Abstract
The tissue engineering field has made great strides in understanding how different aspects of tissue engineered constructs (TECs) and the culture process affect final tendon repair. However, there remain significant challenges in developing strategies that will lead to a clinically effective and commercially successful product. In an effort to increase repair quality, a better understanding of normal development, and how it differs from adult tendon healing, may provide strategies to improve tissue engineering. As tendon tissue engineering continues to improve, the field needs to employ more clinically relevant models of tendon injury such as degenerative tendons. We need to translate successes to larger animal models to begin exploring the clinical implications of our treatments. By advancing the models used to validate our TECs, we can help convince our toughest customer, the surgeon, that our products will be clinically efficacious. As we address these challenges in musculoskeletal tissue engineering, the field still needs to address the commercialization of products developed in the laboratory. TEC commercialization faces numerous challenges because each injury and patient is unique. This review aims to provide tissue engineers with a summary of important issues related to engineering tendon repairs and potential strategies for producing clinically successful products.
Keywords: Tendon tissue engineering, tendon injury, translation, commercialization
Current Tissue Engineering Applications for Tendon Repair
Tendon injury remains common and successful repair remains a significant challenge in orthopaedics.
Injury Incidence
The most commonly injured tendons include the flexor and extensor tendons of the hand (incidence of 4.83 and 18/100,000 per year, respectively)1, the Achilles tendon (12–18/100,000 per year)1, 2, and the rotator cuff tendons (3.73/100,000 per year)1.
Surgical Tendon Repair
Surgical repair of these tendon injuries does not consistently restore function. The failure rate for flexor tendon repair is 4 to 13% with the most common cause of failure being overloaded sutures3–5. For the Achilles tendon (AT), open operative repairs have a re-rupture rate of roughly 2–4%6, 7. However, the failure rate of open surgery is approximately double that for percutaneous repair (4.3% and 2.1%, respectively) and postoperative casting followed by functional bracing significantly reduces re-rupture, when compared to bracing alone (2.4% versus 12.2%, respectively)6. Rotator cuff repair outcomes vary tremendously, with failure rates ranging from 11 to 95% two years following repair 8–10. Similar to the AT, rotator cuff repair can also require an immobilization period. Patients treated for tendon injuries can be immobilized from physiologic loads during healing which makes them more susceptible to joint stiffness and muscle atrophy11, 12. Inconsistencies in traditional repair outcomes necessitate alternative treatments like tissue engineering which has the potential to better restore tendon function after injury.
Flexor Tendon
Tendon grafting is commonly required to repair an injury to a flexor tendon. However, there is a lack of suitable graft material and controversy remains as to which materials are best suited for flexor tendon repair (i.e. extrasynovial vs. intrasynovial flexor tendons). Additionally, most grafting procedures lead to adhesions which limit joint mobility13. Kryger et. al.14 used acellularized allogenic tendon as a scaffold material. Grafts were re-cellularized with epitenon tenocytes, tendon sheath fibroblasts, bone marrow-derived mesenchymal stem cells (MSCs), or adipose derived MSCs and then implanted into a flexor profundus tendon defect in the rabbit. Constructs re-cellularized using each of the four cell types showed viability at 6 weeks unlike acellular controls which remained acellular at 4 and 8 weeks. Although this group did not evaluate tendon adhesions, Hasslund et. al.13 have used a mouse model to examine tendon gliding. The results of their study demonstrated that there was little biomechanical advantage to using autografts for flexor digitorum longus repair as compared to devitalized allografts.
Achilles Tendon (AT)
Repair of AT injuries is often difficult due to the demanding mechanical environment and the fact that the remaining AT tissue is often frayed and unable to bear appreciable loads15–17. Our group has studied aspects of this problem by attempting to repair controlled rabbit AT defects with two types of tissue engineered constructs (TECs). We first created these TECs by suspending 1×105 MSCs/ml in either a low (1.3 mg/ml) or high (2.6mg/ml) concentration of type I collagen gel and then contracted each TEC around two end posts for two weeks18. Low and high collagen concentration TECs were then implanted into contralateral full-width, full-thickness defects in the lateral half of the rabbit AT. While varying TEC collagen concentration did not significantly affect repair biomechanics at 12 weeks post-surgery, the average maximum force and stiffness of these TEC-based repairs were 50% and 60% of normal AT values, respectively18. The corresponding average maximum stress and modulus of these repairs were both 85% of normal AT values18 and both repair groups exceeded the largest peak in vivo forces (IVFs) we have previously recorded in the rabbit AT (19% of failure force)19.
However, our method is not the only approach being taken to improve AT repair. AT defects have also been repaired using bioabsorbable polymer scaffolds and small intestinal submucosa (SIS)20, 21. Ouyang et. al.20 infused knitted Poly-Lactic-Co-Glycolic Acid (PLGA) scaffolds with 0.3ml fibrin glue alone or mixed with 1 million bone marrow stromal cells to fill 1 cm long defects in the rabbit AT. Compared to acellular controls, the cellular repairs increased collagen types I and III and achieved 87% of normal tendon stiffness by 12 weeks. Sato et. al.21 contrasted AT repair using TECs containing either poly–N–acetyl–D–glucosamine (chitin), poly-ε-caprolactone (PCL), polylactic acid (PLA), or chitin/PCL composite. Chitin was not able to maintain sufficient strength in vivo and PCL supported little formation of new fibrous tissue. However, PLA and chitin/PCL composite repairs sustained loads to failure which were roughly 61% and 85%, respectively, of untreated control values at 26 weeks, respectively. In another study, Badylak and co-workers wrapped SIS around a 1.5cm long, full-width, full-thickness AT defect created in a canine model22, 23. By 12 weeks post-surgery, the SIS repair tissue midsubstance sustained greater mechanical force than the proximal musculotendinous origin and distal bony insertion. Consequently, the biomechanical properties of the neotendon repair tissue could not be determined beyond that threshold (approximately 1000N). While SIS and other bioabsorbable scaffolds have already positively influenced AT repair, new strategies are still needed to more effectively repair a rotator cuff injury.
Rotator Cuff
Rotator cuff repair presents a significant clinical challenge because the tendons are subject to high mechanical loads and often have undergone significant degeneration at the time of surgery. Tissue engineers have focused on augmenting suture fixation with various biologic scaffolds including collagen-rich extracellular matrices such as dermis (i.e. GraftJacket Regenerative Tissue Matrix 24–27, TissueMend Soft Tissue Repair Matrix24, and Zimmer Collagen Repair Patch28) and SIS (i.e. Restore Orthobiologic Soft Tissue Implant24, 28 and CuffPatch Bioengineered Tissue Reinforcement24). Augmentation grafts increase suture fixation strength as compared to un-augmented repairs25, and possess a similar biochemical composition to that of tendon24. However, because of the differences between the elastic moduli of grafts and native tendon, the biological scaffolds likely serve a limited mechanical role in rotator cuff repair24. While these scaffolds have been used clinically, there is little evidence that they improve healing of rotator cuff tendons24, 26, 27. In fact, augmenting ovine infraspinatus tendon injuries with SIS (Restore Orthobiologic Soft Tissue Implant) or an acellular porcine dermal patch (Zimmer Collagen Repair Patch) did not significantly improve repair biomechanics at 9 or 24 weeks post-surgery28.
As an alternative approach to rotator cuff repair, researchers have investigated augmenting the tendon-to-bone insertion site with either a fibrin clot or various growth factor treatments. Thomopoulos et. al.29 attempted to repair a rat supraspinatus tendon injury with a fibrin clot. However, they found no biomechanical benefit when compared to untreated controls. Adding a fibrin clot actually decreased repair tissue material properties at 3 weeks post-surgery. By contrast, Rodeo et. al.30 administered an osteoinductive bone protein extract (contains BMP-2-7, TGFβ-1-3, and FGF) using a type I collagen sponge carrier to repair the sheep rotator cuff. When implanted at the interface, the growth-factor infused repairs generated a tendon-to-bone insertion site with greater load to failure than when sponge alone was implanted. In spite of the stronger insertion, the biomechanical results for these groups were still not statistically different after normalizing the results by tissue volume30, 31. Given the increases in repair tissue structural properties found in comparison to untreated and/or sham controls30–32, it is important to recognize that growth factor treatments promote large amounts of scar tissue rather than regenerated tendon31, 33.
Patellar Tendon (PT)
Although the incidence of PT rupture is relatively low (0.68/100,000 per year)1, the patellar tendon has served as a reproducible model for studying natural healing34 and alternative treatment strategies such as tissue engineered constructs (TECs)35–42. In our earliest studies, we created TECs by contracting high concentrations (1, 4 or 8 million cells/ml) of autologous bone marrow-derived mesenchymal stem cells (MSCs) from New Zealand White rabbits in collagen gels around sutures35, 36. When implanted into the PT defect with bone troughs in the patella and tibia, these MSC-gel-suture TECs produced modest improvements in repair stiffness and strength compared to natural healing35, 36. However, these treatments also resulted in ectopic bone in 28% of all repairs at 6, 12, and 26 weeks post surgery35. To eliminate ectopic bone formation and further enhance tendon repair, we 1) reduced cell concentration to 1×105 MSCs/ml, 2) contracted the cell-gel constructs around two end posts to decrease TEC volumetric contraction, and 3) removed the suture which stress shielded the cells. Although ectopic bone formation was eliminated, repair stiffness and maximum force values were still only 26–30% of normal PT values at 12 weeks post-surgery43. To further stimulate tendon repair, we replaced the collagen gel with a collagen sponge composed primarily of bovine type I collagen. At 12 weeks, the MSC-sponge TEC repairs matched the tangent stiffness of the normal PT failure curve up to 100N41, which was equivalent to the maximum in vivo force (IVF) recorded for the rabbit PT during inclined hopping, the most vigorous of the activities of daily living (ADLs) that we studied44. Encouraged by this improvement, we introduced mechanical stimulation in culture based on Functional Tissue Engineering principles45–47. MSC-collagen sponge TECs were mechanically stimulated for two weeks in culture (2.4% strain for 100 cycles in 8 hours/day) and when implanted, produced repair tissue that matched normal PT tangent stiffness up to 150N or 50% greater than measured IVF39, 44. While these results are encouraging, the normal PT in the goat can sustain in vivo forces up to 40% of normal PT failure loads48. Thus, our repairs do not meet potential IVF or provide a safety factor.
Other researchers have also employed the PT defect model to evaluate tissue engineering strategies. Hankemeier et. al.49 used a mixture of human bone marrow stromal cells and liquid fibrin glue to repair a PT defect in immunodeficient rats. At 20 days post-surgery, their TECs repaired the PT with mean collagen fibril diameters, type I collagen mRNA and type I/III collagen mRNA ratio that were not statistically different from normal tendon. However, these repairs were biomechanically inferior to normal PT 49. Using a different approach, Karaoglu et. al.50 implanted small intestinal submucosa (SIS) on the anterior and posterior surfaces of a central-third PT defect in the rabbit. At 12 weeks post-surgery, the repair showed fewer adhesions between the PT and the fat pad and increased repair tissue structural properties when compared to untreated controls (natural healing). However, the material properties of these SIS-treated repairs remained substantially inferior to normal PT values50. Finally, Lyras et. al.51 repaired the central-third defect in the rabbit PT with platelet-rich plasma (PRP) gel. While PRP played an important role in early tendon healing, this treatment did not significantly improve repair biomechanics by 28 days post-surgery compared to untreated controls (natural healing)51. Thus, these treatments may improve a repair’s molecular biology and structure, but restoring normal PT biomechanics is more difficult to achieve.
Summary
The tissue engineering field has made great strides in understanding how different aspects of the TEC and the culture process affect final tendon repair. However, these different repair results from different tendon models raises questions about what fundamental differences are that give rise to these wide-ranging repair outcomes, even when similar approaches are utilized. In extra-articular, acutely-injured tendon models, groups have been able to: 1) match and even exceed the peak IVFs recorded for ADLs; 2) mimic ultimate mechanical properties; and 3) recreate structural features and biological activity patterns of normal tendon18, 38, 39, 41. However, improvements in repair biomechanics and biology have been much more difficult to achieve when tissue engineering strategies are attempted in the rotator cuff model30, 31. We need to understand the fundamental differences in how these models respond to these treatments if tissue engineering is to be a useful clinical treatment modality.
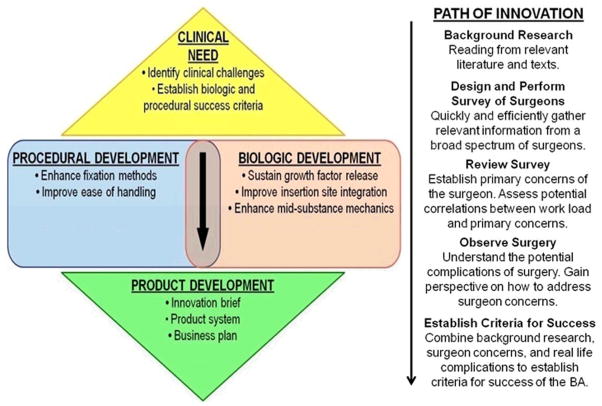
There are still many questions and challenges that face those working in tendon tissue engineering. We need to improve the final repair in the “functional” loading region while also building in a safety factor to account for more strenuous activities. We need to improve tissue quality by restoring more normal fibril distributions. As tendon repair outcomes continue to improve in animal models, we need to: 1) develop more clinically relevant injury models, and 2) produce functional tendon repairs at earlier time points that will ultimately return patients to their pre-injury activities earlier after injury. One of the biggest challenges still facing tissue engineers today is the injury model used to test TEC repairs. For most current pre-clinical injury models, tendons are acutely ruptured or damaged and then immediately repaired. This in no way mimics the clinical scenario where an injury and its repair are separated in time. We need to develop injury models that better mimic the clinical condition so we can fully understand how the TECs perform. Finally, we need strategies to better design TECs that result in functional tendon repair at earlier time points. In that regard, our group is developing a tissue engineering decision tree or TEDT (Fig. 1). This tree incorporates several features, including: 1) a predictive equation that models individual and interactive effects of different factors used to create and mature the TEC; 2) a rational set of evaluation milestones to track the progress of the TEC and repair as it matures in vitro and in vivo; and 3) design goals that determine when our final tendon repair is “good enough.” For example, our goal for repairing the central PT defect in the rabbit model is to match the normal PT failure curve up to at least 100% of peak IVFs by 6 weeks after surgery and at least 250% of peak IVFs by 12 weeks post implantation. Our TEDT will not only speed the process of tissue engineering, but also reduce the cost as we strive to commercialize cell-scaffold based products.
Figure 1. Tissue Engineering Decision Tree (TEDT).
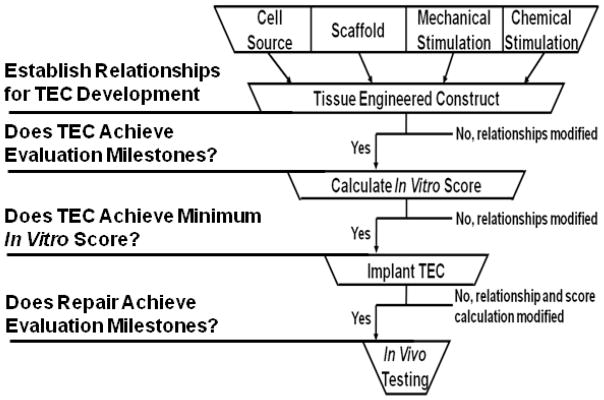
With a near infinite number of possible TEC factors, we need to identify a process to limit those possibilities. We propose to establish relationships among TEC factors and evaluation milestones during all experimental stages and halt experiments that will not lead to improved repair since each successive experimental stage increases time and cost. The goal of our process is reduce the investment (time and money) required for creating effective repairs.
Utilizing Signals and Pathways from Normal Development to Improve Tendon Tissue Engineering Repair
Improving repair outcomes after tendon injury is limited by our lack of understanding of how tendons normally develop. Better understanding how normal development differs from inadequate or impaired adult tendon healing may provide TEC design criteria and evaluation milestones. These criteria can then be used to create TECs that ultimately improve and speed repair. Unfortunately, researchers have still not identified a tendon-specific marker, making it difficult to understand normal tendon development. While not truly tendon-specific, Scleraxis (Scx) is one marker that may be necessary for normal tendon formation52. Scx is a basic helix-loop-helix transcription factor expressed in the sclerotome during early development53. In murine embryogenesis (approximately 21 day gestation), Scx and Sox9 (a transcription factor important for chondrogenesis) act in a coordinated fashion to determine tendon vs. cartilage cell fate, respectively54. Scx and Sox9 expression overlap in the murine sclerotome during early embryonic development. By E11.5, Scx expression surrounds the skeletal primordia where Sox9 is expressed. Sox9 remains in the skeleton while Scx continues to be expressed between the muscle and skeleton where tendons condense (E15.5)54. In fact, Scx may have a pivotal role during tendon condensation, especially in tendons that generate large forces. Murchinson et al.55 have shown that Scx null mice possess severe deformities in force-transmitting tendons. However, Scx null mice show no alterations in anchoring tendons, which carry less force. Scx is not necessary for these tendons to form attachments since tendons in null mice still span from muscle to bone. However, Scx is necessary for appropriate condensation into a mature tendon55. Further study is required to understand mechanisms responsible for tendon formation and the influence of loading environment and body location.
Although growth factor signaling plays an important role in tendon development, healing, and repair, the pathways regulating normal development are not fully understood. Three signaling pathways have been found to regulate aspects of tenogenesis and will be the focus in this review. These pathways include FGF-, TGFβ-, and GDF-signaling56–58.
FGF Signaling
FGF4 and FGF8 are expressed during embryogenesis in the myotome and can induce formation of Scx-expressing progenitor cells in the sclerotome in regions near the myotendinous junction56. When FGF4 is absent, there is a decrease in Scx, tenascin, and FGF8 expression59. However, when FGF4 is reintroduced, only Scx and tenascin are upregulated. Therefore, FGF4 and FGF8 may both aid in cell condensation at the myotendinous junction during tendon development.
TGFβ Signaling
Kuo et. al.57 found that TGF-β1 was not present in the tertiary bundles of chick tendon but was modestly expressed in the endotenon and near the myotendinous junction. TGF-β2 and TGF-β3 were also detected throughout the tertiary bundles and endotenon but only modestly expressed at the muscle attachment. Murine knockouts of these isoforms reveal that TGFβ signaling is essential for limb tendon formation60. Scx expression is severely disrupted in these knockouts as well, suggesting that TGFβ signaling regulates Scx expression.
GDF Signaling
GDF5 and GDF6 knockouts exhibit altered tendon structure and composition, as do GDF7 knockouts to a lesser extent58, 61, 62. GDF5 expression is also upregulated in GDF7 knockouts, suggesting that coordinated expression of these GDF isoforms is necessary for proper tendon formation.
Understanding the expression of tenogenic markers during normal development and natural healing may allow for the isolation and identification of true tendon-specific progenitor cell populations, if they exist. Knowing how these cells condense to form a tendon during development and if/how they behave during natural healing, may provide potential targets to improve repair. If biologists, engineers, and surgeons can learn how to utilize or stimulate these cells during healing, we may ultimately improve healing.
Creation of a Degenerative Tendon Model to Test Tendon TECs
Tendon degeneration or tendinosis63 is seen clinically in approximately 97% of tendon injuries64. Surgeons still debate clinical factors in tendon degeneration, including its onset, the mechanisms involved in its pathogenesis, and the most successful methods for its treatment15, 65. Many investigators66, 67 believe mechanical overuse is a primary factor in the etiology. The working theory is that overuse leads to microtrauma, eliciting both local inflammation and oxidative stress to resident tenocytes68–70. Further, studies have suggested that microscopic damage to collagen fibers can result in an “underuse” environment for the cell, eliciting catabolic signaling and subsequent ECM degradation71. The tendon’s response to multiple microtraumatic events may cause an imbalance in the matrix turnover process, leading to altered production of several matrix metalloproteinases (MMPs)65. Based on mechanical overuse models demonstrating increased expression of inflammatory mediators as well as MMPs72, 73, investigators have suggested that multiple acute inflammatory responses may drive the pathogenesis of degeneration15, 74. For instance, mechanical overloading of tendon explants and tendon fibroblasts in culture increases expression of MMP-1, MMP-13, interleukin-1β (IL-1β), and prostaglandin E2 (PGE2)73, 75, 76. PGE2 delivery to tendon fibroblasts in culture produces decreased collagen synthesis and cellular proliferation77. Administration of IL-1β increases MMP production in numerous tissues including tendon76, 78. Investigators contend this imbalance in matrix turnover leads to collagen matrix disruption and cell phenotype changes, ultimately resulting in degenerative characteristics such as collagen matrix disruption/disorganization, tenocyte hypercellularity, tenocyte nuclear hypertrophy and rounding, mucoid degeneration, neo-vascularization, and small nerve ingrowth (Fig. 2)74, 79.
Figure 2.
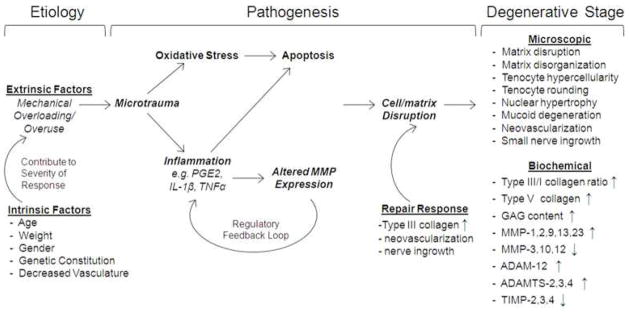
Schematic of potential mechanisms leading to tendon degeneration15, 68, 69, 74.
Unfortunately, the majority of tendon preclinical injury models do not recreate the degenerative condition in a sustainable way. Instead, these models mimic the uncommon situation in which an acute traumatic injury or tear occurs to healthy tendon. Researchers have attempted to develop animal models of tendon degeneration by employing overuse67, 72, 80, collagenase injections81, and cytokine treatments82. Collagenase has been directly injected into tendons of rats72, rabbits83, and horses81. Such treatments disrupt collagen architecture within the tissue and induce hypercellularity. However, their effects depend on injection location and concentration. PGE2 has also been injected multiple times into the rabbit PT82. While PGE2 disrupted the collagen organization, the study did not investigate long-term sustainability of the disruption82. A controllable and sustainable model of tendon degeneration is needed to begin assessing repairs of these degenerative injuries.
Translation of Discoveries to Larger Animal Models
The tissue engineering field needs to challenge our current knowledge base and begin the translation process. The translation process faces many obstacles that can slow progress. Overcoming these obstacles requires that we address several questions. 1) What animal/tissue model or models should be used, and what criteria should be chosen to make these choices? Our group is exploring the translation of tendon and ligament tissue engineering using the sheep model. We have selected the sheep based on several biomechanical and geometric similarities between the sheep and human. When exposed to the same motion paths, both sheep and human knees are loaded throughout the gait cycle84. The intact knee force curves in the sheep and human display similar shapes. Despite the differences between the species, the complex interactions that govern the shape of these knee force curves (e.g., attachment site location, bone geometry, and soft tissue mechanics) appear to be conserved between these models. By contrast, other groups prefer the porcine model85, 86 because the healing process is more similar to humans than for a ruminant87. It is likely that different animal models will be needed to study different aspects of tendon repair and replacement so as to fully assess the potential of different TEC strategies. 2) Does knowledge gained by studying fundamental questions in a more cost-efficient and smaller animal directly translate to the larger animal? Establishing in vitro and in vivo correlates between smaller species (e.g. from mouse to rabbit models) could not only speed the discovery of pre-clinical tissue engineering therapies, but also demonstrates potential for applying these discoveries to even larger species (sheep, porcine, canine). We can test the broader principles that might someday permit rapid, inexpensive experiments to be conducted in the smaller murine model and then strategically apply these principles across multiple species. 3) If our discoveries do not directly translate, what is the process required to adjust the data so the findings can ultimately be mapped across species in a time- and cost-effective manner? This question may be the most difficult and will likely require the coordinated sharing of data among laboratories. Such a plan will likely require large multi-center grants involving teams of investigators targeting important clinical problems using common and controlled experimental designs and methodologies.
This issue is best understood by considering an example. As methods are established to scale up to a larger animal model, what changes must be made in culture techniques or scaffold selection to maintain cellular viability and phenotype throughout the culture process? Increasing scaffold volume can limit the ability of centrally-located cells to exchange nutrients and waste. This problem might be alleviated by introducing perfusion culture, by layering multiple constructs together, or by introducing channels in the scaffold to permit easier access to nutrients. However, each of these modifications can also affect our ability to directly apply the data collected in the smaller animal models. How will perfusion change how cells respond to their local environment? If multiple, smaller volume TECs are used to fill an injury site, how do shear forces and sliding between the TECs affect the healing response? Will adding channels into the scaffold improve nutrient exchange, alter the local cellular mechanical environment and/or change the effect of different treatment combinations? Our field needs to address these and other issues as we strive to create TECs with a likelihood of clinical impact.
Issues Facing Tendon Tissue Engineering Prior to Clinical Use
In addition to the challenges of scaling up tissue engineering technologies to larger animal models, the field also faces the task of convincing our toughest customer, the surgeon, that our product will work. The primary obstacle our field must overcome is that much of our knowledge has been gained from defect and injury models with little to no clinical relevance. One of the most glaring examples is in developing treatments for rotator cuff injuries. By the time a surgeon repairs a torn rotator cuff, the injury has become chronic with degenerative changes to the tendon. The muscle has retracted with the potential for fatty infiltration and scar formation, which can lead to poor outcome irrespective of tendon healing79. With increased muscle retraction, the sutures, suture anchors and degenerative tendon must carry more force, leading to the risk of re-injury. The re-injury rate can increase even more with early motion and aggressive rehabilitation. Our role as tissue engineers will be to determine how our technologies can function in this environment and improve not only the quality of the repair but also the quality of life for the patient.
The explicit use of TECs has yet to be defined, but there are multiple scenarios that need to be considered. First, the TEC could be used as a biological augmentation to induce healing of a degenerative tendon. Current animal studies have examined augmented healing in acutely injured tendons18, 20, 22, 23, 29–32, 39, 41, 49–51. For example, our group has found that TECs produce repair tissue that matches normal failure load up to 150% of peak IVFs for a range of ADLs.39 However, in the chronic, degenerative tendon environment having abnormal cellular phenotype and ECM, not only are the biologic factors vastly different than in an acute injury, the mechanical environment is different as well. These mechanical differences reflect appreciable and likely irreversible changes in the upstream muscle88. Second, the TEC could be used as a load-bearing structure to fill either a midsubstance or tendon-to-bone defect. In this scenario, the mechanical requirements would be different from patient to patient based on the size of the tear, the quality of the tendon ends, and the amount of muscle retraction. Will a single TEC be sufficient to accommodate all possible problems or are multiple TECs needed to allow the surgeon to choose the most appropriate repair option?
In these scenarios, the TEC could be exposed to a richly vascular extra-articular environment, a more hostile intra-articular environment relying on diffusion for nutrition, or some combination. Each environment could dramatically influence repair quality. In particular, how does exposure to a specific environment affect TEC function and ultimately repair quality? Are different TECs necessary for different repair environments? These are just some of the questions and concerns that clinicians have in trying to evaluate whether or not to try a tissue engineered product. While it is not possible for the tissue engineering field to test every possible clinical scenario or even have models that would mimic all aspects of the clinical problem, it is incumbent on the field to test TECs across a variety of injuries, locations and mechanical demands to devise strategies for applying tissue engineered repair methodologies across multiple clinical scenarios.
Commercialization of Tendon Tissue Engineered Strategies
One of the greatest challenges facing investigators working in musculoskeletal tissue engineering lies in the commercialization of products developed in the laboratory. Commercialization of TECs for tendon repair has numerous challenges because each injury is unique. TECs can be designed as load-bearing structures for complete tendon rupture or augmentations of conventional repairs or graft replacements.
Developing load-bearing TECs for complete tendon rupture
Completely replacing a ruptured tendon in a patient with a load-bearing TEC is particularly difficult for several reasons. 1) The TEC must tolerate very large and impulsive forces and stresses during even modest activities of daily living. Ker et. al.89 estimated that peak stresses can approach 100 MPa during vigorous exercise. Such large forces/stresses only allow for a small safety factor in the resulting design and can expose the TECs to excessive deformations and even rupture early after return to activity. 2) Scaffold materials must be selected that meet regulatory restrictions. Only a small subset of FDA-approved synthetic materials are currently available for surgery. Introducing these materials can lead to increased inflammation and rejection after surgery. Biologically-derived materials like tendon autografts and allografts are also attractive options with a long history in orthopaedics. However, autografts pose the problem of donor site morbidity and allografts may face the potential of longer-term rejection90, 91. Both graft types also have such dense ECM structure that investigators cannot readily infuse cells and/or growth factors into them before surgery. Other biological scaffolds like collagen gels and sponges permit more rapid infusion but they are often too compliant and weak to transmit muscle forces. 3) Regardless of choice, each TEC scaffold material must not be so stiff as to shield the infused cells or the joint, leading to altered host and implanted cell phenotypes and potential remodeling of surrounding structures.
Augmenting conventional repairs and replacements
This problem is somewhat less challenging as a scaffold will not need to resist the muscle forces but serve to “augment” the existing repair/replacement method. Consequently, more open porous scaffolds can be chosen that are FDA-approved and capable of encapsulating cells and/or growth factors. Yet such cell-scaffold, growth factor-scaffold or combined strategies also face challenges. 1) Cells must retain a phenotype that may not only vary within the tendon itself (i.e. midsubstance vs. insertion site) but also among tendon types (i.e. rotator cuff vs. PT) and location of tendon (i.e. intra-articular vs. extra-articular). 2) Growth factors may need to be tethered to the scaffold in a manner that controls their release to intrinsic host cells or cells introduced in the TEC. 3) Cells and growth factors may need to be tailored to the acute or chronic wound site and to the particular patient profile in order to produce a successful repair or regeneration.
Translating any tendon repair therapy to patients will ultimately require a multidisciplinary team of investigators and product development specialists (Fig. 3). Surgeons will need to carefully define the clinical problem including the frequency and consequences of an injury as well as the major limitations of traditional repair strategies and causes of re-rupture. Product development experts will need to determine the market potential and inadequacies of current treatments. Surgeons, biologists, engineers, material scientists and designers will need to design the implant to achieve the desired biological effect using acceptable materials and design specifications that ensure retained functionality for demanding clinical constraints like arthroscopy. Only by working together will we increase the likelihood of creating viable solutions after traumatic injury or repetitive motion disorders.
Figure 3.
A roadmap for product development used by our group to bring a tissue engineered TEC from the bench top to bedside including device development for surgical implantation and business plan. Mary Beth Privitera, MoD, Co-Director of the University of Cincinnati Medical Device Innovation and Entrepreneurship Program, and Jeffrey Johnson, PhD, Director of the University of Cincinnati Research Design Innovation and Entrepreneurship Program, helped with the creation this figure.
Acknowledgments
This research was supported by NIH grants AR46574 and AR56943. The authors wish to thank Gino Bradica, PhD, Shun Yoshida, MD, Heather Powell, PhD, Hani Awad, PhD, Matthew Dressler, PhD, Abhishek Jain, MS and Greg Boivin DVM for their contributions to our previous research. The authors also wish to thank Mary Beth Privitera, MoD, Co-Director of the University of Cincinnati Medical Device Innovation and Entrepreneurship Program, and Jeffrey Johnson, PhD, Director of the University of Cincinnati Research Design Innovation and Entrepreneurship Program, for helping with the creation of the roadmap figure.
References
- 1.Clayton RAE, Court-Brown CM. The epidemiology of musculoskeletal tendinous and ligamentous injuries. Injury. 2008;39(12):1338–44. doi: 10.1016/j.injury.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 2.Leppilahti J, Puranen J, Orava S. Incidence of achilles tendon rupture. Acta Orthop Scand. 1996;67(3):277–9. doi: 10.3109/17453679608994688. [DOI] [PubMed] [Google Scholar]
- 3.Zhao C, Moran SL, Cha SS, Kai-Nan-An, Amadio PC. An analysis of factors associated with failure of tendon repair in the canine model. J Hand Surg. 2007;32(4):518–25. doi: 10.1016/j.jhsa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 ofthe fingers during early mobilization. J Hand Surg. 1999;24 B(3):275–80. doi: 10.1054/jhsb.1998.0212. [DOI] [PubMed] [Google Scholar]
- 5.Baktir A, Turk CY, Kabak S, Sahin V, Kardas Y. Flexor tendon repair in zone 2 followed by early active mobilization. J Hand Surg. 1996;21 B(5):624–8. doi: 10.1016/s0266-7681(96)80145-8. [DOI] [PubMed] [Google Scholar]
- 6.Khan RJK, Fick D, Keogh A, Crawford J, Brammar T, Parker M. Treatment of acute achilles tendon ruptures: A meta-analysis of randomized, controlled trials. Journal of Bone and Joint Surgery - Series A. 2005;87(10):2202–10. doi: 10.2106/JBJS.D.03049. [DOI] [PubMed] [Google Scholar]
- 7.Strauss EJ, Ishak C, Jazrawi L, Sherman O, Rosen J. Operative treatment of acute achilles tendon ruptures: An institutional review of clinical outcomes. Injury. 2007;38(7):832–8. doi: 10.1016/j.injury.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. Journal of Bone and Joint Surgery - Series A. 2004;86(2):219–24. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Harryman DT, II, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., III Repairs of the rotator cuff. correlation of functional results with integrity of the cuff. Journal of Bone and Joint Surgery - Series A. 1991;73(7):982–9. [PubMed] [Google Scholar]
- 10.Lafosse L, Brozska R, Toussaint B, Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. Journal of Bone and Joint Surgery - Series A. 2007;89(7):1533–41. doi: 10.2106/JBJS.F.00305. [DOI] [PubMed] [Google Scholar]
- 11.Sharma P, Maffulli N. Biology of tendon injury: Healing, modeling and remodeling. Journal of Musculoskeletal Neuronal Interactions. 2006;6(2):181–90. [PubMed] [Google Scholar]
- 12.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: Differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125(1):106–13. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 13.Hasslund S, Jacobson JA, Dadali T, Basile P, Ulrich–Vinther M, Søballe K, et al. Adhesions in a murine flexor tendon graft model: Autograft versus allograft reconstruction. Journal of Orthopaedic Research. 2008;26(6):824–33. doi: 10.1002/jor.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kryger GS, Chong AKS, Costa M, Pham H, Bates SJ, Chang J. A comparison of tenocytes and mesenchymal stem cells for use in flexor tendon tissue engineering. J Hand Surg. 2007;32(5):597–605. doi: 10.1016/j.jhsa.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology (UK) 2006;45(5):508–21. doi: 10.1093/rheumatology/kel046. [DOI] [PubMed] [Google Scholar]
- 16.Greca FH, Ramos EJB, Dallolmo VC, Silva APG, Mima WH, Okawa L, et al. Evaluation of porcine small intestinal submucosa in achilles tendon repair. Journal of Applied Research. 2005;5(1):115–23. [Google Scholar]
- 17.Fredberg U, Stengaard-Pedersen K. Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation: Review. Scandinavian Journal of Medicine and Science in Sports. 2008;18(1):3–15. doi: 10.1111/j.1600-0838.2007.00746.x. [DOI] [PubMed] [Google Scholar]
- 18.Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Butler DL. Effects of cell-to-collagen ratio in stem cell-seeded constructs for achilles tendon repair. Tissue Eng. 2006;12(4):681–9. doi: 10.1089/ten.2006.12.681. [DOI] [PubMed] [Google Scholar]
- 19.West JR, Juncosa N, Galloway MT, Boivin GP, Butler DL. Characterization of in vivo achilles tendon forces in rabbits during treadmill locomotion at varying speeds and inclinations. J Biomech. 2004;37(11):1647–53. doi: 10.1016/j.jbiomech.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang HW, Goh JCH, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit achilles tendon. Tissue Eng. 2003;9(3):431–9. doi: 10.1089/107632703322066615. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Maeda M, Kurosawa H, Inoue Y, Yamauchi Y, Iwase H. Reconstruction of rabbit achilles tendon with three bioabsorbable materials: Histological and biomechanical studies. Journal of Orthopaedic Science. 2000;5(3):256–67. doi: 10.1007/s007760050161. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert TW, Stewart-Akers AM, Simmons-Byrd A, Badylak SF. Degradation and remodeling of small intestinal submucosa in canine achilles tendon repair. Journal of Bone and Joint Surgery - Series A. 2007;89(3):621–30. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 23.Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for achille’s tendon repair in a dog model. J Biomed Mater Res. 1995;29(8):977–85. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 24.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair: Biomechanical, biochemical, and cellular properties. Journal of Bone and Joint Surgery - Series A. 2006;88(12):2665–72. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 25.Barber FA, Herbert MA, Boothby MH. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy - Journal of Arthroscopic and Related Surgery. 2008;24(1):20–4. doi: 10.1016/j.arthro.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. Journal of Shoulder and Elbow Surgery. 2010;19(2 SUPPL):104–9. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Snyder SJ, Arnoczky SP, Bond JL, Dopirak R. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket matrix. Arthroscopy - Journal of Arthroscopic and Related Surgery. 2009;25(3):329–33. doi: 10.1016/j.arthro.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson GP, Breur GJ, Van Sickle D, Yao JQ, Kim J, Blanchard CR. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. Journal of Shoulder and Elbow Surgery. 2007;16(5 SUPPL):S184–90. doi: 10.1016/j.jse.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Thomopoulos S, Soslowsky LJ, Flanagan CL, Tun S, Keefer CC, Mastaw J, et al. The effect of fibrin clot on healing rat supraspinatus tendon defects. Journal of Shoulder and Elbow Surgery. 2002;11(3):239–47. doi: 10.1067/mse.2002.122228. [DOI] [PubMed] [Google Scholar]
- 30.Rodeo SA, Potter HG, Kawamura S, Turner AS, Hyon JK, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. Journal of Bone and Joint Surgery - Series A. 2007;89(11):2485–97. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 31.Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop. 2008;466(3):622–33. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeherman HJ, Archambault JM, Rodeo SA, Turner AS, Zekas L, D’Augusta D, et al. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. Journal of Bone and Joint Surgery - Series A. 2008;90(10):2206–19. doi: 10.2106/JBJS.G.00742. [DOI] [PubMed] [Google Scholar]
- 33.Gulotta LV, Rodeo SA. Growth factors for rotator cuff repair. Clin Sports Med. 2009;28(1):13–23. doi: 10.1016/j.csm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Butler DL, Gooch C, Kinneberg KR, Boivin GP, Galloway MT, Nirmalanandhan VS, et al. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nature protocols. 2010;5(5):849–63. doi: 10.1038/nprot.2010.14. [DOI] [PubMed] [Google Scholar]
- 35.Awad HA, Boivin GP, Dressler MR, Smith FNL, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. Journal of Orthopaedic Research. 2003;21(3):420–31. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 36.Awad HA, Butler DL, Boivin GP, Smith FNL, Malaviya P, Huibregtse B, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5(3):267–77. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 37.Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. Journal of Orthopaedic Research. 2004;22(5):998–1003. doi: 10.1016/j.orthres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13(6):1219–26. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 39.Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12(8):2291–300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 40.Juncosa-Melvin N, Boivin GP, Gooch C, Galloway MT, West JR, Dunn MG, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue Eng. 2006;12(2):369–79. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 41.Shearn JT, Juncosa-Melvin N, Boivin GP, Galloway MT, Goodwin W, Gooch C, et al. Mechanical stimulation of tendon tissue engineered constructs: Effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng. 2007;129(6):848–54. doi: 10.1115/1.2800769. [DOI] [PubMed] [Google Scholar]
- 42.Nirmalanandhan VS, Juncosa-Melvin N, Shearn JT, Boivin GP, Galloway MT, Gooch C, et al. Combined effects of scaffold stiffening and mechanical preconditioning cycles on construct biomechanics, gene expression, and tendon repair biomechanics. Tissue Engineering - Part A. 2009;15(8):2103–11. doi: 10.1089/ten.tea.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Sklenka AM, et al. Effects of cell-to-collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng. 2005;11(3–4):448–57. doi: 10.1089/ten.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 44.Juncosa N, West JR, Galloway MT, Boivin GP, Butler DL. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. J Biomech. 2003;36(4):483–8. doi: 10.1016/s0021-9290(02)00459-1. [DOI] [PubMed] [Google Scholar]
- 45.Guilak F, Butler DL, Goldstein SA, Mooney D. Functional Tissue Engineering. 2003 [Google Scholar]
- 46.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: The role of biomechanics. J Biomech Eng. 2000;122(6):570–5. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 47.Butler DL, Shearn JT, Juncosa N, Dressler MR, Hunter SA. Functional tissue engineering parameters toward designing repair and replacement strategies. Clin Orthop. 2004;427(SUPPL):S190–9. doi: 10.1097/01.blo.0000144858.65450.d2. [DOI] [PubMed] [Google Scholar]
- 48.Korvick DL, Cummings JF, Grood ES, Holden JP, Feder SM, Butler DL. The use of an implantable force transducer to measure patellar tendon forces in goats. J Biomech. 1996;29(4):557–61. doi: 10.1016/0021-9290(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 49.Hankemeier S, Hurschler C, Zeichen J, Van Griensven M, Miller B, Meller R, et al. Bone marrow stromal cells in a liquid fibrin matrix improve the healing process of patellar tendon window defects. Tissue Engineering - Part A. 2009;15(5):1019–30. doi: 10.1089/ten.tea.2008.0046. [DOI] [PubMed] [Google Scholar]
- 50.Karaoglu S, Fisher MB, Woo SL, Fu Y, Liang R, Abramowitch SD. Use of a bioscaffold to improve healing of a patellar tendon defect after graft harvest for ACL reconstruction: A study in rabbits. Journal of Orthopaedic Research. 2008;26(2):255–63. doi: 10.1002/jor.20471. [DOI] [PubMed] [Google Scholar]
- 51.Lyras DN, Kazakos K, Verettas D, Botaitis S, Agrogiannis G, Kokka A, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129(11):1577–82. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 52.Cserjesi P, Brown D, Ligon K, Lyons G, Copeland N, Gilbert D, et al. Scleraxis: A basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995 Jan 01;121(4):1099–110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 53.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: Tendons and musculoskeletal development in flies and vertebrates. Development. 2010 Sep 01;137(17):2807–17. doi: 10.1242/dev.047498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asou Y, Nifuji A, Tsuji K, Shinomiya K, Olson E, Koopman P, et al. Coordinated expression of scleraxis and Sox9 genes during embryonic development of tendons and cartilage. J Orthop Res. 2002 Jan 01;20(4):827–33. doi: 10.1016/S0736-0266(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 55.Murchison N, Price B, Conner D, Keene D, Olson E, Tabin C, et al. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007 Jan 01;134(14):2697–708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 56.Brent A, Tabin C. FGF acts directly on the somitic tendon progenitors through the ets transcription factors Pea3 and erm to regulate scleraxis expression. Development. 2004 Jan 01;131(16):3885–96. doi: 10.1242/dev.01275. [DOI] [PubMed] [Google Scholar]
- 57.Kuo C, Petersen B, Tuan R. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev Dyn. 2008 May 01;237(5):1477–89. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006 Apr 01;24(4):831–41. doi: 10.1002/jor.20092. [DOI] [PubMed] [Google Scholar]
- 59.Edom-Vovard F, Schuler B, Bonnin M, Teillet M, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Developmental Biology. 2002 Jan 01;247(2):351–66. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- 60.Pryce B, Watson S, Murchison N, Staverosky J, Dünker N, Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFB signaling are essential for tendon formation. Development. 2009 Jan 01;136(8):1351–61. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mikic B, Schalet B, Clark R, Gaschen V, Hunziker E. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the achilles tendon. J Orthop Res. 2001 Jan 01;19(3):365–71. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- 62.Mikic B, Rossmeier K, Bierwert L. Sexual dimorphism in the effect of GDF-6 deficiency on murine tendon. J Orthop Res. 2009 Dec 01;27(12):1603–11. doi: 10.1002/jor.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy. 1998;14(8):840–3. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 64.Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. The Journal of bone and joint surgery American volume. 1991 Dec 01;73(10):1507–25. [PubMed] [Google Scholar]
- 65.Riley G. Chronic tendon pathology: Molecular basis and therapeutic implications. Exp Rev Mol Med. 2005 Mar 24;7(5):1–25. doi: 10.1017/S1462399405008963. [DOI] [PubMed] [Google Scholar]
- 66.Sun H, Li Y, Fung D, Majeska R, Schaffler M, Flatow E. Coordinate regulation of IL-1β and MMP-13 in rat tendons following subrupture fatigue damage. Clinical Orthopaedics and Related Research. 2008 Jan 01;466(7):1555–61. doi: 10.1007/s11999-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, et al. Overuse activity injures the supraspinatus tendon in an animal model: A histologic and biomechanical study. Journal of Shoulder and Elbow Surgery. 2000;9(2):79–84. [PubMed] [Google Scholar]
- 68.Sharma P, Maffulli N. Tendon injury and tendinopathy: Healing and repair. Journal of Bone and Joint Surgery - Series A. 2005;87(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Murrell G. The basic science of tendinopathy. Clin Orthop Relat Res. 2008 Jan 01;:1–11. doi: 10.1007/s11999-008-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Longo U, Oliva F, Olivia F, Denaro V, Maffulli N. Oxygen species and overuse tendinopathy in athletes. Disability and Rehabilitation. 2008 Jan 01;30(20–22):1563–71. doi: 10.1080/09638280701785643. [DOI] [PubMed] [Google Scholar]
- 71.Arnoczky S, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: Is it the over-stimulation or the under-stimulation of tendon cells? International Journal of Experimental Pathology. 2007 Jan 01;:217–66. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jelinsky S, Lake S, Archambault J, Soslowsky L. Gene expression in rat supraspinatus tendon recovers from overuse with rest. Clin Orthop Relat Res. 2008 Jul 01;466(7):1612–7. doi: 10.1007/s11999-008-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flick J, Devkota A, Tsuzaki M, Almekinders L, Weinhold P. Cyclic loading alters biomechanical properties and secretion of PGE 2 and NO from tendon explants. Clinical Biomechanics. 2006;21(1):99–106. doi: 10.1016/j.clinbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 74.Riley G. Tendinopathy—from basic science to treatment. Nat Clin Pract Rheumatol. 2008 Feb 01;4(2):82–9. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 75.Yang G, Im H, Wang J. Repetitive mechanical stretching modulates IL-1β induced COX-2, MMP-1 expression, and PGE production in human patellar tendon fibroblasts. Gene. 2005 Dec 19;363:166–72. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Archambault J, Tsuzaki M, Herzog W, Banes A. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002 Jan 01;20(1):36–9. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 77.Cilli F, Khan M, Fu F, Wang J. Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clinical Journal of Sport Medicine. 2004;14(4):232. doi: 10.1097/00042752-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Bolon B, Campagnuolo G, Zhu L, Duryea D, Zack D, Feige U. Interleukin-1beta and tumor necrosis factor-alpha produce distinct, time-dependent patterns of acute arthritis in the rat knee. Vet Pathol. 2004 May 01;41(3):235–43. doi: 10.1354/vp.41-3-235. [DOI] [PubMed] [Google Scholar]
- 79.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clinical Orthopaedics and Related Research. 2003;(415):111–20. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 80.Lake SP, Ansorge HL, Soslowsky LJ. Animal models of tendinopathy. Disabil Rehabil. 2008;30(20–22):1530–41. doi: 10.1080/09638280701785460. [DOI] [PubMed] [Google Scholar]
- 81.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. Journal of Orthopaedic Research. 2005 Jan;23(1):84–92. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Khan MH, Li Z, Wang JH. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clin J Sport Med. 2005 Jan;15(1):27–33. doi: 10.1097/00042752-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Stone D, Green C, Rao U, Aizawa H, Yamaji T, Niyibizi C, et al. Cytokine-induced tendinitis: A preliminary study in rabbits. J Orthop Res. 1999 Mar 01;17(2):168–77. doi: 10.1002/jor.1100170204. [DOI] [PubMed] [Google Scholar]
- 84.Tapper JE, Fukushima S, Azuma H, Sutherland C, Marchuk L, Thornton GM, et al. Dynamic in vivo three-dimensional (3D) kinematics of the anterior cruciate ligament/medial collateral ligament transected ovine stifle joint. Journal of Orthopaedic Research. 2008;26(5):660–72. doi: 10.1002/jor.20557. [DOI] [PubMed] [Google Scholar]
- 85.Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy - Journal of Arthroscopic and Related Surgery. 2010;26(9 SUPPL 1):S49, S57–e156. doi: 10.1016/j.arthro.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fan H, Liu H, Toh SL, Goh JCH. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30(28):4967–77. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 87.Mueller XM, Tevaearai HT, Jegger D, Tucker O, Von Segesser LK. Are standard human coagulation tests suitable in pigs and calves during extracorporeal circulation? Artif Organs. 2001;25(7):579–84. doi: 10.1046/j.1525-1594.2001.025007579.x. [DOI] [PubMed] [Google Scholar]
- 88.Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. Journal of Bone and Joint Surgery - Series A. 2005;87(12 I):2662–70. doi: 10.2106/JBJS.D.02421. [DOI] [PubMed] [Google Scholar]
- 89.Ker RF, Alexander RM, Bennett MB. Why are mammalian tendons so thick? J Zool. 1988;216:309–24. [Google Scholar]
- 90.Adachi N, Ochi M, Uchio Y, Sakai Y, Kuriwaka M, Fujihara A. Harvesting hamstring tendons for ACL reconstruction influences postoperative hamstring muscle performance. Arch Orthop Trauma Surg. 2003;123(9):460–5. doi: 10.1007/s00402-003-0572-2. [DOI] [PubMed] [Google Scholar]
- 91.Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26(4):661–81. doi: 10.1016/j.csm.2007.06.010. [DOI] [PubMed] [Google Scholar]