Abstract
Human CD4+CD39+ regulatory T (Treg) cells hydrolyze exogenous ATP and participate in immunosuppressive adenosine production. They contain two T-cell subsets whose role in mediating suppression is not understood. Frequencies of both CD4+CD39+ subsets were evaluated in peripheral blood lymphocytes of 57 cancer patients and in tumor infiltrating lymphocytes (TILs) of 6 patients. CD4+CD39+ and CD4+CD39neg T cells isolated using immunobeads and cell sorting were cultured under various conditions. Their conversion into CD39+FOXP3+CD25+ or CD39+FOXnegCD25neg cells was monitored by multiparameter flow cytometry. Hydrolysis of exogenous ATP was measured in luminescence assays.
Two CD4+CD39+ cell subsets differing in expression of CD25, FOXP3, CTLA-4, CD121a, PD-1, LAP, GARP and the cytokine profile accumulated with equal frequencies in the blood and tumor tissues of cancer patients. The frequency of both subsets was significantly increased in cancer. CD39 expression levels correlated with the subsets' ability to hydrolyze ATP. Conventional CD4+CD39neg T cells incubated with IL-2 + TGF-β expanded to generate CD4+CD39+FOXP3+ Treg cells, while CD4+CD39+FOXP3negCD25neg subset cells stimulated via the TCR and IL-2 converted to FOXP3+CTLA4+CD25+ TGF-β-expressing Treg cells. Among CD4+CD39+ Treg cells, the CD4+CD39+FOXP3negCD25neg subset serves as a reservoir of cells able to convert to Treg cells upon activation by environmental signals.
Keywords: CD4+CD39+ Treg cells, CD39 expression, ATP hydrolysis, immune suppression, head and neck squamous cell cancer (HNSCC)
Introduction
Human tumor cells have evolved numerous strategies to avoid and escape from host-mediated antitumor immune responses. Among these strategies, the accumulation of regulatory T (Treg) cells at the tumor site and in the peripheral circulation has recently attracted significant attention. The ability of Treg cells to effectively suppress functions of effector T (Teff) cells responsible for anti-tumor responses and thus to contribute to tumor progression has been evaluated in patients with different cancers [1–3]. In aggregate, current data show that the frequency and suppressor activity of Treg cells are increased in the blood of cancer patients relative to those in age- and sex-matched healthy controls (HCs) [4]. Mechanisms utilized by Treg cells to induce Teff cells suppression have been extensively examined and appear to be diverse and dependent on the environmental context [5]. Today, the role of Treg cells in cancer progression remains controversial. In human cancer associated with pre-existing chronic inflammatory conditions such as, e.g., colon carcinoma, the presence of Treg cells has been related to improved prognosis [3]. In many other solid tumors, however, Treg cells accumulations predict poor outcome [6]. The origin of Treg cells accumulating in the tumor milieu is also unclear. Current experimental evidence indicates that conventional T (Tconv) cells can differentiate into adaptive or inducible (i) Treg cells under specific environmental stimuli [7]. These results suggest that Treg cells, like other CD4+ T cells, are characterized by plasticity which is determined by the local environment [8].
Murine and human Treg cells were reported to express an ectonucleoside triphosphate diphosphohydrolase-1 or CD39, which is responsible for hydrolysis of exogenous of ATP to ADP and AMP [9, 10]. Subsequent hydrolysis of AMP to adenosine is mediated by 5'nuclotidase, CD73, which in mice is also expressed on the surface of Treg cells [9, 10] but in humans is not detectable by flow cytometry on the surface of Treg cells in the peripheral blood [11]. We have recently demonstrated that CD39 is a functional marker on Treg cells, which links them to the ATP breakdown and potentially to the production of immunosuppressive adenosine [12]. We and others have recently described the presence of two subsets of CD4+CD39+ T cells in the human peripheral blood based on CD25 and FOXP3 expression [12]. One subset is FOXP3+CD25+ and suppresses proliferation of autologous CD4+ Tconv cells, the other is FOXP3negCD25neg and does not mediate suppression. Both subpopulations are CD45RO+CD45RAneg T cells and are present in about equal proportions in the peripheral circulation of HCs [12]. Further, the presence of both subsets has been described in infected knee joints by Moncrieffe et al. [13], and in the circulation of patients with renal allograft rejection by Dwyer et al. [11].
Although the plasticity and heterogeneity of Treg cells have been previously recognized [14], the origin and the role of these two CD4+CD39+ Treg cells subsets in disease remain unclear. The possibility that these two subsets represent functionally distinct Treg cells subsets introduces the notion of the “division of labor” among human Treg cells. Here, we examine phenotypic and functional differences between these subsets, consider their origin from Tconv, and evaluate the potential mechanisms used by these cells for the control of immune responses in patients with head and neck squamous cell carcinoma (HNSCC).
Results
Phenotype and cytokine profile of CD4+CD39+ T cells
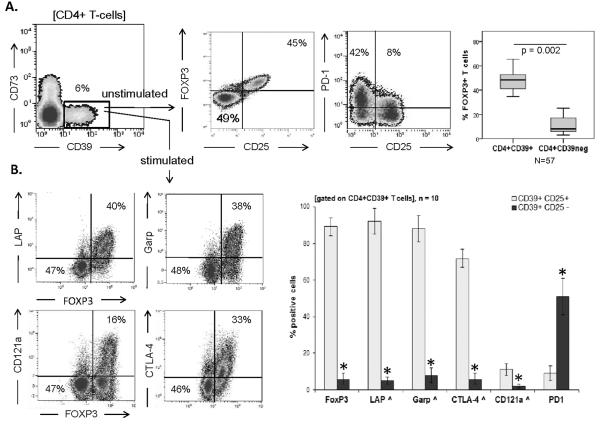
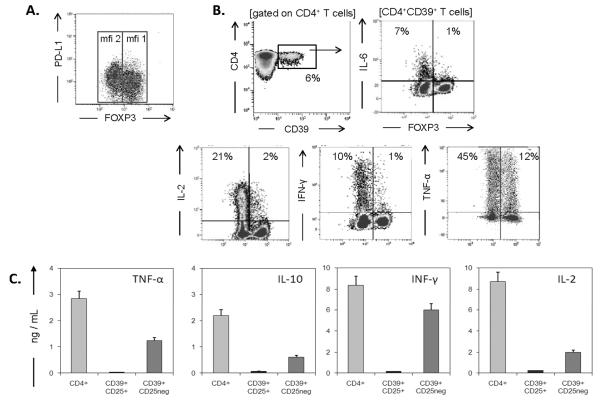
In the peripheral blood of HCs, CD4+CD39+ T cells account for about 6% of total CD4+ T cells (Figure 1A). Confirming our previously reported data, CD4+CD39+ cells contained two phenotypically-distinguishable cell subsets: CD4+CD39+FOXP3+CD25+ and CD4+CD39+FOXP3negCD25neg [12]. In the following text, these cell populations are referred to as `SUBSET 1' (CD25+FOXP3+), and `SUBSET 2' (CD25negFOXP3neg), respectively. Among CD4+CD39+ T cells, the mean frequency (± SD) of FOXP3+ cells was 48 ± 7%, and it was only 11 ± 7% among CD4+CD39neg T cells (Figure 1A). After short-term stimulation (6 h) by SEB, only `SUBSET 1' cells became positive for markers characterizing Treg cells such as TGF-β-associated LAP and GARP as well as IL-1-receptor CD121a and intracellular CTLA4 (Figure 1B). Following stimulation, only `SUBSET 2' cells expressed Th1 cytokines and low concentrations of IL-10, as shown by flow cytometry and Luminex assays (Figure 2). Interestingly, expression of PD-1 on SUBSET 2 cells under unstimulated ex vivo conditions was higher (p < 0.02) as compared with the CD39+FOXP3+ subset (Figure 1A). PD-L1 was expressed on both subsets of CD4+CD39+ lymphocytes with no significant difference in the MFI (Figure 2A). “SUBSET 2” cells with higher levels of PD-1 expression may be susceptible to suppression by “SUBSET 1” cells. In the presence of anti-PD-1 Abs, expression of TNF-α in SUBSET 2 cells was significantly increased (Figure S1). Of note, in the peripheral blood of HCs, CD4+CD39+ were CD73neg by flow cytometry.
Figure 1. Phenotypic features of CD4+CD39+ cells.
(A) PBMCs from HCs were isolated, gated as shown. FOXP3 and PD-1 expression by the CD25+FOXP3+ subset and % of FOXP3+ expression by CD4+CD39+ and CD4+CD39neg T cells were examined by flow cytometry. Box plots show quartiles for 25, 50, 75 as boxes, and values for 0% and 100% as whiskers. (B) CD4+CD39+ T cells were stimulated with SEB for 6 h. Co-expression of TGF-β-associated LAP, GARP, IL-1-receptor CD121a, CTLA4 with FOXP3 was determined. Data are representative (contour plots) or means + SD (bar graphs) of 10 independent experiments each performed using cells from a different HC *p < 0.05 for A&B. All p values in this and other figures were determined using Kruskal-Wallis and two-tailed exact Wilcoxon-Mann Whitney tests.
Figure 2. Expression of PD-L1 and the cytokine profile in CD4+CD39+ T cells.
(A) PD-L1 and FOXP3 co-expression on unstimulated CD4+CD39+ T cells isolated from PBMCs of HCs. (B) PBMCs obtained from HCs were stimulated with SEB for 6 h in the presence of Brefeldin A and stained for intracellular IL-6, IL-2, IFN-γ and TNF-α expression in CD4+CD39+ T cells. Density plots are representative for more than 30 individual experiments each performed with cells of a different HC; (C) Subsets of CD4+CD39+ and CD4+CD39neg cells were single-cell sorted, stimulated with OKT3/anti-CD28 Ab-coated beads and cultured. Supernatants were collected after 48 h, and cytokine concentrations were measured by Luminex. The data are means ± SD for three cultures. CD4+CD39neg Tconv cells served as a positive reference for cytokine measurements.
Surface expression of ecto ATPase, CD39, and ATP-hydrolysis
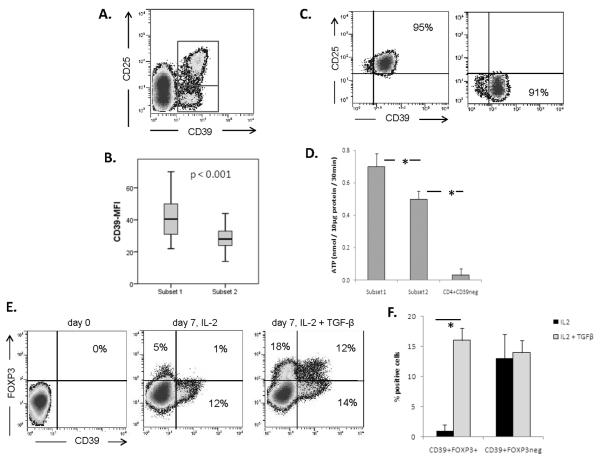
As shown in Figure 3, the two subsets of CD4+CD39+ T cells differed in the expression level of surface CD39 (p < 0.001). The mean fluorescence intensity (MFI) of CD39 was significantly greater in `SUBSET 1' (40 ± 9) than that in `SUBSET 2' (23 ± 5) (n=76 determinations). We, therefore, tested the ability of sorted CD4+CD39+ subsets to hydrolyze exogenous ATP. As expected, `SUBSET 1' cells hydrolyzed exogenous ATP significantly more effectively than `SUBSET 2' cells (p < 0.05). No ATP was hydrolyzed by CD4+CD39neg T cells (Figure 3). The data confirm that CD39 expression levels on the cell surface correlate with its enzymatic activity, i.e., the ability to hydrolyze ATP.
Figure 3. Expression levels of CD39 and hydrolysis of exogenous ATP.
(A) The two CD4+CD39+ subsets are distinguished by CD25 expression; (B) Mean fluorescence intensity (MFI) of CD39 expression in CD25+FOXP3+ cells and CD25negFOXP3neg cells is shown (n=3 for each subset). (C) The purity of CD4+CD39+ cells single cell-sorted into CD25+ and CD25neg cells is shown in a representative plot. (D) The utilization of exogenous ATP by T cells in Subsets 1 and 2. The data are means ± SD from 3 independent experiments with cells of different HCs (*p < 0.05). (E) Induction of CD39+FOXP3+ Treg cells in vitro from separated CD4+CD39neg Tconv cells stimulated via TCR and cultured in the presence of IL-2 (50 IU/mL) ± TGF-β (10ng/mL). (F) Bar graphs show the percentages of CD39+FOXP3+ Treg cells induced in the presence of IL-2 alone or IL-2 and TGF-β. The data are means ± SD from 5 independent experiments with cells of different HCs.
Induction of CD39+FOXP3+ T-cell expansion by exogenous TGF-β
When isolated CD4+CD39neg Tconv cells) were cultured in the presence of IL-2 alone, they expanded with an increase in cells expressing the `SUBSET 2' phenotype (Figure 3E). However, in the presence of exogenous TGF-β (10ng/mL), the proportion of expanding `SUBSET 1' cells considerably increased on day 7. Thus, in the presence of TGF-β in the culture, CD39negFOXP3neg Tconv cells up-regulated and co-expressed CD39 and FOXP3 markers on a substantial proportion of expanding cells (16 ± 3%; means ± SD). It is important to note that in the presence of exogenous TGF-β, a subset of these cells (18% in Figure 3E) up-regulated FOXP3 independently of CD39 expression. No CD39+ T cells were seen on day 3 of culture indicating that the induction of CD39 is relatively slow, as it takes at least 4–5 days under the culture conditions used (data not shown). These results suggest that TGF-β is one of the factors necessary for co-induction of CD39 and FOXP3 expression in expanding Tconv cells. In preliminary experiments, we observed that concentrations of exogenous TGF-β influence the rate of conversion (data not shown). As early apoptosis of “SUBSET 2” cells in culture rather than conversion could be responsible for gains in “SUBSET 1,” we performed absolute cell counts in the presence of a trypan blue dye. No cell death but rather increased cell numbers on day 3 relative to day 1 counts were observed, ruling out early apoptosis as a potential explanation for increases in numbers of CD4+CD39+FOXP3+ cells.
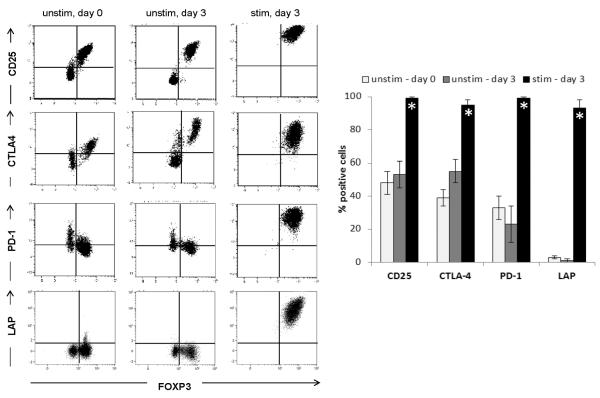
Conversion of `SUBSET2' cells to `SUBSET 1' cells
In unstimulated, freshly isolated CD4+CD39+ T cells the two subsets are readily identifiable based on their expression of the markers commonly associated with Treg cells: CD25, FOXP3, CTLA4, and PD-1 (Figure 4, first column). Unstimulated cells showed a minor up-regulation of CD25 and CTLA4 in `SUBSET 1' cells only (second column). However, upon stimulation with SEB, `SUBSET 2' cells became CD25+FOXP3+PD-1+. Importantly, they also expressed TGF-β associated LAP on their surface which indicates their suppressive function (third column). These results were not altered by the addition of exogenous TGF-β to the culture (data not shown). The data are consistent with the conclusion that in the presence of activation signals, `SUBSET 2' cells acquire phenotypic markers of Treg cells.
Figure 4. Conversion of CD39+FOXP3negCD25neg cells into CD39+FOXP3+CD25+ Treg cells.
Unstimulated CD4+CD39+ T cells consisted of two subpopulations, which differed in their expression of CD25, FOXP3, CTLA4 and PD-1. The cell phenotype was unchanged after a 3-day culture + IL-2 (50 IU/mL) without stimulation. After 3 day stimulation with SEB, all CD39+ T cells expressed Treg cell -associated markers CD25, FOXP3, CTLA4 and PD-1 as well as LAP. Dot plots are gated on CD4+CD39+ T cells. Bar graphs present data (means ± SD) from 4 independent experiments with cells of different HCs (* p < 0.05).
CD4+CD39+ T-cell subsets in the peripheral circulation of patients with cancer
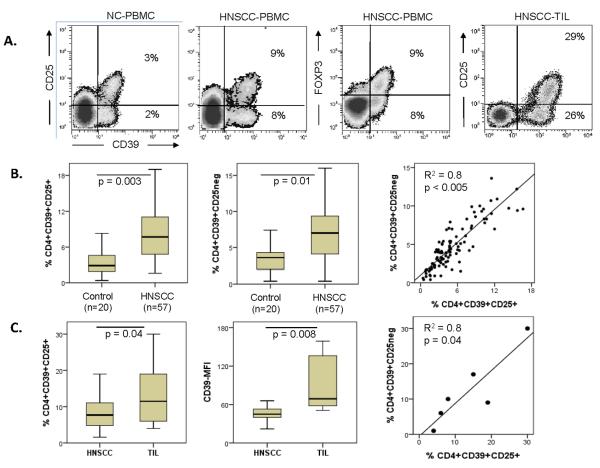
In the peripheral blood of patients with head and neck squamous cell carcinoma (HNSCC), similar to HCs, both subsets of CD4+CD39+ T cells were readily identifiable as well-defined populations by flow cytometry (Figure 5A). In CD39+ T cells, CD25 expression coincided with that of FOXP3. The frequency of `SUBSET 1' and `SUBSET 2' cells was significantly increased in patients with HNSCC relative to that in HCs (Figure 5B), and the percentages of both CD4+CD39+ T-cell subsets significantly correlated with one another (R2 = 0.8, p < 0.005). These data indicate that the two CD4+CD39+ T-cell subsets are present in equal proportions in the peripheral circulation of patients with HNSCC, and that the frequency of both subsets is elevated in the patients relative to that in HCs (p ≤ 0.01).
Figure 5. Frequency of the two CD4+CD39+ T-cell subsets in the peripheral blood of HNSCC patients vs. HCs.
(A) Both subsets of CD4+CD39+ T cells are readily identified as distinct populations by flow cytometry. Representative data for assays performed with cells of 57 HNSCC patients; (B) Box plots show the frequency of both subsets, CD39+CD25+ and CD39+CD25neg, in the circulation of patients with HNSCC relative to HCs. Additionally, correlations are shown for the frequency of both subsets in the peripheral blood of HNSCC patients. (C) Treg cells in tumor infiltrating lymphocytes (TIL). The frequency and MFI for both subsets of CD4+CD39+ Treg cells found in TIL and the correlation of the subset frequencies are shown. TILs were obtained from six HNSCC patients.
CD4+CD39+ T-cell subsets in tumor infiltrating lymphocytes (TILs)
TILs were collected from tumor tissues of six HNSCC patients. In all samples, both subsets were found in about equal proportions (R2=0.8, p=0.04, Figure 5C). In TILs, the mean frequency (± SD) of “SUBSET 1” T cells was 14 ± 10% and that of “SUBSET 2” cells was 12 ± 10%. These frequencies were higher (p = 0.04) than those seen in the circulation of HNSCC patients (Figure 5C). Also, expression of CD39 was significantly elevated in TILs relative to that in circulating CD4+ T cells of HNSCC patients (Figure 5C), suggesting that also the ability to hydrolyze exogenous ATP is increased in TILs. As in PBMCs, we also found in TILs that only `SUBSET 1' cells expressed LAP, GARP, CTLA4 and CD121a (data not shown). In aggregate, these results suggest that suppressor functions of TILs are enhanced relative to those of Treg cells in the peripheral circulation as we have previously reported [1].
Discussion
The co-existence side-by-side of two subsets of CD4+CD39+ T cells in the peripheral blood of HCs and patients with HNSCC is intriguing and raises the question of their role in health and disease. We and others have previously shown that only the CD39+CD25+FOXP3+ subset contains cells able to suppress proliferation of autologous CD4+ T cells [12, 15]. Yet, the CD39+CD25negFOXP3neg subset contains cells able to hydrolyze exogenous ATP. Thus, it is potentially useful in protecting tissue cells from ATP-mediated toxicity [16] and in mediating immune suppression via the adenosinergic pathway [17, 18].
The phenotypic and functional characteristics of the two CD4+CD39+ T-cell subsets that we identified are summarized in Table 1. While both subsets are CD39+ and are able to hydrolyze exogenous ATP, the `SUBSET 1' cells express higher levels of CD39 and, consequently, are more effective in producing 5'AMP and eventually immunosuppressive adenosine. The fact that both subsets express CD39, which, unlike the transcription factor FOXP3 [19], is a stable functional surface marker, implies that both have the potential to mediate suppression. While the two subsets have many features in common, there are some differences that emphasize functional diversity. Specifically, the two subsets can be distinguished by the selective expression of TGF-β-associated surface markers (LAP and GARP), IL-1-receptor CD121a, PD-1 and intracellular CTLA4 in `SUBSET 1' cells only. We confirm the report of Moncrieffe et al. on distinct cytokine profiles in these two subsets [13]. In this respect, the ability of `SUBSET 2' cells to produce IFN-γ, IL-2 and TNF-α suggests they resemble activated CD39neg effector T cells. The `SUBSET 2' cells seem to occupy an intermediate place between CD4+ Tconv cells and differentiated CD4+CD39+FOXP3+ Treg cells. We speculated that they might convert into the `SUBSET 1' Treg cells under favorable conditions. Indeed, this hypothesis is supported by our observation that all `SUBSET 2' cells readily adopt the Treg cell phenotype and function upon stimulation via the TCR (Figure 3). This transformation includes up-regulation of Treg cells-associated surface markers FOXP3, CD25, PD-1 and intracellular CTLA4 as well as TGF-β-associated LAP. Further, this process appears to be independent of exogenous TGF-β, as this cytokine is continuously produced by CD4+CD39+CD25+ Treg cells, which are also present in the culture and which are positive for LAP and GARP. In contrast, with isolated CD4+CD39neg cells, induction of the CD39+FOXP3+ subset required exogenous TGF-β as well as more time, and it was only successful in up to 20% of the starting population.
Table 1.
Phenotypic and functional characteristics of the two CD4+CD39+ T cell subsets
| SUBSET 1 (CD25+FOXP3+) | SUBSET 2 (CD25negFOXP3neg) | |
|---|---|---|
| Frequency | ||
| NC | 3.4 ± 1.9 % | 3.5 ± 1.5 % |
| HNSCC | 6.6 ± 3.8 % | 5.5 ± 3.3 % |
| TIL | 13.6 ± 9.8 % | 12.1 ± 10.1 % |
| Phenotype (in common) | CD39+ CD45RO+PD-L1+ CD45RAneg CD26neg CCR7neg |
CD39+ CD45RO+PD-L1+ CD45RAneg CD26neg CCR7neg |
| Phenotype (differences) | CD25+ CCR4+ FOXP3+ GARP+ LAP+ CD121a+CTLA4+ CD127negCD49dnegPD-1neg |
CD25neg CCR4neg FOXP3neg GARPneg LAPneg CD121anegCTLA4neg CD127+CD49d+PD-1+ |
| Functions | ||
| ATP hydrolysis | yes (strong) | yes (moderate) |
| Cytokine expression a | TGF-β-associated LAP and GARP | IL-6, IL-2, IFN-γ, TNF-α |
| Cytokine production a | not detected | IL-2, IFN-γ, TNF-α, IL-10 |
| Suppression | Suppress proliferation and cytokine expression in Teff [12] | Do not suppress proliferation or cytokine expression [12]. Potential to produce immune-suppressive adenosine |
After activation with SEB for 6 h (expression).
Our data are in agreement with recent observations that differentiated T cells show considerable plasticity enabling them to convert into other lines of differentiation under appropriate environmental conditions [8, 20]. The model we propose based on our in vitro data with human cells fits well with that previously proposed for murine cells [8]. In a simplified form, it explains how suppression of immune responses by Treg cells is kept under a stringent cellular control. Accumulations of both subsets of CD4+CD39+ T cells in situ and in the blood of patients with cancer may, therefore, be explained by the ability of soluble factors present in the tumor microenvironment to induce and promote conversion of CD39neg Tconv cells to the intermediate CD39+FOXPnegCD25neg population, which then proceeds to differentiate into activated and fully suppressive CD39+FOXP3+CD25+ Treg cells. The proposed linear model of Treg cells differentiation contrasts with a stochastic model, in which environmental stimuli at random determine the fate of differentiating Treg cells. However, our data suggest that the CD4+CD39+FOXP3negCD25neg T-cell subset could serve as a reservoir of non-activated Treg cells, which can readily differentiate into fully functional Treg cells. The consistently maintained equal frequency of the two subsets in the peripheral blood of HCs and patients with cancer as well as patients with HIV-1 infection (our unpublished data) implies that the potential conversion of `SUBSET 2' cells into `SUBSET 1' cells is biologically important. Further, the significant increase in the frequency of both subsets among TILs, again preserving the equal ratio of the two, emphasizes the role of the tumor microenvironment in regulating the Treg cells frequency and functions in orderly rather than stochastic manner.
The ability of CD4+ T cells to convert into various lineages (Treg cells /Th1/Th2/Th17) has been described [14]. However, the potential for reciprocal polarity between Treg cells and Th17 cells in the human tumor microenvironment remains uncertain. Th17 cells, like FOXP3+ Treg cells, are long-lived memory T cells, which are associated with chronic inflammation and which accumulate in human tumors [2, 21]. The polarizing factors essential for Th17 differentiation are IL-2, TGF-β and myeloid antigen presenting cells (APCs) [2], and these are similar to conditions required for FOXP3+ Treg cells differentiation. There may be, however, other preferential polarization pathways that favor Treg cells over Th17 cells. For example, our results show that CD4+CD39+CD25neg `SUBSET 2' T cells readily convert into FOXP3+ Treg cells, while CD4+CD39neg Tconv cells are relatively resistant to conversion. In the same context, others show that CD4+CD39+ T cells resist polarization to Th17 cells [11, 13]. Plasticity of CD4+ T cells may be a common phenomenon, but it appears that certain subsets of CD4+CD45RO+ memory T cells are more easily polarized to Treg cells than others.
The high levels of PD-1 expression on the surface of `SUBSET 2' cells and the presence of PD-L1 on the surface of `SUBSET 1' cells strongly suggests that the two CD4+CD39+ subsets interact and perhaps self-regulate their functions. In this context, it is possible to speculate that once activated, PD-L1+ Treg cells have the capability to regulate the differentiation and proliferation of `SUBSET 2' cells, depending on the need for suppression in the local microenvironment. A role of the PD-1/PD-L1 pathway in this interaction between the two subsets is intriguing and deserves further evaluation.
Overall, based on ex vivo examination of CD39+ Treg cells in several different diseases and disease sites, including cancer, autoimmune conditions, HIV-1 infection and transplant rejection [11, 13, 22, 23], it appears that these ATP-hydrolyzing, adenosine-producing cells have attributes enabling them to interact, rapidly respond to environmental changes and to mediate several distinct functions, i.e., ATP removal on the one hand and suppression of immune functions on the other. The view that emerges is that of a multifunctional Treg cells regulated by the microenvironment in which they reside or to which they are recruited.
Materials and Methods
Healthy donors and cancer patients
Peripheral venous blood samples were obtained from 20 healthy control subjects (HCs) and 57 patients with head and neck squamous cell carcinoma (HNSCC). All patients were seen in the Outpatient Clinic of the Department of Otolaryngology at the University of Pittsburgh Medical Center between April 2010 and March 2011, and all subjects signed an informed consent approved by the Institutional Review Board of the University of Pittsburgh (IRB #991206). The HC cohort included 5 females and 15 males with a mean age of 50 ± 5 years (range: 39 – 69 years). The patient cohort included 15 females and 42 males with a mean age of 57 ± 9 years (range: 31 – 78 years). All patients underwent surgery for removal of the primary tumor, and 10 patients additionally received adjuvant radio-chemotherapy prior to phlebotomy for this study. Tumor infiltrating lymphocytes were obtained from additional six HNSCC patients whose tumors were removed at surgery and available for research under the signed consent.
Collection of peripheral blood mononuclear cells
Buffy coats obtained from healthy volunteers (70–80mL) were purchased from the central Blood Bank of Pittsburgh. Blood samples from cancer patients (30–40mL) were drawn into heparinized tubes and centrifuged on Ficoll-Hypaque gradients (GE Healthcare Bioscience). Peripheral blood mononuclear cells (PBMCs) were recovered, washed in AIM-V medium (Invitrogen) and immediately used for experiments. Tumor samples were dissociated before Ficoll-Hypaque gradients.
Cell isolation
CD4+CD39+ and CD4+CD39neg T-cell populations were separated by magnetic beads as previously described [12]. Briefly, negative selection of CD4+ cells was performed using a commercially available biotin-conjugated antibody cocktail specific for the lineage antigens. Next, CD39+ T cells were isolated from the CD4+ T-cell population using biotinconjugated anti-CD39 Abs and magnetic beads coated with anti-biotin Abs. Cell separation was performed using reagents purchased from Miltenyi in an AutoMACS according to the manufacturer's protocol. For functional assays, CD4+ T cells were separated on AutoMACS and stained for relevant surface markers, followed by single-cell sorting into CD39+CD25+ and CD39+CD25neg cells using MoFlow (DAKO cytomation). Viability of sorted cells was measured using a trypan blue dye.
Flow cytometry reagents
The following anti-human monoclonal antibodies (mAb) were used for staining: CD39-FITC/PE/PC7, FOXP3-FITC/PE (clone PCH101), PD-1-PE, PD-L1-PE, LAP-PE, IL-6-PE, GARP-APC, TNF-α-eFluor450 (all eBioscience), CD19-ECD, CD14-ECD, CD45RO-ECD, CD4-PC5, CD8-PC5 (all Beckman Coulter), IFN-γ-APCCy7 (Biolegend), CD25-PE (Miltenyi), CD4-AF700, IL-2-APC, CTLA4-PE (all BD Biosciences), CD121a-PE (R&D Systems) including their respective isotypes, which served as negative controls for surface as well as intracellular staining. All Abs were pre-titrated using activated as well as non-activated PBMCs to determine the optimal staining dilution for each.
Surface and intracellular staining
To measure cytokine expression levels, PBMCs obtained from HCs or cancer patients were stimulated with OKT3/anti-CD28 Ab-coated beads (ratio 1:10, Miltenyi) in 200μL RPMI medium in the presence of Brefeldin A (10μg/mL) for 6 h. Cells were washed and incubated with mAbs specific for each surface marker in 50μL PBS for 30 min at room temperature in the dark. For intracellular staining of cytokines, FOXP3, and CTLA4 cells were fixed for 30 min and washed twice with permeabilization buffer using a commercially available FOXP3 staining kit (eBioscience) as previously described [22]. For measurements of surface CD121a, LAP and GARP, the same conditions were applied without the addition of Brefeldin A.
Flow cytometry
Flow cytometry was performed using an EPICS XL-MCL flow cytometer equipped with Expo32 software and, for cytokine expression assays, a Gallios Cytometer equipped with Expo32 and Kaluza software (all Beckman Coulter). The acquisition and analysis gates were restricted to the lymphocyte gate based on characteristic properties of the cells in the forward and side scatter. At least 1 × 105 events were acquired for analysis and, where applicable, gates were restricted to the CD4+, CD4+CD39+, or CD4+CD39+CD25neg T-cell subsets.
Cytokine production
Various T-cell subpopulations (0.2 × 106 cells/well), including isolated CD4+, CD4+CD39+CD25+, and CD4+CD39+CD25neg T-cell subsets were isolated from PBMCs by magnetic beads and single cell sorting. Cells were then stimulated with OKT3/anti-CD28 Ab-coated beads (ratio 1:1) and cultured in 200μL aliquots of AIM-V medium. On day 2 and 5, supernatants were collected for determination of IL-2, IL-10, IFN-γ and TNF-α cytokine production by Luminex technology (Invitrogen) according to the manufacturer's instructions.
ATP-hydrolysis
CD4+CD39+CD25+ and CD4+CD39+CD25neg cell populations (25 × 103 per well) obtained from HCs were incubated in flat-bottomed 96-well plates for 30 min with 20μM exogenous ATP (Sigma-Aldrich). The concentration of unhydrolyzed ATP was determined by measuring the frequency of luminescent events (CPM) in a luciferase-based detection system (ATP Lite Luminescence System, Perkin-Elmer). Plates were examined in a Microplate Scintillation and Luminescence Counter (Packard). The average count was determined from triplicate wells and the percentage of ATP hydrolysis was determined by using the following formula: ((CPM ATPalone) - (CPM ATPsample) / (CPM ATPalone + CPM ATP for cellsalone)) × 10.
Induction and conversion of Treg cells
CD4+CD39neg and CD4+CD39+ T cells were separated by magnetic beads as described above and separately incubated in 96-well-plates with 200μL RPMI media supplemented with L-glutamine, streptomycin/penicillin and 10% fetal bovine serum (FBS), as well as IL-2 (50IU/mL) ± TGF-β (10ng/mL, R&D Systems). Cells were stimulated with staphylococcal enterotoxin B (SEB, Sigma-Aldrich), and expression of Treg cells -specific markers was monitored. Controls included unstimulated wells for CD4+CD39+ T cells. Media, cytokines and SEB were exchanged after 48 h.
Statistics
Averages were calculated as means. For non-parametric distribution of samples, p-values were calculated by Kruskal-Wallis and two-tailed exact Wilcoxon-Mann-Whitney tests using SPSS software (Version 19, IBM). Correlations were calculated by the Spearman test. P values < 0.05 and R2 values > 0.5 were considered to be significant.
Supplementary Material
Acknowledgements
This study was in part supported by NIH grant PO1 CA109688 (T.L.W.) and by the Pittsburgh-Essen-Partnership Program (P.J.S.). This project used the Immunologic Monitoring and Cellular Products Laboratory (IMCPL) and the Core Facility for Flow Cytometry at University of Pittsburgh Cancer Center.
Abbreviations
- (ATP)
adenosine triphosphate
- (ADP)
adenosine diphosphate
- (AMP)
adenosine monophosphate
- (TGF-β)
transforming growth factor beta
- (LAP)
lysosomal acid phosphatase
- (GARP)
glycoprotein A repetitions predominant
- (SEB)
staphylococcal enterotoxin B
- (PD-L1)
program death ligand 1
- (IFN)
interferon
- (IL)
interleukin
- (TNF)
tumor necrosis factor
- (PBMCs)
peripheral blood mononuclear cells
- (HNSCC)
head and neck squamous cell carcinoma
Footnotes
Conflict of Interest. The authors declare no financial or commercial conflict of interest.
References
- [1].Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin.Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- [2].Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- [3].Gounaris E, Blatner NR, Dennis K, Magnusson F, Gurish MF, Strom TB, Beckhove P, et al. T-regulatory cells shift from a protective anti-inflammatory to a cancer-promoting proinflammatory phenotype in polyposis. Cancer Res. 2009;69:5490–5497. doi: 10.1158/0008-5472.CAN-09-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- [5].Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int.Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- [6].Bindea G, Mlecnik B, Fridman WH, Galon J. The prognostic impact of anti-cancer immune response: a novel classification of cancer patients. Semin Immunopathol. 2011;33:335–340. doi: 10.1007/s00281-011-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bergmann C, Strauss L, Zeidler R, Lang S, Whiteside TL. Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Res. 2007;67:8865–8873. doi: 10.1158/0008-5472.CAN-07-0767. [DOI] [PubMed] [Google Scholar]
- [8].Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- [10].Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schuler PJ, Harasymczuk M, Schilling B, Lang S, Whiteside TL. Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J Immunol Methods. 2011;369:59–68. doi: 10.1016/j.jim.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, Eaton S, Wedderburn LR. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T-cell population. J Immunol. 2010;185:134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T-cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- [15].Ndhlovu LC, Leal FE, Eccles-James IG, Jha AR, Lanteri M, Norris PJ, Barbour JD, et al. A novel human CD4+ T-cell inducer subset with potent immunostimulatory properties. Eur J Immunol. 2010;40:134–141. doi: 10.1002/eji.200939258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- [17].Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- [18].Whiteside TL, Mandapathil M, Schuler P. The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg cells) Curr Med Chem. 2011;18:5217–5223. doi: 10.2174/092986711798184334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat.Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bailey-Bucktrout SL, Bluestone JA. Regulatory T cells: stability revisited. Trends Immunol. 2011;32:301–306. doi: 10.1016/j.it.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, Moyer J, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schulze Zur Wiesch J, Thomssen A, Hartjen P, Toth I, Lehmann C, Meyer-Olson D, Colberg K, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol. 2011;85:1287–1297. doi: 10.1128/JVI.01758-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Law JP, Hirschkorn DF, Owen RE, Biswas HH, Norris PJ, Lanteri MC. The importance of Foxp3 antibody and fixation/permeabilization buffer combinations in identifying CD4+CD25+Foxp3+ regulatory T cells. Cytometry A. 2009;75:1040–1050. doi: 10.1002/cyto.a.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.