Abstract
The pancreatic hormone amylin acts in the central nervous system (CNS) to decrease food intake and body weight. We hypothesized that amylin action in the CNS promotes energy expenditure by increasing the activity of the sympathetic nervous system. In mice, ip administration of amylin significantly increased c-Fos immunoreactivity in hypothalamic and brainstem nuclei. In addition, mice treated with intracerebroventricular (icv) amylin (0.1 and 0.2 nmol) exhibited a dose-related decrease in food intake and body weight, measured 4 and 24 hours after treatment. The icv injection of amylin also increased body temperature in mice. Using direct multifiber sympathetic nerve recording, we found that icv amylin elicited a significant and dose-dependent increase in sympathetic nerve activity (SNA) subserving thermogenic brown adipose tissue (BAT). Of note, icv injection of amylin also evoked a significant and dose-related increase in lumbar and renal SNA. Importantly, icv pretreatment with the amylin receptor antagonist AC187 (20 nmol) abolished the BAT SNA response induced by icv amylin, indicating that the sympathetic effects of amylin are receptor-mediated. Conversely, icv amylin-induced BAT SNA response was enhanced in mice overexpressing the amylin receptor subunit, RAMP1 (receptor-activity modifying protein 1), in the CNS. Our data demonstrate that CNS action of amylin regulates sympathetic nerve outflow to peripheral tissues involved in energy balance and cardiovascular function.
Amylin, also known as islet amyloid polypeptide, is a 37-amino-acid peptide hormone, originally isolated from pancreatic amyloid deposits that occur in diabetes (1, 2). Later, it was found that amylin is a physiological product of the pancreatic β-cells (3, 4) and is stored and cosecreted with insulin in response to nutrient stimulus after ingestion of food (5, 6). Interestingly, secretion of amylin and its plasma concentrations positively correlate with the degree of body adiposity (7).
Accumulating evidence suggests that amylin plays an essential role in the regulation of energy homeostasis and possibly acting as an adiposity signal. A single systemic administration of amylin causes anorexia and weight loss in rats (8–11) and mice (12, 13). Conversely, rats treated with a selective antagonist of the amylin receptor, AC187, exhibited a dose-dependent increase in food intake (14). Amylin also decreased food intake and caused weight loss after a single intracerebroventricular (icv) administration (15). In addition to these short-term effects, amylin appears to participate in the long-term control of food intake and body weight. Indeed, chronic systemic or icv infusion of amylin lowers food intake, body weight, and fat mass in rats (16, 17). Conversely, long-term inhibition of brain amylin receptors by chronic icv infusion of AC187 increased food intake, body weight, and adiposity in rats (18, 19).
The strength of amylin-induced anorexia and weight loss when administered centrally relative to systemic administration indicates that the effects of this hormone on food intake and body weight are centrally mediated. Consistent with this notion, amylin was found in the brain and circulating amylin was shown to cross the blood-brain barrier through a specific and saturable transport system (20, 21). Moreover, binding sites for amylin are present in several brain regions that are involved in the control of food intake such as the area postrema, nucleus tractus solitarii, and hypothalamus (22–25). However, the exact mechanisms by which central nervous system (CNS) action of amylin regulates peripheral metabolism remain elusive.
Amylin receptor is a heterodimer arising from the interaction of two proteins, namely calcitonin receptor (CTR) and receptor activity-modifying protein (RAMP) (26, 27). RAMPs (comprising RAMP1, RAMP2, and RAMP3) are small membrane proteins (28, 29) that are expressed in several brain regions including the brainstem and hypothalamus (30). Importantly, CTR was found to be codistributed with RAMP proteins, particularly RAMP1 and RAMP2, in several brain nuclei (31).
The aim of the present study was to test whether amylin action in the CNS modulates the activity of the sympathetic nervous system, an important regulatory mechanism of several physiological functions including metabolism and energy balance (32). We therefore determined the effect of CNS amylin on regional sympathetic nerve activity (SNA) in mice. In addition, we examined whether the effects are receptor-mediated and tested the relevance of RAMP1 for CNS action of amylin.
Materials and Methods
Animals
Male 12- to 16-week-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, Maine) and male and female nestin-human RAMP1 (hRAMP1) transgenic mice and littermate controls (our own colony) were studied. The nestin-hRAMP1 transgenic mice were produced by mating nestincre mice with GFPflox-hRAMP1 mice, in which an upstream GFP cDNA includes a translational stop sequence and a polyadenylation signal that prevents expression of hRAMP1. Thus, hRAMP1 expression is dependent on Cre recombinase excision of GFP at flanking loxP sites. Genotype of the mice was determined by PCR as described previously (33). Animals were housed in a temperature-, humidity-, and light-controlled room (12-hour light, 12-hour dark cycle), with free access to tap water and chow, except when the mice were fasted. All procedures were approved by the University of Iowa Animal Care and Use Committee.
c-Fos immunohistochemistry
C57BL/6 mice were handled and trained for ip injections for 1 week. Mice were fasted for 3 hours before receiving vehicle (saline) or amylin (10 μg/kg; Bachem, Torrance, California) ip. The dosage of amylin (10 μg/kg) was based on previous experiments demonstrating that this is the lowest effective dose of amylin after ip administration in mice (13). Two hours after injection, mice were deeply anesthetized with ketamine (91 mg/kg) and xylazine (9.1 mg/kg) and perfused transcardially with saline followed by fixation solution containing 4% paraformaldehyde. Brains were dissected and postfixed in 4% paraformaldehyde solution at 4°C overnight and then incubated in a 30% sucrose solution at 4°C overnight.
Each brain was cut into 40 μm pieces, and free-floating sections (with sections from vehicle- and amylin-treated mice incubated together in the same vial) were washed to remove the sucrose and then treated with 0.3% H2O2 for 30 minutes to block endogenous peroxidase activity. After washing, sections were blocked for 1 hour with 3% normal goat serum followed by incubation with anti–c-Fos antibody (1:10 000, PC38; Calbiochem, La Jolla, California) diluted in 1% normal goat serum at 4°C overnight. Sections were washed in PBS, incubated 1 hour at room temperature with a biotinylated goat antirabbit IgG (1:200, BA-1000; Vector Laboratories, Burlingame, California), washed, and then incubated in ABC solution (Vectastain Elite ABC Kit, PK-6100; Vector Laboratories) for 1 hour at room temperature. Sections were rinsed in PBS, incubated in diaminobenzidine (Vector Laboratories; SK-4100) for 1 minute, rinsed again, mounted onto cleaned slides, air-dried for 2 to 3 days, and coverslipped using mounting media (Permount, SP15-500; Fisher Scientific, Pittsburgh, Pennsylvania).
c-Fos–immunoreactive cells were analyzed using comparable sections throughout the hypothalamus and brainstem. Quantification of c-Fos was performed using a counting grid to avoid double counting or missing cells. The position of the counting grid was delineated through adjacent landmarks. Both sides of the brain at 1 to 2 representative levels of each region were quantified (depending on the size of the region analyzed). Cell counts were performed in 6 mice per group.
The icv cannulation
Mice were anesthetized with ip administration of ketamine (91 mg/kg) and xylazine (9.1 mg/kg) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, California). Mouse ear bars, carefully inserted into the ears, and a mouse head holder (Kopf, model 926) were used to secure the mouse head. Under aseptic conditions, the skull was exposed by an incision and the tooth bar adjusted until lambda and bregma were leveled (approximately 2.0 mm below horizontal zero). A stainless steel cannula (25 gauge, 9 mm length) was implanted into the right lateral cerebral ventricle at coordinates 3.0 mm depth below the skull surface, 0.3 mm anterior to bregma, and 1.0 mm lateral to the midline. The cannula was fixed on the skull with dental acrylic and 1 anchoring screw. An obturator protruding 0.5 mm past the tip of the guide cannula was inserted. Mice were allowed at least 1 week of recovery before experimentation. At the end of each experiment, an Evans blue dye was injected icv before the mouse was killed to ensure that the cannula was correctly positioned in the lateral ventricle. The brain was harvested, and the diffusion of the dye throughout the ventricles was verified.
Food intake and body weight study
One week after icv cannulation, mice were housed in individual cages and allowed to acclimate for 1 week. Mice were fasted overnight before icv administration of vehicle (saline) or amylin (0.1 or 0.2 nmol). Body weight and food intake were recorded before icv injections as well as 4 and 24 hours after. The dosage of amylin for icv treatment was selected based on pilot studies identifying the lowest effective dose of icv amylin on food intake and sympathetic nerve traffic in mice.
Body temperature measurement
We used a radio-telemetric system to continuously measure body temperature in conscious freely moving mice. Mice were anesthetized with ip injection of a ketamine/xylazine cocktail. A transmitter (TA10TA-F20 from Data Sciences International, New St Paul, Minnesota) was implanted into the peritoneal cavity of each animal followed by icv cannulation as described above. Mice were then allowed to recover for at least 1 week in individual cages. Mice were fasted overnight before icv injection of vehicle (saline) or amylin (0.2 nmol). Body temperature was collected every 5 minutes and analyzed using Data Science Dataquest software.
Recording of sympathetic nerve traffic
Mice equipped with an icv cannula were anesthetized with ip injection of ketamine (91 mg/kg) and xylazine (9.1 mg/kg). The trachea was cannulated so that each mouse could spontaneously breathe oxygen-rich air. A catheter was inserted into the right jugular vein for maintenance of anesthesia with α-chloralose (12 mg/kg/h). Rectal temperature was maintained at 37.5°C using a temperature-controlled surgical table and a heating lamp.
Multifiber recording was used to assess regional SNA. Using a dissecting microscope, a nerve fascicle subserving brown adipose tissue (BAT), hind limb (for lumbar SNA), or kidney (for renal SNA) was isolated. A bipolar platinum-iridium electrode (Cooner Wire, Chatsworth, California) was suspended under the nerve and secured with silicone gel (Kwik-Cast, WPI, Sarasota, Florida). The nerve signal was amplified and filtered as described previously (34).
Baseline SNA was recorded for 10 minutes, followed by icv injection of vehicle (saline, 2 μl) or amylin (0.1 or 0.2 nmol). In some mice, the specific antagonist of the amylin receptor AC187 (20 nmol; US Biologicals, Salem, Massachusetts) was administered icv 10 minutes before icv injection of vehicle or amylin (0.2 nmol). SNA responses to different treatments were recorded continuously for 240 minutes. At the end of the study, mice were euthanized with a lethal dose of ketamine/xylazine. The integrated voltage after death (background noise) was subtracted from the total integrated voltage to calculate real SNA.
Statistical analysis
Results are displayed as mean ± SEM. SNA data are expressed as percent change from baseline (with 0% as baseline). Data were analyzed using Student's t test or 1- or 2-way ANOVA with or without repeated measures. When ANOVA reached significance, a post hoc comparison was made using Fisher's test. A P value < .05 was considered to be statistically significant.
Results
Peripheral amylin modulates neuronal activity
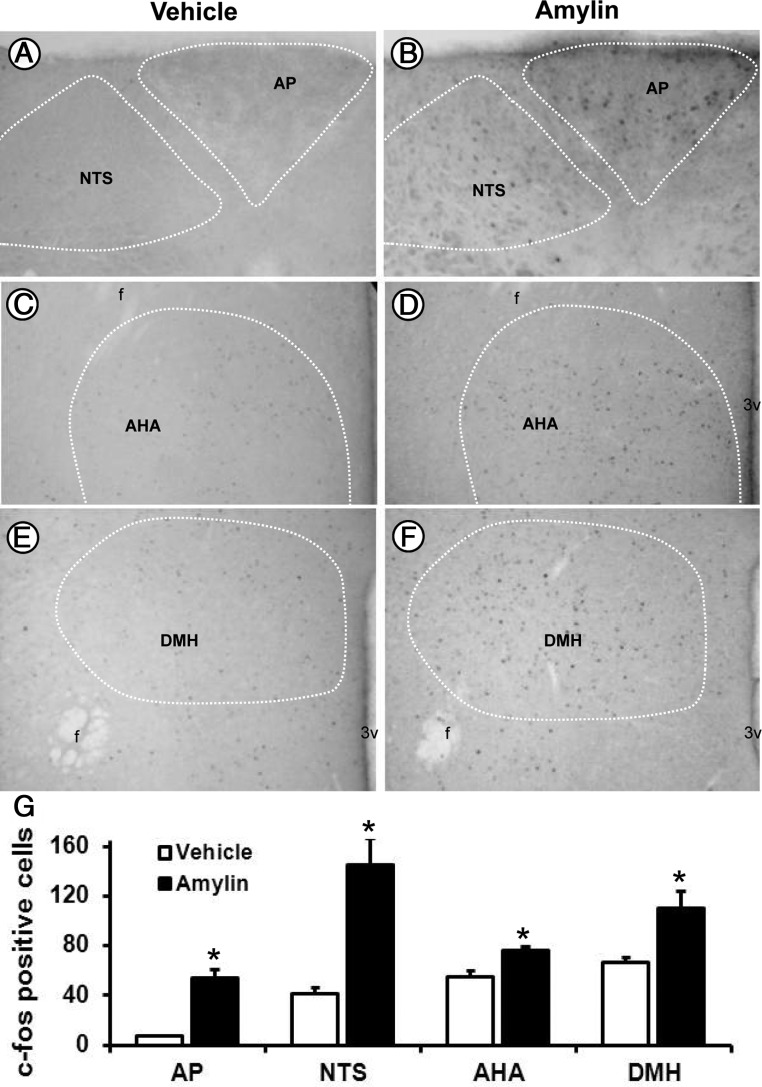
We used c-Fos immunoreactivity as a readout of cellular activation in the brain in response to systemic administration of amylin. Consistent with previous reports (23, 35), we found that in mice, ip injection of amylin (10 μg/kg) caused a significant (P < .01 vs vehicle) increase in c-Fos immunoreactivity in the brainstem nuclei with a 7-fold increase in the area postrema and a 3.5-fold increase in the nucleus tractus solitarii (Figure 1, A, B, and G). In the hypothalamus, amylin treatment significantly (P < .01 vs vehicle) increased the number of c-Fos–immunoreactive cells mainly in the anterior hypothalamic area (Figure 1, C, D, and G) and dorsomedial hypothalamic nucleus (Figure 1, E, F, and G).
Figure 1.
Peripheral amylin increases c-Fos in the mouse brain. A–F, Representative photomicrographs depicting the effect of ip administration of amylin on c-Fos immunoreactivity in the area postrema (AP) and the nucleus tractus solitarii (NTS, A and B), anterior hypothalamic area (AHA, C and D), and dorsomedial hypothalamic nucleus (DMH, E and F) in C57BL/6J mice. G, Comparison of the number of immunoreactive c-Fos neurons in the brainstem and hypothalamic nuclei. *, P < .05 vs vehicle (n = 6 per group). The fornix (f) and third ventricle (3v) are indicated in the photomicrographs.
CNS amylin alters food intake, body weight, and body temperature in mice
Next, we investigated the effect of a single icv injection of amylin on food intake, body weight, and body temperature in C57BL/6J mice. The icv administration of amylin caused a significant (P < .001) and dose-related decrease in food intake, measured 4 and 24 hours after treatment (Figure 2A). The icv injection of amylin also reduced the 4- and 24-hour body weight changes relative to vehicle control treatment (Figure 2B). Moreover, mice treated with icv amylin (0.2 nmol) exhibited a significant increase in body temperature, particularly during the first 4 hours after treatment (Figure 2C). These results demonstrate the effectiveness of icv amylin at this dosage to alter energy homeostasis in mice.
Figure 2.
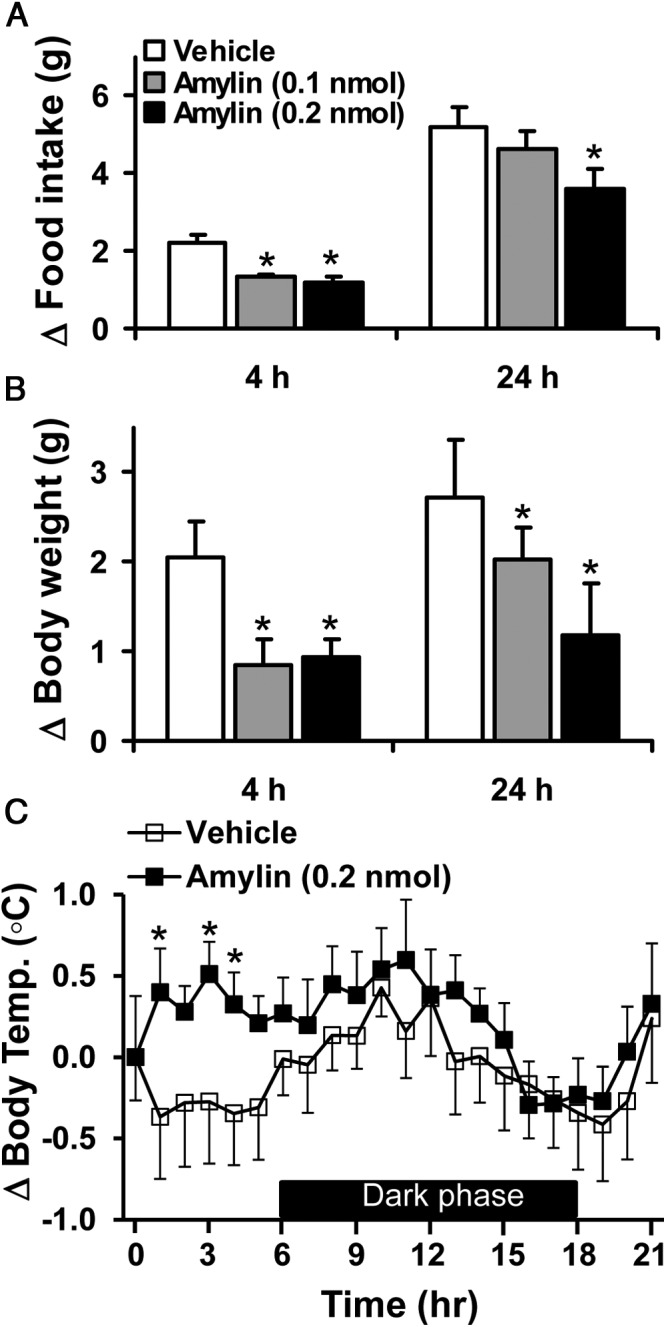
Anorectic and weight-reducing effects of CNS amylin. A and B, Effects of icv injection of amylin (0.1 and 0.2 nmol) on 4- and 24-hour food intake (A) and body weight gain (B) in fasted C57BL/6J mice. C, Effect of icv amylin (0.2 nmol) on body temperature in fasted C57BL/6J mice. *, P < .05 vs vehicle (n = 5–9 per group).
Regional sympathetic nerve activation to CNS amylin
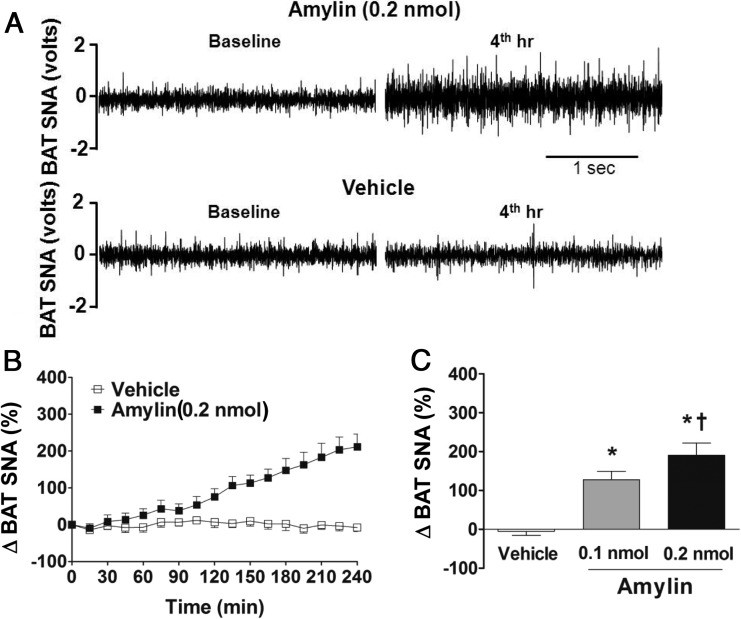
Using direct multifiber recording, we found that icv administration of amylin caused a slow increase in SNA subserving the thermogenic BAT in mice, with ∼200% increase in the fourth hour after the 0.2-nmol dose (Figure 3, A–C). The lower dose of amylin (0.1 nmol) also caused BAT sympathetic activation that was significantly different from vehicle-treated and 0.2 nmol amylin-treated groups (Figure 3C), indicating that icv amylin increases BAT SNA in a dose-dependent manner.
Figure 3.
CNS amylin increases thermogernic sympathetic nerve traffic. A, Representative neurograms of BAT SNA at baseline and 4 hours after icv vehicle and amylin (0.2 nmol). B, Time course of BAT SNA response to icv vehicle and amylin (0.2 nmol). C, Average of last hour of BAT SNA after icv vehicle and amylin (0.1 and 0.2 nmol). *, P < .05 vs vehicle; †, P < .05 vs 0.1 nmol amylin (n = 6–8 per group).
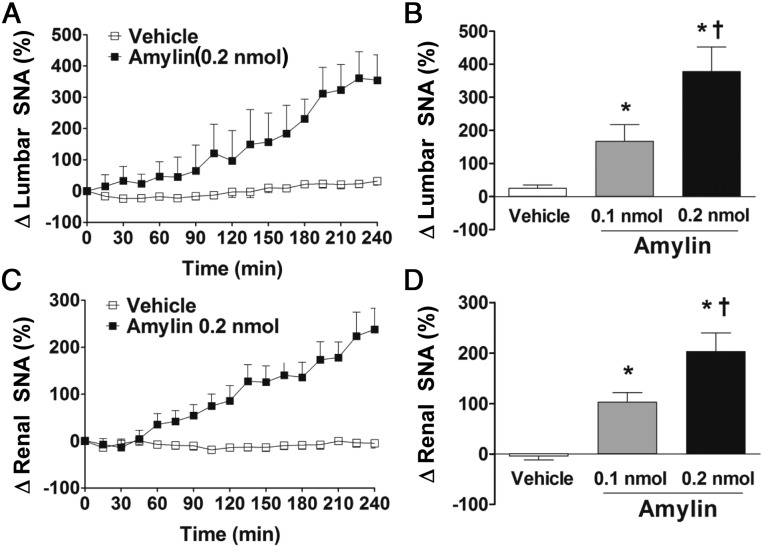
To test whether the increase in BAT SNA response to CNS action of amylin is specific to the BAT, we tested the response of nerves subserving other beds. Of note, icv injection of amylin evoked a robust and dose-related increase in lumbar SNA (Figure 4, A and B) with ∼380% increase in the fourth hour after the 0.2-nmol dose. The icv amylin also increased renal SNA in a dose-dependent manner, reaching ∼190% in the fourth hour after the 0.2-nmol dose (Figure 4, C and D). The difference in time course and potency of the icv amylin-induced increase in regional SNA is consistent with the concept that regional sympathetic traffic is differentially regulated (36).
Figure 4.
CNS amylin increases regional sympathetic nerve traffic. Effects of icv administration of amylin (0.1 and 0.2 nmol) on lumbar (A and B) and renal (C and D) SNA in C57BL/6J mice. A and C, Time course of SNA responses to icv vehicle and amylin (0.2 nmol). B and D, Average of last hour of SNA after icv vehicle and amylin (0.1 and 0.2 nmol). *, P < .05 vs vehicle; †, P < .05 vs 0.1 nmol amylin (n = 6–8 per group).
Receptor-mediated sympathetic nerve activation to CNS amylin
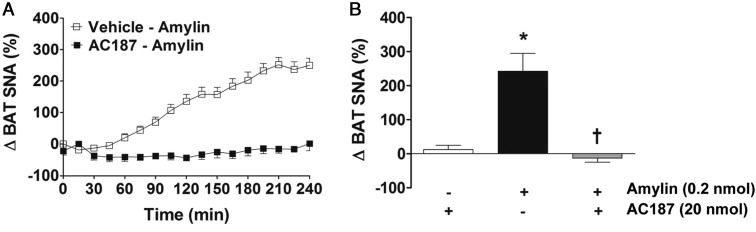
Next, we examined the role of the amylin receptor in mediating the SNA response to amylin. For this, we pretreated mice icv with a specific antagonist of the amylin receptor, AC187, 10 minutes before icv administration of amylin. As shown in Figure 5, icv pretreatment with AC187 (20 nmol) abolished the increase in BAT SNA induced by amylin. These data suggest that CNS amylin increases sympathetic nerve outflow by activating amylin receptors.
Figure 5.
Amylin-induced sympathetic activation is receptor-mediated. A, Time course of BAT SNA response to icv amylin (0.2 nmol) with or without icv pretreatment with AC187 (20 nmol) in C57BL/6J mice. B, Comparison of BAT SNA response (average of last hour of recording) to amylin in presence or absence of AC187. *, P < .05 vs vehicle-AC187 group; †, P < .05 vs vehicle-amylin group (n = 5 per group).
Enhanced sympathetic action of amylin in mice overexpressing RAMP1 in the CNS
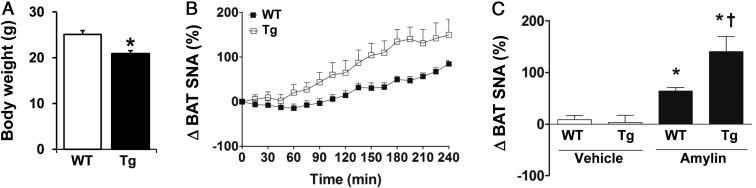
We used the nestin-hRAMP1 mouse model to further test the role of amylin receptors in the central action of amylin. Consistent with our previous report (37), nestin-hRAMP1 transgenic mice exhibited lower body weight relative to littermate wild-type controls (Figure 6A). The body weight was significantly (P < .05) lower in both male (23.3 ± 0.7 g) and female (19.5 ± 0.7 g) transgenic mice when compared with controls (27.9 ± 0.5 g and 23.5 ± 1.0 g, respectively).
Figure 6.
Relevance of RAMP1 for CNS action of amylin. A, Body weight of transgenic (Tg) mice expressing hRAMP1 in the CNS and wild-type (WT) littermate controls. B, Time course of BAT SNA response to icv administration of amylin (0.1 nmol) in transgenic hRAMP1 mice and WT controls. C, Comparison of BAT SNA response (average of last hour of recording) to vehicle and amylin between Tg and WT mice. *, P < .05 vs WT or vehicle group; †, P < .05 vs WT-amylin group (n = 6–8 per group).
As depicted in Figure 6, B and C, icv injection of 0.1 nmol amylin increased BAT SNA by ∼64% in control mice. This response evoked by icv amylin was not different in control male mice (66% ± 10%) relative to control female mice (59% ± 12%). Notably, the BAT sympathetic activation induced by icv amylin was substantially enhanced (P < .05) in mice overexpressing hRAMP1 in the CNS. The icv administration of 0.1 nmol amylin increased BAT SNA by ∼140% (P < .02) in hRAMP1 transgenic mice (Figure 6, B and C). The BAT SNA response to icv amylin was similarly enhanced in male (145% ± 45%) and female (135% ± 47%) transgenic mice. Together, these data highlight the importance of RAMP1 protein in mediating the regulation of the sympathetic nervous system by CNS action of amylin.
Discussion
In the current study, we demonstrate that icv administration of amylin at doses that alter energy homeostasis (decreases food intake and body weight and increases body temperature) caused a significant and dose-dependent increase in sympathetic outflow to the thermogenic BAT, hind limb, and kidney. This action of amylin is mediated by the amylin receptor, because the BAT sympathetic response was blocked with the amylin receptor antagonist AC187. Our data also reveal a key role of CNS RAMP1 in the control of sympathetic nerve traffic by amylin. Indeed, amylin-induced BAT sympathetic activation was significantly enhanced in transgenic mice overexpressing hRAMP1, a critical subunit of the amylin receptor. Our data highlight the importance of CNS amylin for the control of regional sympathetic nerve traffic.
The ability of peripheral administration of amylin to modulate neuronal activity in the brain, as indicated by c-Fos immunoreactivity, further support the notion that amylin possesses properties of an adiposity signal acting in the CNS to regulate energy homeostasis in a manner similar to other adiposity signals such as leptin and insulin (38). The area postrema, which is devoid of a functional blood-brain barrier, has been proposed to serve as a gate for amylin to alter neuronal activity (38). Amylin-binding sites are present in this region, and electrophysiological studies provided direct evidence that amylin potently activates area postrema neurons (39). Direct microinjection of amylin into the area postrema caused a dose-dependent decrease in food intake, whereas amylin receptor blockade in this nucleus, using AC187, increased food intake (38). Moreover, the anorectic effects of amylin are abolished in rats that have the area postrema/nucleus tractus solitarii lesioned (38). However, lesioning other nuclei such as the lateral parabrachial nucleus also reduced the anorectic effect of amylin (40). In addition, microinjection of amylin into the hypothalamus produced a dose-dependent anorectic effect, which was more pronounced than after area postrema administration (41). This raises the possibility that CNS amylin may regulate energy homeostasis through an anatomically distributed network of amylin receptor-containing neurons.
Amylin-induced sympathetic activation to tissues such as BAT and hind limb is consistent with the role of amylin in the regulation of energy homeostasis and its weight-reducing effect. Indeed, CNS action of amylin has been shown to decrease body weight in several species (38) and confirmed in the present study in mice. In contrast, pharmacological blockade of amylin receptor in the CNS increases food intake, body weight, and fat mass (18). Evidence that amylin increases energy expenditure derives from the observation that rats treated with amylin had a more pronounced decrease in fat mass than those pair fed (42). This was further supported by the demonstration that amylin treatment caused a marked increase in body temperature and oxygen consumption in rats (43, 44). In the present study, we confirmed the effect of amylin on body temperature in mice. Of note, blockade of sympathetic transmission with propranolol, a β-adrenoceptor antagonist, reversed amylin-induced thermogenic and hyperthermic responses (44). Together, these findings indicate that the sympathetic nervous system is critically involved in mediating the effect of amylin on energy expenditure.
Our study revealed a stimulatory effect of amylin on sympathetic nerve traffic to the kidney, a critical organ for arterial pressure control. Interestingly, subjects with essential hypertension were found to be hyperamylinemic (45, 46). In addition, plasma concentrations of amylin correlates with blood pressure levels and metabolic syndrome in patients with varying degrees of adiposity (47). A prohypertensive action of amylin was further supported by the demonstration that acute iv administration of amylin raises arterial pressure that can be reversed with ganglionic blockade (48). However, other studies have shown that amylin treatment either has no effect or lowers blood pressure in rats and humans (49–51). Thus, additional studies are required to clarify the hemodynamic effects of amylin and the significance of amylin-induced renal sympathetic activation.
Our finding of enhanced amylin-induced BAT sympathetic activation in nestin-hRAMP1 transgenic mice emphasizes the importance of RAMP proteins for amylin receptor signaling in the CNS. Interestingly, amylin was found to increase neuronal c-Fos in the brain regions that express RAMPs and CTR (31). Our finding is also consistent with our previous study demonstrating an enhanced metabolic response to amylin in nestin-RAMP1 transgenic mice (37). These mice also exhibited elevated energy expenditure, leading to lower fat mass and body weight (37). Importantly, we showed that blocking the sympathetic nervous system signaling by antagonizing β-adrenergic receptors reversed the elevated gene expression level of these genes in BAT of nestin-hRAMP1 mice, which was associated with increased weight gain in these transgenic animals (37). Thus, RAMP1 is a critical determinant of the sympathetic and metabolic responses to amylin, and overexpression of RAMP1 in the CNS enhances the action of amylin.
Amylin and its analogs have recently emerged as potential antiobesity drugs. In particular, the demonstration that amylin can restore or enhance leptin sensitivity in obese patients has stimulated interest in the CNS-mediated physiological effects of amylin and its role in the pathogenesis of obesity (52). These clinical developments underscore the importance of understanding the underlying mechanisms that mediate the effect of amylin on energy homeostasis. Understanding the mechanisms by which amylin controls the sympathetic nerve traffic to tissues involved in the regulation of energy homeostasis such as BAT will offer the tantalizing possibility that it may be possible to selectively enhance those beneficial thermogenic, weight-reducing sympathetic actions of amylin.
Acknowledgments
Current address for C.F.-S.: Universidade Federal Fluminense, Department of Basic Sciences, Nova Friburgo, RJ 28.625-650, Brazil.
This study was supported by grants from National Institutes of Health (HL084207 to K.R. and NS075599 to A.F.R.), American Diabetes Association (1-11-BS-127 to K.R.), and the University of Iowa Fraternal Order of Eagles Diabetes Center (to K.R.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2263
- BAT
- brown adipose tissue
- CNS
- central nervous system
- CTR
- calcitonin receptor
- hRAMP1
- human RAMP1
- icv
- intracerebroventricular
- RAMP
- receptor activity-modifying protein
- SNA
- sympathetic nerve activity.
References
- 1. Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–831 [DOI] [PubMed] [Google Scholar]
- 2. Clark A, Cooper JG, Lewis CE, et al. Islet amyloid formed from diabetes-associated peptide may be pathogenic in type-2 diabetes. Lancet. 1987;2:231–234 [DOI] [PubMed] [Google Scholar]
- 3. Johnson KH, O'Brien TD, Hayden DW, et al. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic β-cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 1988;130:1–8 [PMC free article] [PubMed] [Google Scholar]
- 4. Lukinius A, Wilander E, Westermark GT, Engström U, Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989;32:240–244 [DOI] [PubMed] [Google Scholar]
- 5. Butler PC, Chou J, Carter WB, et al. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes. 1990;39:752–756 [DOI] [PubMed] [Google Scholar]
- 6. Butler PC, Chou J, Wang YN, et al. Amylin is co-secreted with insulin in man. Clin Res. 1990;38:A307 [Google Scholar]
- 7. Pieber TR, Roitelman J, Lee Y, Luskey KL, Stein DT. Direct plasma radioimmunoassay for rat amylin-(1-37): concentrations with acquired and genetic obesity. Am J Physiol. 1994;267:E156–E164 [DOI] [PubMed] [Google Scholar]
- 8. Balasubramaniam A, Renugopalakrishnan V, Stein M, Fischer JE, Chance WT. Syntheses, structures and anorectic effects of human and rat amylin. Peptides. 1991;12:919–924 [DOI] [PubMed] [Google Scholar]
- 9. Chance WT, Balasubramaniam A, Stallion A, Fischer JE. Anorexia following the systemic injection of amylin. Brain Res. 1993;607:185–188 [DOI] [PubMed] [Google Scholar]
- 10. Young AA, Cooper GJS, Carlo P, Rink TJ, Wang MW. Response to Intravenous Injections of Amylin and Glucagon in Fasted, Fed, and Hypoglycemic Rats. Am J Physiol. 1993;264:E943–E950 [DOI] [PubMed] [Google Scholar]
- 11. Arnelo U, Permert J, Adrian TE, Larsson J, Westermark P, Reidelberger RD. Chronic infusion of islet amyloid polypeptide causes anorexia in rats. Am J Physiol Reg Integ Comp Physiol. 1996;40:R1654–R1659 [DOI] [PubMed] [Google Scholar]
- 12. Morley JE, Flood JF. Amylin Decreases Food-Intake in Mice. Peptides. 1991;12:865–869 [DOI] [PubMed] [Google Scholar]
- 13. Bhavsar S, Watkins J, Young A. Synergy between amylin and cholecystokinin for inhibition of food intake in mice. Physiol Behav. 1998;64:557–561 [DOI] [PubMed] [Google Scholar]
- 14. Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J. Amylin receptor blockade stimulates food intake in rats. Am J Physiol Reg Integ Comp Physiol. 2004;287:R568–R574 [DOI] [PubMed] [Google Scholar]
- 15. Lutz TA, Rossi R, Althaus J, Del Prete E, Scharrer E. Amylin reduces food intake more potently than calcitonin gene-related peptide (CGRP) when injected into the lateral brain ventricle in rats. Peptides. 1998;19:1533–1540 [DOI] [PubMed] [Google Scholar]
- 16. Mack C, Wilson J, Athanacio J, et al. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference, and body weight. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1855–R1863 [DOI] [PubMed] [Google Scholar]
- 17. Rushing PA, Hagan MM, Seeley RJ, Lutz TA, Woods SC. Amylin: a novel action in the brain to reduce body weight. Endocrinology. 2000;141:850–853 [DOI] [PubMed] [Google Scholar]
- 18. Rushing PA, Hagan MM, Seeley RJ, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142:5035–5038 [DOI] [PubMed] [Google Scholar]
- 19. Cancello R, Tounian A, Poitou Ch, Clément K. Adiposity signals, genetic and body weight regulation in humans. Diabetes Metab. 2004;30:215–227 [DOI] [PubMed] [Google Scholar]
- 20. Banks WA, Kastin AJ, Maness LM, Huang W, Jaspan JB. Permeability of the blood-brain barrier to amylin. Life Sci. 1995;57:1993–2001 [DOI] [PubMed] [Google Scholar]
- 21. Banks WA, Kastin AJ. Differential permeability of the blood-brain barrier to two pancreatic peptides: Insulin and amylin. Peptides. 1998;19:883–889 [DOI] [PubMed] [Google Scholar]
- 22. Vanrossum D, Menard DP, Fournier A, Stpierre S, Quirion R. Autoradiographic Distribution and Receptor-Binding Profile of [I-125] Bolton Hunter-Rat Amylin Binding-Sites in the Rat-Brain. J Pharmacol Exp Ther. 1994;270:779–787 [PubMed] [Google Scholar]
- 23. Rowland NE, Crews EC, Gentry RM. Comparison of Fos induced in rat brain by GLP-1 and amylin. Regul Pept. 1997;71:171–174 [DOI] [PubMed] [Google Scholar]
- 24. Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567 [DOI] [PubMed] [Google Scholar]
- 25. Paxinos G, Chai SY, Christopoulos G, et al. In vitro autoradiographic localization of calcitonin and amylin binding sites in monkey brain. J Chem Neuroanat. 2004;27:217–236 [DOI] [PubMed] [Google Scholar]
- 26. Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–242 [DOI] [PubMed] [Google Scholar]
- 27. Leuthäuser K, Gujer R, Aldecoa A, et al. Receptor-activity-modifying protein 1 forms heterodimers with two G-protein-coupled receptors to define ligand recognition. Biochem J. 2000;351:347–351 [PMC free article] [PubMed] [Google Scholar]
- 28. Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Amylin receptors: molecular composition and pharmacology. Biochem Soc Trans. 2004;32:865–867 [DOI] [PubMed] [Google Scholar]
- 29. Udawela M, Hay DL, Sexton PM. The receptor activity modifying protein family of G protein coupled receptor accessory proteins. Semin Cell Dev Biol. 2004;15:299–308 [DOI] [PubMed] [Google Scholar]
- 30. Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPS. Pharmacol Ther. 2006;109:173–197 [DOI] [PubMed] [Google Scholar]
- 31. Barth SW, Riediger T, Lutz TA, Rechkemmer G. Peripheral amylin activates circumventricular organs expressing calcitonin receptor A/B subtypes and receptor-activity modifying proteins in the rat. Brain Res. 2004;997:97–102 [DOI] [PubMed] [Google Scholar]
- 32. Dulloo AG. Biomedicine. A sympathetic defense against obesity. Science. 2002;297:780–781 [DOI] [PubMed] [Google Scholar]
- 33. Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahmouni K, Morgan DA, Morgan GM, et al. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest. 2004;114:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riediger T, Zuend D, Becskei C, Lutz TA. The anorectic hormone amylin contributes to feeding-related changes of neuronal activity in key structures of the gut-brain axis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R114–R122 [DOI] [PubMed] [Google Scholar]
- 36. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557 [DOI] [PubMed] [Google Scholar]
- 37. Zhang Z, Liu X, Morgan DA, et al. Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes. 2011;60:1063–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–471 [DOI] [PubMed] [Google Scholar]
- 39. Riediger T, Schmid HA, Lutz T, Simon E. Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1833–R1843 [DOI] [PubMed] [Google Scholar]
- 40. Becskei C, Grabler V, Edwards GL, Riediger T, Lutz TA. Lesion of the lateral parabrachial nucleus attenuates the anorectic effect of peripheral amylin and CCK. Brain Res. 2007;1162:76–84 [DOI] [PubMed] [Google Scholar]
- 41. Chance WT, Balasubramaniam A, Zhang FS, Wimalawansa SJ, Fischer JE. Anorexia following the intrahypothalamic administration of amylin. Brain Res. 1991;539:352–354 [DOI] [PubMed] [Google Scholar]
- 42. Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the β-cell hormone amylin in diet-induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147:5855–5864 [DOI] [PubMed] [Google Scholar]
- 43. Bouali SM, Wimalawansa SJ, Jolicoeur FB. In vivo central actions of rat amylin. Regul Pept. 1995;56:167–174 [DOI] [PubMed] [Google Scholar]
- 44. Osaka T, Tsukamoto A, Koyama Y, Inoue S. Central and peripheral administration of amylin induces energy expenditure in anesthetized rats. Peptides. 2008;29:1028–1035 [DOI] [PubMed] [Google Scholar]
- 45. Kailasam MT, Parmer RJ, Tyrell EA, Henry RR, O'Connor DT. Circulating amylin in human essential hypertension: heritability and early increase in individuals at genetic risk. J Hypertens. 2000;18:1611–1620 [DOI] [PubMed] [Google Scholar]
- 46. Novials A, Rodriguez-Mañas L, Chico A, El Assar M, Casas S, Gomis R. Amylin and hypertension: association of an amylin -G132A gene mutation and hypertension in humans and amylin-induced endothelium dysfunction in rats. J Clin Endocrinol Metab. 2007;92:1446–1450 [DOI] [PubMed] [Google Scholar]
- 47. Hou X, Sun L, Li Z, et al. Associations of amylin with inflammatory markers and metabolic syndrome in apparently healthy Chinese. PLoS One. 2011;6:e24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haynes JM, Hodgson WC, Cooper ME. Rat amylin mediates a pressor response in the anaesthetised rat: implications for the association between hypertension and diabetes mellitus. Diabetologia. 1997;40:256–261 [DOI] [PubMed] [Google Scholar]
- 49. Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity (Silver Spring). 2010;18:1739–1746 [DOI] [PubMed] [Google Scholar]
- 50. Seth R, Knight WD, Overton JM. Combined amylin-leptin treatment lowers blood pressure and adiposity in lean and obese rats. Int J Obes (Lond). 2011;35:1183–1192 [DOI] [PubMed] [Google Scholar]
- 51. Young AA, Rink TJ, Wang MW. Dose response characteristics for the hyperglycemic, hyperlactemic, hypotensive and hypocalcemic actions of amylin and calcitonin gene-related peptide-I (CGRPα) in the fasted, anaesthetized rat. Life Sci. 1993;52:1717–1726 [DOI] [PubMed] [Google Scholar]
- 52. Roth JD, Maier H, Chen S, Roland BL. Implications of Implications of amylin receptor agonism: integrated neurohormonal mechanisms and therapeutic applications. Arch Neurol. 2009;66:306–310 [DOI] [PubMed] [Google Scholar]