Abstract
Background:
Osteosarcoma is the most common primary bone sarcoma and affects all ages. There are substantial differences in management and outcomes for patients who have localized disease compared with distant spread at the time of diagnosis. Our goal was to examine potential risk factors predictive of metastatic disease at presentation.
Methods:
The Surveillance, Epidemiology, and End Results (SEER) Program database was used to identify all patients diagnosed with osteosarcoma from 2000 to 2008 and to classify each patient as having metastatic or localized disease at the time of diagnosis. Patient-based characteristics, tumor characteristics, and county-level socioeconomic measures were analyzed to determine which factors were predictive of an increased rate of distant metastatic disease at presentation. These factors were analyzed as univariate characteristics as well as in a multivariate logistic regression model.
Results:
We identified 2017 cases of high-grade osteosarcoma, and 464 (23.0%) of the patients presented with metastatic disease. In the unadjusted logistic regression analysis, patients had increased odds of metastatic disease at presentation if they had an age of sixty years or more (odds ratio [OR] = 2.22; 95% confidence interval [CI], 1.71 to 2.89), had a tumor located in the axial skeleton (OR = 2.47; 95% CI, 1.88 to 3.26), and lived in a county with low socioeconomic status (OR = 1.59; 95% CI, 1.08 to 2.35). These factors remained significant when combined in multivariate models controlling for age, location, and socioeconomic status. For patients with recorded tumor size information (n = 1398), the odds of metastasis at presentation increased by 10% with each additional centimeter of tumor size (OR = 1.10; 95% CI, 1.08 to 1.13). When the patients with missing tumor size information were excluded, socioeconomic status was no longer a significant risk factor for metastasis at presentation in the multivariate model.
Conclusions:
Osteosarcoma patients with advanced age, a tumor in the axial skeleton, a larger tumor size, and a residence in a less affluent county were more likely to have metastatic disease at presentation.
Level of Evidence:
Prognostic Level II. See Instructions for Authors for a complete description of levels of evidence.
Osteosarcoma is the most common primary sarcoma of bone and affects patients of every age. An estimated 15% to 20% of individuals with osteosarcoma have identifiable metastases at initial presentation1-3, and these patients have a far worse prognosis compared with patients with localized disease only1,3-10. There is a paucity of data regarding clinical characteristics associated with a greater risk of distant metastatic disease at diagnosis5.
Because osteosarcoma is a rare tumor, with an estimated incidence of 1.7 to 4.4 per million per year depending on age11, obtaining a meaningful number of patients to study is challenging. We utilized the Surveillance, Epidemiology, and End Results (SEER) Program database (maintained by the National Cancer Institute), which is a commonly used tool for the analysis of rare cancers. The SEER Program now collects data from seventeen geographically variable cancer registries and represents approximately 26% of the U.S. population.
The purpose of the present investigation was to analyze patient characteristics (age, sex, and race), tumor characteristics (location, histologic subtype, and size), and socioeconomic measures obtained at the county level (median family income, number of persons below the poverty line, percentage of high school graduates, and rural or urban setting) to identify factors associated with an increased rate of distant metastatic disease at presentation. This information could provide insights into individual, tumor, and social factors that are associated with the development of metastatic osteosarcoma prior to diagnosis.
Materials and Methods
We identified all cases of osteosarcoma listed in the SEER Program database from 2000 to 2008. This time period reflects data collection from all seventeen current SEER registries and provides consistency in both the population of interest and the methods used to diagnose metastatic disease. The database maintained by the SEER Program is publicly available and does not contain unique patient identifiers. The SEER*Stat application (version 7.0.9) was used to determine frequency rates and variables of interest.
Data Elements
Patient characteristics of interest included age, sex, and race. Patient age is recorded in the SEER database as a categorical variable in five-year intervals, beginning at zero years and ending at eight-five years or more. The incidence of osteosarcoma follows a bimodal distribution with respect to age, with different histologic subtypes predominating in younger and older age groups. We therefore categorized patients into three distinct age groups of zero to twenty-four years, twenty-five to fifty-nine years, and sixty years or older to reflect this variability, as has been done previously11. Race was characterized as white, black, or other.
We were also interested in tumor-specific characteristics, specifically histologic subtype, location, and size. The histologic subtype (according to the International Classification of Diseases for Oncology, Third Revision [ICD-O-3]) and location are recorded in the SEER database at the time of diagnosis. Tumor size is recorded as a continuous variable to the nearest millimeter.
Osteosarcoma in the elderly population has a higher proportion of post-radiation osteosarcoma and malignant transformation from Paget disease compared with that in other age groups11. Although we were able to identify cases of osteosarcoma associated with Paget disease, post-radiation osteosarcoma was not listed as a specific subtype. The SEER database does contain the order of malignancy (e.g., first primary, second of two or more primaries, etc.). In an attempt to identify post-radiation osteosarcoma, we distinguished between patients presenting with their first primary malignancy and those with a prior malignancy. A similar approach has been taken in a previous investigation11.
The anatomic site information in the SEER database is relatively nonspecific and does not indicate the specific bone involved or the location within that bone. We categorized the location as the axial skeleton (pelvis, spine, and ribs), extremities (long and short bones of the upper and lower extremities), or other (mandible, skull, and other atypical locations).
The SEER database also includes several socioeconomic measures pertaining to the patient’s county of residence; these include median family income, the percentage of individuals below the poverty line, the percentage of individuals at least twenty-five years old who have less than twelve years of education, and rural or urban setting (based on the 2000 U.S. census). We used some of these socioeconomic status (SES) variables to calculate a composite score for comparison, as has been done in previous investigations12-14. Briefly, we compiled the SES data for all patients with osteosarcoma and categorized the resulting income, poverty, and education data for the county of residence into quartiles. We then assigned each quartile a number from 1 to 4, with a higher number reflecting higher income, less poverty, or more education. For median family income, the quartiles were (1) <$46,450, (2) $46,450 to $51,400, (3) $51,410 to $63,550, and (4) >$63,550. For the percentage of individuals below the poverty line, the quartiles were (1) >17.90%, (2) 12.44% to 17.90%, (3) 8.41% to 12.43%, and (4) <8.41%. For the percentage of individuals at least twenty-five years old with less than twelve years of education, the quartiles were (1) >28.77%, (2) 18.82% to 28.77%, (3) 15.16% to 18.81%, and (4) <15.16%. Each of the three categories was given the same weight and the categories were combined to form a composite score12-14. Patients with the lowest possible combination of SES variables (lowest quartile within each variable) were then compared with the remainder of the sample. Population density can be effectively divided into counties in a metropolitan area (urban) and counties not in a metropolitan area (rural) on the basis of Rural-Urban Continuum Codes developed by the U.S. Department of Agriculture15.
Our outcome of interest was a dichotomous variable representing the presence of metastatic disease at the time of diagnosis. We included patients with a staging of “distant” disease (n = 468) in this category. Patients whose staging was categorized as “localized” or “regional” (n = 1633) were considered to have no evidence of distant metastasis. Patients with an entry that was blank or “unstaged” (n = 146) were excluded.
Statistical Analysis
We first examined the frequency of various histologic subtypes among all patients with osteosarcoma. Descriptive statistics and univariate methods (chi-square tests) were then used to examine the proportion of patients presenting with localized disease or distant metastatic disease according to key factors including age, race, sex, tumor location and size, and socioeconomic measures. Finally, a series of regression models were used to examine the association between presentation with distant metastatic disease and an array of patient and county-level measures including sex, age, race, histologic subtype, tumor location, tumor size, composite SES score, rural or urban setting, and history of cancer. All statistical calculations were performed with use of SAS software (version 9.3; SAS Institute, Cary, North Carolina).
Model Selection
The initial logistic regression analysis involved simple univariate modeling for each predictor variable. Multivariate models were then created by means of a stepwise selection method using only those predictor variables with a substantial measure of association (p < 0.1). The first multivariate model utilized the entire sample of 2017 patients with high-grade osteosarcoma by excluding tumor size as a predictor. This model included age, tumor location, and composite SES score as significant predictors. A second, separate multivariate model utilized a more limited sample of 1398 patients (excluding 619 with missing data for size) and included age, tumor location, and tumor size as significant predictors. In addition, SES was retained in this model as a possible risk factor, given its significant measure of association in the multivariate analysis of the entire cohort. A third model including all of the analyzed variables was then created to verify the stability of our findings.
Missing Data
Two of the predictor variables used in the univariate analysis and the regression modeling had missing data. Specifically, one of the 2017 patients with high-grade osteosarcoma had a missing value for geographic setting (rural or urban) and 619 had a missing value for tumor size. The entire identified cohort of 2017 patients was utilized for any portion of the analysis that did not include use of these variables. When these variables were part of a univariate analysis or regression model, entries with missing data were excluded from that specific analysis.
We also performed a sensitivity analysis to investigate the effect of our handling of missing data. A summary variable was created to indicate whether or not a patient’s tumor size was recorded. This “missing size” variable was used in a regression analysis of the entire cohort to determine whether its inclusion had any effect on the odds ratio (OR) estimates.
Source of Funding
Some of the investigators received salary support from the National Institutes of Health.
Results
The SEER registry contained 2101 cases of osteosarcoma diagnosed from 2000 to 2008. Including all histologic subtypes, the overall rate of distant metastatic disease at presentation was 22.3%. A detailed examination of the various histologic subtypes revealed that patients with intraosseous well-differentiated osteosarcoma and parosteal osteosarcoma were less likely to present with distant metastasis compared with many other osteosarcoma subtypes (Table I). Furthermore, these two subtypes have a less aggressive clinical course compared with the other high-grade subtypes and are treated differently. For these reasons, we chose to exclude intraosseous well-differentiated osteosarcoma and parosteal osteosarcoma (n = 84) from the remainder of the analysis, leaving a final cohort of 2017 cases of high-grade osteosarcoma.
TABLE I.
Histologic Subtype of Osteosarcoma and Metastatic Disease at Presentation, 2000 to 2008
| Subtype | No. | Metastatic Disease at Presentation (no. [%]) |
| Total | 2101 | 468 (22.3) |
| Osteosarcoma, not otherwise specified | 1401 | 350 (25.0) |
| Chondroblastic osteosarcoma | 312 | 59 (18.9) |
| Fibroblastic osteosarcoma | 120 | 18 (15.0) |
| Telangiectatic osteosarcoma | 73 | 16 (21.9) |
| Osteosarcoma in Paget disease | 25 | 9 (36.0) |
| Small-cell osteosarcoma | 19 | 3 (15.8) |
| Central osteosarcoma | 35 | 4 (11.4) |
| Intraosseous well-differentiated osteosarcoma | 5 | 0 (0) |
| Parosteal osteosarcoma | 79 | 4 (5.1) |
| Periosteal osteosarcoma | 21 | 3 (14.3) |
| High grade surface osteosarcoma | 11 | 2 (18.2) |
The majority of the 2017 cases occurred in adolescents, which is consistent with previous data (Fig. 1)11. The percentage of patients with distant metastatic disease at presentation differed according to age at diagnosis (Fig. 2). Specifically, metastatic disease at diagnosis was more common among patients with an age of sixty years or more (37.1%) than among patients with an age of less than sixty years (20.2%, p < 0.001). Substantial disparities in the rate of metastatic disease also existed when patients were stratified according to age, tumor location, tumor size, median family income, and composite SES score (Table II). Specifically, an increased rate of metastatic disease at presentation was associated with an age of sixty years or more (p < 0.001), an axial location (p < 0.001), greater tumor size (p < 0.001), the lowest quartile of income (p = 0.011), and the lowest twelfth of the composite SES score (p = 0.019).
Fig. 1.
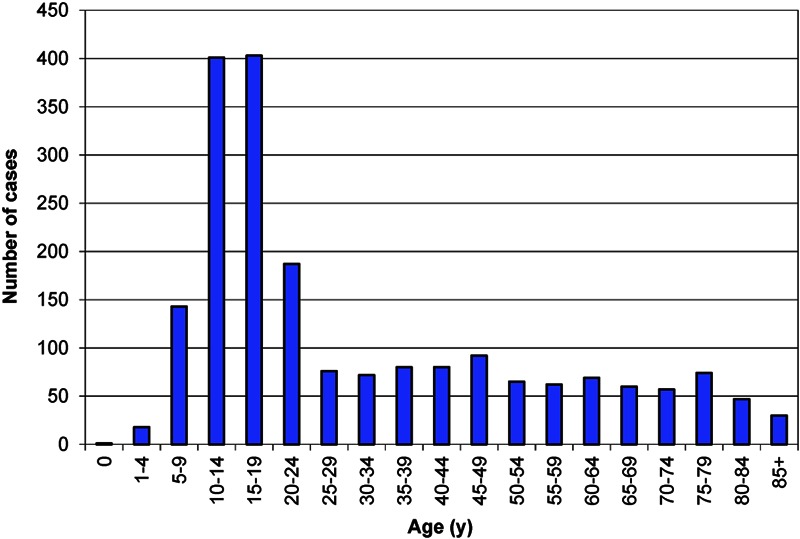
Number of high-grade osteosarcoma cases from 2000 to 2008 according to age at diagnosis.
Fig. 2.
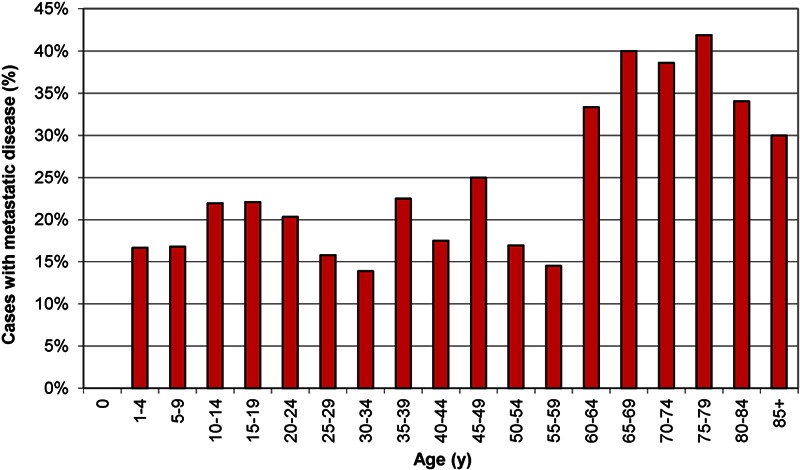
Percentage of high-grade osteosarcoma cases with distant metastatic disease at presentation from 2000 to 2008 according to age at diagnosis.
TABLE II.
Univariate Analysis of Patient Characteristics and Metastatic Disease at Presentation with High-Grade Osteosarcoma, 2000 to 2008
| Category | No. | Metastatic Disease at Presentation (no. [%]) | P Value* |
| Age in yr | <0.001 | ||
| 0-24 | 1153 | 242 (21.0) | |
| 25-59 | 527 | 97 (18.4) | |
| 60-85+ | 337 | 125 (37.1) | |
| Sex | 0.196 | ||
| Male | 1099 | 265 (24.1) | |
| Female | 918 | 199 (21.7) | |
| Race | 0.708 | ||
| White | 1545 | 362 (23.4) | |
| Black | 289 | 63 (21.8) | |
| Other | 183 | 39 (21.3) | |
| Location | <0.001 | ||
| Extremity | 1445 | 300 (20.8) | |
| Axial | 267 | 105 (39.3) | |
| Other | 305 | 59 (19.3) | |
| Size† | <0.001 | ||
| ≤5 cm | 333 | 32 (9.6) | |
| >5 to 10 cm | 594 | 107 (18.0) | |
| >10 cm | 471 | 145 (30.8) | |
| Income | 0.011 | ||
| Lowest quartile | 339 | 96 (28.3) | |
| All others | 1678 | 368 (21.9) | |
| Poverty | 0.983 | ||
| Lowest quartile | 505 | 116 (23.0) | |
| All others | 1512 | 348 (23.0) | |
| Education | 0.300 | ||
| Lowest quartile | 498 | 123 (24.7) | |
| All others | 1519 | 341 (22.4) | |
| Composite SES‡ | 0.019 | ||
| Lowest twelfth | 127 | 40 (31.5) | |
| All others | 1890 | 424 (22.4) | |
| Rural or urban§ | 0.597 | ||
| Rural | 162 | 40 (24.7) | |
| Urban | 1854 | 424 (22.9) |
For the proportion of metastatic disease at presentation.
619 observations eliminated because of missing data.
SES = socioeconomic status.
One observation eliminated because of missing data.
Because of the higher proportion of metastatic disease at diagnosis among older patients, we conducted additional analyses to study patients with an age of sixty years or more in greater detail (Table III). The rate of metastasis of axial tumors at presentation was >50% in this population. No increase in the risk of metastatic disease at presentation was revealed in patients with a history of previous malignancy or patients with Paget disease. The rate of metastasis at presentation was slightly lower in older patients with Paget disease (31.8%) than in older patients with a different histologic subtype (37.5%), but the difference was not significant (p = 0.596). However, because of the limited number of cases of osteosarcoma in patients with Paget disease (n = 25 in the entire cohort, n = 22 in patients with an age of sixty years or more), the study was not adequately powered to detect a true difference.
TABLE III.
Univariate Analysis of the Characteristics of Patients Sixty Years of Age or Older and Metastatic Disease at Presentation with High-Grade Osteosarcoma, 2000 to 2008
| Category | No. | Metastatic Disease at Presentation (no. [%]) | P Value* |
| Sex | 0.191 | ||
| Male | 165 | 67 (40.6) | |
| Female | 172 | 58 (33.7) | |
| Race | 0.910 | ||
| White | 271 | 99 (36.5) | |
| Black | 43 | 17 (39.5) | |
| Other | 23 | 9 (39.1) | |
| Married | 0.318 | ||
| Yes | 190 | 76 (40.0) | |
| No | 136 | 47 (34.6) | |
| Location | 0.003 | ||
| Extremity | 145 | 51 (35.2) | |
| Axial | 85 | 44 (51.8) | |
| Other | 107 | 30 (28.0) | |
| Size† | <0.001 | ||
| ≤5 cm | 64 | 8 (12.5) | |
| >5 to 10 cm | 75 | 24 (32.0) | |
| >10 cm | 72 | 30 (41.7) | |
| Income | 0.039 | ||
| Lowest quartile | 57 | 28 (49.1) | |
| All others | 280 | 97 (34.6) | |
| Composite SES‡ | 0.087 | ||
| Lowest twelfth | 20 | 11 (55.0) | |
| All others | 317 | 114 (36.0) | |
| Rural or urban | 0.372 | ||
| Rural | 39 | 17 (43.6) | |
| Urban | 298 | 108 (36.2) | |
| First cancer | 0.089 | ||
| Yes | 215 | 87 (40.5) | |
| No | 122 | 38 (31.1) | |
| Paget osteosarcoma | 0.596 | ||
| Yes | 22 | 7 (31.8) | |
| No | 315 | 118 (37.5) |
For the proportion of metastatic disease at presentation.
126 observations were eliminated because of missing data.
SES = socioeconomic status.
The univariate logistic regression models revealed increased odds of metastatic disease at presentation among patients with an age of sixty years or more (OR = 2.22; 95% confidence interval [CI], 1.71 to 2.89), patients with a tumor in the axial skeleton (OR = 2.47; 95% CI, 1.88 to 3.26), patients with a large tumor (OR = 1.10; 95% CI, 1.08 to 1.13; for each 1-cm increase in size), and patients with the lowest composite SES score (OR = 1.59; 95% CI, 1.08 to 2.35) (Table IV). The estimated ORs based on age, tumor location, and tumor size remained stable when more variables were added to the model.
TABLE IV.
Odds Ratios for Risk of Presentation with Metastatic Disease*
| Variable | Model 1 (Unadjusted) | Model 2† | Model 3‡ | Model 4§ |
| N = 2017 | N = 1398 | N = 1397 | ||
| Age in yr | ||||
| 0-24 | Ref | Ref | Ref | Ref |
| 25-59 | 0.85 (0.65-1.10) | 0.80 (0.60-1.05) | 0.73 (0.51-1.04) | 0.74 (0.52-1.06) |
| 60-85+ | 2.22 (1.71-2.89) | 2.06 (1.55-2.76) | 1.72 (1.17-2.53) | 1.63 (1.06-2.50) |
| Sex | ||||
| Female | Ref | Ref | ||
| Male | 1.15 (0.93-1.42) | 1.15 (0.87-1.53) | ||
| Race | ||||
| White | Ref | Ref | ||
| Black | 0.91 (0.67-1.23) | 0.98 (0.64-1.48) | ||
| Other | 0.89 (0.61-1.28) | 0.85 (0.53-1.38) | ||
| Location | ||||
| Extremity | Ref | Ref | Ref | Ref |
| Axial | 2.47 (1.88-3.26) | 2.21 (1.64-2.96) | 2.89 (1.96-4.25) | 3.03 (2.03-4.51) |
| Other | 0.92 (0.67-1.25) | 0.77 (0.55-1.08) | 0.74 (0.45-1.22) | 0.77 (0.46-1.27) |
| Size | ||||
| For each 1-cm increase | 1.10 (1.08-1.13) | 1.10 (1.07-1.12) | 1.10 (1.07-1.13) | |
| Composite SES | ||||
| Not lowest twelfth | Ref | Ref | Ref | Ref |
| Lowest twelfth | 1.59 (1.08-2.35) | 1.61 (1.08-2.40) | 1.26 (0.74-2.14) | 1.28 (0.74-2.21) |
| Rural or urban | ||||
| Urban | Ref | Ref | ||
| Rural | 1.11 (0.76-1.61) | 0.87 (0.51-1.48) | ||
| Histology | ||||
| Osteosarcoma, NOS | Ref | Ref | ||
| Chondroblastic | 0.70 (0.52-0.95) | 0.73 (0.49-1.08) | ||
| Fibroblastic | 0.53 (0.32-0.89) | 0.73 (0.38-1.38) | ||
| Telangiectatic | 0.84 (0.48-1.49) | 1.02 (0.52-2.00) | ||
| Paget | 1.69 (0.74-3.86) | 1.29 (0.35-4.73) | ||
| Small cell | 0.56 (0.16-1.94) | 0.25 (0.03-2.02) | ||
| Central | 0.39 (0.14-1.10) | 0.65 (0.22-1.95) | ||
| Periosteal | 0.50 (0.15-1.71) | 0.74 (0.16-3.42) | ||
| High-grade surface | 0.67 (0.14-3.10) | 0.78 (0.14-4.36) | ||
| First cancer | ||||
| Yes | Ref | Ref | ||
| No | 1.23 (0.90-1.67) | 1.01 (0.63-1.64) |
The values are given as the odds ratio, with the 95% confidence interval in parentheses. NOS = not otherwise specified, and SES = socioeconomic status.
Logistic regression controlling for age, tumor location, and SES.
Logistic regression controlling for age, tumor location, tumor size, and SES.
Logistic regression controlling for age, sex, race, tumor location, tumor size, SES, rural or urban setting, histology, and sequence of malignancy.
The composite SES score lost significance as a risk factor when tumor size was included in the multivariate model. Because of missing data, the sample size decreased by 619 entries in the models in which size was included. A possible explanation for the loss of significance of SES as a predictor variable is the fact that the rate of metastasis at presentation in the group with the lowest SES score was higher in the patients who were eliminated (eighteen of thirty-nine, 46%) compared with those who were included (twenty-two of eighty-eight, 25%) (p = 0.018). Furthermore, individuals for whom the tumor size had not been recorded were more likely to have metastatic disease at presentation (29.1% compared with 20.3%, p < 0.001).
The sensitivity analysis that involved the entire cohort of 2017 cases and included the summary variable representing whether or not a patient had a recorded value for tumor size yielded estimates that were similar to those of the primary analysis. Multivariate analysis controlling for missing size information in addition to age, tumor location, and composite SES score revealed that missing size information was predictive of metastasis at presentation (OR = 1.46; 95% CI, 1.16 to 1.82). However, the OR estimates for an age of sixty years or more (OR = 2.04; 95% CI, 1.53 to 2.73), an axial tumor location (OR = 2.10; 95% CI, 1.56 to 2.84), and a low composite SES score (OR = 1.62; 95% CI, 1.08 to 2.42) were similar to those in the analysis that eliminated cases with missing tumor size information.
Discussion
Analysis of the SEER database from 2000 to 2008 revealed that 23.0% cases of high-grade osteosarcoma presented with distant metastatic disease at the time of initial diagnosis. Greater age, axial tumor location, larger tumor size, and residence in the least affluent counties were all associated with greater odds of metastatic disease at diagnosis.
It has been demonstrated previously, and it is well accepted, that patients with metastatic disease at initial presentation have a poorer prognosis than those with localized disease3-10. Bacci et al. reported an overall survival rate of 94% at two years for osteosarcoma patients with localized disease compared with 55% for those with metastatic disease at presentation4. Similarly, Bielack et al. found five-year overall survival rates of 70.1% and 31.6% and ten-year overall survival rates of 64.4% and 26.7% for patients with localized and metastatic disease at presentation, respectively5.
Bielack et al. also found that presentation with metastatic disease was associated with larger tumor size, an axial tumor location, and a longer history of symptoms5. To our knowledge, that is the only previous report that includes discussion of clinical risk factors for presentation with metastatic disease. The present study builds on their results in a number of ways. First, it involves a larger cohort and one that is a representative cross-section of the U.S. population. Second, we were able to treat tumor size as a continuous variable and determine that each 1-cm increase in size was associated with a 10% increase in the odds of presenting with a metastasis. Finally, we were able to include socioeconomic measures in addition to tumor and patient characteristics. Taken together, our analysis confirmed and expanded on the conclusions from the single prior investigation by Bielack et al. and clearly identified populations at risk for metastatic osteosarcoma at presentation.
An age of sixty years or more was an independent risk factor for metastatic disease at the time of diagnosis. Many previous studies have revealed an association of increasing age with a poorer prognosis in patients with osteosarcoma5-7,10,16,17. However, there is substantial uncertainty and debate regarding whether age itself is a risk factor or is simply a surrogate for other, more important, aspects of the disease. For instance, older patients have greater percentages of axial and large tumors, both of which are associated with a poorer prognosis16,18-21. Greater angiogenesis in the tumors in older individuals may also contribute to the greater prevalence of early metastasis18. This age group is also subject to different subtypes of osteosarcoma, specifically osteosarcoma associated with Paget disease and post-radiation osteosarcoma, whose behavior may differ from that of conventional osteosarcoma11. Older individuals can also have more medical comorbidities, complicating the chemotherapeutic options and reducing treatment responses6. In the present study, an age of sixty years or more was an independent risk factor for distant metastatic disease at presentation even when controlling for tumor location, size, and histology.
We determined that an axial tumor location and larger tumor size were independent risk factors for metastasis at presentation. Similar to the situation involving advancing age, prior research on osteosarcoma revealed that tumors in the axial skeleton and large tumors were associated with a poor prognosis5,8-10,16,21. The inferior oncologic results in these cases can be partially explained by the difficulty in performing surgical resection and obtaining adequate margins3,5. In addition, tumors in the axial skeleton are typically in closer proximity to large venous sinuses, which may increase the likelihood of metastatic disease19,20. Furthermore, tumors in the axial skeleton often grow undetected well past the time that tumors in other locations would have been noticed and evaluated. This suggests that increased time to presentation may contribute to an increased rate of metastasis at presentation for both tumors in the axial skeleton and large tumors, as the possibility of distant disease increases as neoplastic cells continue to divide untreated over time. However, we are aware of no conclusive evidence to date that delay in diagnosis is associated with decreased survival5,22. Advanced patient age, tumor size, and tumor location cannot easily be modified by the surgeon, oncologist, or primary care provider. Any unexplained skeletal mass, especially one with a history of rapid enlargement or increasing pain, should be evaluated without delay. Tumors in the axial skeleton pose a difficulty precisely because they tend to be identified late in the disease course because of a lack of symptoms or a palpable mass. More common malignancies, such as breast, prostate, and colon cancer, can be identified prior to the onset of symptoms by the use of various screening strategies. Osteosarcoma, however, is so rare that any screening studies utilizing current technology would not be effective or justifiable.
Our finding that patients residing in counties with the lowest composite SES score were at higher risk for metastasis at presentation is a new observation that adds to previous research. Unlike patient age and tumor size and location, which are inherent characteristics of the patient and tumor, the SES score is a reflection of the environment in which the patient resides. It reflects the context in which an individual patient is evaluated and treated rather than the underlying disease process. Socioeconomic status is a concept that combines many individual details, social factors, and local infrastructure. A lower SES score may reflect an area with less access to medical care. It could also reflect an overall reluctance or inability of residents to seek care in a timely manner, resulting in delays in diagnosis. The composite SES score in the present study does not account for many factors that could influence an individual’s socioeconomic status. However, it remains worthwhile to use the available data to investigate whether differences at the community level influence disease presentation in individuals. Similar derivations of SES measures in the SEER database have been used in previous investigations12,13.
Although the extent of patient-level information available in the SEER database is limited, some inferences may be reasonably made on the basis of the county-level data. For instance, a county with a low SES score could be representative of an area with diminished resources and reduced access to care. We found that those individuals living in the poorest and least educated counties demonstrated increased odds of developing metastatic disease prior to diagnosis. This may suggest that there is a critical level of collective understanding and infrastructure below which the risk of metastasis at presentation increases. Other investigations have revealed low socioeconomic status to be a risk factor for presentation with advanced disease in several other types of cancer23-27. Osteosarcoma should be considered as another entity that may benefit from increased efforts in communities with limited resources.
Although the present study was not intended to guide treatment after diagnosis, our findings do have some potential implications for clinical practice. The identification of high-risk groups may help providers in counseling individuals regarding the likelihood of discovering metastatic disease at the time of diagnosis, as the rate in identified high-risk groups was much higher than the overall rate of 20%. It is not clear from this study whether members of these high-risk groups would benefit from closer pulmonary surveillance or aggressive treatment of indeterminate pulmonary nodules, but the high rate of metastasis at presentation suggests that patients with the risk factors identified in the study would be ideal participants in further investigations of these questions.
The study has several limitations. First, the use of a large national database, while providing an ample number of cases to analyze, is not without accompanying restrictions. We were not able to confirm the accuracy of the histologic diagnoses or identification of metastatic disease. In addition, we did not have complete information regarding the size or specific location of the tumors reported. Second, we relied on county-level data to draw conclusions regarding an individual’s socioeconomic status. Although this strategy is justifiable, it does not account for variations in income, poverty, or education within the area of interest. Finally, we did not investigate the treatments used or oncologic outcomes in the sample cohort. That was not the purpose of the present study, but it represents an important area for further research.
In summary, advanced age, an axial tumor location, increasing tumor size, and residence in less affluent counties were all associated with a greater probability of having distant metastatic disease at the time of presentation with osteosarcoma.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Acknowledgments
Note: The authors acknowledge Yubo Gao, PhD, for assistance with the statistical analysis.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Mialou V, Philip T, Kalifa C, Perol D, Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles AS, Hartmann O. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005 Sep 1;104(5):1100-9 [DOI] [PubMed] [Google Scholar]
- 2.Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999 Oct 15;86(8):1602-8 [DOI] [PubMed] [Google Scholar]
- 3.Kager L, Zoubek A, Pötschger U, Kastner U, Flege S, Kempf-Bielack B, Branscheid D, Kotz R, Salzer-Kuntschik M, Winkelmann W, Jundt G, Kabisch H, Reichardt P, Jürgens H, Gadner H, Bielack SS; Cooperative German-Austrian-Swiss Osteosarcoma Study Group Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003 May 15;21(10):2011-8 [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, Briccoli A, Rocca M, Ferrari S, Donati D, Longhi A, Bertoni F, Bacchini P, Giacomini S, Forni C, Manfrini M, Galletti S. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003 Jul;14(7):1126-34 [DOI] [PubMed] [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002 Feb 1;20(3):776-90 [DOI] [PubMed] [Google Scholar]
- 6.Grimer RJ, Cannon SR, Taminiau AM, Bielack S, Kempf-Bielack B, Windhager R, Dominkus M, Saeter G, Bauer H, Meller I, Szendroi M, Folleras G, San-Julian M, van der Eijken J. Osteosarcoma over the age of forty. Eur J Cancer. 2003 Jan;39(2):157-63 [DOI] [PubMed] [Google Scholar]
- 7.Jawad MU, Cheung MC, Clarke J, Koniaris LG, Scully SP. Osteosarcoma: improvement in survival limited to high-grade patients only. J Cancer Res Clin Oncol. 2011 Apr;137(4):597-607 Epub 2010 Jun 01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, Schwarz R, von Hochstetter AR, Salzer-Kuntschik M, Berdel WE, Jürgens H, Exner GU, Reichardt P, Mayer-Steinacker R, Ewerbeck V, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003 Jan 15;21(2):334-41 [DOI] [PubMed] [Google Scholar]
- 9.Ozaki T, Flege S, Liljenqvist U, Hillmann A, Delling G, Salzer-Kuntschik M, Jürgens H, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002 Feb 15;94(4):1069-77 [PubMed] [Google Scholar]
- 10.Clark JC, Dass CR, Choong PF. A review of clinical and molecular prognostic factors in osteosarcoma. J Cancer Res Clin Oncol. 2008 Mar;134(3):281-97 Epub 2007 Oct 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009 Apr 1;115(7):1531-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cormier JN, Xing Y, Gor BJ, Chan W. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006 Mar 15;106(6):1276-85 [DOI] [PubMed] [Google Scholar]
- 13.Sun M, Abdollah F, Liberman D, Abdo A, Thuret R, Tian Z, Shariat SF, Montorsi F, Perrotte P, Karakiewicz PI. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer. 2011 Sep 15;117(18):4277-85 Epub 2011 Mar 08 [DOI] [PubMed] [Google Scholar]
- 14.Robert SA, Strombom I, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, Remington PL. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004 Jul;15(4):442-50 [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute Surveillance epidemiology and end results: county attributes. 2012 Apr 16. http://seer.cancer.gov/seerstat/variables/countyattribs/. Accessed 2012 Aug 5
- 16.Harting MT, Lally KP, Andrassy RJ, Vaporciyan AA, Cox CS, Jr, Hayes-Jordan A, Blakely ML. Age as a prognostic factor for patients with osteosarcoma: an analysis of 438 patients. J Cancer Res Clin Oncol. 2010 Apr;136(4):561-70 Epub 2009 Sep 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrari S, Bertoni F, Mercuri M, Picci P, Giacomini S, Longhi A, Bacci G. Predictive factors of disease-free survival for non-metastatic osteosarcoma of the extremity: an analysis of 300 patients treated at the Rizzoli Institute. Ann Oncol. 2001 Aug;12(8):1145-50 [DOI] [PubMed] [Google Scholar]
- 18.Ek ET, Ojaimi J, Kitagawa Y, Choong PF. Outcome of patients with osteosarcoma over 40 years of age: is angiogenesis a marker of survival? Int Semin Surg Oncol. 2006;3:7 Epub 2006 Mar 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahey M, Spanier SS, Vander Griend RA. Osteosarcoma of the pelvis. A clinical and histopathological study of twenty-five patients. J Bone Joint Surg Am. 1992 Mar;74(3):321-30 [PubMed] [Google Scholar]
- 20.Kawai A, Huvos AG, Meyers PA, Healey JH. Osteosarcoma of the pelvis. Oncologic results of 40 patients. Clin Orthop Relat Res. 1998 Mar;348:196-207 [PubMed] [Google Scholar]
- 21.Spanier SS, Shuster JJ, Vander Griend RA. The effect of local extent of the tumor on prognosis in osteosarcoma. J Bone Joint Surg Am. 1990 Jun;72(5):643-53 [PubMed] [Google Scholar]
- 22.Brasme JF, Morfouace M, Grill J, Martinot A, Amalberti R, Bons-Letouzey C, Chalumeau M. Delays in diagnosis of paediatric cancers: a systematic review and comparison with expert testimony in lawsuits. Lancet Oncol. 2012 Oct;13(10):e445-59 [DOI] [PubMed] [Google Scholar]
- 23.Smith EC, Ziogas A, Anton-Culver H. Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer. 2012 Dec 15;118(24):6179-87 Epub 2012 Jun 26 [DOI] [PubMed] [Google Scholar]
- 24.Marlow NM, Halpern MT, Pavluck AL, Ward EM, Chen AY. Disparities associated with advanced prostate cancer stage at diagnosis. J Health Care Poor Underserved. 2010 Feb;21(1):112-31 [DOI] [PubMed] [Google Scholar]
- 25.Macleod U, Mitchell ED, Burgess C, Macdonald S, Ramirez AJ. Risk factors for delayed presentation and referral of symptomatic cancer: evidence for common cancers. Br J Cancer. 2009 Dec 3;101(Suppl 2):S92-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford SM, Sauerzapf V, Haynes R, Forman D, Jones AP. Social and geographical factors affecting access to treatment of colorectal cancer: a cancer registry study. BMJ Open. 2012;2(2):e000410 Epub 2012 Apr 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyratzopoulos G, Abel GA, Barbiere JM, Brown CH, Rous BA, Greenberg DC. Variation in advanced stage at diagnosis of lung and female breast cancer in an English region 2006-2009. Br J Cancer. 2012 Mar 13;106(6):1068-75 Epub 2012 Mar 01 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest


