere to brainstem and spinal cord following removal of M1 and/or S1 in infant monkeys showed no differences when compared to CST output patterns in adult monkeys following similar lesions [99]. Thus, recovery of infants and adults did not occur by establishing new cortical output connections from the undamaged contralesional sensorimotor areas.
A major question arises from the extensive research carried out on effects of lesions to motor cortex in nonhuman primates through the 1980s: What is the mechanism for recovery of voluntary movement control, especially for fine dexterous movements of the hand and fingers? Sherrington et al. suggested that since ablation of the arm area in the M1 of one hemisphere produces only temporary paralysis and that further ablations in M1 of the same hemisphere and the other hemisphere (and of S1) do not reinstate the paralysis, the function of M1 had been taken over at a subcortical level [40, 59]. They also observed that stimulation of perilesional cortex did not produce upper limb movements, further suggesting that undamaged M1 did not take over function of the damaged region. In contrast, the smaller lesions induced by Glees and Cole [38] showed that recovery of hand function following ablations of the arm area of M1 was associated with undamaged parts of M1 becoming able to produce arm movements when stimulated. Similarly, Nudo et al. have demonstrated that reorganization of perilesional cortex associated with postlesion training of skillful hand movements and concurrent cortical stimulation in squirrel monkeys is associated with better recovery of hand movements [74–76, 78, 87]. It is important to note that in the studies by Nudo et al., the brain lesions were very small compared to previous studies where the entire arm area of M1 or the entire precentral gyrus was intentionally removed. Importantly, however, these contemporary studies suggest that recovery is stimulated by postlesion training/therapy and is accompanied by cortical reorganization in the perilesional cortex as well as altered connectivity from ventral premotor cortex to S1, which implicates a role for S1 in recovery from damage to M1. Surprisingly, ventral premotor cortex connectivity to perilesional M1 regions was not changed, although perilesional M1 neurons are thought to alter motor maps to permit control over muscle groups that were originally controlled by the lesioned area.
Another important question is whether independent digit movements can recover after a complete lesion of the M1 arm/hand area. Many of the classical studies in the first half of the 20th century involved large lesions where the investigators purposefully damaged the areas deep within the central sulcus to ensure that there were no surviving M1 neurons. For example, Ogden and Franz [79] stated: “To destroy the motor zone lying concealed with the central fissure the white hot cautery was pushed about 6 to 8 mm into the brain substance and carried close to and parallel with the fissure.” It seems highly likely that such a procedure would also have damaged neurons of the adjacent S1, yet they reported full recovery of grasping and all fine motor functions of the contralesional arm associated with constraining the less impaired ipsilesional arm and extensive rehabilitation training. Unfortunately, like many studies at this time, there were no quantitative measures or techniques that forced the monkey to use fully independent digit movements for precision grasping of objects. Ogden and Franz [79] also reported that a monkey that did not receive constraint of the less impaired limb and intensive therapy did not show good recovery of grasping and only used power-type grasps (using all digits), which is consistent with the more recent findings [84].
It is generally accepted that recovery of independent digit movements and precision grip are mediated by monosynaptic connections from M1 to hand motor neurons in the spinal cord [55, 57]. However, Murata et al. recently reported that recovery of independent digit movements and precision grip was possible after lesion of the M1 hand/digit areas with intensive daily training of the impaired contralesional limb combined with restraint of the less impaired ipsilesional limb [71]. They used ibotenic acid rather than surgical removal of the area to produce these lesions and evaluated reacquisition of precision grip using a dexterity (Kluver) board apparatus with the smallest well being 1-cm diameter. Monkeys were trained before the lesion to acquire food pellets from this well successfully on 1000 trials on two consecutive days. Mean prelesion success rate was about 80% on this well and 83–100% on larger wells. Postlesion performance in the last three days (more than 10 weeks after the lesion) returned to a 60% success rate on the smallest well and 78–100% on the other wells. They used video analysis to qualitatively assess type of grip used. They also noted how postlesion recovery began with gripping raisins between the tip of the index and on the proximal phalanx of thumb, but progressed to grip between the tips of the thumb and index.
We have also reported recovery of independent digit movements and precision grasping using a dexterity board apparatus with a smallest well of 1 cm in diameter in rhesus monkeys with much larger lesions including most of the arm areas of M1, premotor cortex and M2 (Fig. 5) [21]. This work represents an advance over the earlier lesion studies on macaque monkeys that relied primarily on success rates in target acquisition to estimate motor performance rather than temporal, spatial and kinetic measures to quantitatively evaluate the reaching kinematics and hand coordination in both the transport and manipulation phases of grasping [19, 86]. In the lesions we have studied, some of the digit representations in the depths of the central sulcus were spared (Figs. 5(a) and 5(b)). However, there was no intensive daily pre- or postlesion training in these monkeys as in the studies discussed above [71, 74]. Testing in our work was approximately at weekly intervals prelesion and exactly weekly intervals for the first two months postlesion with only 25 trials with each hand on the dexterity board apparatus (and 15 trials with each hand on another apparatus). No physical constraint of the ipsilesional limb was imposed, but the testing apparatus forced the use of the contralesional limb [86]. Thus, although our intent was to evaluate “spontaneous recovery” we recognize that the limited forced use of the impaired limb likely provided some therapy once/week and may have stimulated use of that hand in the monkey’s cage as indicated by observation and a “learned nonuse test” in which either hand could be used to acquire food pellets [20]. However, we did not evaluate location of the gripping surface on the thumb or report on performance in the smallest well as was reported by Murata et al. [71]. To investigate this aspect of grip, we have recently reviewed our video recordings and found that monkeys with lesions of arm areas of M1, M1 + LPMC and M1 + LPMC + M2 (Fig. 5) did return to using precision grip (e.g., Fig. 6) and were successful on smaller wells if they were also successful on those wells during prelesion testing. Moreover, there was clear postlesion evidence of manipulation of the pellet while in precision grip to produce a more secure gripping position between the tips of the thumb and index in these monkeys (e.g., Fig. 6). Thus, the monkeys recover impressive ability for precision grip and manipulation of a very small fairly rigid object (0.5-mm food pellet) that is likely more difficult to manipulate than the raisin treat used by Murata et al. [71]. An important question in this work is whether M1 neurons deep in the central sulcus are damaged in these lesions and, thus, may subserve recovery of independent finger movements and precision grip. We are currently addressing this issue using combined surgical removal of the M1 arm area and ibotenic acid injected deep along the central sulcus arm area.
Fig. 6.
Performance of precision grasping and manipulation by a monkey with a lesion to arm areas of M1 + LPMC (SDM48 − extent of lesion shown in Fig. 6(c) of McNeal et al. [64]. The sequence of video frames shows precision grasp of a food pellet between the tips of the index finger and thumb (a) followed by manipulation the food pellet (b)–(d). Once the pellet is removed from well C (diameter of 19mm) of the modified dexterity board, it is manipulated to a more secure location on the palmar surface of the distal phalanx of the index. The times shown in each frame represent the time since initial contact with the dexterity board (i.e., 0.12 s spent manipulating the pellet’s position on the fingertip by moving the thumb).
An important issue relevant to control of independent finger movements and M1 lesions is that a large body of work suggests that M1 areas controlling an individual finger are distributed throughout the M1 hand area, rather than being localized to separate areas [114]. This is supported by anatomical and physiological evidence concerning widespread inputs to and outputs from M1 hand area neurons (e.g., [15, 25, 94]) and studies of M1 neuron recording showing that activation is distributed throughout M1 hand area [95]. Moreover, short-term inactivation of small regions within medial, intermediate and lateral portions of the hand area in rhesus monkeys showed effects that were not isolated to single fingers and, in general, appeared to be stochastic rather than systematic in their effects on different digits [97]. This distributed organization of M1 neurons controlling the digits means that only large lesions will damage neurons controlling all movements of any one digit and that independent digit movements are likely to recover in the case of small lesions by reorganization of perilesional areas as discussed above. This is also consistent with observations in stroke patients that voluntary contractions of muscles to move a single digit were accompanied by inappropriate contractions in muscles acting on additional digits due to decreased ability to selectively activate certain muscles and suppress activation of other muscles [96]. Indeed, Lang and Schieber [53] concluded that spared cerebral motor areas and other descending pathways allow activation of finger muscles after motor cortex or CST lesions, but do not provide highly selective control due to damage of M1 output.
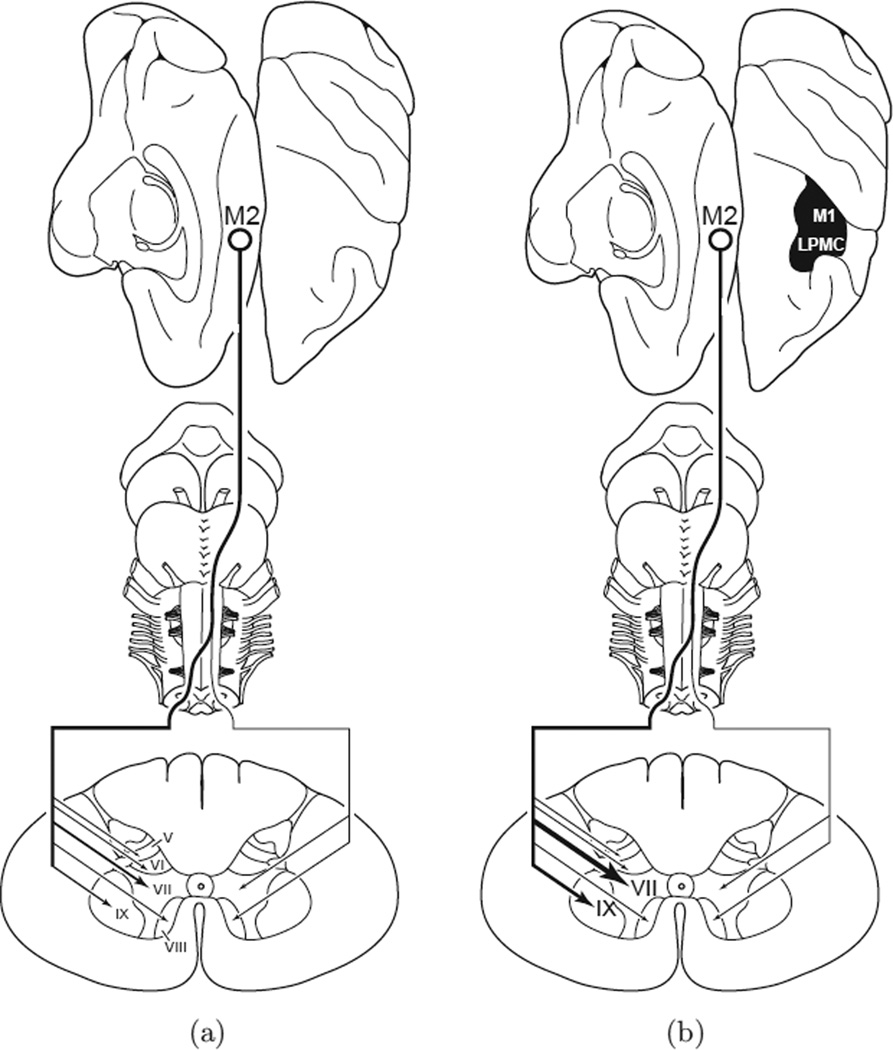
Overall, given that in many human brain lesions such as those arising from middle cerebral artery (MCA) stroke, which often damage the lateral aspect of M1 and premotor cortex, it seems likely that recovery must depend on reorganization in non-injured brain areas, either subcortical as surmised by Sherrington and colleagues and/or nearby cortical premotor areas as suggested by others [22]. It is this latter possibility that has primarily driven our recent work in which effects of lesions of most of the arm areas of M1 and lateral premotor cortex have been surgically removed to partially simulate the effects of a large middle cerebral artery stroke. In these studies, we have shown that behavioral deficits increase with lesion volume, especially as the lesion is expanded to include the medial motor areas and medial prefrontal areas [21]. Consistent with and expanding on previous work over the past 100+ years, substantial recovery of fine hand motor function, including precision grasp, occurs even when the lesion includes medial premotor areas. However, we have convincingly shown that when damage is limited to lateral cortical motor areas, which have been shown by others to provide the bulk of CST connections onto interneurons in lamina VII and motor neurons in lamina IX [26, 63], one mechanism of recovery includes enhancement of CST connections from the medially located supplementary motor area (M2) in spinal cord laminae that contain neurons which have lost substantial input from lateral motor areas (Fig. 7) [64]. Importantly, this mechanism appears to correlate strongly with recovery of hand/digit fine motor function for grasping small food targets and gross arm function in the form of accurate, fast reaching movements to these targets. Moreover, a deficit of fine hand movement control is re-established for a few weeks if the M2 arm area is lesioned using ibotenic acid (after recovery from the M1/LPMC lesion), strongly suggesting that the M2 arm area is partially responsible for recovery [64]. Reorganization of corticofugal outputs to enhance connections onto brainstem motor nuclei is also likely, and we are currently studying these output connections in the pons where there appears to be a selective increase in M2 connections onto some nuclei.
Fig. 7.
Summary diagram illustrating the main findings of McNeal and colleagues [64]. The left diagram (a) illustrates the corticospinal projection from the supplementary motor cortex (M2) in the control experiments. This projection originates from the medial wall of the hemisphere (top, hinged to left from dorsal view of cerebral cortex on right) and most descending fibers cross the midline at inferior brainstem levels (middle) ending in the spinal cord (bottom). The relative intensity of the projection to spinal cord laminae is indicated by line thickness and arrow size. Denser terminal projections are represented by increased line thickness and arrow head size. Progressively lighter terminal projections are indicated by progressively thinner lines and arrowheads. The right diagram (b) illustrates the M2 corticospinal projection in the brain injury experiments after motor recovery of dexterous upper extremity movements. The lesion is located on the dorsal view of the hemisphere (blackened area) and involved the arm representation of the primary motor cortex (M1) and adjacent part of the lateral premotor cortex (LPMC). Extensive enhancement of the contralateral projection to lamina VII and IX occurred following the lateral motor cortical injury but not in other contralateral or ipsilateral laminae. (Fig. 13 — Wiley-Liss, Inc., J Comput Neurol, used with permission.)
Another important finding of our work in collaboration with our colleagues at the University of North Dakota is that recovery after lesions to motor and premotor areas in the nonhuman primate is associated with long term activation of microglia and macrophages in the perilesional cortex and cervical spinal cord that continues for up to one year after the lesion [72, 73]. Moreover, marked increases in brain derived neurotrophic factor (BDNF) and its receptor subtypes were also observed in the perilesional area and cervical spinal cord, suggesting that a long-term contribution of neurotrophic factors in the recovery process is associated with establishing enhanced connections between CST fibers from M2 and ventral horn motor neurons. Whether these processes can be enhanced with certain pharmaceutical or physical therapies is an important question. For example, Nogo is a key axonal growth inhibitory protein and pharmaceutical blockade of this protein induces axonal sprouting and function recovery in stroke [56]. Axonal growth stimulators are also targets for current research (for a review of these issues, see [11]).
There are clear and potentially important implications of this work for human patients with brain injury due to stroke or trauma. First, it appears that recovery is possible even after relatively large lesions affecting lateral cortical motor areas if the output fibers of other motor areas such as the medially located supplementary motor cortex are spared. Indeed, middle cerebral artery occlusion is the most common form of stroke and the arm/hand region of M1 and its descending projection fibers are often destroyed [12]. In contrast, M2 resides in the territory of the anterior cerebral artery which is spared in greater than 97% of first time stroke victims [7]. However, this situation does not preclude the possibility of the descending fibers from M2 being injured because they eventually pass through subcortical white matter regions [66] supplied by branches of the middle cerebral artery [101, 109]. Therefore, the application of MRI techniques such as diffusion tensor imaging to quantify whether the descending M2 fibers are spared following lateral cortical injury should reveal whether enhancement of M2 corticospinal connections promotes recovery of hand functions in patients. However, our work has also shown remarkable recovery of hand function after lesions that also include the arm areas of M2 (Fig. 5(c)) and adjacent pre-SMA (see Figs. 2 and 3 of [21]). Thus, reorganization of other cortical (e.g., cingulate motor areas M3 and/or M4 and parietal cortex) or subcortical motor nuclei may also contribute to recovery under such conditions. Finally, we have also shown that many of these monkeys recover to perform consistently at levels equal to, or even better than during prelesion training. This is likely due to continued task practice since large lesions of motor cortical areas do not appear to abolish well-established motor habits [52] or the ability to learn new hand motor tasks ([92] as reported by Lashley [52]). Collectively, such findings provide considerable support for the idea that favorable recovery is possible following substantial cortical brain damage in nonhuman primates. The clinical question of how best to promote such a recovery in human patients with typically larger lesions using physical rehabilitation techniques (i.e., task performance), brain stimulation (transcranial DC stimulation, repetitive transcranial magnetic stimulation or epidural stimulation) (for a review, see [88]), and pharmaceutical techniques [11] either singly or in combination [3, 28, 80] remains a high priority in the pursuit to enhance the recovery process following motor cortex injury.
8. Conclusions
It is clear from early classical and more recent work that nonhuman primates are able to recover contralesional movement control after small and large lesions of frontal motor cortical areas, especially with some type of intense rehabilitation (e.g., [6, 71, 79]) or even less intense task practice that involves minimal forced use of the impaired limb [21]. Indeed we have observed a very poor recovery of upper limb movements in only one monkey who received a very large lesion affecting the dorsal frontal lobe motor areas and medial prefrontal cortex that also included a large volume of white matter damage [21]. It is quite possible that this monkey would have shown better recovery with intense rehabilitation such as that provided by Ogden and Franz [79]. However, the other monkeys in our study in which the lesion spared at least some cortical motor areas (i.e., cingulate or M2) as well as parts of M1 deep in the central sulcus showed good recovery that was associated with return to prelesion skill levels, or greater manipulation skill levels [21]. In contrast, humans with lesions that affect cortical motor areas commonly do not show such good recovery, especially in terms of grasping and manipulating small objects. Possible reasons for poorer recovery in these patients include: (1) greater subcortical white matter damage disrupting descending corticofugal projections arising from apparently spared motor areas, as well as subcortical damage interrupting the many longitudinally orientated corticocortical axonal pathways that interconnect distant parts of the cortical mantle and subserve the reaching and grasping process (i.e., parietal and frontal areas), (2) greater cortical functional specialization and hand dominance in humans, as reflected in the more developed CST [17, 58] which may affect ability of nonlesioned motor areas to remodel inputs/outputs to take over function of damaged areas, (3) stronger interhemispheric inhibition (associated with greater lateralization) in humans such that undamaged motor areas in the lesioned hemisphere are greatly inhibited and less able to drive neuroplasticity following the lesion and (4) greater effects of emotional depression in humans, leading to lower motivation during rehabilitation.
Subcortical white matter damage is likely one of the most important factors limiting recovery in humans. There are several reports that surgical lesions to frontal lobe cortical motor areas in humans for treatment of cancer, epilepsy and arteriovenous malformations produce only minor or no lasting motor deficits [13, 65, 91]. Damage to white matter is minimized in such surgeries, but can be much greater when the lesion is due to stroke or traumatic injury. Importantly, several recent studies have shown that the integrity of the corticospinal tract at the level of the internal capsule is a strong predictor of motor function recovery after stroke [60, 89, 100]. Thus, even in the case of cortical strokes when there is no loss of blood supply to the internal capsule, damage to the white matter just below the cortical lesion, not gray matter, may be a primary determinant of motor function recovery. Indeed, our work suggests that M2 can substitute functionally after damage to M1 and LPMC if the M2 output fibers are not damaged [64]. Furthermore, in our studies one monkey (SDM64) demonstrated slower and poorer recovery than other monkeys receiving similar (M1 + LPMC arm area) lesions [21] that was apparently due to greater white matter damage that unintentionally disrupted the descending corticofugal fibers arising from M2 that was verified with a tract tracer experiment (unpublished observations).
There are many important implications for future experimentation to improve the recovery prognosis for upper limb motor function in humans. The effects of the various therapeutic techniques discussed above (forced task practice, pharmacological treatments, cortical stimulation) on the neuroplastic response of M2 after a large lesion in the MCA territory that produces poor spontaneous recovery in monkeys would be one useful study. Development of controlled ischemic and hemorrhagic models of MCA stroke in monkeys that are similar to those used in rats would also be helpful because the recovery process from such strokes may differ from surgical ablation, although it will clearly be difficult to control extent of damage in monkeys due to the extensive arterial territory supplied by the MCA [18, 36, 90, 111]. Studies in nonhuman primates are also recommended for preclinical testing of neuroprotective agents [32] and will also be helpful for studies of rehabilitation effects because of the similarity of upper limb use to humans.
After review of more than 100 years of research conducted on the recovery of upper limb movement in nonhuman primates, it is clear that our understanding of the motor recovery process continues to develop. The early work showed that there are many potential neural systems other than the frontal motor cortex capable of effective participation in motor recovery. Although some advances have been made, we are still faced with the daunting task of identifying all neural systems that support the recovery process. Obtaining large groups of patients with isolated injury to distinct cortical motor areas, primarily limited to the gray matter, are conceivably improbable even with access to large patient populations. However, due to the structural homologies of the nonhuman primate and human brain that correlate to the highly developed control of distal upper limb movements [17, 58], there remains great potential to identify contributing factors that lead to specific motor deficits, pinpoint the mechanisms supporting favorable recovery, and implement potential rehabilitative interventions following isolated motor cortex injury in the nonhuman primate model.
Acknowledgments
Supported by National Institutes of Health grant NS 046367. The assistance of staff in the Hardin Library for Health Sciences Rare Book Room at the University of Iowa is gratefully acknowledged.
References
- 1.Armstrong DM, Drew T. Electromyographic responses evoked in muscles of the forelimb by intracortical stimulation in the cat. J Physiol. 1985;367:309–326. doi: 10.1113/jphysiol.1985.sp015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard JW, Woolsey CN. A study of localization in the cortico-spinal tracts of monkey and rat. J Comp Neurol. 1956;105:25–50. doi: 10.1002/cne.901050103. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt E, Nagpal A, Greer KH, Grunewald TK, Steele JL, Wiemiller JW, Lewis SM, Carey JR. Effect of finger tracking combined with electrical stimulation on brain reorganization and hand function in subjects with stroke. Exp Brain Res. 2007;182:435–447. doi: 10.1007/s00221-007-1001-5. [DOI] [PubMed] [Google Scholar]
- 4.Black P, Cianci SN, Markowitz RS. Differential recovery of proximal and distal motor power after cortical lesions. Trans Am Neurol Assoc. 1971;96:173–177. [PubMed] [Google Scholar]
- 5.Black P, Markowitz RS, Cianci SN. In search of the motor engram: A behavioral study of somatotopic localization in motor cortex of monkey. Trans Am Neurol Assoc. 1974;99:188–190. [PubMed] [Google Scholar]
- 6.Black P, Markowitz RS, Cianci SN. Recovery of motor function after lesions in motor cortex of monkey. Ciba Found Symp. 1975;34:65–83. doi: 10.1002/9780470720165.ch5. [DOI] [PubMed] [Google Scholar]
- 7.Bogousslavsky J, Regli F. Anterior cerebral artery territory infarction in the Lausanne Stroke Registry. Clinical and etiologic patterns. Arch Neurol. 1990;47:144–150. doi: 10.1001/archneur.1990.00530020040012. [DOI] [PubMed] [Google Scholar]
- 8.Brinkman C. Lesions in supplementary motor area interfere with a monkey’s performance of a bimanual coordination task. Neurosci Lett. 1981;27:267–270. doi: 10.1016/0304-3940(81)90441-9. [DOI] [PubMed] [Google Scholar]
- 9.Brinkman C. Supplementary motor area of the monkey’s cerebral cortex: Short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buys EJ, Lemon RN, Mantel GW, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmichael ST. Targets for neural repair therapies after stroke. Stroke. 2010;41:S124–S126. doi: 10.1161/STROKEAHA.110.597146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky J. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007;24:97–103. doi: 10.1159/000103123. [DOI] [PubMed] [Google Scholar]
- 13.Chamoun RB, Mikati MA, Comair YG. Functional recovery following resection of an epileptogenic focus in the motor hand area. Epilepsy Behav. 2007;11:384–388. doi: 10.1016/j.yebeh.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Thaler D, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. II. The timing and selection of learned movements. Exp Brain Res. 1995;102:461–473. doi: 10.1007/BF00230650. [DOI] [PubMed] [Google Scholar]
- 15.Cheney PD, Fetz EE. Comparable patterns of muscle facilitation evoked by individual corticomotoneuronal (CM) cells and by single intracortical microstimuli in primates: Evidence for functional groups of CM cells. J Neurophysiol. 1985;53:786–804. doi: 10.1152/jn.1985.53.3.786. [DOI] [PubMed] [Google Scholar]
- 16.Clarke E, O’Malley CD. The human brain and spinal cord. Berkeley: University of California Press; 1968. [Google Scholar]
- 17.Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, Maier I, Martin J, Nudo RJ, Ramon-Cueto A, Rouiller EM, Schnell L, Wannier T, Schwab ME, Edgerton VR. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Arceuil HE, Duggan M, He J, Pryor J, de Crespigny A. Middle cerebral artery occlusion in Macaca fascicularis: Acute and chronic stroke evolution. J Med Primatol. 2006;35:78–86. doi: 10.1111/j.1600-0684.2006.00147.x. [DOI] [PubMed] [Google Scholar]
- 19.Darling WG, Peterson CR, Herrick JL, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Measurement of coordination of object manipulation in nonhuman primates. J Neurosci Methods. 2006;154:38–44. doi: 10.1016/j.jneumeth.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Darling WG, Pizzimenti MA, Rotella DL, Hynes SM, Ge J, Stilwell-Morecraft KS, Vanadurongvan T, McNeal DW, Solon-Cline KM, Morecraft RJ. Minimal forced use without constraint stimulates spontaneous use of the impaired upper extremity following motor cortex injury. Exp Brain Res. 2010;202:529–542. doi: 10.1007/s00221-010-2157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darling WG, Pizzimenti MA, Rotella DL, Peterson CR, Hynes SM, Ge J, Solon K, McNeal DW, Stilwell-Morecraft KS, Morecraft RJ. Volumetric effects of motor cortex injury on recovery of dexterous movements. Exp Neurol. 2009;220:90–108. doi: 10.1016/j.expneurol.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denny-Brown D. Disintegration of motor function resulting from cerebral lesions. J Nerv Ment Dis. 1950;112:1–45. [PubMed] [Google Scholar]
- 23.Denny-Brown D, Botterell EH. The motor functions of agranular frontal cortex. Res Publ Assoc Res Nerv Ment Dis. 1948;27:235–345. [PubMed] [Google Scholar]
- 24.Devanne H, Cassim F, Ethier C, Brizzi L, Thevenon A, Capaday C. The comparable size and overlapping nature of upper limb distal and proximal muscle representations in the human motor cortex. Eur J Neurosci. 2006;23:2467–2476. doi: 10.1111/j.1460-9568.2006.04760.x. [DOI] [PubMed] [Google Scholar]
- 25.Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: Representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- 26.Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16:6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisner-Janowicz I, Barbay S, Hoover E, Stowe AM, Frost SB, Plautz EJ, Nudo RJ. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008;100:1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang PC, Barbay S, Plautz EJ, Hoover E, Strittmatter SM, Nudo RJ. Combination of NEP 1–40 treatment and motor training enhances behavioral recovery after a focal cortical infarct in rats. Stroke. 2010;41:544–549. doi: 10.1161/STROKEAHA.109.572073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrier D. The Functions of the Brain. New York: G. P. Putnam’s Sons; 1886. [Google Scholar]
- 30.Ferrier D, Yeo GF. A record of experiments on the effects of lesion of different regions of the cerebral hemispheres. Philos Trans Royal Soc Lond. 1884;175:479–564. [Google Scholar]
- 31.Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- 32.Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, Finklestein SP, Pangalos MN, Poole M, Stiles GL, Ruffolo RR, Walsh FL. Missing steps in the STAIR case: A Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- 33.Finger S. Origins of Neuroscience: A History of Explorations into Brain Function. New York: Oxford University Press; 1994. [Google Scholar]
- 34.Flourens P. Examen de la phrenologie. Paris: Paulin; 1843. [Google Scholar]
- 35.Fritsch G, Hitzig E. Über die elektrische Erregbarkeit des Grosshirns. Arch Anat Physiol. 1870;37:300–332. [Google Scholar]
- 36.Fukuda S, del Zoppo GJ. Models of focal cerebral ischemia in the nonhuman primate. ILAR J. 2003;44:96–104. doi: 10.1093/ilar.44.2.96. [DOI] [PubMed] [Google Scholar]
- 37.Fulton J, Kennard M. A study of flaccid and spastic paralysis produced by lesions of the cerebral cortex in primates. Res Publ Assoc Res Nerv Ment Dis. 1934;13:158–210. [Google Scholar]
- 38.Glees P, Cole J. Recovery of skilled motor functions after small repeated lesions of motor cortex in macaque. J Neurophysiol. 1950;13:137–148. [Google Scholar]
- 39.Graham Brown T, Sherrington CS. Note on the functions of the cortex cerebri. J Physiol (Lond) 1913;46(suppl):xxii. [Google Scholar]
- 40.Grunbaum ASF, Sherrington CS. Observations on the physiology of the cerebral cortex of the anthropoid apes. Proc Royal Soc Lond. 1903;72:152–155. [Google Scholar]
- 41.Halsband U, Passingham R. The role of premotor and parietal cortex in the direction of action. Brain Res. 1982;240:368–372. doi: 10.1016/0006-8993(82)90239-6. [DOI] [PubMed] [Google Scholar]
- 42.Halsband U, Passingham RE. Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis) Behav Brain Res. 1985;18:269–277. doi: 10.1016/0166-4328(85)90035-x. [DOI] [PubMed] [Google Scholar]
- 43.Hamuy TP. Retention and performance of “skilled movements” after cortical ablations in monkeys. Bull Johns Hopkins Hosp. 1956;98:417–444. [PubMed] [Google Scholar]
- 44.Hepp-Reymond MC, Trouche E, Wiesendanger M. Effects of unilateral and bilateral pyramidotomy on a conditioned rapid precision grip in monkeys (Macaca fascicularis) Exp Brain Res. 1974;21:519–527. doi: 10.1007/BF00237170. [DOI] [PubMed] [Google Scholar]
- 45.Horsley V, Schafer E. A record of experiments upon the functions of the cerebral cortex. Philos Trans Royal Soc Lond, Ser B. 1888;179:1–45. [Google Scholar]
- 46.Jacobsen C. Influence of motor and premotor area lesions upon the retention of skilled movements in monkeys and chimpanzees. Res Publ Assoc Res Nerv Ment Dis. 1934;13:225–247. [Google Scholar]
- 47.Kennard M. Age and other factors in motor recovery from precentral lesions in monkeys. Am J Physiol. 1936;115:138–146. [Google Scholar]
- 48.Kennard M. Relation of age to motor impairment in man and in subhuman primates. Arch Neurol Psychiatry. 1940;44:377–397. [Google Scholar]
- 49.Kennard M. Reorganization of motor function in the cerebral cortex of monkeys deprived of motor and premotor areas in infancy. J Neurophysiol. 1938;1:477–496. [Google Scholar]
- 50.Kennard M. A cortical reorganization of motor function: Studies on series of monkeys of various ages from infancy to maturity. Arch Neurol Psychiatry. 1942;48:227–240. [Google Scholar]
- 51.Knapp HD, Taub E, Berman AJ. Effect of deafferentation on a conditioned avoidance response. Science. 1958;128:842–843. doi: 10.1126/science.128.3328.842. [DOI] [PubMed] [Google Scholar]
- 52.Lashley KS. Studies of cerebral function in learning: V. The retention of motor habits after destruction of the so-called motor areas in primates. Arch Neurol Psychiatry. 1924;12:249–276. [Google Scholar]
- 53.Lang CE, Schieber MH. Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol. 2004;91:1722–1733. doi: 10.1152/jn.00805.2003. [DOI] [PubMed] [Google Scholar]
- 54.Lawrence DG, Hopkins DA. The development of motor control in the rhesus monkey: Evidence concerning the role of corticomotoneuronal connections. Brain. 1976;99:235–254. doi: 10.1093/brain/99.2.235. [DOI] [PubMed] [Google Scholar]
- 55.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Lee J-K, Kim J-E, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lemon RN. Neural control of dexterity: What has been achieved? Exp Brain Res. 1999;128:6–12. doi: 10.1007/s002210050811. [DOI] [PubMed] [Google Scholar]
- 58.Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: Organizational differences for motor specialization? Muscle Nerve. 2005;32:261–279. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- 59.Leyton ASF, Sherrington CS. Observations on the excitable cortex of the chimpanzee, orangutan and gorilla. Exp Physiol. 1917;11:135–222. [Google Scholar]
- 60.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74:280–287. doi: 10.1212/WNL.0b013e3181ccc6d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu CN, Chambers WW. An experimental study of the cortico-spinal system in the Monkey (Macaca Mulatta). The spinal pathways and preterminal distribution of degenerating fibers following discrete lesions of the pre- and postcentral gyri and bulbar pyramid. J Comp Neurol. 1964;123:257–283. doi: 10.1002/cne.901230209. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Rouiller EM. Mechanisms of recovery of dexterity following unilateral lesion of the sensorimotor cortex in adult monkeys. Exp Brain Res. 1999;128:149–159. doi: 10.1007/s002210050830. [DOI] [PubMed] [Google Scholar]
- 63.Maier MA, Armand J, Kirkwood PA, Yang HW, Davis JN, Lemon RN. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: An anatomical and electrophysiological study. Cereb Cortex. 2002;12:281–296. doi: 10.1093/cercor/12.3.281. [DOI] [PubMed] [Google Scholar]
- 64.McNeal DW, Darling WG, Ge J, Stilwell-Morecraft KS, Solon KM, Hynes SM, Pizzimenti MA, Rotella DL, Vanadurongvan T, Morecraft RJ. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518:586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mikuni N, Ikeda A, Yoneko H, Amano S, Hanakawa T, Fukuyama H, Hashimoto N. Surgical resection of an epileptogenic cortical dysplasia in the deep foot sensorimotor area. Epilepsy Behav. 2005;7:559–562. doi: 10.1016/j.yebeh.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 66.Morecraft RJ, Herrick JL, Stilwell-Morecraft KS, Louie JL, Schroeder CM, Ottenbacher JG, Schoolfield MW. Localization of arm representation in the corona radiata and internal capsule in the nonhuman primate. Brain. 2002;125:176–198. doi: 10.1093/brain/awf011. [DOI] [PubMed] [Google Scholar]
- 67.Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Gedney M, Ge J, Schroeder CM, Van Hoesen GW. Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol. 2007;500:134–165. doi: 10.1002/cne.21165. [DOI] [PubMed] [Google Scholar]
- 68.Morecraft RJ, Tanji J. Cingulofrontal interactions and cingulate skeletomotor areas. In: Vogt BA, editor. Cingulate Neurobiology and Disease. New York: Oxford University Press; 2009. pp. 113–144. [Google Scholar]
- 69.Morecraft RJ, Van Hoesen GW. Convergence of limbic input to the cingulate motor cortex in the rhesus monkey. Brain Res Bull. 1998;45:209–232. doi: 10.1016/s0361-9230(97)00344-4. [DOI] [PubMed] [Google Scholar]
- 70.Mott F, Halliburton W. Localisation of function in the lemur’s brain. Proc Royal Soc Lond Ser B. 1908;80:136–147. [Google Scholar]
- 71.Murata Y, Higo N, Oishi T, Yamashita A, Matsuda K, Hayashi M, Yamane S. Effects of motor training on the recovery of manual dexterity after primary motor cortex lesion in macaque monkeys. J Neurophysiol. 2008;99:773–786. doi: 10.1152/jn.01001.2007. [DOI] [PubMed] [Google Scholar]
- 72.Nagamoto-Combs K, McNeal DW, Morecraft RJ, Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- 73.Nagamoto-Combs K, Morecraft RJ, Darling WG, Combs CK. Long-term gliosis and molecular changes in the cervical spinal cord of the rhesus monkey after traumatic brain injury. J Neurotrauma. 2010;27:565–585. doi: 10.1089/neu.2009.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nudo R, Milliken G, Jenkins W, Merzenich M. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9:740–747. doi: 10.1016/s0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 76.Nudo RJ, Friel KM. Cortical plasticity after stroke: Implications for rehabilitation. Revue Neurologique. 1999;155:713–717. [PubMed] [Google Scholar]
- 77.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 78.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 79.Ogden R, Franz SI. On cerebral motor control: The recovery from experimentally produced hemiplegia. Psychobiology. 1917;1:33–47. [Google Scholar]
- 80.Page SJ, Elovic E, Levine P, Sisto SA. Modified constraint-induced therapy and botulinum toxin A: A promising combination. Am J Phys Med Rehabil. 2003;82:76–80. doi: 10.1097/00002060-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 81.Passingham R, Perry H, Wilkinson F. Failure to develop a precision grip in monkeys with unilateral neocortical lesions made in infancy. Brain Res. 1978;145:410–414. doi: 10.1016/0006-8993(78)90878-8. [DOI] [PubMed] [Google Scholar]
- 82.Passingham RE. Cues for movement in monkeys (Macaca mulatta) with lesions in premotor cortex. Behav Neurosci. 1986;100:695–703. doi: 10.1037//0735-7044.100.5.695. [DOI] [PubMed] [Google Scholar]
- 83.Passingham RE. Premotor cortex: Sensory cues and movement. Behav Brain Res. 1985;18:175–185. doi: 10.1016/0166-4328(85)90073-7. [DOI] [PubMed] [Google Scholar]
- 84.Passingham RE, Perry VH, Wilkinson F. The long-term effects of removal of sensorimotor cortex in infant and adult rhesus monkeys. Brain. 1983;106:675–705. doi: 10.1093/brain/106.3.675. [DOI] [PubMed] [Google Scholar]
- 85.Penfield W, Welch K. The supplementary motor area of the cerebral cortex: A clinical and experimental study. Arch Neurol Psychiatry. 1951;66:289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- 86.Pizzimenti MA, Darling WG, Rotella DL, McNeal DW, Herrick JL, Ge J, Stilwell-Morecraft KS, Morecraft RJ. Measurement of reaching kinematics and prehensile dexterity in nonhuman primates. J Neurophysiol. 2007;98:1015–1029. doi: 10.1152/jn.00354.2007. [DOI] [PubMed] [Google Scholar]
- 87.Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, Stowe AM, Quaney BM, Nudo RJ. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: A feasibility study in primates. Neurol Res. 2003;25:801–810. doi: 10.1179/016164103771953880. [DOI] [PubMed] [Google Scholar]
- 88.Plow EB, Carey JR, Nudo RJ, Pascual-Leone A. Invasive cortical stimulation to promote recovery of function after stroke: A critical appraisal. Stroke. 2009;40:1926–1931. doi: 10.1161/STROKEAHA.108.540823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qiu M, Darling WG, Morecraft RJ, Ni CC, Rajendra J, Butler AJ. White matter integrity is a stronger predictor of motor function than BOLD response in patients with stroke. Neurorehabil Neural Repair. 2011;25:275–284. doi: 10.1177/1545968310389183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roitberg B, Khan N, Tuccar E, Kompoliti K, Chu Y, Alperin N, Kordower JH, Emborg ME. Chronic ischemic stroke model in cynomolgus monkeys: Behavioral, neuroimaging and anatomical study. Neurol Res. 2003;25:68–78. doi: 10.1179/016164103101200950. [DOI] [PubMed] [Google Scholar]
- 91.Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75:62–68. doi: 10.3171/jns.1991.75.1.0062. [DOI] [PubMed] [Google Scholar]
- 92.Rothmann M. Ueber die physiologische Wertung der cortico-spinalen (Pyramiden) Bahn. Arch f Anat u Physiol (Physiol Abt) 1907:217–227. [Google Scholar]
- 93.Rouiller EM, Yu XH, Moret V, Tempini A, Wiesendanger M, Liang F. Dexterity in adult monkeys following early lesion of the motor cortical hand area: The role of cortex adjacent to the lesion. Eur J Neurosci. 1998;10:729–740. doi: 10.1046/j.1460-9568.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 94.Sato KC, Tanji J. Digit-muscle responses evoked from multiple intracortical foci in monkey precentral motor cortex. J Neurophysiol. 1989;62:959–970. doi: 10.1152/jn.1989.62.4.959. [DOI] [PubMed] [Google Scholar]
- 95.Schieber MH, Hibbard LS. How somatotopic is the motor cortex hand area? Science. 1993;261:489–492. doi: 10.1126/science.8332915. [DOI] [PubMed] [Google Scholar]
- 96.Schieber MH, Lang CE, Reilly KT, McNulty P, Sirigu A. Selective activation of human finger muscles after stroke or amputation. Adv Exp Med Biol. 2009;629:559–575. doi: 10.1007/978-0-387-77064-2_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: Effects on individuated finger movements. J Neurosci. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schneider C, Zytnicki D, Capaday C. Quantitative evidence for multiple widespread representations of individual muscles in the cat motor cortex. Neurosci Lett. 2001;310:183–187. doi: 10.1016/s0304-3940(01)02105-x. [DOI] [PubMed] [Google Scholar]
- 99.Sloper JJ, Brodal P, Powell TP. An anatomical study of the effects of unilateral removal of sensorimotor cortex in infant monkeys on the subcortical projections of the contralateral sensorimotor cortex. Brain. 1983;106:707–716. doi: 10.1093/brain/106.3.707. [DOI] [PubMed] [Google Scholar]
- 100.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 101.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: Cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 102.Taub E, Ellman SJ, Berman AJ. Deafferentation in monkeys: Effect on conditioned grasp response. Science. 1966;151:593–594. doi: 10.1126/science.151.3710.593. [DOI] [PubMed] [Google Scholar]
- 103.Taub E, Miller NE, Novack TA, Cook EW, 3rd, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 104.Taub E, Morris DM. Constraint induced movement therapy to enhance recovery after stroke. Current Atherosclerosis Reports. 2011;3:279–286. doi: 10.1007/s11883-001-0020-0. [DOI] [PubMed] [Google Scholar]
- 105.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: A new family of techniques with broad application to physical rehabilitation — A clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 106.Thaler D, Chen YC, Nixon PD, Stern CE, Passingham RE. The functions of the medial premotor cortex. I. Simple learned movements. Exp Brain Res. 1995;102:445–460. doi: 10.1007/BF00230649. [DOI] [PubMed] [Google Scholar]
- 107.Travis AM. Neurological deficiencies after ablation of the precentral motor area in Macaca mulatta. Brain. 1955;78:155–173. doi: 10.1093/brain/78.2.155. [DOI] [PubMed] [Google Scholar]
- 108.Travis AM. Neurological deficiencies following supplementary motor area lesions in Macaca mulatta. Brain. 1955;78:174–198. doi: 10.1093/brain/78.2.174. [DOI] [PubMed] [Google Scholar]
- 109.van der Zwan A, Hillen B, Tulleken CA, Dujovny M, Dragovic L. Variability of the territories of the major cerebral arteries. J Neurosurg. 1992;77:927–940. doi: 10.3171/jns.1992.77.6.0927. [DOI] [PubMed] [Google Scholar]
- 110.Vilensky JA, Gilman S. Lesions of the precentral gyrus in nonhuman primates: A premedline bibliography. Int J Primatol. 2002;23:1319–1333. [Google Scholar]
- 111.West GA, Golshani KJ, Doyle KP, Lessov NS, Hobbs TR, Kohama SG, Pike MM, Kroenke CD, Grafe MR, Spector MD, Tobar ET, Simon RP, Stenzel-Poore MP. A new model of cortical stroke in the rhesus macaque. J Cereb Blood Flow Metab. 2009;29:1175–1186. doi: 10.1038/jcbfm.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wiesendanger M. Postlesion recovery of motor and sensory cortex in the early twentieth century. J Hist Neurosci. 2011;20:42–57. doi: 10.1080/09647041003775446. [DOI] [PubMed] [Google Scholar]
- 113.Wolf SL, Winstein CJ, Miller PJ, Taub E, Uswatte G, Morris D, Giuliani C, Llight KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper limb extremity function 3 to 9 months affer stroke: The EXCITE randomized clinical trial. JAMA. 2006;296:20095–20104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 114.Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM. Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis. 1951;30:238–264. [PubMed] [Google Scholar]