Key Points
p65 is an important factor in hematopoiesis through the regulation of hematopoietic stem cell function and lineage commitment.
p65 controls the expression of genes encoding key factors that promote hematopoietic stem cell homeostasis.
Abstract
Hematopoiesis is a tightly regulated process resulting in the production of blood cells. Self-renewal and differentiation of hematopoietic stem cells (HSCs) are key processes in hematopoietic development. Disruption of these steps can lead to altered cell distribution and disease. To investigate the role of the nuclear factor-κB subunit RelA/p65 in the regulation of HSCs in vivo, we generated mice lacking RelA/p65 in the hematopoietic compartment. Using this model system, we show that loss of p65 severely impairs HSC function and occurs in conjunction with increased hematopoietic stem and progenitor cell cycling, extramedullary hematopoiesis, and differentiation defects. Gene array studies of phenotypic HSCs indicate the up-regulation of genes normally expressed in lineage restricted cells, as well as the down-regulation of genes involved in HSC maintenance and homeostasis. We hypothesize that changes in gene expression in p65-deficient cells lead to decreased self-renewal and differentiation efficiency of hematopoietic stem and progenitor cells. These studies demonstrate that p65 is an important regulator of hematopoiesis through the transcription of genes involved in HSC fate.
Introduction
Hematopoiesis is the process by which all cellular components of the blood develop. This process begins with the hematopoietic stem cell (HSC), a rare cell type that can give rise to all blood cell lineages. Key components of HSC biology include the ability to self-renew and to differentiate. These properties are essential for HSCs to maintain themselves and to support hematopoiesis over the lifetime of an organism.1 The balance between self-renewal and differentiation is tightly regulated and dependent on several signaling pathways that regulate the expression of key factors in HSC homeostasis.1,2
HSCs have been purified from the bone marrow and characterized using phenotypic markers.2 HSCs are negative for lineage markers, with most residing in the lineage-negative, c-kit+, Sca-1+ (LSK) fraction, and are further enriched in the Flk2- fraction (LSKF) of bone marrow.3 HSCs localize to specialized niches where cells such as osteoblasts and perivascular cells secrete factors required for maintenance.4 Investigation into the functions of the endosteal and vascular components of the niche and how each regulates HSC function is ongoing.4,5 However, it is known that HSC quiescence is maintained by many factors, including thrombopoietin, which is crucial for HSC dormancy and function.6 On stimulation, HSCs proliferate and undergo differentiation to give rise to multipotent progenitors (MPPs), which then give rise to lineage-restricted progenitors.2 HSCs express a number of transcripts originally thought to be specific to lineage-restricted cells, suggesting that the transcription of lineage associated genes may prime cells for downstream fates.2,7 Defining pathways involved in the transcriptional regulation of factors important in HSC fate is an important issue in stem cell biology.
Many transcription factors control hematopoietic cell fate. LMO2 is required for hematopoiesis and interacts with a variety of factors including Gata-2 and Scl-1 to promote transcription of genes involved in HSC fate.8 Both Scl-19 and Gata-210 are required for the maintenance of HSCs and are sometimes overexpressed in acute myeloid leukemia, correlating with poor prognosis.11 The Ikaros family of transcription factors is also required for HSC function12 through the recruitment of corepressors.13 Other transcription factors, although not required for the initiation of HSCs, influence their fate. Notch1 is implicated in HSC self-renewal14 and, when inactivated in HSCs in mice, results in a myeloproliferative disorder,15 whereas PU.116 and C/EBPα17 regulate lineage commitment of hematopoietic stem and progenitor cells (HSPCs). Overall, the regulation of genes involved in HSC fate is carefully controlled by a variety of transcription factors during hematopoiesis.
The nuclear factor (NF)-κB family of transcription factors is comprised of 5 members: p65 (RelA), RelB, c-Rel, p50/p105 (nuclear factor [NF]-κB1), and p52/p100 (NF-κB2). These proteins share a conserved Rel homology domain, which controls DNA binding, dimerization and interaction with inhibitory IκB proteins. In the classical NF-κB signaling pathway, p50 and p65 are held in an inactive state by IκBα. On stimulation, activation of the IκB kinase (IKK) complex occurs, causing IKKβ to phosphorylate IκBα, resulting in its ubiquitination and proteasomal degradation. In the alternative pathway, IKKα homodimers are activated and subsequently phosphorylate p100, resulting in proteolytic processing to p52 and release of the p52/RelB dimer. NF-κB is then able to translocate to the nucleus and regulate the expression of specific genes, including those encoding cytokines and cytokine receptors and inflammatory and antiapoptotic proteins.18 NF-κB is important in the development of various cell types, including the liver19 and secondary lymphoid organs.20 Inappropriate or extended activation of NF-κB can lead to excessive inflammation, prolonged survival of damaged cells, and evasion of apoptosis, allowing for oncogenic transformation.18,21
NF-κB is known to be an important regulator of both differentiation and function of lineage-committed hematopoietic cells. Studies have revealed involvement of NF-κB in the survival of developing lymphocytes.22,23 Mice lacking c-Rel24 and animals engrafted with rela−/− fetal liver cells display defects in lymphocyte function and proliferation.25-27 Transplantation of ikkb−/− fetal liver cells in mice results in apoptosis of common lymphoid progenitors (CLPs) and DN3 T-cell precursors undergoing selection.28 Genetic deletion of IKKβ,29,30 IκBα,31 and RelB32 in animals results in granulocytosis, splenomegaly, and impaired immune response, indicating an important role for NF-κB in myeloid cell development as well.
Little evidence describing a requirement for NF-κB in HSPCs exists. Mice engrafted with rela−/− fetal liver cells develop all lineages of hematopoietic cells and appear normal.20 However, c-rel−/− rela−/− fetal liver cells display decreased engraftment efficiency due to defects in short-term reconstitution, and death of recipients is caused by severe anemia and granulocytosis.33 More recent results show that simultaneous systemic deletion of p52 and RelB dramatically decreases HSC function due to intrinsic and extrinsic factors.34 Further studies examining the role of NF-κB in HSPCs are lacking.
We set out to determine the role of the NF-κB subunit p65 in HSC fate. Using a conditional deletion mutant of p65 in the hematopoietic compartment, we find that loss of p65 results in a severe decrease in HSC function, coupled with the increased proliferation and accumulation of phenotypic HSPCs in the bone marrow. These defects occur in conjunction with extramedullary hematopoiesis and differentiation defects. Gene array studies on p65−/− LSKF cells indicate the up-regulation of genes normally expressed in lineage restricted cells and the down-regulation of genes involved in HSC maintenance and homeostasis. Taken together, these data show that p65 is required for the maintenance of HSCs through the regulation of genes involved in hematopoietic cell fate.
Methods
Animals
Vav-Cre [B6.Cg-Tg(Vav1-cre)A2Kio/J], Mx-Cre [B6.Cg-Tg(Mx1-cre)1Cgn/J], PepBoy (B6.SJL-PtprcaPepcb/BoyJ), and B6 (C57BL/6J) mice were obtained from Jackson Laboratories. p65 conditional mice have been described.35 B6 mice received daily intraperitoneal injections of compound A (10 mg/kg) or vehicle for 2 weeks. Male and female animals were 8 to 12 weeks of age upon analysis, except transplant recipients, and housed in accordance with protocols approved by the University of North Carolina Institutional Animal Care and Use Committee.
Tissue processing
Bone marrow was flushed from femurs and tibias. Spleens were gently disrupted with frosted glass slides. Cells were subjected to red blood cell lysis on ice for 5 minutes and passed through a 70-μm filter. Blood was collected from the submandibular or tail veins.
Flow cytometry
Cells were Fc blocked where appropriate and stained with antibodies (supplemental Table 1 on the Blood website). To detect apoptosis, cells were stained for surface markers followed by Annexin V (eBiosciences) following the manufacturer’s protocol. Samples were collected on a Beckman-Coulter CyAn ADP and analyzed using TreeStar FlowJo.
Histopathology
Tissues were fixed in 10% saline-buffered formalin, decalcified where appropriate, paraffin embedded, sectioned, and stained with hematoxylin and eosin or the Reticulin-Nuclear Fast Red Stain Kit (Dako). Images were captured using an Olympus BX51 at ×40 for hematoxylin and eosin–stained spleen sections and ×400 for hematoxylin and eosin– or ×200 for reticulin-stained bone sections, respectively.
Complete blood cell counts
Blood was collected via cardiac puncture into EDTA-coated tubes. Samples were analyzed immediately on a Heska’s Animal Blood Counter.
Immunoblotting
Cells were resuspended in lysis buffer (Cell Signaling Technologies) supplemented with protease and phosphatase inhibitor cocktails (Sigma) and incubated on ice. Equal amounts of clarified lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, blocked in Tris-buffered saline with 0.05% Tween-20 and 5% nonfat milk, and incubated with the indicated antibodies overnight. Blots were incubated with secondary antibody for 1 hour at room temperature and developed with enhanced chemiluminescence detection reagent (GE, Buckinghamshire, UK). Primary antibodies include p65 and β-actin (Cell Signaling Technologies).
Quantitative real-time polymerase chain reaction
RNA was isolated using TRIzol reagent (Invitrogen) and subject to reverse transcription (SuperScript III kit; Invitrogen). Transcript levels were normalized to GusB. All Taqman primers were obtained from Applied Biosystems (Foster City, CA).
Bone marrow transplantation
Bone marrow was injected into the tail vein of lethally irradiated (950 cGy, 1.68 cGy/s, 137Cs source) 6- to 8-week-old male PepBoy recipients 6 to 20 hours after irradiation. CD45.1 and CD45.2 expression on peripheral blood cells was analyzed by flow cytometry 20 weeks after transplant and with GraphPad Prism. HSC frequency was calculated with L-Calc software (www.stemcell.com/en/Products/All-Products/LCalc-Software.aspx; StemCell Technologies).
Colony formation
Lineage-Flk2−Sca-1+c-kit+ cells were resuspended in methylcellulose media with cytokines (stem cell factor [SCF], interleukin [IL]-3, IL-6, and erythropoietin; StemCell Technologies M3434), and aliquoted into 24-well plates (100 cells per well). Cells were incubated for 7 to 10 days.
Cell proliferation
Animals were administered 10 μg 5-ethynyl-2′-deoxyuridine (EdU) (Click-iT EdU Flow Cytometry Assay kit; Invitrogen) per gram of body weight via intraperitoneal injection 18 hours prior to harvest. Bone marrow was stained as described, and EdU was detected following the manufacturer’s protocol.
Hematopoietic cell homing
Bone marrow cells (2 × 107) were injected into the tail vein of lethally irradiated (950 cGy, 1.68 cGy/s, 137Cs source) 8-week-old male CD45.1 recipients. Recipients were irradiated 24 hours prior to injection. Engraftment was measured 48 hours after injection.
Gene expression analysis
Lineage-Flk2−Sca-1+c-kit+ cells were sorted into buffer RLT (Qiagen) with 1% β-mercaptoethanol. RNA was extracted with the RNEasy Kit (Qiagen), amplified and labeled using the Nugen Ovation Pico WTA System V2, and hybridized to Affymetrix Mouse GeneChip 1.0 ST arrays. Data were normalized using robust multichip average in Partek Genomics Suite. A heat map of genes with a fold-change of ≥1.5 and P ≤ .01 was generated using hierarchical clustering. The data discussed in this publication have been deposited in the National Center for Biotechnology Information's Gene Expression Omnibus (GEO) and are accessible through GEO Series accession number GSE45755 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45755).
Gene set enrichment analysis
Gene set enrichment analysis was performed using Gene Set Enrichment Analysis software (http://www.broadinstitute.org/gsea) using the gene set as permutation type, 1000 permutations, and Signal2Noise ratio of classes as metric for ranking genes.
Statistics
Student's t tests (2-tailed) were performed using Microsoft Excel.
Results
Generation of hematopoietic p65-null mice
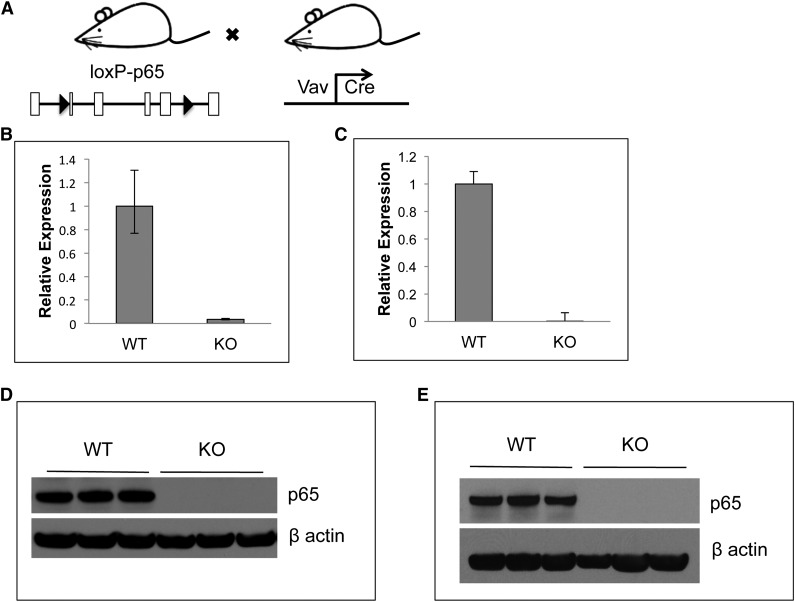
Conventional rela(p65)−/− animals die at embryonic day 15 due to liver apoptosis.19 To examine the role of p65 in hematopoiesis, conditional knockouts35 were crossed with mice expressing Cre under the control of the vav1 promoter. Vav is an adaptor protein expressed in all hematopoietic cells,36 including stem cells.37 The resulting progeny (p65hem−/− mice) are hematopoietic null for p65 (Figure 1A). To ensure complete deletion of p65, expression was examined by quantitative real-time PCR. p65 mRNA was not detected in whole bone marrow (Figure 1B) or in LSKF cells, a population highly enriched for HSCs (Figure 1C).3 Finally, p65 protein was ablated in whole bone marrow (Figure 1D) and splenocytes (Figure 1E) of p65hem−/− mice, but not in VCre+p65WT/WT counterparts (herein defined as wild-type). These data show that p65 is efficiently deleted from the hematopoietic compartment in this model.
Figure 1.
Characterization of p65 hematopoietic null mice. (A) Breeding scheme for the generation of p65hem−/− mice. p65 expression in (B) whole bone marrow cells or (C) HSCs (lineage-Flk2−Sca-1+c-kit+ fraction) of p65hem−/− or VCre+ (wild-type) mice was analyzed by quantitative reverse transcriptase-polymerase chain reaction. (D) Whole bone marrow or (E) whole splenocytes from p65hem−/− or wild-type mice were analyzed for p65 protein expression.
Loss of p65 results in accumulation of phenotypic HSCs and a decrease in progenitors
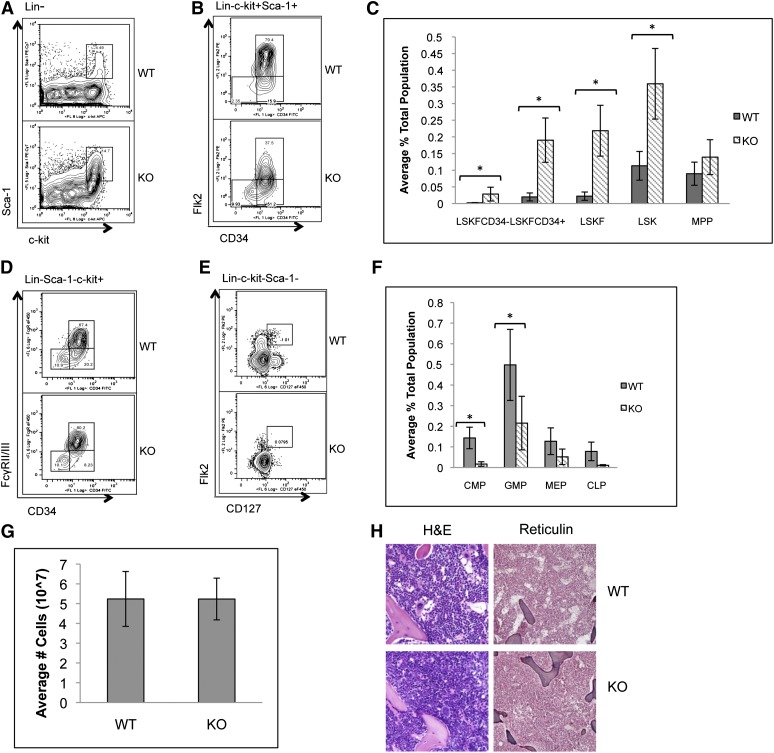
To determine whether the loss of p65 in the hematopoietic compartment leads to defects in HSC function, HSPCs were examined. The frequency of bone marrow LSK cells, as well as phenotypic HSCs including LSKFCD34+ and LSKFCD34− cells, increased, whereas the frequency of LSKFlk2+CD34+ cells did not change in the absence of p65 (Figure 2A-C). An accumulation of LSKCD48+CD150+ cells was also observed, whereas a twofold decrease in LSKCD150+CD48− cells was noted (supplemental Figure 2). Conversely, the percentage of common myeloid progenitors (CMPs), granulocyte/monocyte progenitors (GMPs), megakaryocyte/erythrocyte progenitors (MEPs), and CLPs decreased (Figure 2D-F). No significant differences in bone marrow cellularity (Figure 2G), morphology, or fibrosis (Figure 2H) were observed.
Figure 2.
p65 deletion results in deregulation of hematopoietic stem and progenitor cell homeostasis. Bone marrow cells from wild-type or p65hem−/− mice were analyzed for the frequency of (A) LSK cells and (B) HSPCs by flow cytometry (n ≥ 7). (C) The average percentage of cells is displayed. Bone marrow cells from wild-type or p65hem−/− mice were analyzed for (D) myeloid (n ≥ 7) or (E) lymphoid (n = 4) progenitor cell frequency using flow cytometry. (F) The average percentage of cells is displayed. (G) The average total number of cells recovered from the femurs and tibias of mice from each genotype is displayed (n ≥ 10). (H) Femur sections were stained with hematoxylin and eosin or reticulin to monitor changes in morphology or fibrosis. Images are representative of staining from 3 different animals per genotype. *P ≤ .005.
Accumulation of phenotypic HSCs in the absence of p65 is due to intrinsic factors and is inducible
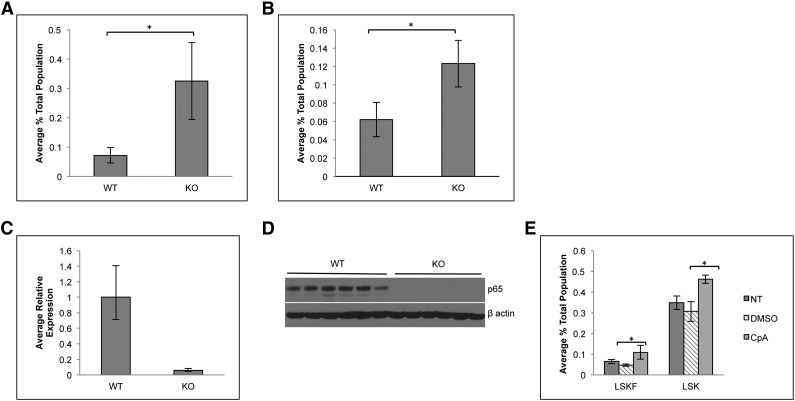
To determine whether the accumulation of phenotypic HSCs is due to cell intrinsic factors, bone marrow cells from p65hem−/− or wild-type mice were transplanted into lethally irradiated CD45.1 recipients. Twenty weeks after transplant, the percentage of LSKF cells increased by threefold in the bone marrow of animals receiving p65−/− cells (Figure 3A). The effect of p65 deletion in hematopoietic cells was also examined using an inducible model, in which the Mx1 promoter drives the expression of Cre on induction by poly(I:C).38 Recipients were engrafted with cells from Mx1-Cre+ p65fl/fl mice or littermate controls, at which point Cre expression was induced. At 29 weeks after induction, the excision of p65 was nearly complete in total bone marrow of recipients that received Mx1-Cre+ cells (Figure 3C-D). Deletion of p65 correlated with an increase in bone marrow LSKF cells (Figure 3B), indicating that the accumulation of phenotypic HSCs in the bone marrow is due to intrinsic factors. Last, a twofold accumulation of phenotypic HSCs was also induced by the pharmacologic inhibition of IKKβ,39 an activator of NF-κB, in nontransgenic mice (Figure 3E). Taken together, these data show that the accumulation of phenotypic HSCs in the bone marrow is due to cell intrinsic factors that are regulated by p65 to maintain homeostasis.
Figure 3.
Accumulation of LSKF cells in the absence of p65 is due to cell intrinsic factors. (A) 5 × 106 whole bone marrow cells from wild-type or p65hem−/− mice were injected into lethally irradiated recipients. Twenty weeks after transplant, the frequency of bone marrow LSKF cells was monitored by flow cytometry (n = 5). All recipients displayed >98% CD45.2+ peripheral blood cells. (B) 3 × 106 whole bone marrow cells from Mx1-Cre+p65fl/fl mice or littermate controls were transplanted into lethally irradiated recipients. Four weeks after transplant (engraftment of >95% CD45.2 cells), recipients were injected with 250 μg poly(I:C) 3 times, every other day. Twenty-nine weeks after induction, the frequency of bone marrow CD45.2+ LSKF cells was analyzed by flow cytometry (n = 6). p65 expression was examined in whole bone marrow cells from animals receiving poly(I:C) by (C) quantitative reverse transcriptase-polymerase chain reaction and (D) immunoblot. *P ≤ .005. (E) Bone marrow was harvested from nontransgenic animals treated with an IKKβ inhibitor (compound A) 16 hours after the last injection and analyzed by flow cytometry (n = 3 NT, n = 6 DMSO and CpA).
Loss of p65 results in the localization of LSKF cells to the spleen and extramedullary hematopoiesis
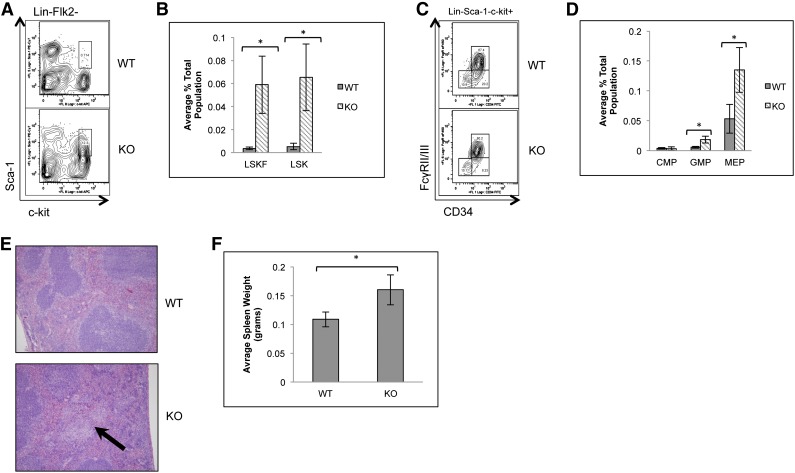
Due to the increased frequency of bone marrow LSKFCD34− and CD34+ cells in the absence of p65, localization of LSKF cells to the spleen was also examined. Spleens of p65hem−/− animals showed a significant increase in LSK cells, the majority of which were Flk2 negative (Figure 4A-B). Changes in common myeloid (Figure 4C-D) and lymphoid progenitors (data not shown) were not observed, whereas GMP and MEPs were increased 5- and 2.5-fold, respectively (Figure 4D). p65hem−/− mice also displayed extramedullary hematopoiesis (Figure 4E) and splenomegaly (Figure 4F), indicating that p65 is important for the regulation of HSC localization in vivo.
Figure 4.
Loss of p65 results in the accumulation of HSPCs in the spleen. (A) Splenocytes from wild-type or p65hem−/− mice were analyzed for HSC frequency using flow cytometry. (B) The average number of cells per genotype is displayed (n ≥ 7). (C) Myeloid progenitors from wild-type or p65hem−/− mice were analyzed by flow cytometry. (D) The average number of cells per genotype is displayed (n ≥ 7). (E) Spleens from wild-type or p65hem−/− mice were stained with hematoxylin and eosin for histological analysis. The arrow indicates a region of extramedullary hematopoiesis. Image is representative of staining from 3 different animal per genotype. (F) Average weight of spleens from wild-type or p65hem−/− mice are displayed (n ≥ 10). *P ≤ .005.
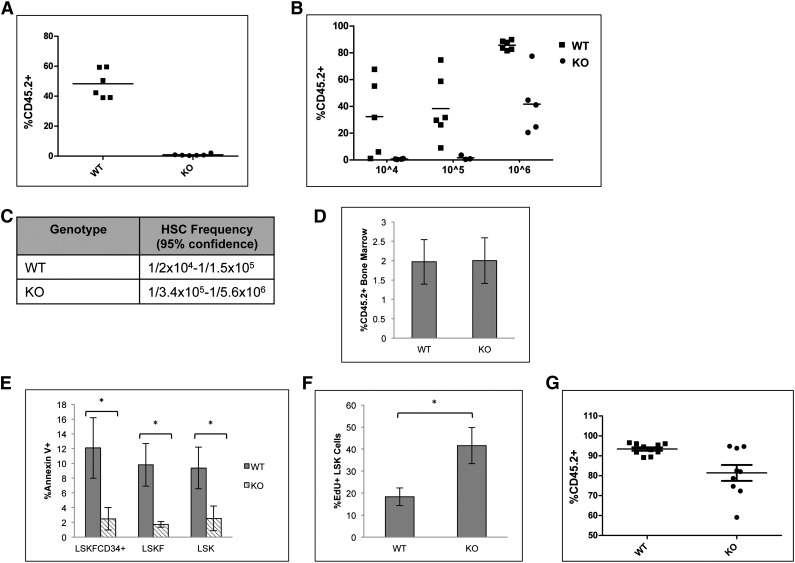
Deletion of p65 impairs HSC repopulation ability in vivo
A competitive repopulation assay was implemented to test the repopulation potential of p65−/− HSCs. Animals were transplanted with equal numbers of test and competitor cells, and engraftment was measured 20 weeks after transplant. Surprisingly, cells lacking p65 were not able to reconstitute transplant recipients in a competitive situation (Figure 5A). Limiting dilution experiments indicate that the absence of p65 causes a severe decrease in the number of functional HSCs in the bone marrow (Figure 5B-C). Importantly, levels of chimerism were examined in mice receiving either wild-type of p65−/− bone marrow 48 hours after transplant and were found to be equivalent (Figure 5D),40 indicating that loss of p65 does not affect the homing efficiency of hematopoietic cells.
Figure 5.
Deletion of p65 greatly reduces the repopulation ability of HSCs. (A) 1 × 106 test cells (CD45.2) from wild-type or p65hem−/− mice were injected into lethally irradiated recipients along with 1 × 106 healthy competitor cells (CD45.1). Engraftment was determined by virtue of CD45.1 and CD45.2 markers on peripheral blood cells (and bone marrow, data not shown) of recipients 20 weeks after transplant. (B) Varying numbers of test cells (CD45.2) from wild-type or p65hem−/− mice were injected into lethally irradiated recipients along with 2 × 105 healthy competitor cells (CD45.1). Engraftment was determined by virtue of CD45.1 and CD45.2 markers on peripheral blood cells (and bone marrow, data not shown) of recipients 20 weeks after transplant. (C) The frequency of functional HSCs was calculated using L-Calc software. (D) 2 × 107 whole bone marrow cells from wild-type or p65hem−/− mice were injected into the lateral tail vein of lethally irradiated mice. Engraftment of donor cells in the bone marrow of recipients was analyzed 48 hours after transplant (n = 4). (E) Apoptosis of HSPCs from p65hem−/− mice or littermates was analyzed by Annexin V staining (n ≥ 7). (F) Wild-type or p65hem−/− mice were injected with EdU 18 hours prior to harvest, and the proliferative index of HSPCs was analyzed by flow cytometry (n = 5). (G) Bone marrow was isolated from primary recipients reconstituted with 106 test cells and 105 competitor cells (B), and then 3 × 106 cells were injected into lethally irradiated secondary recipients. Engraftment was monitored 20 weeks after transplant. (n = 2 donors per group with 85% to 90% CD45.2+ cells, 10 recipients per group). *P ≤ .005.
The accumulation of LSKF cells and defects in repopulation by p65-null HSCs may be due to death of HSCs or progenitors. p65 induces survival in the presence of inflammatory cytokines, such as tumor necrosis factor α,19 and previous work has suggested that IKKβ activity is key for the survival of hematopoietic progenitors.30 Therefore, apoptosis of HSPCs from p65hem−/− mice or littermates was measured. Surprisingly, significantly fewer p65−/− HSPCs underwent apoptosis than those from littermate controls (Figure 5E), whereas proliferation of p65-null HSPCs was increased (Figure 5F). Bone marrow chimeras are stable for >6 months (data not shown), suggesting that HSC self-renewal is not abolished by the loss of p65. However, a moderate decrease in repopulation by p65−/− HSCs was seen on transplantation into secondary recipients, suggesting that self-renewal ability is compromised (Figure 5G). These data indicate that p65 is important in the regulation of factor necessary for HSC self-renewal.
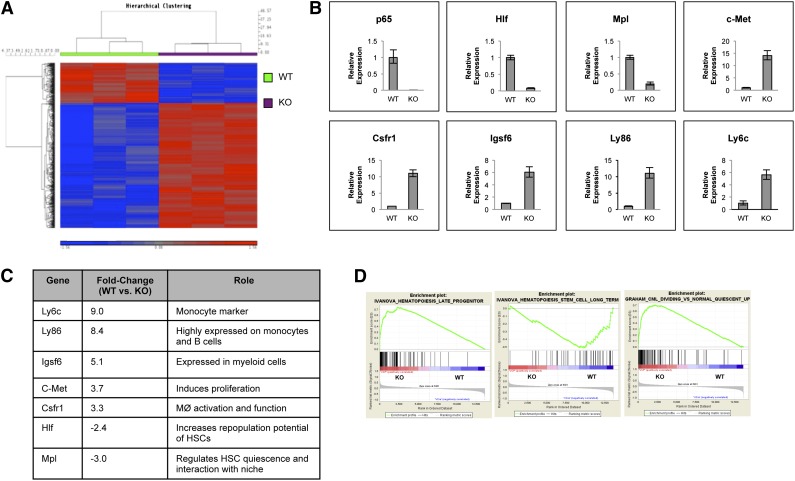
Deletion of p65 in the hematopoietic compartment alters the expression of genes involved in HSC fate
To address the impact of p65 deletion in HSCs, gene expression of sorted KLSF cells from wild-type or p65hem−/− mice was analyzed. Data show that loss of p65 resulted in significant changes in gene expression (Figure 6A), the majority of which encode a myeloid differentiation program, including Ly6c, Csfr1, Igsf6 (Figure 6B-C), and others. Another factor, c-Met, involved in the mobilization of hematopoietic progenitors,41 was up-regulated in p65−/− LSKF cells. Importantly, genes such as Mpl and hepatic leukemia factor (Hlf), which are involved in HSC homeostasis, were down-regulated in the absence of p65 (Figure 6B-C). Gene enrichment analysis42 indicates that the gene expression pattern of p65−/− LSKF cells resembles that of late hematopoietic progenitors43 (Figure 6D, middle) more closely than long-term HSCs43 (Figure 6D, left). p65−/− LSKF cells also correlate strongly with a proliferative, rather than quiescent, program44 (Figure 6D, right). Collectively, changes in gene expression in p65−/− LSKF cells suggest a potential shift toward hematopoietic progenitors, correlating with a decrease in the number of functional HSCs (Figure 5A-C) and increased proliferation (Figure 5F). These data show that p65 is an important regulator of genes involved in both the maintenance and function of HSCs.
Figure 6.
p65 is an important regulator of gene expression in HSPCs. (A) RNA from sorted LSKF cells of individual wild-type or p65hem−/− mice was used for microarray analysis. A heat map indicating changes of ≥1.5-fold with P ≤ .01 includes 689 genes. (B) Changes in gene expression were confirmed by quantitative reverse transcriptase-polymerase chain reaction. Data are representative of 3 separate experiments. (C) A representative table of relevant changes in gene expression. (D) The gene expression profile of p65−/− HSCs was compared with those of (top) long-term HSCs, (middle) late hematopoietic progenitors, and (bottom) dividing hematopoietic cells by Gene Set Enrichment Analysis using curated gene lists.
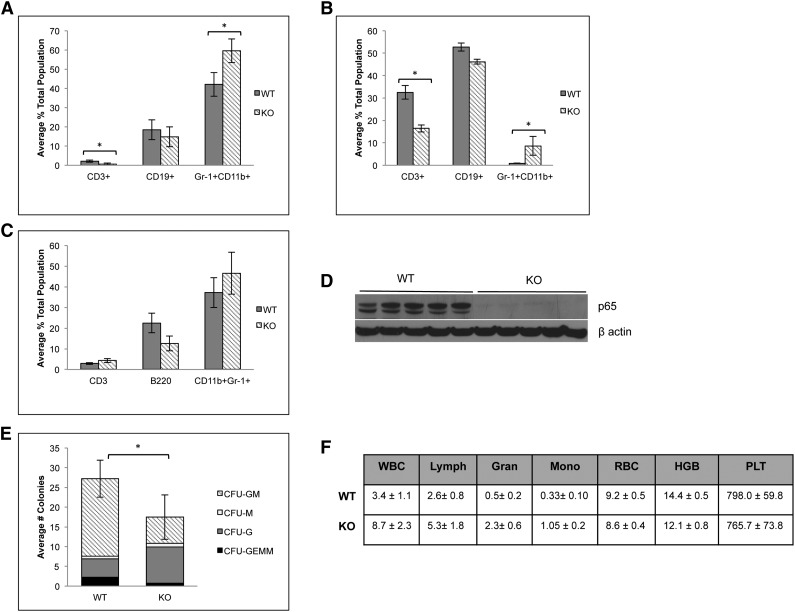
p65 is involved in differentiation of HSPCs
Because of the significant defect in HSC function in the absence of p65, lineage-committed cells were analyzed to assess downstream defects in hematopoietic development. p65hem−/− animals develop basic lineages of differentiated hematopoietic cells in their bone marrow (Figure 7A) and spleens (Figure 7B). A decrease in the percentage of CD3+ T cells was observed in the bone marrow and spleens as expected,25 although the percentage of CD3+CD4+ and CD3+CD8+ T cells did not change (data not shown). A moderate increase in CD11b+Gr-1+ neutrophils was observed in both the bone marrow and spleens of p65hem−/− animals. p65−/− HSCs are also capable of repopulating all lineages of hematopoietic cells in bone marrow (Figure 7C) and spleens (data not shown) of transplant recipients, both of which are void of p65 expression (Figure 7D and data not shown). Notably, reconstitution coincides with an accumulation of LSKF cells in the bone marrow (Figure 3A), as seen in the p65hem−/− mice (Figure 2A-C). Sorted p65−/− LSKF cells also show decreased differentiation potential in a cytokine-rich environment in vitro, as well as a shift toward granulocyte-colony forming cells (Figure 7E). Last, p65hem−/− mice exhibit altered blood cell counts, displaying a myelomonocytic phenotype (Figure 7F), with a substantial increase in total white blood cells, along with the accumulation of neutrophils in the spleen (Figure 7B). These results show that loss of p65 in the hematopoietic compartment results in excessive production of granulocytic cells. Together, these data indicate that p65 is an important regulator of early hematopoiesis, impacting the function of cells through decreased ability to differentiate into lineage-restricted cells.
Figure 7.
Absence of p65 alters the differentiation of HSPCs. (A) Bone marrow or (B) spleens of p65hem−/− mice or wild-type controls were analyzed for the frequency of lineage-committed cells by flow cytometry (n ≥ 7). (C) 5 × 106 whole bone marrow cells from p65hem−/− mice or littermate controls were transplanted into lethally irradiated recipients. Twenty weeks after transplant, bone marrow was harvested, and the frequency of lineage-committed cells was analyzed by flow cytometry (top; n = 5). All recipients displayed >98% engraftment by CD45.2 cells. (D) Bone marrow was analyzed for the expression of p65 by immunoblotting. (E) One hundred LSKF cells were cultured in methylcellulose media with cytokines for 7 days. The average number of colonies per well is displayed. Data are representative of 3 separate experiments. *P < .005. (F) A complete blood panel was performed on peripheral blood collected via cardiac puncture from p65hem−/− and wild-type counterparts (n ≥ 7).
Discussion
We explored the role of the NF-κB subunit p65 in HSC function. Data reveal that p65 is a key regulator of hematopoietic development through the regulation of genes involved in HSC homeostasis and function. HSCs lacking p65 showed defects in repopulation ability (Figure 5A-B, H), coinciding with a twofold decrease in the percentage of bone marrow LSKCD150+CD48− cells (supplemental Figure 2). Surprisingly, this defect correlated with a striking accumulation of LSKFCD34− and CD34+ cells, as well as LSKCD150+CD48+, in the bone marrow of p65hem−/− animals (Figure 2A; supplemental Figure 2). Proliferation of the HSPC fraction is also affected by the loss of p65, in which the number of dividing LSK increases by threefold (Figure 5F), and are protected from apoptosis (Figure 5E). Changes in gene expression include up-regulation of factors involved in differentiation and proliferation and down-regulation of factors that promote HSC quiescence (Figure 7B), suggesting a shift toward hematopoietic progenitors. p65-null HSCs can support long-term hematopoiesis in animals lacking p65 in the hematopoietic compartment, as well as transplant recipients reconstituted with p65-null cells (Figure 7A-B) but show a further decrease in repopulation potential on secondary transplantation, indicating diminished self-renewal (Figure 5G). These data show an important role for p65 in the regulation of HSCs.
Loss of p65 also affects the fate of MPPs. p65hem−/− animals display splenomegaly (Figure 4F) and neutrophilia (Figure 7B,F). Concurrently, extramedullary hematopoiesis is observed (Figure 4E), along with a modest accumulation of GMPs in the spleen (Figure 4D) and a reduction in the percentage of bone marrow myeloid progenitors (Figure 2C-D). p65−/− LSKF cells also form fewer colonies when cultured in the presence of cytokines, with an increase in the frequency of granulocyte colony-forming units (Figure 7E). These findings parallel changes in gene expression in p65−/− LSKF cells, in which genes associated with a myeloid differentiation program are up-regulated.
Recent evidence indicates that noncanonical NF-κB signaling is important for HSC self-renewal and function. Whole body RelB/p52 knockout animals display a severe decrease in the number of functional HSCs in their bone marrow. Although the number of HSPCs increased by approximately twofold in p52/RelB−/− mice, the number of functional HSCs decreased significantly, correlating with poor engraftment in transplant recipients.34 Interestingly, our data show that loss of p65 results in similar defects in HSC repopulation, although the phenotype of HSCs lacking p65 is far less severe (Figures 5B and 7A-B). Importantly, protein expression of both p100 and RelB is low in the bone marrow and is not affected by the deletion of p65 (supplemental Figure 1A).45 Proliferation of RelB/p52−/− HSPCs increased through the up-regulation of cyclin D1 and down-regulation of the cell cycle inhibitor p27.34 Although p65−/− HSPCs also proliferate at a higher rate (Figure 5F), gene expression data show that loss of p65 modulates the expression of several genes specific to cell fate rather than cell cycle regulators (Figure 7B). Gene expression analysis of the RelB/p52−/− HSCs may help to further elucidate functionally distinct roles for these NF-κB subunits.
Additional evidence for impaired HSPC function was uncovered in mice engrafted with c-rel/rela−/− fetal liver cells. Animals injected with c-rel/rela−/− cells succumb to engraftment failure, severe anemia, and granulocytosis due to a defect in precursor populations required for short-term engraftment, as well dysfunctional granulocyte development.33 Due to the short-term duration of these experiments, effects of c-rel/rela deletion on HSC self-renewal were not analyzed. However, data indicate a decreased in repopulation potential and ability of c-rel/rela−/− fetal liver cells to differentiate into lineage-committed cells.33
Numerous changes in gene expression were uncovered through the analysis of LSKF cells from wild-type and p65hem−/− mice. The majority of changes involve the up-regulation of factors expressed in lineage-committed cells, such as myeloid factors Ly6c,46 Csfr1,47 and Igsf6.48 c-Met, involved in the mobilization of HSPCs,41 was also up-regulated in p65−/−LSKF cells. Notably, Mpl, which encodes the thrombopoietin receptor, was down-regulated in the absence of p65. Thrombopoietin is one of many factors required to maintain HSCs in a quiescent state, and the inhibition of MPL in vivo results in a decreased number of quiescent HSCs.6 Thus, the down-regulation of Mpl in the absence of p65 could at least partially reduce the function of HSCs. Concurrently, Hlf is also down-regulated. HLF is known to activate LMO2,49 which is essential in HSC function,50 and overexpression in primitive human HSCs increased the reconstitution ability in NOD/SCID mice.49 Although the effects of Hlf deletion in HSCs is not known, it is probable that HLF is an important factor in cell function. These changes in gene expression in p65-null LSKF cells suggest a shift toward progenitors (Figure 6D), consistent with increased proliferation (Figure 5F) and decreased long-term reconstitution potential (Figure 5A-B).
Although the order of events is not clear, we hypothesize that loss of p65 leads to the cycling of HSCs, inducing a progenitor-like state and resulting in a severe decrease in long-term repopulation potential due to a decrease in self-renewal. Concurrently, the up-regulation of genes normally expressed in lineage-restricted cells leads to skewed differentiation and abnormal distribution of hematopoietic cells. Changes in lineage-restricted populations may be due to overcompensation in response to inefficient differentiation of HSPCs, or p65 may influence fate in both HSCs and specific lineage-committed cell types separately. Interestingly, deletion of p65 modulates the expression of both p100 and RelB in splenocytes (supplemental Figure 1B) but not in the bone marrow (supplemental Figure 1B), indicating a potential role for p65 in the regulation of cell differentiation through the up-regulation of additional NF-κB subunits.45 Deletion of p65 specifically within myeloid progenitors has not been studied and may uncover a mechanism by which these changes occur, further elucidating the link between transcriptional control of HSC and lineage-committed cell fates by p65. Together, these data show that p65 is an important regulator of genes involved in the fate of HSCs and is necessary for the maintenance of homeostasis during hematopoiesis.
Supplementary Material
Acknowledgments
The authors thank Aaron Ebbs, Amanda Rinkenbaugh, José Roques, Mark Ross, and Charlene Santos for technical assistance, Dr George Fedoriw for the evaluation of pathology, and Dr Joel Parker for help with gene expression analysis.
This work was supported by National Cancer Institute Center Core Support grant P30CA06086 (University of North Carolina Flow Cytometry Core Facility), National Institutes of Health grants CA75080 (National Cancer Institute), AI35098 (National Institutes of Allergy and Immunological Diseases), CA73756 (National Cancer Institute), and CA138937 (National Cancer Institute), and the Samuel Waxman Cancer Research Foundation (to A.S.B.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.J.S. performed research, collected data, and made the figures; and S.J.S. and A.S.B. designed the research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Albert S. Baldwin, Lineberger Comprehensive Cancer Center, 450 West Dr, Chapel Hill, NC 27599; e-mail: albert_baldwin@med.unc.edu.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169(2):338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98(25):14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10(3):201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- 5.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3(1):137–147. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 8.Landry J-R, Bonadies N, Kinston S, et al. Expression of the leukemia oncogene Lmo2 is controlled by an array of tissue-specific elements dispersed over 100 kb and bound by Tal1/Lmo2, Ets, and Gata factors. Blood. 2009;113(23):5783–5792. doi: 10.1182/blood-2008-11-187757. [DOI] [PubMed] [Google Scholar]
- 9.Lacombe J, Herblot S, Rojas-Sutterlin S, et al. Scl regulates the quiescence and the long-term competence of hematopoietic stem cells. Blood. 2010;115(4):792–803. doi: 10.1182/blood-2009-01-201384. [DOI] [PubMed] [Google Scholar]
- 10.Tsai FY, Keller G, Kuo FC, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371(6494):221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 11.Vicente C, Conchillo A, García-Sánchez MA, Odero MD. The role of the GATA2 transcription factor in normal and malignant hematopoiesis. Crit Rev Oncol Hematol. 2012;82(1):1–17. doi: 10.1016/j.critrevonc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Nichogiannopoulou A, Trevisan M, Neben S, Friedrich C, Georgopoulos K. Defects in hemopoietic stem cell activity in Ikaros mutant mice. J Exp Med. 1999;190(9):1201–1214. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18(11):3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 2002;21(21):3295–3313. doi: 10.1038/sj.onc.1205318. [DOI] [PubMed] [Google Scholar]
- 15.Klinakis A, Lobry C, Abdel-Wahab O, et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature. 2011;473(7346):230–233. doi: 10.1038/nature09999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nerlov C, Graf TPU. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998;12(15):2403–2412. doi: 10.1101/gad.12.15.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh HC, Gooya J, Renn K, Friedman AD, Johnson PF, Keller JR. C/EBPalpha determines hematopoietic cell fate in multipotential progenitor cells by inhibiting erythroid differentiation and inducing myeloid differentiation. Blood. 2006;107(11):4308–4316. doi: 10.1182/blood-2005-06-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 19.Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274(5288):782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 20.Alcamo E, Hacohen N, Schulte LC, Rennert PD, Hynes RO, Baltimore D. Requirement for the NF-kappaB family member RelA in the development of secondary lymphoid organs. J Exp Med. 2002;195(2):233–244. doi: 10.1084/jem.20011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassères DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25(51):6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 22.Gerondakis S, Banerjee A, Grigoriadis G, Vasanthakumar A, Gugasyan R, Sidwell T, Grumont RJ. NF-κB subunit specificity in hemopoiesis. Immunol Rev. 2012;246(1):272–285. doi: 10.1111/j.1600-065X.2011.01090.x. [DOI] [PubMed] [Google Scholar]
- 23.Bottero V, Withoff S, Verma IM. NF-kappaB and the regulation of hematopoiesis. Cell Death Differ. 2006;13(5):785–797. doi: 10.1038/sj.cdd.4401888. [DOI] [PubMed] [Google Scholar]
- 24.Grumont RJ, Rourke IJ, O’Reilly LA, Strasser A, Miyake K, Sha W, Gerondakis S. B lymphocytes differentially use the Rel and nuclear factor kappaB1 (NF-kappaB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J Exp Med. 1998;187(5):663–674. doi: 10.1084/jem.187.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horwitz BH, Scott ML, Cherry SR, Bronson RT, Baltimore D. Failure of lymphopoiesis after adoptive transfer of NF-kappaB-deficient fetal liver cells. Immunity. 1997;6(6):765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- 26.Doi TS, Takahashi T, Taguchi O, Azuma T, Obata Y. NF-kappa B RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J Exp Med. 1997;185(5):953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prendes M, Zheng Y, Beg AA. Regulation of developing B cell survival by RelA-containing NF-kappa B complexes. J Immunol. 2003;171(8):3963–3969. doi: 10.4049/jimmunol.171.8.3963. [DOI] [PubMed] [Google Scholar]
- 28.Senftleben U, Li ZW, Baud V, Karin M. IKKbeta is essential for protecting T cells from TNFalpha-induced apoptosis. Immunity. 2001;14(3):217–230. doi: 10.1016/s1074-7613(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 29.Greten FR, Arkan MC, Bollrath J, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130(5):918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mankan AK, Canli O, Schwitalla S, Ziegler P, Tschopp J, Korn T, Greten FR. TNF-alpha-dependent loss of IKKbeta-deficient myeloid progenitors triggers a cytokine loop culminating in granulocytosis. Proc Natl Acad Sci USA. 2011;108(16):6567–6572. doi: 10.1073/pnas.1018331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-kappa B activation, enhanced granulopoiesis, and neonatal lethality in I kappa B alpha-deficient mice. Genes Dev. 1995;9(22):2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 32.Weih F, Carrasco D, Durham SK, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 33.Grossmann M, Metcalf D, Merryfull J, Beg A, Baltimore D, Gerondakis S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci USA. 1999;96(21):11848–11853. doi: 10.1073/pnas.96.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Xiu Y, Ashton J, Xing L, Morita Y, Jordan CT, Boyce BF. Noncanonical NF-κB signaling regulates hematopoietic stem cell self-renewal and microenvironment interactions. Stem Cells. 2012;30(4):709–718. doi: 10.1002/stem.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180(4):2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 36.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33(2):314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 37.Almarza E, Segovia JC, Guenechea G, Gómez SG, Ramírez A, Bueren JA. Regulatory elements of the vav gene drive transgene expression in hematopoietic stem cells from adult mice. Exp Hematol. 2004;32(4):360–364. doi: 10.1016/j.exphem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269(5229):1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 39.Ziegelbauer K, Gantner F, Lukacs NW, et al. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br J Pharmacol. 2005;145(2):178–192. doi: 10.1038/sj.bjp.0706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams GB, Chabner KT, Alley IR, et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 41.Tesio M, Golan K, Corso S, et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011;117(2):419–428. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- 42.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 44.Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 45.Finco TS, Baldwin AS. Mechanistic aspects of NF-kappa B regulation: the emerging role of phosphorylation and proteolysis. Immunity. 1995;3(3):263–272. doi: 10.1016/1074-7613(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 46.Jutila DB, Kurk S, Jutila MA. Differences in the expression of Ly-6C on neutrophils and monocytes following PI-PLC hydrolysis and cellular activation. Immunol Lett. 1994;41(1):49–57. doi: 10.1016/0165-2478(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 47.Sasmono RT, Ehrnsperger A, Cronau SL, et al. Mouse neutrophilic granulocytes express mRNA encoding the macrophage colony-stimulating factor receptor (CSF-1R) as well as many other macrophage-specific transcripts and can transdifferentiate into macrophages in vitro in response to CSF-1. J Leukoc Biol. 2007;82(1):111–123. doi: 10.1189/jlb.1206713. [DOI] [PubMed] [Google Scholar]
- 48.Bates EE, Kissenpfennig A, Péronne C, Mattei MG, Fossiez F, Malissen B, Lebecque S. The mouse and human IGSF6 (DORA) genes map to the inflammatory bowel disease 1 locus and are embedded in an intron of a gene of unknown function. Immunogenetics. 2000;52(1-2):112–120. doi: 10.1007/s002510000259. [DOI] [PubMed] [Google Scholar]
- 49.Shojaei F, Trowbridge J, Gallacher L, et al. Hierarchical and ontogenic positions serve to define the molecular basis of human hematopoietic stem cell behavior. Dev Cell. 2005;8(5):651–663. doi: 10.1016/j.devcel.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Yamada Y, Warren AJ, Dobson C, Forster A, Pannell R, Rabbitts TH. The T cell leukemia LIM protein Lmo2 is necessary for adult mouse hematopoiesis. Proc Natl Acad Sci USA. 1998;95(7):3890–3895. doi: 10.1073/pnas.95.7.3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.