Abstract
Diastolic dysfunction is a prognosticator for future cardiovascular events that demonstrates a strong correlation with obesity. Pharmacological inhibition of dipeptidylpeptidase-4 (DPP-4) to increase the bioavailability of glucagon-like peptide-1 is an emerging therapy for control of glycemia in type 2 diabetes patients. Accumulating evidence suggests that glucagon-like peptide-1 has insulin-independent actions in cardiovascular tissue. However, it is not known whether DPP-4 inhibition improves obesity-related diastolic dysfunction. Eight-week-old Zucker obese (ZO) and Zucker lean rats were fed normal chow diet or diet containing the DPP-4 inhibitor, linagliptin (LGT), for 8 weeks. Plasma DPP-4 activity was 3.3-fold higher in ZO compared with Zucker lean rats and was reduced by 95% with LGT treatment. LGT improved echocardiographic and pressure volume-derived indices of diastolic function that were impaired in ZO control rats, without altering food intake or body weight gain during the study period. LGT also blunted elevated blood pressure progression in ZO rats involving improved skeletal muscle arteriolar function, without reducing left ventricular hypertrophy, fibrosis, or oxidative stress in ZO hearts. Expression of phosphorylated- endothelial nitric oxide synthase (eNOS)Ser1177, total eNOS, and sarcoplasmic reticulum calcium ATPase 2a protein was elevated in the LGT-treated ZO heart, suggesting improved Ca2+ handling. The ZO myocardium had an abnormal mitochondrial sarcomeric arrangement and cristae structure that were normalized by LGT. These studies suggest that LGT reduces blood pressure and improves intracellular Cai2+ mishandling and cardiomyocyte ultrastructure, which collectively result in improvements in diastolic function in the absence of reductions in left ventricular hypertrophy, fibrosis, or oxidative stress in insulin-resistant ZO rats.
Epidemiological studies indicate that two-thirds of Americans are overweight or obese, and this epidemic is associated with increased cardiovascular-related morbidity and mortality (1). The obese population has a high incidence of insulin resistance, which is an important risk factor for progression to cardiac dysfunction and diabetes. Therapeutic strategies are needed that both improve glycemia and have favorable direct or indirect effects on cardiovascular outcomes, including diastolic function. In this regard, the role of incretin signaling is being increasingly recognized. The gut-derived incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotrophic peptide play an important role in both postprandial and long-term glucose homeostasis by enhancing glucose-stimulated insulin secretion and suppressing glucagon release (2). The exopeptidase, dipeptidylpeptidase-4 (DPP-4), which circulates in the plasma, rapidly degrades circulating GLP-1 and glucose-dependent insulinotrophic peptide, which limits the half-life of these hormones to about 2 minutes. The recent development of incretin enhancer therapies based on GLP-1 receptor (GLP-1R) agonism or DPP-4 inhibition to prolong the half-life of GLP-1 are established therapies for glycemic reduction in diabetic patients. Importantly, emerging evidence suggests that augmentation of GLP-1 using GLP-1 analogs or DPP-4 inhibitors may improve cardiovascular outcomes (3–6). The notion that incretin enhancer therapies may have direct beneficial effects in the heart and vasculature (4, 7) is supported by recent evidence confirming the presence of GLP-1Rs in cardiomyocytes, the endocardium, and coronary endothelial and smooth muscle cells (8), as well as DPP-4 in the coronary microvasculature (9). Indeed, GLP-1Rs and membrane-bound DPP-4 are distributed throughout the systemic vasculature as well. Mice with genetic deletion of the GLP-1R exhibit left ventricular (LV) hypertrophy (LVH) and diastolic and systolic dysfunction (10), and GLP-1 analog therapy improved diastolic and systolic dysfunction in a mouse model of obesity (11). Studies of the cardioprotective benefits of DPP-4 inhibitory therapy in rodents and humans and have focused on models of myocardial infarction and atherosclerosis (4). Nonetheless, there are only a limited number of studies on the effects of DPP-4 inhibition on ventricular function. A recent study reported that DPP-4 inhibition improved cardiac function in diabetic rats (9). However, the effects of DPP-4 inhibitors on in vivo cardiac diastolic function in the setting of obesity associated with insulin resistance has not been examined. Diastolic dysfunction is often the earliest functional cardiac abnormality associated with obesity (12–14), and there is a high prevalence (40%) of moderate or severe diastolic dysfunction in the early phase of type 2 diabetes (T2D) (15).
Linagliptin (LGT) is a potent, long acting, and highly specific DPP-4 inhibitor (16) that was recently approved for treatment of T2D. Although LGT has undergone extensive clinical testing to determine efficacy for treatment of glycemic reduction in T2D, little is known concerning the potential of LGT to blunt the severity of diastolic dysfunction in prediabetic states of obesity-related cardiomyopathy. To test this notion, we used insulin-resistant Zucker obese (ZO) rats with established diastolic dysfunction (17). A leptin receptor mutation in the ZO rat prevents hypothalamic binding of leptin resulting in severe obesity. At an early age, rats exhibit metabolic abnormalities, such as hyperinsulinemia and dyslipidemia, which contribute to mild hypertension and an abnormal cardiac phenotype characterized by myocardial interstitial fibrosis, steatosis, abnormal mitochondrial ultrastructure and biogenesis, and diastolic dysfunction (17, 18), cardiovascular manifestations that are seen in obese humans with cardiorenal metabolic syndrome (19). In the present investigation, we tested whether an 8-week treatment with LGT could ameliorate progression of an already established abnormal cardiac phenotype in ZO rats. Here, we report that LGT reduces the severity of in vivo diastolic dysfunction in ZO rats.
Materials and Methods
Methods
Zucker lean (ZL) and ZO rats were purchased from Charles River, Inc (Raleigh, North Carolina) and cared for in accordance with National Institutes of Health guidelines. All procedures were approved in advance by the Institutional Animal Care and Use Committee of the University of Missouri. LGT, (R)-8-(3-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin2-ylmethyl)-3,7-dihydro-purine-2,6-dione (20), was added to rat chow so that the final concentration in chow was 83-mg LGT/kg−1 to achieve a dose and plasma level of approximately 4 mg/kg−1 per day−1 and 100nM, respectively. This dose is effective at reducing plasma DPP-4 activity and had been used in a previous study in rats to examine glucose-independent effects on the vasculature (21). Four groups of rats were used, and they include ZL control (ZLC), ZL treated with LGT (ZLL), ZO control (ZOC), and ZO treated with LGT (ZOL). To obtain initial and final body weight and weight gained during the 8-week experiment, rats were weighed immediately before the start of the experiment at 8 weeks of age and at weekly intervals thereafter.
Telemetric blood pressure (BP) monitoring
Under isoflurane anesthesia (2% isoflurane in a stream of air containing 40% O2), a subset of 7-week-old ZL and ZO rats were implanted with an abdominal aorta catheter attached to a radiotransmitter (TA11PA-C40; Data Sciences International, St Paul, Minnesota) as previously described (22). After a 3-week recovery, mean arterial pressure (MAP) was monitored in 300-second bins every 15 minutes for 3 12-hour light and 3 12-hour dark cycles (sampling rate, 1000 Hz), and telemetry data were analyzed post hoc.
Echocardiography
Echocardiography was performed on isoflurane-anesthetized 16-week-old rats in a left lateral decubitus position using a GE Vivid i system with an 11.5-MHz phased-array pediatric probe. Details of the echocardiographic procedures are reported in the Supplemental material, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Cardiac catheterization and pressure volume (PV) analysis
The protocol for cardiac catheterization and PV analysis has been previously described in detail (22, 23).
Determination of vasomotor responses
A first order (1A) arteriole of the gastrocnemius feed artery was isolated from the medial head of the gastrocnemius as previously described (24) and placed in ice-cold buffer (see details in Supplemental material). Isolation and equilibration procedures are described in detail in Supplemental material. Vasomotor responses were determined in 1 arteriole per animal. Endothelium-dependent and endothelium-independent dilation of 1A arterioles was determined in response to acetylcholine and the nitric oxide (NO)-donor sodium nitroprusside. At the end of each experiment, the physiological salt solution bath solution was replaced with Ca2+-free physiological salt solution to determine maximal passive diameter.
Biochemical examination
At the end of the 8-week treatment period, a venous blood sample was collected from a subset of fasting rats in each treatment group, and plasma was stored at −80°C for glucose and insulin assay and homeostatic model assessment of insulin resistance (HOMA-IR) as previously described (17). DPP-4 activity assay in plasma, and the myocardium was determined using fluorogenic substrate, H-Ala-Pro-AFC, and citrate synthase activity was measured in mitochondrial fractions as previously described (17). Reactive oxygen species (ROS) were measured as previously described (17). Whole-cell homogenates were used for immunoblots (17). Details of biochemical assays are given in Supplemental material.
Gravimetric assessment of ventricular hypertrophy
Rats were weighed and euthanized via exsanguination under isoflurane anesthesia (see above). At autopsy, the LV and septum (LV+S) were dissected intact. To assess LVH, the LV+S weight was normalized to tibia length (TL) rather than body weight, because the significant differences in body weight between ZL and ZO rats confound such an analysis.
3-Nitrotyrosine (3-NT) immunohistochemistry
To detect the presence of reactive nitrogen species (RNS), LV tissue was fixed, embedded in paraffin, and immunostained with an antibody to 3-NT (AB5411; 1:150 dilution; Millipore, Billerica, Massachusetts) as previously described (17).
Ultrastructure analysis with transmission electron microscopy
Details of LV tissue preparation, sectioning, staining, and viewing are as previously described (25). A JOEL 1400-EX transmission electron microscope (Joel Ltd, Tokyo, Japan) was used to review 3 fields randomly chosen per rat to obtain 3 ×2000 images/LV.
Statistical analysis
Results are reported as the mean ± SE. Two-way ANOVA and post hoc t tests (Holm-Sidak) were performed to examine differences in outcomes between control and LGT treated ZL and ZO groups (Sigma Plot 12.0; Systat Software, Chicago, Illinois). All differences were considered significant when P < .05. Twenty-four-hour ambulatory mean arterial BP (MAP) of 10-, 12-, and 14-week-old rats, corresponding to 2, 4, and 6 weeks of treatment, respectively, were compared by 2-way repeated measures ANOVA and paired comparisons by Holm-Sidak post hoc tests.
Results
Effect of LGT on plasma and tissue DPP-4 activity
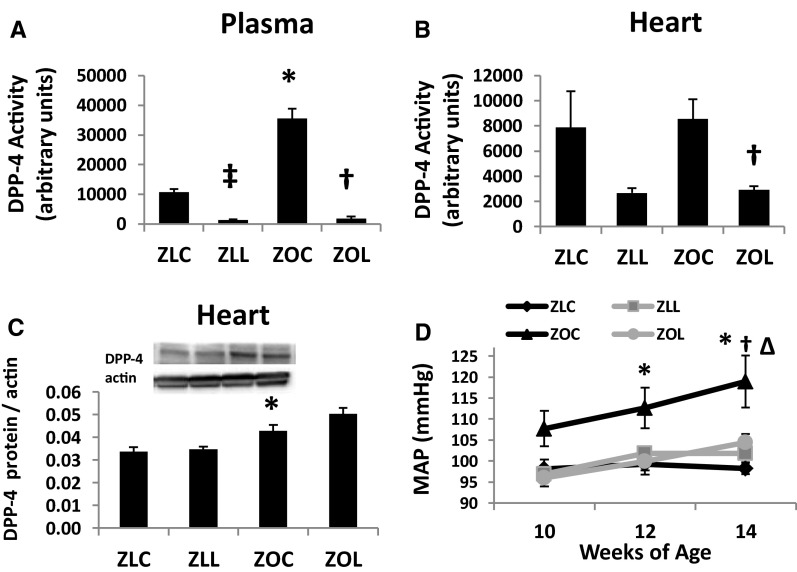
Plasma DPP-4 activity was 3.3-fold higher in ZOC compared with ZLC rats (Figure 1A) and was significantly decreased by LGT. DPP-4 activity in the heart of control ZL and ZO rats did not differ (P > .05), but ZOL rats exhibited significant reduction in DPP-4 activity (P < .05) (Figure 1B). Myocardial expression of DPP-4 protein was elevated in ZOC and ZOL rats compared with lean counterparts (Figure 1C).
Figure 1.
Linagliptin (LGT) reduces DPP-4 activity in the plasma (A) and myocardium (B) of Zucker Lean (ZL) and Obese rats. C, DPP-4 protein expression is elevated in control- and LGT-treated ZO rat hearts compared to lean counterparts. D, Twenty four hour ambulatory mean arterial pressure (MAP) of Zucker rats. Symbols indicate differences in MAP between treatment groups within a single time period. P < .05, *, ZLC vs ZOC; ‡, ZLC vs ZLL; †, ZOC vs ZOL; and Δ, ZOC vs ZLL.
LGT effects on BP, body weight, systemic metabolic parameters, and LVH
Twenty-four-hour ambulatory MAP of 10-, 12-, and 14-week-old rats, corresponding to 2, 4, and 6 weeks of treatment, respectively, are shown in Figure 1D. Between 10 and 14 weeks of age, MAP increased with age in ZLL, ZOC, and ZOL rats but not in ZLC (P < .05). Although ZOC rats tended to have higher MAP at 10 weeks of age compared with ZLC rats, significant differences were detected only at 12 and 14 weeks (P < .05). By 14 weeks of age, ZO rats treated with LGT had significantly lower MAP compared with ZOC (P < .05) (Table 1). Similar strain and treatment effects occurred for systolic and diastolic BP at 14 weeks of age (data not shown).
Table 1.
Baseline Parameters of Control and LGT-Treated ZL and ZO Rats
| Parameter | Main effect | P value | ZLC | ZLL | ZOC | ZOL |
|---|---|---|---|---|---|---|
| MAP (mm Hg) | Strain | .012 | 98.3 ± 1.4 (6) | 101.9 ± 2.1 (5) | 118.9 ± 6.2 (6)ab | 104.4 ± 2.0 (4) |
| Treatment | .001 | |||||
| Interaction | .003 | |||||
| Initial body wt (g) | Strain | .001 | 228 ± 6 | 222 ± 7 | 353 ± 9a | 343 ± 9c |
| Treatment | .331 | |||||
| Interaction | .789 | |||||
| Final body wt (g) | Strain | .001 | 380 ± 10 | 374 ± 9 | 594 ± 14a | 570 ± 17c |
| Treatment | .257 | |||||
| Interaction | .490 | |||||
| Body wt gain (%) | Strain | .985 | 67 ± 3 | 69 ± 5 | 69 ± 6 | 67 ± 6 |
| Treatment | .996 | |||||
| Interaction | .665 | |||||
| TL (mm) | Strain | .001 | 38.8 ± 0.2 | 39.1 ± 0.3 | 37.0 ± 0.3a | 37.1 ± 0.4c |
| Treatment | .525 | |||||
| Interaction | .750 | |||||
| LV+S wt (mg) | Strain | .001 | 696 ± 21 | 663 ± 22 | 812 ± 14ab | 735 ± 9c |
| Treatment | .003 | |||||
| Interaction | .211 | |||||
| LV/TL (mg/g) | Strain | .001 | 17.9 ± 0.5 | 17.0 ± 0.5 | 21.9 ± 0.4ab | 19.8 ± 0.3c |
| Treatment | .002 | |||||
| Interaction | .216 |
Radio-telemetry-derived 24-hour ambulatory MAPs shown were measured at 14 weeks of age. Most other parameters were evaluated at the end of the study when rats were 16 weeks old. Values are mean ± SE; n = 11 in each group except when noted otherwise in parentheses. wt, weight.
P < .05, ZLC vs. ZOC.
P < .05, ZOC vs. ZOL.
P < .05, ZLL vs. ZOL.
Body weight of 16-week-old ZOC and ZOL rats was similarly greater than body weight of their respective lean counterparts (Table 1 and Supplemental Figure 1). Percent body weight gain during the 8-week study period was 65 ± 2% and 62 ± 2% for ZLC and ZLL, respectively, and 81 ± 6% and 81 ± 3% for ZOC and ZOL, respectively. LGT treatment had no significant effects on body weight (Supplemental Figure 1) or food consumed (data not shown) within each strain during the test period. The LV of 16-week-old ZOC and ZOL rats is hypertrophic as indicated by increases in the ratio of LV+S mass to TL (Table 1).
Plasma insulin levels and HOMA-IR, but not glucose, were elevated in 16-week-old ZOC and ZOL compared with their lean counterparts (Supplemental Table 1). Although LGT treatment tended to lower blood glucose, insulin, and HOMA-IR, the effects did not reach statistical significance in ZO rats. Serum cholesterol levels were elevated in ZOC and ZOL compared with their lean counterparts. LGT treatment had no effect on serum cholesterol level in ZO rats. Serum triglycerides were elevated in ZOC compared with ZLC. Triglyceride level of ZOL rats were intermediate but did not differ significantly from either ZLC or ZOC. Nonesterified fatty acid (FA) levels were marginally increased in ZOC (P > .05) compared with ZLC, and the levels in ZOL were not different from ZOC (Supplemental Table 1).
LGT treatment improves diastolic function
Echocardiography
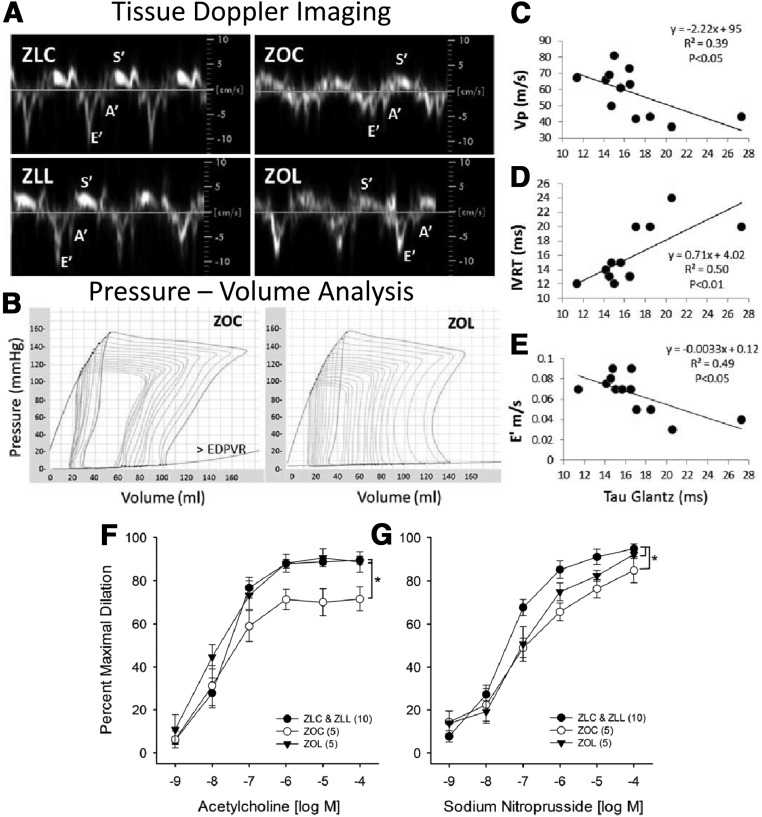
Compared with untreated ZL rats, the control ZO rats exhibited abnormalities in multiple diastolic parameters (Table 2), including decreases in early peak septal annular velocity (E'), the tissue Doppler E' to peak late septal annular velocity (A') ratio (Figure 2A), and mitral inflow propagation velocity (Vp), as well as increases in A', LV filling pressure (E to E' and E to Vp ratios), isovolumic relaxation time (IVRT), and myocardial performance index (Tei index of global cardiac function).
Table 2.
Summary of Cardiac Function Outcomes by Echocardiography and Pressure-Volume Analysis
| Echocardiography parameters | Main effect | P value | ZLC (10) | ZLL (12) | ZOC (11) | ZOL (11) |
|---|---|---|---|---|---|---|
| SWTd (cm) | Strain | .001 | 0.14 ± 0.004 | 0.14 ± 0.01 | 0.18 ± 0.01a | 0.18 ± 0.01c |
| Treatment | .608 | |||||
| Interaction | .677 | |||||
| PWTd (cm) | Strain | .001 | 0.16 ± 0.01 | 0.15 ± 0.01 | 0.20 ± 0.01a | 0.20 ± 0.01c |
| Treatment | .516 | |||||
| Interaction | .224 | |||||
| LVIDd (cm) | Strain | .741 | 0.73 ± 0.02 | 0.70 ± 0.03 | 0.72 ± 0.02 | 0.73 ± 0.02 |
| Treatment | .707 | |||||
| Interaction | .357 | |||||
| LVIDs (cm) | Strain | .631 | 0.30 ± 0.02 | 0.27 ± 0.02 | 0.27 ± 0.02 | 0.28 ± 0.02 |
| Treatment | .696 | |||||
| Interaction | .357 | |||||
| RWT | Strain | .001 | 0.42 ± 0.01 | 0.42 ± 0.02 | 0.53 ± 0.03a | 0.52 ± 0.04c |
| Treatment | .790 | |||||
| Interaction | .646 | |||||
| EF (%) | Strain | .335 | 91 ± 2 | 93 ± 1 | 93 ± 1 | 93 ± 1 |
| Treatment | .350 | |||||
| Interaction | .235 | |||||
| FS (%) | Strain | .540 | 59 ± 3 | 62 ± 2 | 62 ± 2 | 61 ± 3 |
| Treatment | .571 | |||||
| Interaction | .300 | |||||
| LA (cm) | Strain | .001 | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.37 ± 0.01ab | 0.32 ± 0.02 |
| Treatment | .017 | |||||
| Interaction | .030 | |||||
| Ao (cm) | Strain | .018 | 0.28 ± 0.01 | 0.27 ± 0.01 | 0.33 ± 0.02ab | 0.29 ± 0.02 |
| Treatment | .043 | |||||
| Interaction | .221 | |||||
| LA/Ao | Strain | .285 | 1.09 ± 0.04 | 1.12 ± 0.06 | 1.19 ± 0.07 | 1.15 ± 0.10 |
| Treatment | .942 | |||||
| Interaction | .532 | |||||
| E (m/sec) | Strain | .362 | 1.02 ± 0.03 | 0.99 ± 0.02 | 0.98 ± 0.04b | 1.10 ± 0.09c |
| Treatment | .231 | |||||
| Interaction | .068 | |||||
| E' (m/sec) | Strain | .001 | 0.10 ± 0.01 | 0.10 ± 0.004 | 0.05 ± 0.003ab | 0.08 ± 0.01c |
| Treatment | .002 | |||||
| Interaction | .037 | |||||
| A' (m/sec) | Strain | .001 | 0.040 ± 0.002 | 0.046 ± 0.004 | 0.071 ± 0.004ab | 0.056 ± 0.006c |
| Treatment | .222 | |||||
| Interaction | .007 | |||||
| E/E' | Strain | .001 | 11.4 ± 1.2 | 9.9 ± 0.5 | 19.2 ± 1.1ab | 13.9 ± 1.4c |
| Treatment | .001 | |||||
| Interaction | .039 | |||||
| E'/A' | Strain | .001 | 2.46 ± 0.24 | 2.41 ± 0.23 | 0.77 ± 0.07ab | 1.54 ± 0.26c |
| Treatment | .065 | |||||
| Interaction | .035 | |||||
| Vp | Strain | .001 | 60 ± 4 | 63 ± 3 | 38 ± 2ab | 64 ± 6 |
| Treatment | .001 | |||||
| Interaction | .001 | |||||
| E/Vp | Strain | .001 | 1.75 ± 0.12 | 1.62 ± 0.08 | 2.61 ± 0.12ab | 1.78 ± 0.20 |
| Treatment | .001 | |||||
| Interaction | .003 | |||||
| IVRT | Strain | .001 | 11.5 ± 0.6 | 11.7 ± 0.5 | 20.6 ± 0.7ab | 13.5 ± 0.5c |
| Treatment | .001 | |||||
| Interaction | .001 | |||||
| MPI | Strain | .001 | 0.33 ± 0.01 | 0.34 ± 0.01 | 0.46 ± 0.02ab | 0.36 ± 0.02 |
| Treatment | .002 | |||||
| Interaction | .001 | |||||
| Pressure-volume parameters | Main effect | P value | ZLC (9) | ZLL (5) | ZOC (6) | ZOL (8) |
| Slope of EDPVR (mm Hg/μL) | Strain | .001 | 0.0041 ± 0.0005 | 0.0045 ± 0.0004 | 0.0186 ± 0.0038ab | 0.0047 ± 0.0005 |
| Treatment | .002 | |||||
| Interaction | .001 | |||||
| τ (Weiss) (msec) | Strain | .012 | 10.6 ± 0.7 | 9.8 ± 0.9 | 13.6 ± 0.7ab | 10.5 ± 0.4 |
| Treatment | .008 | |||||
| Interaction | .117 | |||||
| τ (Glantz) (msec) | Strain | .001 | 13.4 ± 0.4 | 13.0 ± 1.1 | 19.5 ± 1.7ab | 14.8 ± 0.5 |
| Treatment | .012 | |||||
| Interaction | .001 |
SWTd, septal wall thickness-diastole; PWTd, posterior wall thickness-diastole; RWT, relative wall thickness; LVIDd, LV inner dimension-diastole; LVIDs, LV inner dimension-systole; EF, ejection fraction; FS, fractional shortening; LA, left atrial diameter; Ao, aortic diameter; A′, peak late septal annular velocity; E′, early peak septal annular velocity; IVRT, isovolumic relaxation time; E, velocity of early mitral inflow; E/E' index of LA filling pressure; E/Vp, index of LV filling pressure; MPI, myocardial performance index. Values are mean ± SE. Numbers in parentheses are sample sizes.
P < .05, ZLC vs. ZOC.
P < .05, ZOC vs. ZOL.
P < .05, ZLL vs. ZOL.
Figure 2.
Zucker Obese (ZO) rats exhibit diastolic dysfunction (A–E) and impaired vasomotor reactivity of skeletal muscle arterioles (F and G) which are ameliorated by the DPP-4 inhibitor, linagliptin (LGT). A, Representative tissue doppler images are shown of the early and late (E′ and A′) motion of the septal annulus during diastole and during early systole (S′). Mean group values for E′, A′ and E′/A′ are listed in Table 2. B, Representative pressure-volume loops are shown of ZOC and ZOL during pre-load reduction. C–E, Regression analysis indicates significant relationships between tau (t), the time constant of isovolumic relaxation, generated by PV analysis and echocardiography-derived parameters of early diastolic relaxation. Vp = propagation velocity of mitral inflow; IVRT = isovolumic relaxation time; E′ = velocity of early septal annular wall motion determined by pulse wave echocardiography for the same rats. Data points were plotted from ZOC and ZOL rats that had both cardiac procedures. Lintagliptin treatment improved vasomotor reactivity of skeletal muscle arterioles. Concentration-response curves of gastrocnemius 1A arterioles to acetylcholine (F) and sodium nitroprusside (G). Values are mean ± SE, sample size in parentheses. *, P < .05 for ZOC versus all other groups (F) and ZLC & ZLL versus all other groups (G).
Consistent with gravimetric analysis of ventricular weights (Table 1), the echocardiographic M-mode measures of cardiac wall dimensions, including septal wall thickness, posterior wall thickness, and relative wall thickness were all significantly greater in ZOC and ZOL hearts compared with ZLC. However, LGT treatment in ZO rats did not affect any of these cardiac dimensional parameters (Table 2).
PV analysis
Hemodynamic parameters obtained under steady state conditions are shown in Supplemental Table 2. The load-independent diastolic parameters obtained either under steady state conditions (τ, the time constant of isovolumic relaxation) or after occlusion of the inferior vena cava to reduce preload (slope of the end diastolic PV relationship or end diastolic pressure-volume relationship [EDPVR]) (Table 2) indicate diastolic abnormalities in both the active and passive properties of the LV wall, respectively, in ZOC and amelioration with LGT. On the other hand, load-independent systolic parameters, including end systolic elastance, preload recruitable stroke work, the slope of dP/dtmax vs end diastolic volume, the slope of PV area vs end diastolic volume, and maximum time varying elastance, did not differ significantly among groups, suggesting preserved systolic function (Supplemental Table 3). Previous reports have demonstrated that τ varies indirectly with Vp or E' and directly with IVRT in populations of normal and heart failure subjects (26, 27). In the subset of ZOC and ZOL rats in which we performed both cardiac procedures, we observed similar linear relationships (Figure 2, C–E). τ, Vp, IVRT, and to some extent, E' are all markers of early diastolic function, and our regression analyses suggest reasonably good correspondence between early diastolic parameters derived by noninvasive echocardiography and those obtained by direct catheterization in control and LGT-treated ZO rats. Chronic increases in preload/afterload eventually lead to decreases in LV wall compliance and cardiac output. Increased preload (end diastolic pressure [EDP]) and afterload (MAP) are apparent in ZOC compared with ZLC, because is increased LV wall stiffness (>EDPVR). We also observed decreased cardiac output in ZOC compared with ZLC. There was partial improvement of cardiac output in ZO rats treated with LGT, as well as normalization of EDP (Supplemental Table 2) and LV wall stiffness (slope of the EDPVR) (Table 2). Total peripheral vascular resistance index (TPRI), which is calculated by normalizing MAP to cardiac index, was elevated in ZOC compared with ZLC and was reduced in LGT-treated rats (P = .07) (Supplemental Table 2).
LV fibrosis
As surrogates for interstitial fibrosis, there were no significant changes observed in the protein expression levels of collagen 1 or collagen 3 in myocardial homogenates. Although trends suggest a decrease in the ratio of collagen 3 to collagen 1 in ZOC that was improved with LGT treatment, no significant differences were detected (Supplemental Figure 2).
LGT improves vascular function in resistance arterioles
LGT had no effect on any vascular outcome in ZLL compared with ZLC, so measurements from this group were combined with ZLC. Maximal passive diameters and percent preconstriction with phenylephrine were not different among groups (Supplemental Table 4). Endothelium-dependent vasodilation of gastrocnemius 1A arterioles to acetylcholine was decreased in ZOC compared with ZLC and ZLL, a defect that was abolished in the ZOL group (Figure 2F). Endothelium-independent vasodilation to sodium nitroprusside was also reduced in ZOC compared with ZLC and ZLL. However, this defect was not improved in the ZOL group (Figure 2G). These data are consistent with improved arteriolar endothelial function in ZOL animals despite a maintained reduction in smooth muscle NO sensitivity.
LGT effects on ROS and RNS
Although the accumulation of ROS was increased in ZOC (Supplemental Figure 3), the improvement in diastolic function in the ZOL group was not associated with decreased ROS. Similarly, LGT had no effect on accumulation of RNS in ZO hearts.
LGT effects on Ca+2 cycling protein expression
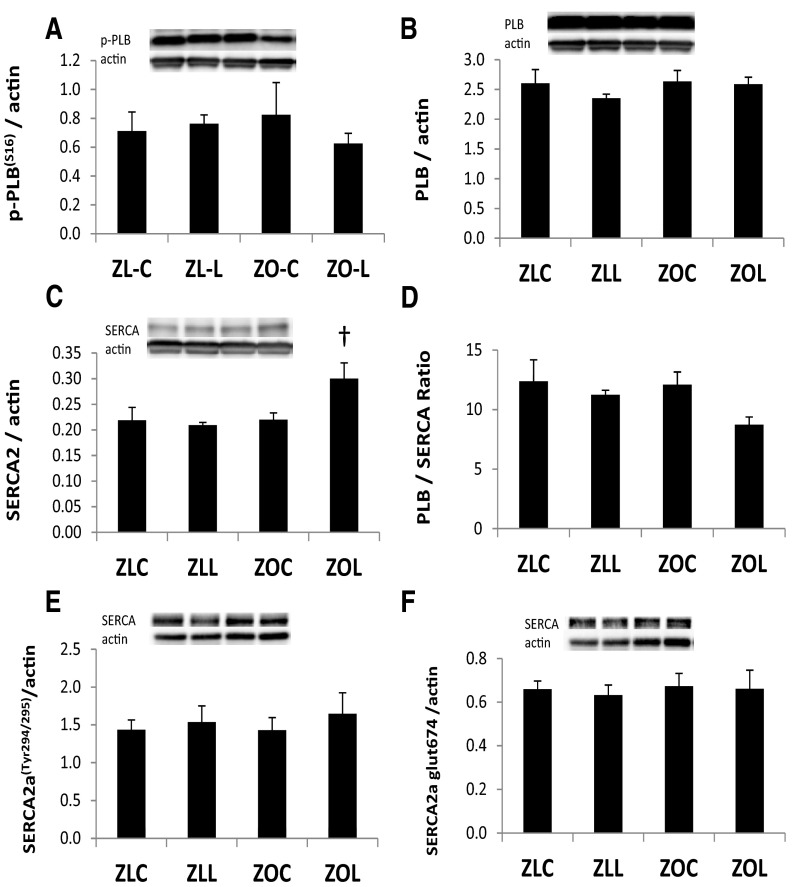
To identify possible compensatory changes or deficiencies in cardiomyocyte Cai2+ cycling, we examined protein expression of sarcoplasmic reticulum calcium ATPase 2a (SERCA2a) and its regulator protein phospholamban (PLB), as well as nitration of the SERCA2a tyrosine294/295 residue and glutathiolation of the cysteine674 residue of SERCA2a. The relative levels of phosphorylated-PLBSer16 and total PLB did not differ among the groups (Figure 3, A and B). SERCA2a pump expression levels were similar in ZOC hearts compared with ZLC, but SERCA2a levels were increased in ZOL (Figure 3C). Figure 3D shows the ratio of total PLB to SERCA2a. Neither the level of nitration of the tyrosine294/295 residue of SERCA2a nor the level of glutathiolation of the cysteine674 residue was altered in ZOC or ZOL (Figure 3, E and F).
Figure 3.
Linagliptin (LGT) effects on myocardial Ca+2 handling proteins. Bar graphs show β-actin normalized protein levels of phosphorylated-PLBser16 (A), total PLB (B), SERCA2a (C), total PLB/SERCA2a ratio (D), phosphorylated-SERCA2aTyr294/295 (E), and glutathiolated SERCA2a (F) at the 674 residue and from LV homogenates control and LGT treated ZL and ZO rats. Representative protein bands with corresponding β-actin bands are shown above bar graphs. P < .05; †, ZOC vs ZOL.
Mitochondrial structure and function
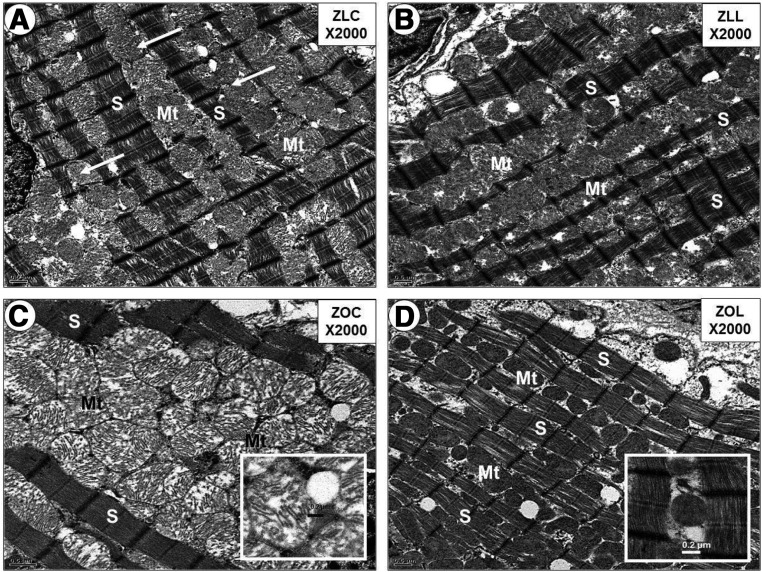
In ZOC hearts, mitochondrial ultrastructure was highly disorganized, and significant damage to mitochondria was observed (Figure 4 and Supplemental Figure 4); LGT treatment resulted in normalization of mitochondrial ultrastructure in the ZOL group. Mitochondrial protein content of electron transport chain subunits I, II, III, and V did not differ between ZLC and ZOC hearts, and LGT treatment did not result in significant changes in these subunits compared with ZOC (Supplemental Figure 5, A and B). Mitochondrial citrate synthase activity was also not different among groups (Supplemental Figure 5C). AMP-activated kinase (AMPK) is a highly conserved serine/threonine kinase that monitors the energy state of the cell and coordinates the metabolic response in the energy-deprived failing heart. No differences were detected in total AMPK protein and levels of phosphorylated-AMPK(T172) in LV whole-cell protein extracts among the groups (Supplemental Figure 5, D–F).
Figure 4.
Linagliptin (LGT) abrogates excessive numbers of intermyofibrillar mitochondria in Zucker Obese (ZO) Rats. Panel A illustrates the normal mitochondrial morphology (arrows) in the intermyofibrillar regions between sarcomeres (S) in the Zucker Lean control (ZLC) rats. Panel B reveals a similar mitochondrial morphology and sarcomere arrangement in the Zucker Lean linagliptin (ZLL) compared to ZLC. Panel C depicts mitochondrial biogenesis in the intermyofibrillar regions between sarcomeres. Note that the marked expansion of intermyofibrillar mitochondria (Mt) distorts the normal sarcomere morphology compared to A, B, and D. Insert in panel C depicts the abnormal mitochondrial morphology consisting of loss of matrix electron density and abnormal cristae compared to the cristae shown in the insert in panel D of linagliptin treated models (ZOL). Panel D demonstrates the normalization – abrogation of mitochondrial biogenesis in the ZO rats treated with linagliptin (ZOL) models. Insert depicts a more electron dense matrix and improved cristae. Magnification ×2000; bar = 0.5μm panels A–D. Magnification ×4000; bar = 0.2μm in insert panels C and D.
Metabolic insulin signaling
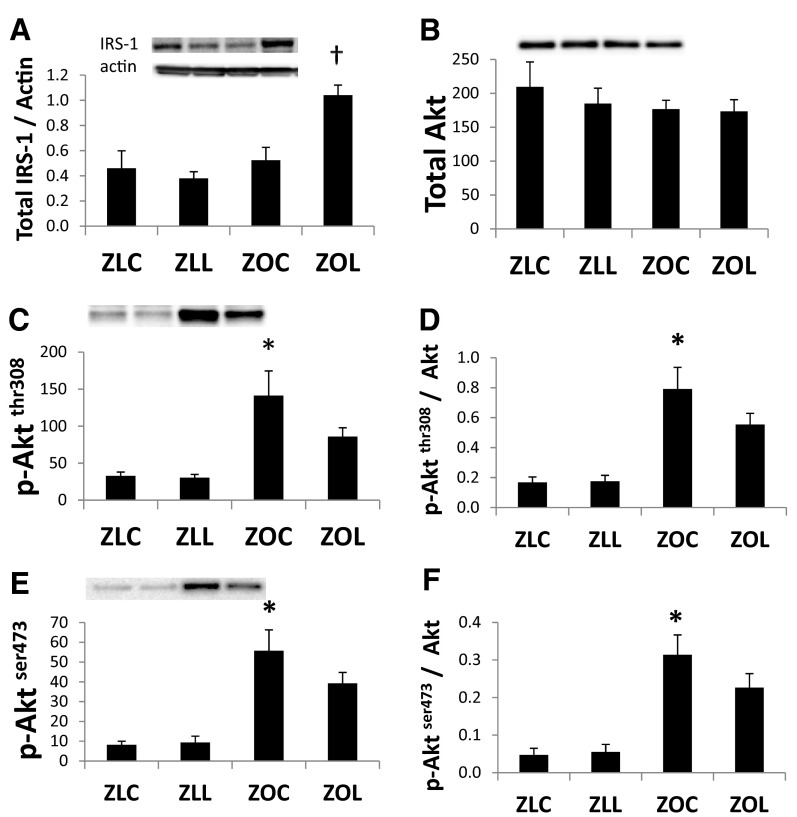
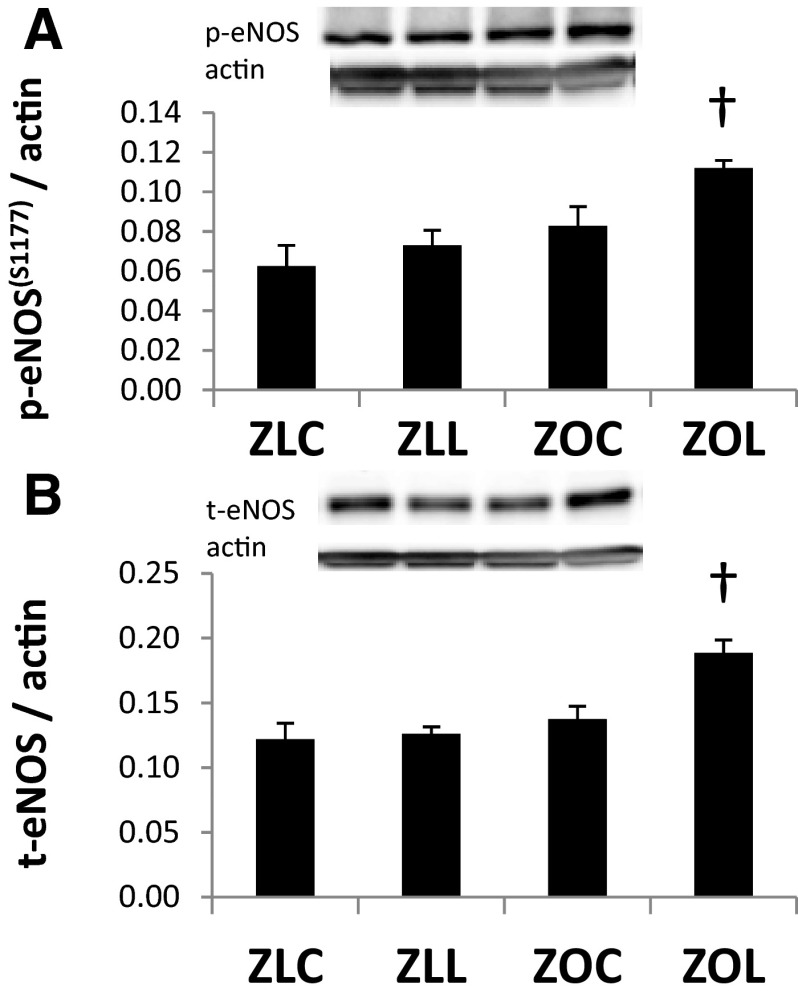
Myocardial insulin receptor substrate-1 (IRS-1) protein levels were not altered in ZOC compared with ZLC, but total IRS-1 was increased after LGT treatment (Figure 5A). There were no differences in total protein kinase B (Akt) expression among the 4 groups (Figure 5B). The phosphorylation of Akt at the threonine308 and serine473 residues was elevated in ZOC hearts compared with ZLC (P < .05) (Figure 5, C and E). The relative expressions of phosphorylated-AktThr308 and phosphorlyated-AktSer473 were both increased in ZOC hearts compared with ZLC (Figure 5, D and F). The hearts of LGT-treated ZO rats tended to have lower levels of phosphorylated Akt. However, the differences did not reach statistical significance. Levels of myocardial phosphorylated-eNOSSer1177 and total endothelial NO synthase (eNOS) protein were not altered in ZOC compared with ZLC. However, both phosphorylated-eNOSSer1177 and total eNOS protein were increased after LGT treatment (Figure 6, A and B).
Figure 5.
Cardiac insulin signaling is improved in the Zucker Obese (ZO) heart. Bar graphs show levels of total IRS-1/actin in myocardial protein extracts from control or LGT-treated Zucker lean (ZL) and obese rats (A). Bar graphs show levels of total Akt (B), phosphorylated-Aktthr308 (C), phosphorylated-Aktser473 (E) and D and F show the ratios of each phosphorylated-Akt to total Akt. Representative bands are shown above bar graphs. Values are mean ± SE. P < .05; *, ZLC vs ZOC; †, ZOC vs ZOL.
Figure 6.
Linagliptin (LGT) treatment increases myocardial eNOS expression in the Zucker Obese (ZO) heart. Bar graphs show levels of phosphorylated-eNOSser1177 (A) and total eNOS protein (B) normalized to actin in myocardial protein extracts. Representative bands are shown above bar graphs. Values are mean ± SE. P < .05; *, ZLC vs ZOC; †, ZOC vs ZOL.
Discussion
The goal of the present study was to determine the early changes that occur in the heart of young insulin-resistant ZO rats that manifest diastolic dysfunction. The major findings in this investigation are that impairments in in vivo cardiac diastolic function in hyperinsulinemic obese rats with a leptin receptor mutation markedly improved with chronic pharmacological inhibition of DPP-4, which occurred in concert with improved vascular endothelial function and a reduction in BP. This is the first study to show that LGT treatment is associated with improved myocardial relaxation and blunted progression of hypertension in an animal model of insulin-resistant obesity cardiomyopathy. This model is also relevant to the emerging pandemic in childhood/adolescent overweight/obesity associated with prediabetic insulin resistance and diastolic dysfunction. Thus, the results of this investigation support the notion that targeted pharmacologic interventions could be useful in ameliorating pathophysiologic abnormalities in diastolic and vascular endothelial dysfunction in the setting of obesity/prediabetes.
We previously demonstrated diastolic dysfunction in 8- to 12-week-old ZO rats (17, 18). In the context of an echocardiographic examination of cardiac function, comprising multiple systolic and diastolic parameters, LGT was effective at improving diastolic parameters, including motion of the LV wall during diastole (E' to A' ratio), as well as parameters measured during the active phase of early diastolic relaxation, including the Vp of mitral inflow and IVRT. Indices of LV filling pressure (E to E' and E to Vp ratios) were elevated in ZOC rats and were normalized in ZO rats treated with LGT. Diastolic parameters derived from PV analysis, including τ and the LV stiffness constant (slope of the EDPVR), indicate diastolic abnormalities in both the active and passive properties of the LV wall, respectively, in ZOC and amelioration with LGT. Moreover, among ZOC and ZOL rats in which we performed both cardiac procedures, there was good correspondence between indices of early diastolic function derived by noninvasive echocardiography (E', Vp, and IVRT) and τ, which was obtained by direct catheterization. Chronic increases in preload/afterload eventually lead to decreases in LV wall compliance and cardiac output. Increased preload (EDP) and afterload (MAP) are apparent in ZOC compared with ZLC, as is increased LV wall stiffness. We also observed decreased cardiac output in ZOC compared with ZLC. There was partial improvement of cardiac output in ZO rats treated with LGT as well as normalization of EDP and LV wall stiffness. The improvement in EDP in ZOL rats was consistent with decreases in the echocardiography-derived indices of LV filling pressure.
The factors contributing to LV diastolic dysfunction include abnormalities of neurohormonal activation, LV passive stiffness, and LV relaxation caused by altered calcium signaling. The cardiac phenotype in ZO rats is characterized, in part, by development of LVH and fibrosis that is initially an adaptive response to progressive increases in preload (volume expansion) and afterload due to increased sympathetic activation (22). In this study, improvement in diastolic function in ZOL hearts was not accompanied by regression of LVH. The dissociation between the improvement in diastolic function and LVH has also been observed in other animal models of cardiomyopathy (28, 29) and humans with metabolic syndrome (30). We also examined changes in collagen expression to determine the effect of LGT on cardiac fibrosis. Collagen deposition in the extracellular compartment primarily comprises 2 subtypes of collagen, type 1 and 3. A shift in the ratio of these collagen subtypes from type 3 to type 1 is thought to be responsible for an increase in chamber stiffness, because collagen type 3 is more compliant than the type 1 isoform (27, 28). Therefore, improved diastolic function after LGT treatment cannot be ascribed to changes in cardiac fibrosis nor LVH.
Myocardial oxidative stress has been linked to diastolic dysfunction in obese and nonobese rodent models of metabolic syndrome (17, 22, 31). Moreover, ROS play a role in myocardial remodeling by activating signaling pathways that mediate hypertrophy, fibrosis, and apoptosis (32). We therefore examined the accumulation of ROS, as determined by superoxide production, and RNS, by immunohistochemical staining for 3-NT. Surprisingly, LGT-mediated improvement in diastolic function was not accompanied by reductions in the levels of either ROS or RNS. In this regard, improvement in diastolic dysfunction independent of reductions in cardiac oxidative stress has previously been reported (33).
We have examined the role of SERCA2a activity in the heart, because SERCA2a determines Ca2+ loading of the sarcoplasmic reticulum, thereby regulating the amount of calcium released and cardiac contractility and relaxation. SERCA2a function is dependent, in part, on its expression level and activity, as well as via interaction with its inhibitor, PLB (34). Consistent with a previous study in Zucker hearts (35), SERCA2a protein levels in ZOC hearts were not different from those in ZLC. However, the levels were significantly elevated by LGT treatment in ZO hearts. Increased SERCA2a and decreased PLB to SERCA2a ratio are reported to be determinants of improved diastolic function (36). We examined the changes in the levels of total PLB in these samples and the ratio of PLB to SERCA2a. PLB level did not change, but the ratio of PLB to SERCA2a tended to decrease after LGT treatment in ZO rats. The phosphorylation of PLB at the serine16 residue, mediated by protein kinase A, is one mechanism by which the negative regulation of SERCA2a by dephosphorylated PLB is relieved (34), and GLP-1 signaling has been shown to activate protein kinase A signaling (37). In the present study, phosphorlated-PLBSer16 was not different in any group. We further examined whether this is reflected in nitrosylation of SERCA2a itself, which has been demonstrated to increase under conditions of oxidative stress (38). This was examined in this study by Western blotting using an antibody directed towards tyrosine294/295 residue of SERCA2a. Similar to 3-NT, accumulation in total protein phosphorylated-SERCA2aTyr294/295 was not affected by LGT. In this regard, the functional SERCA2a level has been reported to be crucial for postischemic myocardial function and salvage during ischemic reperfusion, and the beneficial effect of SERCA2a expression was shown to be independent of oxidative stress (39). Therefore, the improvement of diastolic dysfunction after LGT administration in this study may be due, in part, to changes in SERCA2a protein levels.
Another important factor determining cardiac function, including diastolic dysfunction, is mitochondrial function, which is impacted by a combination of factors, including mitochondrial structural integrity, content/biogenesis, and metabolic activity (40, 41). In this study, ultrastructural integrity of mitochondria markedly improves after LGT, as indicated by improved sarcolemmal and cristae organization. An association between mitochondrial structural abnormalities and diastolic dysfunction in the absence of systolic dysfunction or other features of cardiomyopathy has previously been reported in a murine model of low-dose doxyrubicin-induced diastolic dysfunction (33). Moreover, although oxidative stress has been implicated in development of diastolic dysfunction, it was not a contributing factor in this model. Therefore, the reversal of mitochondrial damage with LGT may be a critical factor contributing to improvement in diastolic dysfunction. In contrast to mitochondrial structural disorganization, we observed no changes in myocardial protein levels of different components of mitochondrial electron transport chain (I–V) and cyclooxygenase4 content and citrate synthase activity. The expression of phosphorylated-AMPK were also not changed in ZOC or LGT-treated rats. Similar results were also reported in another study in ZO hearts (42). In this regard, lipid-induced cardiac dysfunction may occur independently of alterations in mitochondrial content. Indeed, increased accumulation of triglycerides and FA uptake has been reported in the ZO heart despite normal mitochondrial content and long-chain FA oxidation with unaltered activities of citrate synthase and β-hydroxyacyl-coenzyme A dehydrogenase (43).
Insulin metabolic signaling in the heart plays an important role in cardiac substrate metabolism and function (17, 44). In this study, there was an increase in myocardial IRS-1 protein levels in LGT-treated ZO rats, suggesting improved myocardial insulin sensitivity. There were significant increases in both phosphorylated-AktThr308 and phosphorylated-AktSer473 in the myocardium of untreated ZO rats and LGT treatment tended to decrease both phosphorylated forms of Akt. In this respect, activation of Akt has been reported in patients with T2D and LV dysfunction (45–47). In rats, chronic infusion of insulin results in stimulation of AktSer473phosphorylation in the heart (48) and white adipose tissue (49). Similarly, basal levels of phosphorylated-AktSer473 are elevated in the heart of ob/ob mice (47). Although the acute activation of Akt has been considered beneficial, chronic persistent activation of Akt has been associated with pathological hypertrophy and heart failure (50). Akt plays a role in regulating FA uptake in the myocardium, and increased phosphorylation of Akt is associated with elevated FA uptake in insulin-resistant rats fed a high-fat diet (51) as well as obese Zucker rats (43, 52). Persistent activation of Akt has also been shown to impair insulin signaling through phosphorylation of threonine residues in the insulin receptor (53). Therefore, improvement in Akt signaling and IRS-1 protein expression may account for improvement in cardiac function.
The role of GLP-1 in lowering BP has been reported in few long-term animal and human studies (54, 55). Sitagliptin has been shown to decrease BP in hypertensive humans (56) as well as hypertensive (57) and diabetic rats (58). Direct vasodilator effects of DPP-4 inhibition have also been described in vascular rings, and of the varied DPP-4 inhibitors tested, LGT was the most potent compound, followed by alogliptin and vildagliptin. The vasorelaxant effects of alogliptin and LGT have been shown to involve the NO/cyclic guanosine monophosphate pathway (21, 59). In this study, we observed decreased MAP in ZOL rats and an increase in both eNOS and phosphorylated-eNOSSer1177 protein levels in LGT-treated ZO hearts compared with obese rats. Furthermore, the defect in endothelium-dependent vasodilation of skeletal muscle 1A arterioles observed in ZOC rats was abolished by treatment with LGT. This improvement occurred despite reduced vascular smooth muscle sensitivity to NO, assessed via vasodilation to the NO donor sodium nitroprusside. Taken together, these data suggest that the improved endothelium-dependent vasodilation in LGT-treated ZO arterioles occurs as a result of either increased NO bioavailability, to overcome reduced NO sensitivity, or via up-regulation of other endothelium-dependent vasodilator species (ie, prostacyclin, endothelium-derived hyperpolarizing factors). Importantly, the changes noted in arteriolar function correspond with the noted changes in BP. Namely, in ZOC rats, arteriolar dilation was reduced in concert with increased BP, and both were corrected by LGT. In this regard, improvement of vascular endothelial function has been observed in obese Zucker rats and apolipopoteinB null mice by DPP-4 inhibitor treatment (60, 61). Therefore, increased eNOS phosphorylation and arteriolar endothelial function are likely contributors to the beneficial effects of LGT on MAP and cardiac dysfunction in ZO rats. These results suggest that the improvement of cardiac function may be due, in part, to hemodynamic effects of LGT. However, eNOS signaling in the heart has been shown to improve diastolic function, and this may occur without any significant change in ventricular mass (17, 62, 63). Moreover, accumulating evidence suggests that GLP-1 and DPP-4 have direct effects in the heart and coronary circulation (60, 64). It is now established that components of the incretin system are expressed in the myocardium (8) and the coronary circulation (8, 9) and that genetic deletion of the GLP-1 R leads to development of a cardiomyopathic phenotype (10), whereas deletion of DPP-4 confers cardioprotection from experimental manipulations that promote cardiomyopathy (65). Because DPP-4 is not specific for GLP-1, the effects of DPP-4 inhibition on heart function may include effects mediated through regulation of other substrates, such as stromal cell-derived factor-1a (9). Therefore, the beneficial effects of LGT on diastolic function maybe due to a combination of hemodynamic and DPP-4-mediated pleiotropic effects on the myocardium and coronary vasculature.
In summary, cardiac diastolic dysfunction is an independent predictor of death in patients with preserved ejection fraction, and therapies that improve diastolic function are likely to improve survivorship. Although DPP-4 therapies are used primarily for improving glycemia in diabetic patients, clinical trials are currently underway to examine whether there are potential cardiovascular benefits beyond glycemic control. This study supports the concept that therapeutic targeting of DPP-4 with a specific inhibitor may be beneficial in blunting obesity-associated cardiac diastolic dysfunction in the prediabetic state.
Acknowledgments
We thank Brenda Hunter for editorial assistance, Nathan Rehmer for technical and administrative support, and Terry L. Carmack and Lisa D. Watkinson of the Truman Memorial Veterans' Hospital Radiopharmaceutical Sciences Institute for surgical assistance. We also thank the Electron Microscope Core Center at the University of Missouri-Columbia for help with preparation of tissue samples.
This work was supported by Boehringer Ingelheim Pharma (V.G.D.). Additional support was provided by National Institutes of Health Grants RO1-HL073101 and RO1-HL107910 (to J.R.S.), the Veterans Affairs Merit System Grant 0018 (to J.R.S.), and the Department of Veterans Affairs Grant CDA-2BB47 (to A.W.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 1A
- first order
- A'
- peak late septal annular velocity
- Akt
- protein kinase B
- AMPK
- AMP-activated kinase
- BP
- blood pressure
- DPP-4
- dipeptidylpeptidase-4
- E'
- early peak septal annular velocity
- EDP
- end diastolic pressure
- EDPVR
- end diastolic pressure-volume relationship
- eNOS
- endothelial nitric oxide synthase
- FA
- fatty acid
- GLP-1
- glucagon-like peptide-1
- GLP-1R
- GLP-1 receptor
- HOMA-IR
- homeostatic model assessment of insulin resistance
- IRS-1
- insulin receptor substrate-1
- IVRT
- isovolumic relaxation time
- LGT
- linagliptin
- LV
- left ventricular
- LVH
- LV hypertrophy
- LV+S
- LV and septum
- MAP
- mean arterial pressure
- NO
- nitric oxide
- 3-NT
- 3-nitrotyrosine
- PLB
- phospholamban
- PV
- pressure volume
- RNS
- reactive nitrogen species
- ROS
- reactive oxygen species
- SERCA2a
- sarcoplasmic reticulum calcium ATPase 2a
- T2D
- type 2 diabetes
- TL
- tibia length
- Vp
- propagation velocity
- ZL
- Zucker lean
- ZLC
- ZL control
- ZLL
- ZL treated with LGT
- ZO
- Zucker obese
- ZOC
- ZO control
- ZOL
- ZO treated with LGT.
References
- 1. Bender SB, McGraw AP, Jaffe IZ, Sowers JR. Mineralocorticoid receptor-mediated vascular insulin resistance: an early contributor to diabetes-related vascular disease? Diabetes. 2013;62:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drucker DJ. The role of gut hormones in glucose homeostasis. J Clin Invest. 2007;117:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jose T, Inzucchi SE. Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc Dis Res. 2012;9(2):109–116 [DOI] [PubMed] [Google Scholar]
- 4. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jax T. Treatment of patients with diabetes with GLP-1 analogues or DPP-4- inhibitors: a hot topic for cardiologists? Clin Res Cardiol. 2009;98:75–79 [DOI] [PubMed] [Google Scholar]
- 6. Anagnostis P, Athyros VG, Adamidou F, et al. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13:302–312 [DOI] [PubMed] [Google Scholar]
- 7. Scheen AJ. Cardiovascular effects of gliptins. Nat Rev Cardiol. 2013;10:73–84 [DOI] [PubMed] [Google Scholar]
- 8. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 9. Shigeta T, Aoyama M, Bando YK, et al. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851 [DOI] [PubMed] [Google Scholar]
- 10. Gros R, You X, Baggio LL, et al. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003;144:2242–2252 [DOI] [PubMed] [Google Scholar]
- 11. Noyan-Ashraf MH, Shikatani EA, Schuiki I, et al. A glucagon-like peptide-1 analog reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation. 2013;127:74–85 [DOI] [PubMed] [Google Scholar]
- 12. Yoon YS, Uchida S, Masuo O, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–2085 [DOI] [PubMed] [Google Scholar]
- 13. Santos JL, Salemi VM, Picard MH, Mady C, Coelho OR. Subclinical regional left ventricular dysfunction in obese patients with and without hypertension or hypertrophy. Obesity (Silver Spring). 2011;19:1296–1303 [DOI] [PubMed] [Google Scholar]
- 14. Van Putte-Katier N, Rooman RP, Haas L, et al. Early cardiac abnormalities in obese children: importance of obesity per se versus associated cardiovascular risk factors. Pediatr Res. 2008;64:205–209 [DOI] [PubMed] [Google Scholar]
- 15. Poulsen MK, Henriksen JE, Dahl J, et al. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ Cardiovasc Imaging. 2010;3:24–31 [DOI] [PubMed] [Google Scholar]
- 16. Deacon CF, Holst JJ. Linagliptin, a xanthine-based dipeptidyl peptidase-4 inhibitor with an unusual profile for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2010;19:133–140 [DOI] [PubMed] [Google Scholar]
- 17. Zhou X, Ma L, Habibi J, et al. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pulakat L, DeMarco VG, Ardhanari S, et al. Adaptive mechanisms to compensate for overnutrition-induced cardiovascular abnormalities. Am J Physiol Regul Integr Comp Physiol. 2011;301(4):R885–R895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sowers JR, Whaley-Connel AT, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardio Renal Medicine. 2011;1:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckhardt M, Langkopf E, Mark M, et al. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2-ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem. 2007;50:6450–6453 [DOI] [PubMed] [Google Scholar]
- 21. Kröller-Schön S, Knorr M, Hausding M, et al. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res. 2012;96:140–149 [DOI] [PubMed] [Google Scholar]
- 22. DeMarco VG, Johnson MS, Habibi J, et al. Comparative analysis of telmisartan and olmesartan on cardiac function in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2011;300:H181–H190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeMarco VG, Johnson MS, Ma L, et al. Overweight female rats selectively breed for low aerobic capacity exhibit increased myocardial fibrosis and diastolic dysfunction. Am J Physiol Heart Circ Physiol. 2012;302:H1667–H1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol. 2005;98:753–761 [DOI] [PubMed] [Google Scholar]
- 25. Whaley-Connell A, Govindarajan G, Habibi J, et al. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab. 2007;293:E355–E363 [DOI] [PubMed] [Google Scholar]
- 26. Takatsuji H, Mikami T, Urasawa K, et al. A new approach for evaluation of left ventricular diastolic function: spatial and temporal analysis of left ventricular filling flow propagation by color M-mode Doppler echocardiography. J Am Coll Cardiol. 1996;27:365–371 [DOI] [PubMed] [Google Scholar]
- 27. Oki T, Tabata T, Yamada H, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol. 1997;79:921–928 [DOI] [PubMed] [Google Scholar]
- 28. Daniels A, van BM, Janssen BJ, et al. Impaired cardiac functional reserve in type 2 diabetic db/db mice is associated with metabolic, but not structural, remodelling. Acta Physiol (Oxf). 2010;200:11–22 [DOI] [PubMed] [Google Scholar]
- 29. Basu R, Oudit GY, Wang X, et al. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009;297:H2096–H2108 [DOI] [PubMed] [Google Scholar]
- 30. de las Fuentes L, Brown AL, Mathews SJ, et al. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur Heart J. 2007;28:553–559 [DOI] [PubMed] [Google Scholar]
- 31. Johnson MS, DeMarco VG, Heesch CM, et al. Sex differences in baroreflex sensitivity, heart rate variability, and end organ damage in the TGR(mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2011;301(4):H1540–H1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190 [DOI] [PubMed] [Google Scholar]
- 33. Hiraumi Y, Iwai-Kanai E, Baba S, et al. Granulocyte colony-stimulating factor protects cardiac mitochondria in the early phase of cardiac injury. Am J Physiol Heart Circ Physiol. 2009;296:H823–H832 [DOI] [PubMed] [Google Scholar]
- 34. Cerra MC, Imbrogno S. Phospholamban and cardiac function: a comparative perspective in vertebrates. Acta Physiol (Oxf). 2012;205:9–25 [DOI] [PubMed] [Google Scholar]
- 35. Young ME, Guthrie PH, Razeghi P, et al. Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes. 2002;51:2587–2595 [DOI] [PubMed] [Google Scholar]
- 36. Lebeche D, Davidoff AJ, Hajjar RJ. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nat Clin Pract Cardiovasc Med. 2008;5:715–724 [DOI] [PubMed] [Google Scholar]
- 37. Ye Y, Keyes KT, Zhang C, Perez-Polo JR, Lin Y, Birnbaum Y. The myocardial infarct size-limiting effect of sitagliptin is PKA-dependent, whereas the protective effect of pioglitazone is partially dependent on PKA. Am J Physiol Heart Circ Physiol. 2010;298:H1454–H1465 [DOI] [PubMed] [Google Scholar]
- 38. Evangelista AM, Thompson MD, Weisbrod RM, et al. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxid Redox Signal. 2012;17:1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Talukder MA, Kalyanasundaram A, Zuo L, et al. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1426–H1434 [DOI] [PubMed] [Google Scholar]
- 40. Ren J, Pulakat L, Whaley-Connell A, Sowers JR. Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease. J Mol Med. 2010;88:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aroor AR, Mandavia C, Ren J, Sowers JR, Pulakat L. Mitochondria and oxidative stress in the cardiorenal metabolic syndrome. Cardio Renal Medicine. 2012;2:87–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burgmaier M, Sen S, Philip F, et al. Metabolic adaptation follows contractile dysfunction in the heart of obese Zucker rats fed a high-fat “Western” diet. Obesity (Silver Spring). 2010;18:1895–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Holloway GP, Snook LA, Harris RJ, Glatz JF, Luiken JJ, Bonen A. In obese Zucker rats, lipids accumulate in the heart despite normal mitochondrial content, morphology and long-chain fatty acid oxidation. J Physiol. 2011;589:169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pulakat L, Aroor AR, Gul R, Sowers JR. Cardiac insulin resistance and microRNA modulators. Exp Diabetes Res. 2012;2012:654904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haq S, Choukroun G, Lim H, et al. Differential activation of signal transduction pathways in human hearts with hypertrophy versus advanced heart failure. Circulation. 2001;103:670–677 [DOI] [PubMed] [Google Scholar]
- 46. Dutka DP, Pitt M, Pagano D, et al. Myocardial glucose transport and utilization in patients with type 2 diabetes mellitus, left ventricular dysfunction, and coronary artery disease. J Am Coll Cardiol. 2006;48:2225–2231 [DOI] [PubMed] [Google Scholar]
- 47. Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Samuelsson AM, Bollano E, Mobini R, et al. Hyperinsulinemia: effect on cardiac mass/function, angiotensin II receptor expression, and insulin signaling pathways. Am J Physiol Heart Circ Physiol. 2006;291:H787–H796 [DOI] [PubMed] [Google Scholar]
- 49. Ueno M, Carvalheira JB, Tambascia RC, et al. Regulation of insulin signalling by hyperinsulinaemia: role of IRS-1/2 serine phosphorylation and the mTOR/p70 S6K pathway. Diabetologia. 2005;48:506–518 [DOI] [PubMed] [Google Scholar]
- 50. Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. 2011;13:825–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ouwens DM, Diamant M, Fodor M, et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia. 2007;50:1938–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huisamen B, Lochner A. Exercise modulates myocardial protein kinase B/Akt in Zucker obese rats. Heart. 2005;91:227–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morisco C, Condorelli G, Trimarco V, et al. Akt mediates the cross-talk between β-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res. 2005;96:180–188 [DOI] [PubMed] [Google Scholar]
- 54. Hocher B, Reichetzeder C, Alter ML. Renal and cardiac effects of DPP-4 inhibitors—from preclinical development to clinical research. Kidney Blood Press Res. 2012;36:65–84 [DOI] [PubMed] [Google Scholar]
- 55. Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135 [DOI] [PubMed] [Google Scholar]
- 56. Mistry GC, Maes AL, Lasseter KC, et al. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598 [DOI] [PubMed] [Google Scholar]
- 57. Pacheco BP, Crajoinas RO, Couto GK, et al. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–528 [DOI] [PubMed] [Google Scholar]
- 58. Ferreira L, Teixeira-de-Lemos E, Pinto F, et al. Effects of sitagliptin treatment on dysmetabolism, inflammation, and oxidative stress in an animal model of type 2 diabetes (ZDF rat). Mediators Inflamm. 2010;2010:592760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shah Z, Pineda C, Kampfrath T, et al. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol. 2011;55:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mason RP, Jacob RF, Kubant R, et al. Effect of enhanced glycemic control with saxagliptin on endothelial nitric oxide release and CD40 levels in obese rats. J Atheroscler Thromb. 2011;18:774–783 [DOI] [PubMed] [Google Scholar]
- 61. Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2012;59:265–276 [DOI] [PubMed] [Google Scholar]
- 62. Lam CS, Brutsaert DL. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2012;60:1787–1789 [DOI] [PubMed] [Google Scholar]
- 63. Piech A, Massart PE, Dessy C, et al. Decreased expression of myocardial eNOS and caveolin in dogs with hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2002;282:H219–H231 [DOI] [PubMed] [Google Scholar]
- 64. Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55:10–16 [DOI] [PubMed] [Google Scholar]
- 65. Sauvé M, Ban K, Momen MA, et al. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]