Abstract
Exposure of mice to UV radiation results in suppression of the contact hypersensitivity (CHS) response. Here, we report that the UV-induced suppression of CHS is associated with increases in the levels of cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), and PGE2 receptors in the exposed skin. UV radiation-induced suppression of CHS was inhibited by topical treatment of the skin with celecoxib or indomethacin (inhibitors of COX-2) or AH6809 (an EP2 antagonist). Moreover, mice deficient in COX-2 were found to be resistant to UV-induced suppression of CHS. The exposure of wild-typemice to UVB radiation resulted in DNA hypermethylation, increased DNA methyltransferase (Dnmt) activity, and elevated levels of Dnmt1, Dnmt3a, and Dnmt3b proteins in the skin, and these responses were downregulated on topical treatment of the site of exposure after irradiation with indomethacin or EP2 antagonist. Topical treatment of UVB-exposed COX-2-deficient mice with PGE2 enhanced the UVB-induced suppression of CHS as well as global DNA methylation and elevated the levels of Dnmt activity and Dnmt proteins in the skin. Intraperitoneal injection of 5-aza-2′-deoxycytidine (5-Aza-dc), a DNA demethylating agent, restored the CHS response to 2,4-dinitrofluorobenzene in UVB-exposed skin and this was associated with the reduction in global DNA methylation and Dnmt activity and reduced levels of Dnmt proteins. Furthermore, treatment with 5-Aza-dc reversed the effect of PGE2 on UV-induced suppression of CHS in COX-2-deficient mice. These findings reveal a previously unrecognized role for PGE2 in the promotion of UVB-induced immunosuppression and indicate that it is mediated through PGE2 regulation of DNA methylation.
Introduction
It has been shown that UV radiation in the UVB (290–320 nm) range induces immunosuppression in laboratory animals and that xenografted tumor cells are more readily established and grow more rapidly in UV-irradiated mice than mice that have not been UV irradiated [1]. The exposure of the skin to UV radiation triggers the release of prostaglandins (PGs), such as PGD2, PGF2α, and PGE2, which are produced from arachidonic acid by the action of cyclooxygenases (COX) [2,3]. The PGs have been implicated in UV radiation-induced immunosuppression in several studies that show that nonsteroidal anti-inflammatory drugs (NSAIDs), including indomethacin, that exert their effects through COX-2 inhibition can reverse the immunosuppressive effect of UV radiation [4,5]. Among the PG metabolites, PGE2, which is produced abundantly by keratinocytes in UV-exposed skin [6,7], is the major and most effective metabolite generated by COX-2 activity and is considered to be a potent mediator of inflammatory responses. Collectively, these data suggest that PGE2 plays a key role in UV radiation-induced immunosuppression. The mechanisms by which PGE2 induces or promotes UV-induced immunosuppression and the relationship of these mechanisms to those of other proposed mediators of UV-induced immunosuppression have not been elucidated fully, however.
These mechanisms are of considerable clinical interest as UV-induced immunosuppression has been implicated as a risk factor for melanoma and nonmelanoma skin cancers. Epidemiologic and clinical studies suggest a link between inflammation and skin cancer [8] and also suggest that the use of NSAIDs reduces the relative risk for developing skin cancer [9]. NSAIDs exert their anti-inflammatory and anti-tumor promoting effects, in part, by targeting PGs. It is known that UV radiation induces the expression of COX-2 and production of PGE2 in mouse skin. We have demonstrated previously that there is a distinct pattern of DNA hypermethylation in UVB-exposed mouse skin and UVB-induced skin tumors in mice that is associated with elevated expression and activity of the DNA methyltransferase (Dnmt) 1, Dnmt3a, and Dnmt3b [10]. Together, these data suggested the possibility that PGE2 may be involved in the promotion of UVB-induced immunosuppression and that the PGE2 may act to promote the immunosuppression by enhancing the levels of DNA methylation.
To explore these possibilities, we used a combination of genetic and pharmacological approaches to assess the role of PGE2 in standard contact hypersensitivity (CHS) model of UVB-induced immunosuppression. We found that UV-induced up-regulation of COX-2/PGE2 is involved in suppression of the CHS response in UV-exposed mice. The role of PGE2 is supported by the evidence that COX-2-deficient mice are resistant to UVB-induced suppression of CHS. We also show that administration of PGE2 to the mice increases the levels of global DNA methylation in UV-exposed skin and that this increase in DNA methylation is associated with increased expression and activity of Dnmts.
Materials and Methods
Animals
Female C3H/HeN mice (6–7 weeks old) were purchased from Charles River Laboratory (Wilmington, MA). The COX-2-deficient male and female heterozygous mice (COX-2+/-) were used in this study and were bred using heterozygous x heterozygous in our animal resource facility, as described [11]. The breeding pairs were kindly provided by Dr Langenbach of the National Institutes of Environmental Health Sciences (National Institutes of Health). In some experiments, wild-type littermates from this breeding strain were also used in this study. The survival of COX-2-deficient heterozygous mice is similar to that of wild-type mice and there are no gross phenotypical differences as compared with their wild-type counterparts. All mice were maintained under standard housing conditions of a 12-hour dark/12-hour light cycle, a temperature of 24 ± 2°C, and relative humidity of 50 ± 10%. Food and water were provided ad libitum. The animal protocol used in this study was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Antibodies and Reagents
Antibodies were purchased as follows: 5-methylcytosine (5-mC) from Calbiochem, EMD Biosciences (New Jersey, NJ); Dnmt1, Dnmt3a, and Dnmt3b from Imgenec Corporation (San Diego, CA); COX-2, EP2, EP3, and EP4 from Santa Cruz Biotechnology (Dallas, TX); EP1 fromAbcam (Cambridge, MA), and DNA isolation kits were purchased from Qiagen Inc (Valencia, CA). The positive controls of EP1, EP2, EP3, and EP4 for Western blot analysis were obtained from Santa Cruz Biotechnology. The Methylamp Global DNA Methylation Quantification Kit and the EpiQuik DNA Methyltransferase Activity Assay Kit were purchased from Epigentek, Inc (New York, NY). The PGE2 immunoassay kit was purchased from Cayman Chemical (Ann Arbor, MI). All other chemicals of analytical grade were purchased from Sigma-Aldrich Chemical Co (St Louis, MO).
UVB Irradiation of Mice
The dorsal hairs of mice were trimmed or shaved with electric clippers at least 24 hours before UVB exposure. The shaved backs of the mice were UVB irradiated as described earlier [12] using a band of four FS20 UVB lamps (Daavlin; UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UV dosage. The UV lamps emit UVB (280–320 nm; ≈80% of total energy) and UVA (320–375 nm; ≈20% of total energy) with UVC emission being insignificant. The peak emission of UV radiation is at 314 nm. This UV unit enables us to enter the UV dose in millijoules and variations in energy output are compensated automatically so that the desired UV dose is delivered.
UVB-induced CHS Model and Assay
The shaved backs of the mice were exposed to UVB radiation (150 mJ/cm2) on four consecutive days. Twenty-four hours after the last UV exposure, the mice were sensitized by painting 25 µl of 0.5% 2,4-dinitrofluorobenzene (DNFB) in acetone/olive oil (4:1, vol/vol) on the UVB-irradiated skin site. The CHS response was elicited 5 days later by painting both surfaces of both ears of each mouse with 20 µl of 0.2% DNFB in acetone/olive oil (4:1, vol/vol). The ear skin thickness was measured 24 hours after the challenge using an engineer's micrometer (Mitutoyo, Tokyo, Japan) and was compared with the ear thickness just before the challenge, as detailed previously [13,14]. Mice that were not UV irradiated but were sensitized and challenged as described served as a positive control. Mice that were not UV irradiated and received only ear challenge without sensitization with DNFB served as a negative control. During the UV exposure of the mice, the ears of the mice were protected from the UV irradiation. The UV-induced suppression of CHS was determined as described previously [13,14]. Each group consisted of four to five mice.
UVB Irradiation of Mice for the Biochemical Analysis Including Epigenetic Regulators
The shaved backs of the mice were exposed to UVB radiation (150 mJ/cm2) on four consecutive days with or without the treatment with PGE2, COX-2 inhibitors, or EP2 antagonist. Twenty-four hours after the last UV exposure, mice were sacrificed and dorsal skin samples were collected for preparing the samples for biochemical analysis.
Treatment of Mice with PGE2, Indomethacin, Celecoxib, and 5-Aza-2′-deoxycytidine
To assess the effect of PGE2 or COX-2 inhibitors (indomethacin or celecoxib) on UVB-induced suppression of the CHS response, the mice were treated topically with celecoxib (500 µg in 0.2 ml acetone) [15,16], indomethacin (50 µg in 0.2 ml of acetone) [16,17], or AH6809 (25 µg in 0.2 ml of acetone) [16,18], which were applied to the shaved dorsal skin 30 minutes before UVB exposure. PGE2 (50 µg in 0.2 ml of acetone) was applied to the COX-2-deficient mouse skin after UVB exposure [16,17]. Control animals were treated topically with acetone (0.2 ml of acetone). Some mice were administered 5-aza-2′-deoxycytidine (5-Aza-dc; 1 mg/kg body weight) by intraperitoneal (i.p.) injection after exposure to UVB radiation [19]. Two doses of 5-Aza-dc were administered: first dose of 5-Aza-dc was injected i.p. after first UVB exposure of mice and second dose of 5-Aza-dc was administered i.p. after third exposure of UVB irradiation of mice.
Dot-blot Analysis of 5-mC for DNA Methylation
Genomic DNA from skin samples was isolated, and dot-blot analysis was performed as detailed previously [12]. Briefly, genomic DNA (50 ng) was transferred to a positively charged nitrocellulose membrane by vacuum dot-blot analysis (Bio-Dot Apparatus; Bio-Rad, Hercules, CA) and fixed by baking the membrane for 30 minutes at 80°C. After blocking the nonspecific binding sites in blocking buffer, the membrane was incubated with the antibodies specific for 5-mC at room temperature for 1 hour. After washing, the membrane was incubated with HRP-conjugated secondary antibody. The enhanced chemiluminescence reagents were used to detect the circular bands of 5-mC.
Analysis of Global DNA Methylation
Total genomic DNA was extracted from the mouse skin samples using the Qiagen amp DNA Mini Kit (Qiagen Sciences, Valencia, CA) following the manufacturer's protocol. The levels of global DNA methylation were determined using the Methylamp Global DNA Methylation Quantification Kit (Epigentek, Inc) following the manufacturer's instructions.
Assay of Dnmt Activity
Skin samples from different treatment groups were used to prepare nuclear extracts. Nuclear extracts were prepared using the EpiQuik Nuclear Extraction Kit (Epigentek, Inc), and Dnmt activity was determined using the EpiQuik DNA Methyltransferase Activity Assay Kit (Epigentek, Inc) according to the instructions provided by the manufacturer.
Western Blot Analysis
The epidermis was separated from the whole skin as described earlier [20] and pooled from two or three mice in each group (n = 4–5 per group). Lysates of the epidermis were prepared for Western blot analysis as described previously [21]. Proteins (25–60 µg) were resolved on 8% to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membranes were incubated in blocking buffer for 1 hour at room temperature and then incubated with the primary antibodies in blocking buffer overnight at 4°C. The membrane was then washed with phosphatebuffered saline and incubated with HRP-conjugated secondary antibody. Protein bands were visualized using the enhanced chemiluminescence detection reagents. Equal loading of proteins on the gel was verified by reprobing the membrane with anti-β-actin or antihistone H3 antibody.
Immunoassay Analysis of PGE2
Skin samples were homogenized in 100 mM phosphate buffer (pH 7.4) containing 1 mM EDTA and 10 µM indomethacin using a polytron homogenizer (PT3100; Fisher Scientific, Pittsburg, PA). The supernatants were collected for analysis of the levels of PGE2 using the Cayman PGE2 Enzyme Immunoassay Kit (Cayman Chemical) following the manufacturer's protocol.
Statistical Analysis
The statistical significance of difference between the values of control and treatment groups was determined using analysis of variance. P < .05 was considered as statistically significant.
Results
UV-induced Suppression of CHS Is Associated with an Increase in the Levels of COX-2, PGE2, and PGE2 Receptors
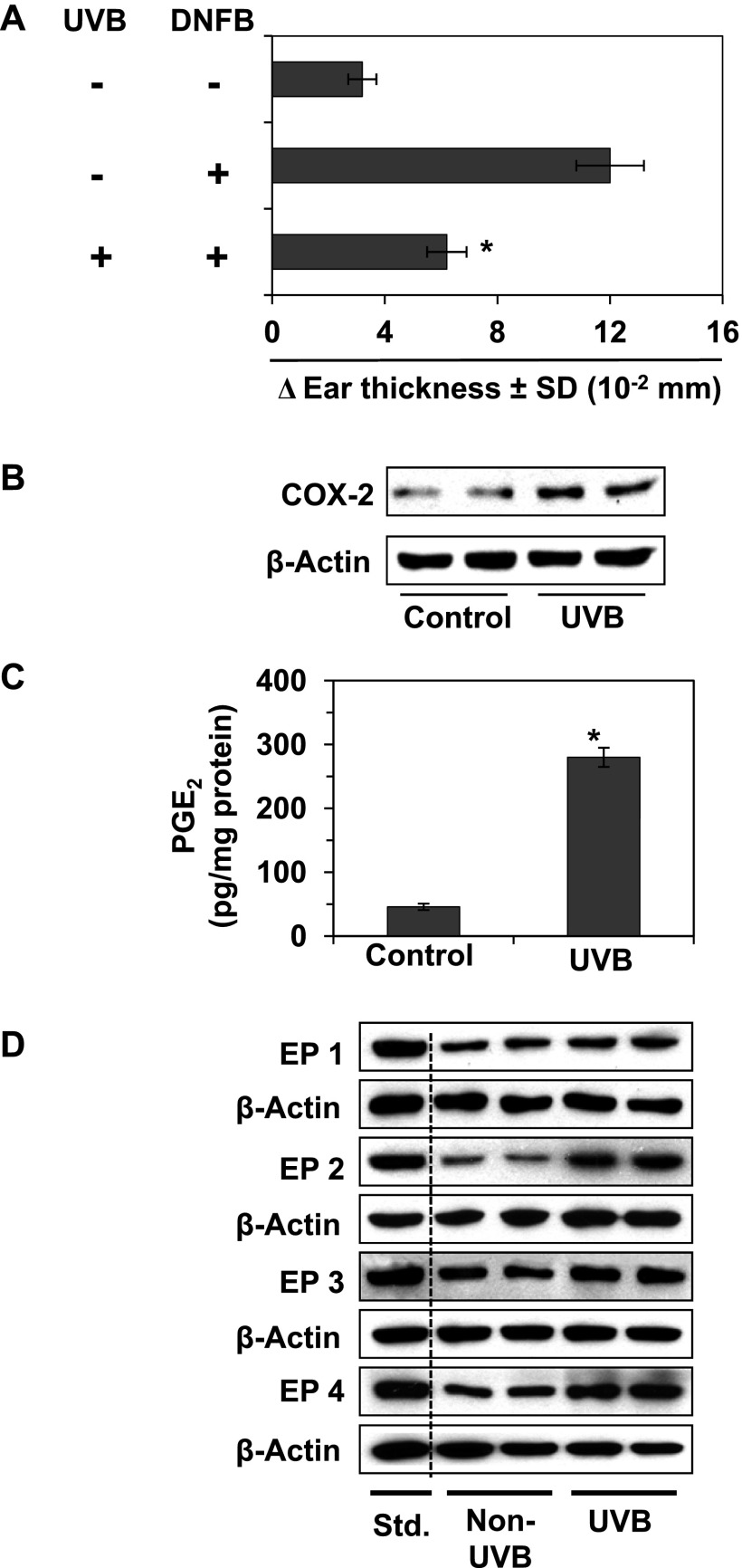
It is well recognized that exposure of mice to UV radiation induces suppression of the immune system or CHS response. We first verified that exposure of C3H/HeN mouse skin to UV radiation on four consecutive days resulted in suppression of the CHS response to DNFB, a skin contact sensitizer (Figure 1A). To determine the mechanism of UV-induced suppression of CHS, we investigated whether UV-induced suppression of CHS is associated with enhancement of the levels of mediators of inflammation, such as COX-2. For this purpose, mice were UVB irradiated for four consecutive days and sacrificed 24 hours after the last UVB irradiation. Skin samples from UVB-irradiated and non-UVB-irradiated mice were collected, and skin lysates were subjected to Western blot analysis. As shown in Figure 1, Western blot analysis revealed that the expression of COX-2 was approximately 2.5-fold higher in UVB-exposed skin than in non-UV-Bexposed mouse skin (Figure 1B). The production of PGE2 metabolite and the expression levels of PGE2 receptors also were higher in the UVB-exposed skin than non-UVB-exposed mouse skin (Figure 1, C and D). Positive controls of EP receptors were used for proper recognition of receptors during gel electrophoresis.
Figure 1.
Effect of UVB radiation on CHS response and the levels of inflammatory mediators. The shaved dorsal skin of female C3H/HeN mice was exposed to UVB radiation (150 mJ/cm2) on four consecutive days as described in Materials and Methods section. (A) The mice were sensitized to DNFB, and the CHS response to application of DNFB on ear skin (challenge) was assessed by measurement of the ear swelling response 24 hours later as described in Materials and Methods section. The change in ear skin thickness is reported in millimeter (x 10-2) as the mean ± SD, with n = 4 to 5 per group. (B–D) The mice were exposed to UVB (150 mJ/cm2) on four consecutive days and then sacrificed 24 hours after the last UVB exposure. Dorsal skin samples from UVB-exposed and non-UVB-exposed mice were collected for analysis. (B) The levels of COX-2 were determined in lysates of the skin using Western blot analysis. (C) The concentration of PGE2 was determined in homogenates of the epidermis using an ELISA kit. PGE2 concentration is expressed in terms of pg/mg protein as the mean ± SD. (D) The levels of expression of the PGE2 receptors (EP1, EP2, EP3, and EP4) were determined by Western blot analysis. To recognize and verify the exact band of EP receptors, a positive control of individual EP receptor was run on the same gel. For B to D, data are shown in duplicates, and each sample was prepared by pooling the skin biopsies from at least two mice in each group. Std., standards or positive controls of EP receptors.
Treatment of Mice with COX-2 Inhibitors Prevents UVB-Induced Suppression of CHS in Mice
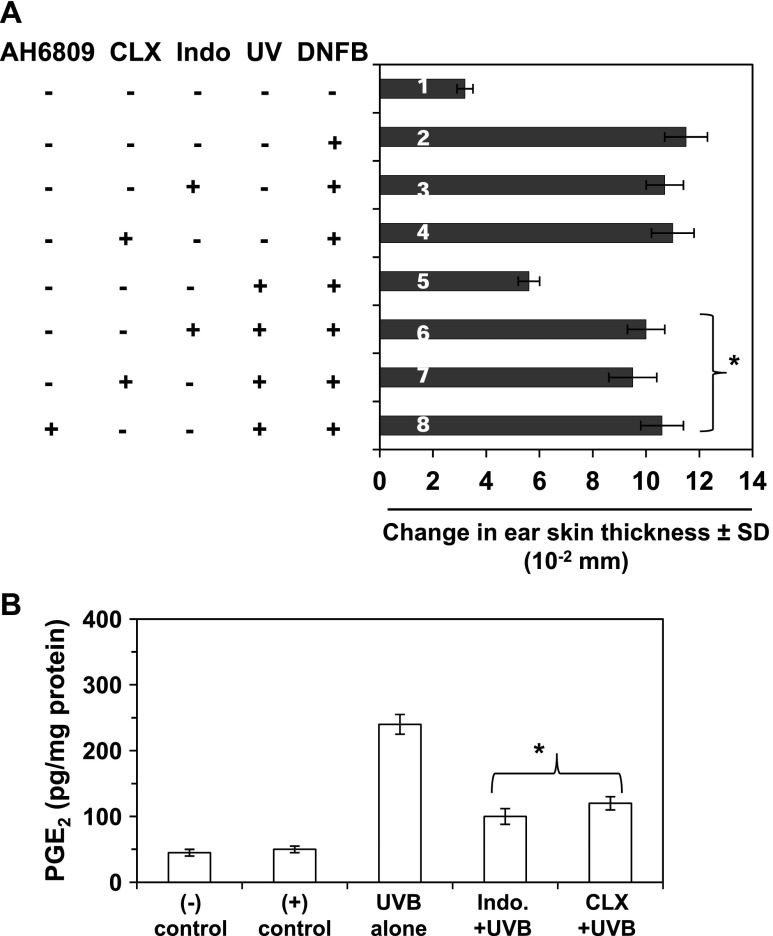
To determine whether the induction of COX-2 in UVB-exposed skin plays a role in UVB-induced suppression of immune reactions, the effect of UVB radiation on CHS was determined in mice that had been administered COX-2 inhibitors. As shown in Figure 2A, topical administration of indomethacin (sixth bar) or celecoxib (seventh bar) before each exposure to UVB resulted in inhibition of UVB-induced suppression of the CHS response in the mice. Importantly, treatment of mice with indomethacin or celecoxib did not affect the CHS response to DNFB in non-UV-irradiated mice (third or fourth bar). We also found that the production of PGE2 was significantly lower (P < .001) in indomethacin- or celecoxib-treated UVB-exposed mouse skin compared to UV-exposed skin that was not treated with these COX-2 inhibitors (Figure 2B). As this suggested a role for PGE2 in UVB-induced immunosuppression, we examined the effect of the EP2 antagonist, AH6809, on UVB-induced suppression of CHS in mice. Topical treatment of the skin site with AH6809 before each exposure of UVB enhanced sensitization to DNFB in UVB-exposed mice (Figure 2A, eighth bar) compared to the UVB-exposed mice that were not treated with the AH6809 (fifth bar).
Figure 2.
COX-2 inhibitors (indomethacin and celecoxib) and an EP2 antagonist (AH6809) inhibit UVB-induced suppression of the CHS response. Mice were exposed to UVB radiation, and the CHS response was measured as described in Figure 1A, except that some mice were treated by topical administration of the indicated agents to the skin 30 minutes before each UVB irradiation as described in Materials and Methods section. The change in ear skin thickness is reported in millimeter (x 10-2) as the mean ± SD, with n = 4 to 5 per group. Mice in treatment group 1 were not sensitized with DNFB but were challenged with DNFB on the ear skin. Mice were treated with indomethacin (sixth bar), celecoxib (seventh bar), or EP2 antagonist (eighth bar). Significant CHS response versus UVB alone-exposed group of mice (fifth bar), *P < .001. Indo, indomethacin; CLX, celecoxib. (B) The levels of PGE2 in the dorsal skin samples were determined using a PGE2 immunoassay kit. Significant inhibition versus UVB alone group, *P < .001. Data are shown as the means ± SD.
Effect of Indomethacin and EP2 Antagonist on DNA Methylation in UVB-Irradiated Mouse Skin
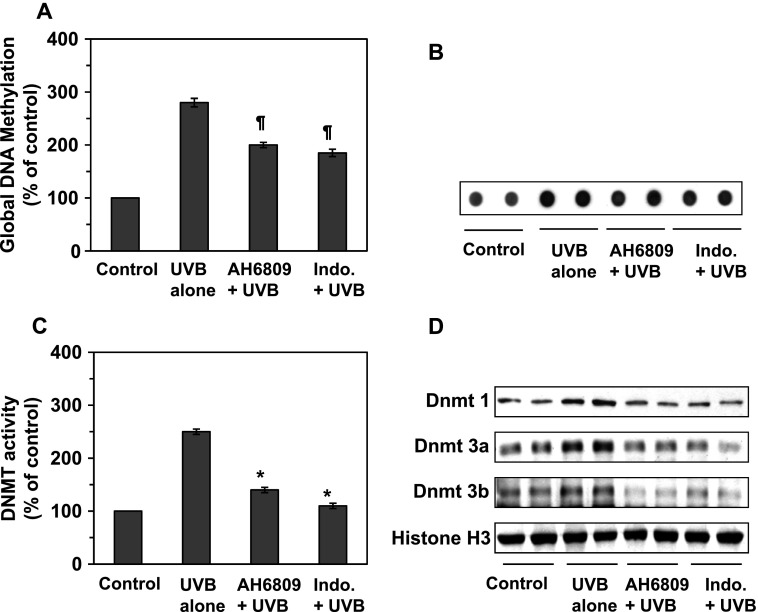
Wepreviously have shown that exposure of the mouse skin to UV radiation results in aberrant DNA hypermethylation in the UVB-exposed skin and UVB-induced skin tumors in mice [10]. PGE2 regulates many fundamental cellular functions and has been implicated in regulation of DNA methylation in fibroblasts [22]. As most of the biologic functions of PGE2 aremediated through the PGE2 receptor EP2, we tested the effects of PGE2 on global DNA methylation by using an EP2 antagonist to block the action of PGE2 in UV-exposed skin. In these experiments, the mice were UVB irradiated on four consecutive days with or without topical application of indomethacin or the EP2 antagonist. The mice were sacrificed 24 hours after the last UVB exposure and skin samples were processed for analysis. Quantitative analysis of the global DNA methylation levels showed that the DNA methylation in the UVB-exposed skin was more than 2.5-fold higher than in the non-UVB-exposed mouse skin (Figure 3A). Topical application of indomethacin or EP2 antagonist before each exposure to UVB resulted in significantly lower levels of global DNA methylation (P < .01) in the mouse skin compared to the levels in mouse skin that was exposed to UVB radiation in the absence of treatment with indomethacin or EP2 antagonist. This observation was further verified by dot-blot analysis in which the levels of DNA methylation were determined using an antibody specific for 5-mC (Figure 3B).
Figure 3.
Indomethacin and EP2 antagonist (AH6809) affect UVB-induced epigenetic regulators in UVB-irradiated mouse skin. Mice were UVB exposed in the presence and absence of topical treatment of themouse skin with indomethacin or EP2 antagonist on four consecutive days. Samples of the dorsal skin were collected 24 hour later. (A, B) Total DNA was extracted from the epidermis. (A) The levels of global DNA methylation were determined in skin samples using a Global DNA Methylation Kit. (B) The levels of 5-mC in the DNA were estimated by dot-blot analysis. (C) Nuclear extracts were prepared and total Dnmt activity was determined using the Dnmt Activity Assay Kit. Data are presented in terms of percentage versus non-UVB-irradiated control skin. (D) The skin samples from the mice described in A were analyzed for the presence of Dnmt1, Dnmt3a, and Dnmt3b proteins using Western blot analysis. Significant inhibition versus the group exposed to UVB but not treated with indomethacin or PGE2 antagonist, *P < .001, ¶P < .01.
Effect of Indomethacin and EP2 Antagonist on Dnmt Activity and Dnmt Proteins in UVB-Irradiated Mouse Skin
As Dnmts play a key role in DNA methylation, we determined the effect of indomethacin on Dnmt activity in UVB-exposed skin. Mice were exposed to UVB radiation on four consecutive days with and without topical application of indomethacin or AH6809. They were then sacrificed 24 hours after the last UV exposure, and skin samples were prepared for the analysis of Dnmt activity and Dnmt proteins. As shown in Figure 3C, Dnmt activity was significantly higher in the UVB-exposed mouse skin (P < .001) than the non-UVB-exposed mouse skin. Furthermore, the Dnmt activity was significantly lower (P < .001) in indomethacin-treated UVB-exposed skin than UVB alone-exposed mouse skin. To determine whether the indomethacin-induced reduction in Dnmt activity was associated with reduced expression of Dnmt proteins, the levels of Dnmt proteins were determined in the skin lysates using Western blot analysis. As shown in Figure 3D, there was a reduction in the levels of Dnmt1, Dnmt3a, and Dnmt3b proteins in the indomethacin-treated UVB-exposed skin as compared with non-indomethacin-treated UVB-exposed skin.
To test directly whether PGE2 promotes UVB-induced suppression of immune reactivity through DNA methylation, we treated mice with EP2 antagonist before each exposure to UVB irradiation and analyzed Dnmt activity and the levels of Dnmt proteins in skin samples. As shown in Figure 3C, the levels of Dnmt activity were significantly lower in the AH6809-treated UVB-exposed mouse skin (P < .001) than in non-AH6809-treated UVB-exposed skin. Similarly, the levels of Dnmt1, Dnmt3a, and Dnmt3b proteins, as determined by Western blot analysis, were reduced in the AH6809-treated UVB-exposed mouse skin (Figure 3D).
COX-2-deficient Mice Are Resistant to UVB-Induced Suppression of CHS
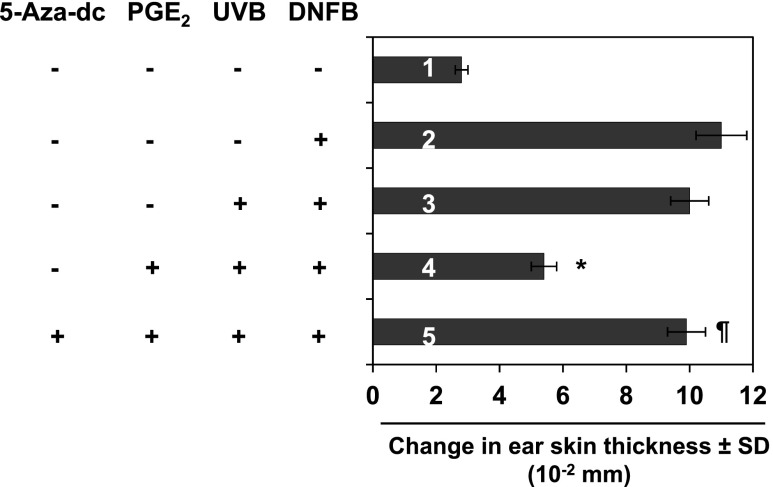
If PGE2 promotes UVB-induced immunosuppression in mice, then PGE2 deficiency in mice should prevent UVB-induced suppression of CHS. Thus, to confirm the role of PGE2 in UVB-induced suppression of CHS, we used COX-2-deficient mice that are unable to produce sufficient PGE2 to adequately suppress the CHS response. The COX-2-deficient mice were subjected to the CHS assay protocol as described in Materials and Methods section. As shown in Figure 4, the sensitization reactions after DNFB challenge (third bar) of the COX-2-deficient mice after UVB exposure on four consecutive days were almost equal to those of mice in the positive control group (second bar), indicating that the COX-2-deficient mice are indeed resistant to UVB-induced suppression of the CHS response. We have also examined the effect of UVB irradiation on the CHS response in wild-type littermates of COX-2-deficient mice. Following CHS protocol, it was observed that UVB irradiation of wild-type littermates resulted in suppression of CHS response to DNFB, as was observed in C3H/HeN mice (data not shown). To further verify the role of PGE2 in UVB-induced immunosuppression, another group of COX-2-deficient mice was exposed to UVB and treated with PGE2 as described in Materials and Methods section. As shown in Figure 4, the COX-2-deficient mice that were UVB irradiated and treated with PGE2 showed a significant suppression of the CHS response (fourth bar) compared with the mice that were not treated with PGE2 but exposed to UVB.
Figure 4.
Effect of UVB irradiation on the CHS response in COX-2-deficient mice. Mice were UVB irradiated on four consecutive days with or without topical application of PGE2 after each UVB exposure and the CHS response assessed 24 hours later, as described in Figure 1 and in Materials and Methods section. The COX-2-deficient mice were resistant to UVB-induced suppression of the CHS response. Topical application of PGE2 after each UVB exposure promoted suppression of CHS in UV-exposed mice (fourth bar) and treatment of the mice with a DNA demethylating agent 5-Aza-dc (1 mg/kg body weight) by i.p. injection after exposure to UVB radiation restored the CHS response in the PGE2-treated COX-2-deficient mice. Significant suppression of CHS versus positive control group (fourth bar), *P < .001. Significant restoration of CHS versus PGE2-induced suppression of CHS, ¶P < .01.
Treatment of Mice with the DNA Demethylating Agent, 5-Aza-dc, Abrogates PGE2-Induced Suppression of CHS in UVB-Exposed COX-2-Deficient Mice
To test whether PGE2 promotes UVB-induced suppression of CHS in mice by promoting DNA methylation, we treated another group of COX-2-deficient mice with PGE2 and then with the DNA demethylating agent 5-Aza-dc following the protocol described in Materials and Methods section. As shown in Figure 4, treatment of COX-2-deficient mice with PGE2 and subsequently with 5-Aza-dc resulted in a full sensitization reaction after treatment of the mice with DNFB (fifth bar), and this CHS response was comparable to that observed in the positive control group (second bar). These data suggest that PGE2-induced DNA methylation might be blocked or that demethylation may occur in the mice treated with 5-Aza-dc resulting in full sensitization and restoration of the CHS response.
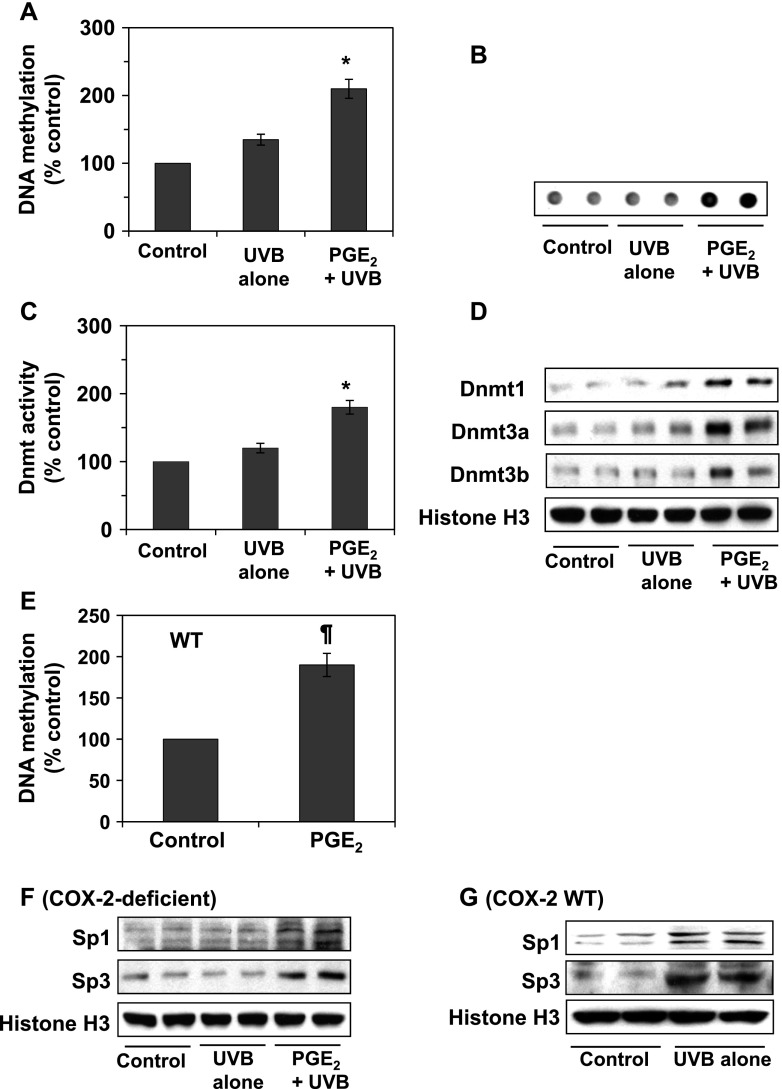
Effect of PGE2 on DNA Methylation, Dnmt Activity, and Dnmt1, Dnmt3a, and Dnmt3b Proteins in UVB-Exposed Skin of COX-2-Deficient Mice
The results of the current study show that inhibition of PGE2 in UVB-exposed skin by topical administration of indomethacin resulted in reduced expression of global DNA methylation and Dnmt activity and lowered the levels of Dnmt proteins (Figure 3). To further verify that PGE2 affects epigenetic regulators on UVB exposure, we used the COX-2-deficient mice. In these experiments, COX-2-deficient mice were exposed to UVB radiation on four consecutive days with or without topical treatment with PGE2, as detailed in Materials and Methods section. Mice were sacrificed 24 hours after the last UVB exposure and skin samples were collected for the analysis of epigenetic biomarkers. As shown in Figure 5A, exposure of COX-2-deficient mice to UVB did not significantly influence DNA methylation. In contrast, the levels of global DNA methylation were significantly higher in the UVB-exposed COX-2-deficient mice that were treated with PGE2 than in the UVB-exposed COX-2-deficient mice that were not treated with PGE2. This effect of PGE2 on DNA methylation was further verified by dot-blot analysis using antibody specific to 5-mC (Figure 5B). We also determined the effects of PGE2 on Dnmt activity and Dnmt proteins (Dnmt1, Dnmt3a, and Dnmt3b) in the skin samples from these treatment groups. We found that the levels of Dnmt activity were not significantly higher in the COX-2-deficient mice that were exposed to UVB compared to the levels in the COX-2-deficient mice that were not exposed to UVB. However, treatment of COX-2-deficient mice with PGE2 resulted in significantly higher (P < .001) levels of Dnmt activity in the UVB-exposed skin of the COX-2-deficient mice compared to the levels of activity in the non-PGE2-treated UVB-exposed COX-2-deficient mice (Figure 5C). Similarly, treatment with PGE2 enhanced the levels of Dnmt1, Dnmt3a, and Dnmt3b proteins in the UVB-exposed COX-2-deficient mice (Figure 5D). Interestingly, the levels of the Dnmt3a protein in the UVB-exposed PGE2-treated COX-2-deficient mouse skin were greater than the levels of the Dnmt1 and Dnmt3b proteins. To further verify that PGE2 is responsible for enhanced DNA methylation, the wild-type littermates of COX-2-deficient mice were treated with PGE2 for four consecutive days, then sacrificed 24 hours after the last treatment of PGE2, and skin samples were collected for the analysis of global DNA methylation levels. It was found that PGE2 treatment significantly enhanced (P < .01) the levels of global DNA methylation in PGE2-treated mouse skin compared to non-PGE2-treated control skin (Figure 5E).
Figure 5.
Effect of UVB irradiation on epigenetic regulators in COX-2-deficient mice. Mice were UVB irradiated on four consecutive days with or without topical application of PGE2 and samples of the dorsal skin were taken 24 hours later, as described in Materials and Methods section (n = 5). (A) The levels of global DNA methylation in the mouse skin were determined using a Global DNA Methylation Assay Kit. In these mice, the UVB irradiation did not significantly affect the levels of global DNA methylation. Topical treatment of the UVB-exposed mouse skin with PGE2 enhanced the levels of global DNA methylation. (B) The levels of 5-mC in the extracted DNA were estimated by dotblot analysis. (C) Total Dnmt activity in nuclear extracts of skin samples was determined using the Dnmt Activity Assay Kit. Topical treatment of the UVB-exposed mouse skin with PGE2 increased Dnmt activity in the skin. Data are presented in terms of percentage versus non-UVB-irradiated control group. Significantly higher activity versus mice exposed to UVB without topical application of PGE2, *P < .001. (D) The levels of Dnmt1, Dnmt3a, and Dnmt3b proteins in the skin samples from the mice described in A were determined using Western blot analysis. (E) The effect of topical treatment of PGE2 on DNA methylation levels in the wild-type littermates was determined. Significant increase versus non-PGE2-treated mouse skin, ¶P < .01. (F, G) The expression of the transcription factors, Sp1 and Sp3, were determined using Western blot analysis. (F) COX-2-deficient mice were resistant to UVB-induced increases in expression of Sp1 and Sp3 and topical application of PGE2 in UVB-exposed COX-2-deficient mice resulted in overexpression of Sp1 and Sp3 transcription factors. (G) UVB irradiation increased the expression of Sp1 and Sp3 in the skin of wild-type littermates of COX-2-deficient mice. Equal nuclear protein loading on gels was verified using anti-histone H3 antibody.
PGE2 Increases Dnmt3a Expression through Increased Expression of Sp1 and Sp3
The expression of Dnmt3a can be regulated by the transcription factors Sp1 and Sp3 [23]. To determine whether the marked PGE2-induced increase in the levels of Dnmt3a protein is associated with up-regulation of Sp1 and/or Sp3, we determined the expression of these transcription factors in the skin of COX-2-deficient mice that had been exposed to UVB with and without treatment with PGE2. Western blot analysis revealed that UVB irradiation did not affect the expression of Sp1 and Sp3 transcription factors in the COX-2-deficient mice. Treatment of UVB-exposed COX-2-deficient mice with PGE2 markedly increased the expression of both transcription factors compared to the expression in either UVB-exposed COX-2-deficient mice that were not treated with PGE2 or non-UVB-irradiated mouse skin, as shown in Figure 5F. In contrast, the expression levels of Sp1 and Sp3 were increased in UVB-irradiated skin of wild-type littermates of COX-2-deficient mice compared to non-UVB-irradiated control skin (Figure 5G). These data suggest that PGE2-mediated increase in Dnmt3a is due, at least in part, to increased expression of the Sp1 and Sp3 transcription factors.
Effect of DNA Demethylating Agent (5-Aza-dc) on UVB-Induced Suppression of CHS in C3H/HeN Mice
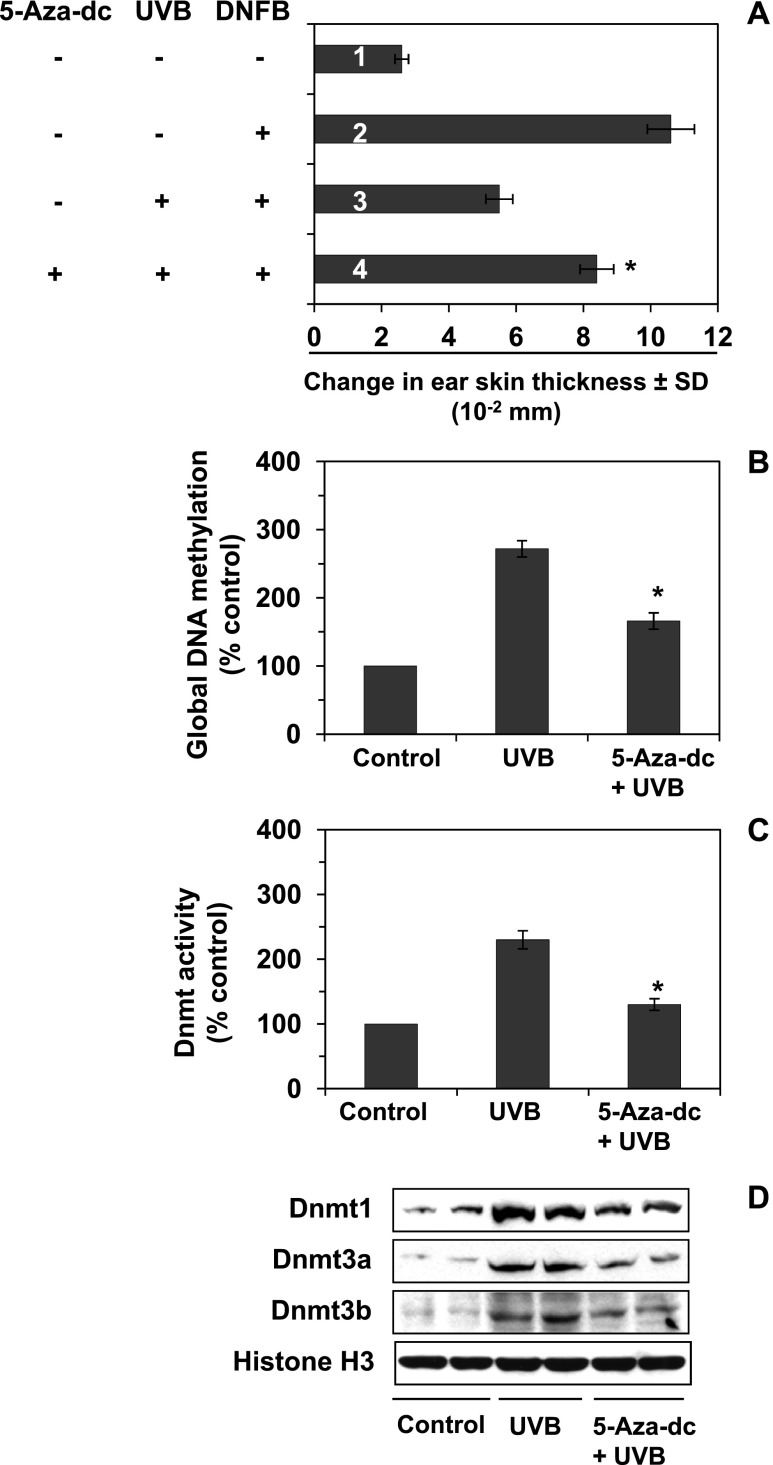
To verify whether PGE2-enhanced DNA methylation and Dnmt activity have a role in UVB-induced CHS in mice, additional experiments were conducted. Mice were exposed to UVB with and without treatment with 5-Aza-dc and the CHS response to DNFB was evaluated, as detailed in Materials and Methods section. As shown in Figure 6A, exposure of the mice to UVB irradiation confirmed significant suppression (P < .001) of CHS (third bar) compared to the positive control group of mice (second bar). Treatment of mice with 5-Aza-dc by i.p. injection after each UVB exposure resulted in significant enhanced sensitization of mouse skin (P < .001) after challenge to DNFB (fourth bar) compared to the CHS response in UVB-exposed mice (third bar) that were not treated with 5-Aza-dc.
Figure 6.
Effect of i.p. treatment of 5-Aza-dc on UVB-induced suppression of CHS and DNA methylation. Mice were UVB irradiated on four consecutive days with or without treatment with 5-Aza-dc. The CHS response was measured as described in Materials and Methods section (n = 4–5). (A) Injection of 5-Aza-dc after UVB irradiation reduced UVB-induced suppression of the CHS response or restored the CHS response in UVB-irradiated mice. For the analysis of epigenetic regulators, shaved mouse skin was UVB irradiated for four consecutive days with and without the treatment of 5-Aza-dc. Skin samples were collected 24 hours after the last UVB exposure. Treatment of mice with 5-Aza-dc reduced (B) the levels of UVB-induced global DNA methylation in UVB-irradiated mouse skin and (C) Dnmt activity in UVB-irradiated mouse skin. Significant difference versus UVB irradiation in the absence of application of 5-Aza-dc, *P < .01; and (D) the levels of Dnmt proteins, as determined by Western blot analysis in UVB-irradiated mouse skin.
Effect of 5-Aza-dc on UVB-Induced DNA Methylation and Dnmt Activity
To examine whether reversal of UVB-induced suppression of CHS by 5-Aza-dc is associated with suppression of DNA methylation and Dnmt activity, additional experiments were conducted. For this purpose, C3H/HeN mice were exposed to UVB on four consecutive days with and without treatment with 5-Aza-dc by i.p. injection, and 24 hours after the last UVB exposure, the mice were sacrificed and skin samples were collected for the analysis of DNA methylation and Dnmt activity. As shown in Figure 6, the results confirmed that in the untreated mice UVB irradiation significantly increased the levels of global DNA methylation (Figure 6B) and Dnmt activity (Figure 6C) in the UVB-exposed mice compared to non-UVB-exposed mouse skin. The levels of global DNA methylation and Dnmt activity were significantly lower (P < .01) in the UVB-exposed mice that were treated with 5-Aza-dc than in the UVB-exposed mice that were not treated with 5-Aza-dc. Western blot analysis of the levels of Dnmt proteins in the skin lysate samples from the same treatment groups revealed that the treatment of mice with 5-Aza-dc resulted in reduced expression of Dnmt1, Dnmt3a, and Dnmt3b proteins compared to the levels in the skin of the non-5-Aza-dc-treated UVB-exposed mice (Figure 6D).
Discussion
UV-induced PGE2 overproduction has been implicated in various stages of tumor development, invasion, and metastasis of tumor cells [8,24]; however, it is not yet clearly understood how PGE2 contributes to the UV-induced immunosuppression and how the action of PGE2 is related to the other proposed mechanisms that have been implicated in this process. In the present study, using a well-established CHS protocol, we show that UV-induced suppression of CHS is associated with the overexpression of COX-2, PGE2, and PGE2 receptors in the mouse skin. Our study shows that treatment of mice with indomethacin, celecoxib, or antagonist of PGE2 receptor EP2 reverses or blocks UVB radiation-induced immunosuppression in mice and thus supports the concept that inhibitors of PGE2 or PGE2 signaling play a crucial role in immunosuppression after UV irradiation. Under similar conditions, exposure of the skin to UVB enhanced expression of Dnmt1, Dnmt3a, and Dnmt3b proteins as well as Dnmt activity, which subsequently increased global DNA methylation. In the reverse situation, treatment of mice with indomethacin or EP2 antagonist (AH6809) decreased the levels of DNA methylation, Dnmt activity, and the protein expressions of Dnmt1, Dnmt3a, and Dnmt3b in UVB-exposed mouse skin. These data suggest that PGE2-mediated DNA methylation may have a role in the suppression of the immune system in UVB-irradiated mice.
To verify the role of PGE2 in UVB-induced immunosuppression, we used COX-2-deficient mice, which were unable to synthesize PGE2 after UVB irradiation. It was observed that COX-2-deficient mice were resistant to UVB-induced suppression of CHS, whereas the treatment of UVB-exposed COX-2-deficient mice with PGE2 resulted in suppression of CHS response, thus suggesting the role of PGE2 in UVB-induced immunosuppression. To establish a link between PGE2 and DNA hypermethylation in UVB-exposed skin, COX-2-deficient mice that were treated with PGE2 were also treated with DNA demethylating agent (5-Aza-dc) after UVB irradiation. The treatment of mice with 5-Aza-dc resulted in full sensitization reaction after DNFB challenge in PGE2-treated COX-2-deficient mice, thus suggesting the role of PGE2-mediated DNA hypermethylation in UVB-induced immunosuppression.
The link between overexpression of PGE2 and DNA methylation on exposure of the skin to UVB irradiation was further verified using COX-2-deficient mice. A significant increase in DNA methylation and Dnmt activity was not observed when COX-2-deficient mice were irradiated multiple times to UVB radiation. However, the treatment of these UVB-irradiated mice with PGE2 resulted in significant greater Dnmt activity, overexpression of various Dnmt proteins (Dnmt1, Dnmt3a, and Dnmt3b), and increased DNA methylation compared to the mice that were exposed to UVB but were not treated with PGE2. Epigenetic alterations including DNA hypermethylation or hypomethylation have been implicated in tumor growth and progression including the skin tumors in mice and humans [10]. The role of inflammatory mediators such as PGE2 in UV irradiation-induced immunosuppression has been indicated by studies showing that NSAIDs that exert their effect through COX-2 inhibition can reverse the immunosuppressive effect of UV radiation [4,5]. We therefore further tried to establish a link between DNA methylation and UVB-induced immunosuppression. For this purpose, C3H/HeN mice were exposed to UVB radiation with or without treatment with a DNA demethylating agent (5-Aza-dc) and CHS response was evaluated. The results demonstrate that treatment ofmice with 5-Aza-dc reversed or inhibited UVB-induced suppression of CHS response to DNFB accompanied with lowering the levels of global DNA methylation, Dnmt activity, and the protein expressions of Dnmt1, Dnmt3a, and Dnmt3b compared to non-5-Aza-dc-treated UVB-exposed mice. It was interesting to note that the expression of Dnmt3a was greater in PGE2-treated mice.
Several immunomodulatory mediators have been shown to play a role in the UV-induced immunosuppression [13,14,25–27]. Previously, we have shown that the UV-induced suppression of CHS is associated with an imbalance of interleukin-10 and interleukin-12 [13] and that the repair of UV-induced DNA damage in the form of cyclobutane pyrimidine dimers and nucleotide excision repair mechanisms play a role in this process [14]. The current study clearly implicates PGE2, which is known to affect multiple immune and inflammatory factors. This suggests that exposure of skin to UVB radiation can induce immune suppression by different events that trigger widespread disruption of immune and inflammatory response. Elucidation of the relative roles of these events and their interactions in the context of dose responses and genetic predisposition requires further study.
The findings of the current studies have significant clinical relevance as PGE2 generation is upregulated in inflammation, infection, and cancer. In general, the outcome of our findings allow us to speculate that changes in PGE2 biosynthesis may contribute to changes in DNA methylation patterns associated with a wide variety of diseases including the various skin diseases associated with chronic exposure to sunlight and other environmental factors. Collectively, the results of our study demonstrate the role of PGE2-mediated DNA methylation in UVB-induced immunosuppression. UV-induced immunosuppression has been considered as a risk factor in nonmelanoma skin cancers caused by excessive exposure to UV radiation [1,28], and the incidence of nonmelanoma skin cancer was reduced in subjects receiving a selective COX-2 inhibitor (celecoxib) compared to subjects receiving placebo [9]. Therefore, the outcome of our study not only provides substantial insight into the intricate roles of PGE2 in UVB-induced immunosuppression and how DNA methylation is regulated by PGE2 in UVB-exposed skin but also provide a rationale for considering the development of new strategies using the inhibitors of PGE2 and demethylating agents alone or in combination for the prevention or treatment of nonmelanoma skin cancers.
Abbreviations
- 5-Aza-dc
5-aza-2′-deoxycytidine
- COX-2
cyclooxygenase-2
- CHS
contact hypersensitivity
- DNFB
2,4-dinitrofluorobenzene
- Dnmt
DNA methyltransferase
- PGs
prostaglandins
Footnotes
This research was financially supported by the National Institutes of Health/National Center for Complementary and Alternative Medicine/National Cancer Institute (CA140197 and AT002536 to S.K.K.) and the Veterans Administration Merit Review Award (1I01BX001410-01 to S.K.K.). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No conflict of interest.
References
- 1.Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carcinogenesis. Proc Natl Acad Sci USA. 1977;74:1688–1692. doi: 10.1073/pnas.74.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beissert S, Granstein RD. UV-induced cutaneous photobiology. Crit Rev Biochem Mol Biol. 1996;31:381–404. doi: 10.3109/10409239609108723. [DOI] [PubMed] [Google Scholar]
- 3.Fischer SM. Is cyclooxygenase-2 important in skin carcinogenesis? J Environ Pathol Toxicol Oncol. 2002).;21:183–191. [PubMed] [Google Scholar]
- 4.Hart PH, Townley SL, Grimbaldeston MA, Khalil Z, Finlay-Jones JJ. Mast cells, neuropeptides, histamine, and prostaglandins in UV-induced systemic immunosuppression. Methods. 2002;28:79–89. doi: 10.1016/s1046-2023(02)00201-3. [DOI] [PubMed] [Google Scholar]
- 5.Chung HT, Burnham DK, Robertson B, Roberts LK, Daynes RA. Involvement of prostaglandins in the immune alterations caused by the exposure of mice to ultraviolet radiation. J Immunol. 1986;137:2478–2484. [PubMed] [Google Scholar]
- 6.Ruzicka T, Walter JF, Printz MP. Changes in arachidonic acid metabolism in UV-irradiated hairless mouse skin. J Invest Dermatol. 1983;81:300–303. doi: 10.1111/1523-1747.ep12519287. [DOI] [PubMed] [Google Scholar]
- 7.Kuwamoto K, Miyauchi-Hashimoto H, Tanaka K, Eguchi N, Inui T, Urade Y, Horio T. Possible involvement of enhanced prostaglandin E2 production in the photosensitivity in xeroderma pigmentosum group A model mice. J Invest Dermatol. 2000;114:241–246. doi: 10.1046/j.1523-1747.2000.0883x.x. [DOI] [PubMed] [Google Scholar]
- 8.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 9.Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, Kang S, Linden KG, Heffernan M, Duvic M, et al. Chemoprevention of non-melanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835–1844. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nandakumar V, Vaid M, Tollefsbol TO, Katiyar SK. Aberrant DNA hypermethylation patterns lead to transcriptional silencing of tumor suppressor genes in UVB-exposed skin and UVB-induced skin tumors of mice. Carcinogenesis. 2011;32:597–604. doi: 10.1093/carcin/bgq282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–1246. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 12.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J Invest Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meeran SM, Katiyar S, Elmets CA, Katiyar SK. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol Cancer Ther. 2006;5:1660–1668. doi: 10.1158/1535-7163.MCT-06-0095. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res. 2010;3:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wulff BC, Thomas-Ahner JM, Schick JS, Oberyszyn TM. Celecoxib reduces the effects of acute and chronic UVB exposure in mice treated with therapeutically relevant immunosuppressive drugs. Int J Cancer. 2010;126:11–18. doi: 10.1002/ijc.24749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun KS, Langenbach R. The prostaglandin E2 receptor, EP2, regulates survivin expression via an EGFR/STAT3 pathway in UVB-exposed mouse skin. Mol Carcinog. 2011;50:439–448. doi: 10.1002/mc.20728. [DOI] [PubMed] [Google Scholar]
- 17.Chun KS, Akunda JK, Langenbach R. Cyclooxygenase-2 inhibits UVB-induced apoptosis in mouse skin by activating the prostaglandin E2 receptors, EP2 and EP4. Cancer Res. 2007;67:2015–2021. doi: 10.1158/0008-5472.CAN-06-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun KS, Lao HC, Trempus CS, Okada M, Langenbach R. The prostaglandin receptor EP2 activates multiple signaling pathways and β-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis. 2009;30:1620–1627. doi: 10.1093/carcin/bgp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia D, Wang D, Kim SH, Katoh H, DuBois RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18:224–226. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. Orally administered green tea polyphenols prevent ultraviolet radiation-induced skin cancer in mice through activation of cytotoxic T cells and inhibition of angiogenesis in tumors. J Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 21.Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by the green tea polyphenol (-)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 22.Huang SK, Scruggs AM, Donaghy J, McEachin RC, Fisher AS, Richardson BC, Peters-Golden M. Prostaglandin E2 increases fibroblast gene-specific and global DNA methylation via increased DNA methyltransferase expression. FASEB J. 2012;26:3703–3714. doi: 10.1096/fj.11-203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jinawath A, Miyake S, Yanagisawa Y, Akiyama Y, Yuasa Y. Transcriptional regulation of the human DNA methyltransferase 3A and 3B genes by Sp3 and Sp1 zinc finger proteins. Biochem J. 2005;385:557–564. doi: 10.1042/BJ20040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 25.Soontrapa K, Honda T, Sakata D, Yao C, Hirata T, Hori S, Matsuoka T, Kita Y, Shimizu T, Kabashima K, et al. Prostaglandin E2-prostaglandin E receptor subtype 4 (EP4) signaling mediates UV irradiation-induced systemic immunosuppression. Proc Natl Acad Sci USA. 2011;108:6668–6673. doi: 10.1073/pnas.1018625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-α in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 27.Walterscheid JP, Nghiem DX, Kazimi N, Nutt LK, McConkey DJ, Norval M, Ullrich SE. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc Natl Acad Sci USA. 2006;103:17420–17425. doi: 10.1073/pnas.0603119103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh MM, Karagas MR, Applebaum KM, Spencer SK, Perry AE, Nelson HH. A role for ultraviolet radiation immunosuppression in non-melanoma skin cancer as evidenced by gene-environment interactions. Carcinogenesis. 2008;29:1950–1954. doi: 10.1093/carcin/bgn160. [DOI] [PMC free article] [PubMed] [Google Scholar]