Abstract
Bariatric surgery elevates serum bile acids. Conjugated bile acid administration, such as tauroursodeoxycholic acid (TUDCA), improves insulin sensitivity, whereas short-circuiting bile acid circulation through ileal interposition surgery in rats raises TUDCA levels. We hypothesized that bariatric surgery outcomes could be recapitulated by short circuiting the normal enterohepatic bile circulation. We established a model wherein male obese rats underwent either bile diversion (BD) or Sham (SH) surgery. The BD group had a catheter inserted into the common bile duct and its distal end anchored into the middistal jejunum for 4–5 weeks. Glucose tolerance, insulin and glucagon-like peptide-1 (GLP-1) response, hepatic steatosis, and endoplasmic reticulum (ER) stress were measured. Rats post-BD lost significantly more weight than the SH rats. BD rats gained less fat mass after surgery. BD rats had improved glucose tolerance, increased higher postprandial glucagon-like peptide-1 response and serum bile acids but less liver steatosis. Serum bile acid levels including TUDCA concentrations were higher in BD compared to SH pair-fed rats. Fecal bile acid levels were not different. Liver ER stress (C/EBP homologous protein mRNA and pJNK protein) was decreased in BD rats. Bile acid gavage (TUDCA/ursodeoxycholic acid [UDCA]) in diet-induced obese rats, elevated serum TUDCA and concomitantly reduced hepatic steatosis and ER stress (C/EBP homologous protein mRNA). These data demonstrate the ability of alterations in bile acids to recapitulate important metabolic improvements seen after bariatric surgery. Further, our work establishes a model for focused study of bile acids in the context of bariatric surgery that may lead to the identification of therapeutics for metabolic disease.
Bariatric surgery has become an important therapeutic option for obesity and nonalcoholic fatty liver disease (1, 2). Weight reduction can be also achieved through lifestyle changes, but the degree of sustained weight loss is relatively small (3). In contrast, roux-en-Y gastric bypass (RYGB) results in greater and more sustained weight loss and is associated with reduced overall mortality despite the immediate perioperative surgical risks (4). Despite the success of RYGB, the mechanisms that explain these improvements remain unclear.
One hypothesized mechanism implicates bile acids and bile acid signaling. Patients who underwent RYGB or vertical sleeve gastrectomy had increased serum bile acid concentrations (5). In a separate study, conjugated bile acid subfractions were all significantly higher in post-RYGB patients compared with weight-matched controls (6). Whereas bile acids are critical for the normal absorption of lipids from the gastrointestinal tract, they also have important signaling actions. Endogenous and exogenous bile acids (eg, 6-ethyl-chenodeoxycholic acid) are recognized ligands for nuclear receptors such as the farnesoid X receptor (FXR) (7–9) and cell surface G-coupled receptors such as the G protein-coupled bile acid receptor 1 or TGR5 (10, 11). There is considerable evidence suggesting that successful bariatric surgical procedures have important effects on levels and actions of bile acids, and bile acids are therefore being targeted as potential therapeutic agents in obesity (12). Consistent with the hypothesis that there is increased bile acid signaling after RYGB, Jansen et al (13) found elevated levels of the FXR target gene, human fibroblast growth factor 19 (FGF), in a cohort of RYGB patients 3 months after surgery. Further, recently intrajejunal taurocholic acid infusion in humans was found to reduce the glycemic response to small intestinal glucose loads and was associated with an increase in the plasma incretin hormone, glucagon-like peptide-1 (GLP-1) (14).
Other surgical procedures also produce important changes in bile acids. In rats, using a quite different procedure in which sections of the ileum are inserted into the jejunum (ileal transposition), we observed increased bile acid levels (taurine and glycine conjugated) concomitant with improvements in body weight and obesity comorbidities (15). One likely explanation is that this alteration in intestinal anatomy moved the ileum, the primary site of bile acid absorption, more proximal, producing significant changes in bile acid dynamics including a short circuiting of the enterohepatic bile acid circulation. The current work directly assessed the impact of altering the path of bile acids in the gastrointestinal tract using a procedure that does not involve gastric restriction or altered nutrient flow. To this end, we established a procedure that produced a total diversion of bile from the main biliary tree to the jejunum using a surgically placed artificial catheter (a procedure we term “bile diversion” or BD). This technique was first used by Manfredini et al (16) who reported improved glucose tolerance in rats with BD. Our experiments using this BD technique in diet-induced obese rats results in weight loss, reduced hepatic steatosis, improved glucose regulation, and elevated bile acids without cholestasis or biliary obstruction. Thus, we are able to recapitulate key benefits of bariatric procedures by a focused direct manipulation of bile.
Materials and Methods
Animals and diets
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. Adult male Long-Evans rats (The Jackson Laboratory, Bar harbor, Maine) were single housed (22 ± 2°C) on a 12-hour light, 12-hour dark cycle. Animals were randomized to chow (Harlan Teklad, Placentia, California), or high-fat (HF) diet (40 kcal % fat; Research Diets, New Brunswick, New Jersey) as we have previously described (15). Animals were provided ad libitum access to diets unless specified as fasting or pair fed.
Surgeries and postoperative care
Under isoflurane anesthesia the BD group had one end of a catheter 1 mm in internal diameter made of polyethylene tubing PE-50 22 gauge (Instech Laboratories, Inc, Plymouth Meeting, Pennsylvania) inserted into the common bile duct and its other end anchored into the middistal jejunum 15 cm distal to the ligament of Treitz unless specifically mentioned (Figure 1A). Control rats did not receive any surgery (Naïve), or underwent Sham (SH) procedures wherein the bile duct was dissected. Postoperatively, rats received analgesia and were returned to the HF diet. In addition for the first 2 weeks postoperatively all rats had ad libitum access to a liquid source of calories as well (Osmolite 1.0; Abbott Nutrition, Columbus, Ohio). Each sham-pair-fed (SH-PF) rat was paired to an individual BD rat and provided food equivalent to the paired BD rat's prior 24-hour intake. Rats were humanely destroyed 4–5 weeks after surgery depending on the experiment. Liver and intestinal biopsies were fixed in formalin for routine histology, frozen section in Tissue-Tek O.C.T. medium (Electron Microscopy Sciences, Fort Washington, Pennsylvania) and flash frozen in liquid nitrogen for quantitative real-time PCR.
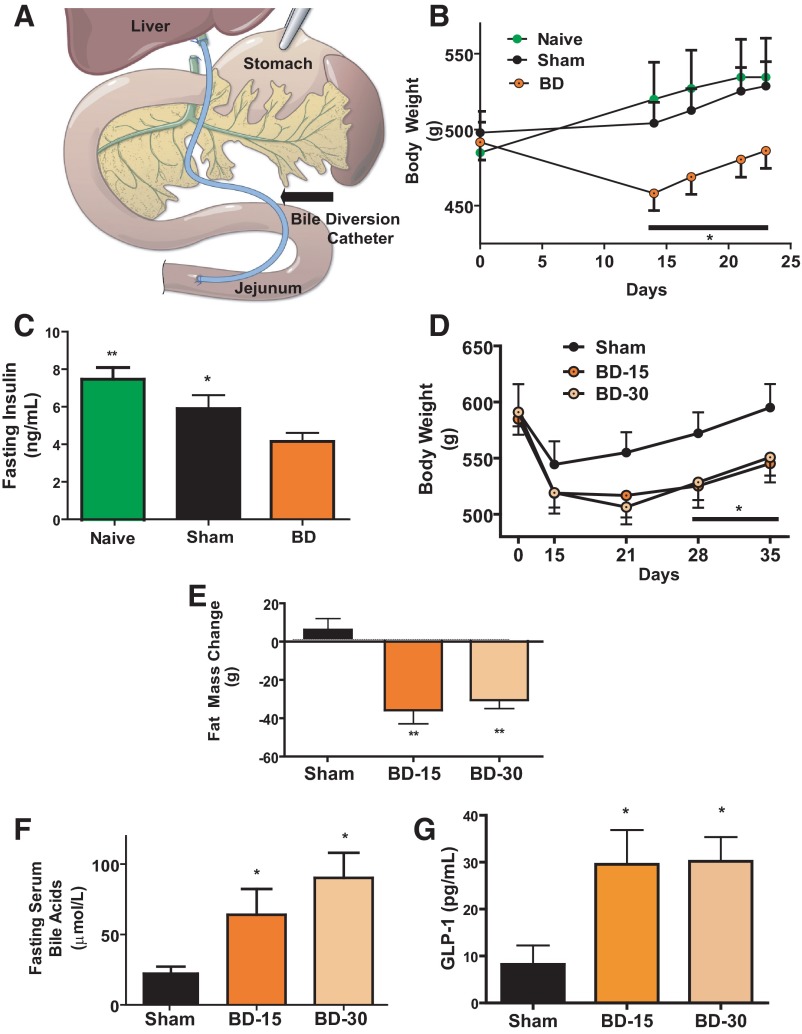
Figure 1.
A, Illustration of BD. Black arrow points to polyethylene catheter. B, Body weight after surgery. Rats in BD had catheter inserted 15 cm distal to ligament of Treitz. This group lost more body weight compared with rats in the SH and Naïve groups. N: BD = 12; SH = 8; Naive = 5 (repeated measures ANOVA: *, P < .05 BD vs SH and Naïve). C, Fasting insulin at 4 weeks. Plasma insulin levels were lower in the BD surgery group compared with rats in the SH surgery and surgery Naïve groups. N: BD = 12; SH = 8; Naive = 5 (ANOVA, post hoc Tukey's; *, P < .05 SH vs BD; **, P < .01 Naïve vs BD). D, Body weight after surgery with different catheter insertion sites. Rats had either SH surgery, BD catheter inserted into the jejunum 15 cm from the ligament of Treitz (BD-15) or 30 cm from the ligament of Treitz surgery (BD-30). The BD-15 and BD-30 groups lost more body weight compared with rats in the SH surgery group. N: SH = 5, BD-15 = 8, BD-30 = 9 (repeated measures ANOVA: *, P < .05 BD-15 and BD-30 vs SH). E, Body composition estimation of fat mass change by magnetic resonance. The weight gained by the SH group had an increase in their proportion of fat mass whereas BD-15 and BD-30 surgery groups lost fat mass over the same time period. N: SH = 5; BD-15 = 8; BD-30 = 9 (]ANOVA, post hoc Tukey's: **, P < .01 for BD-15 and BD-30 vs SH). F, Serum bile acid levels after surgery. Fasting total serum bile acid levels were higher in BD-15 and BD-30 rats compared with SH group. N: SH = 5; BD-15 = 7; BD-30 = 8 (ANOVA, post hoc Tukey's *, P < .05 for BD-15 and BD-30 vs SH). G, Plasma GLP-1 levels after surgery. Postprandial plasma GLP-1 levels (15 min) were higher in the BD-15 and BD-30 rats compared with SH group. N: SH = 5; BD-15 = 7; BD-30 = 8 (ANOVA, post hoc Tukey's: *, P < .05 for BD-15 and BD-30 vs SH).
Bile acid gavage experiment
Obese rats (weight range 560–720 g) were randomized to receive daily gavage (500 mg/kg in a solution of bile acid) of either saline, ursodeoxycholic acid (UDCA; Sigma Aldrich, St Louis, Missouri) or taurine conjugated UDCA (TUDCA, a generous gift from Dr Kenneth Setchell) for 3 weeks. The baseline body weights for all 3 groups were not different. The gavage was performed in the morning just after lights on for 5 days a week for 3 weeks. Rats were humanely destroyed sacrificed the day after the last gavage, and liver tissue was obtained for histology, frozen section, and quantitative real-time PCR. Serum that was used to quantify total bile acids using the 3α-hydroxysteroid dehydrogenase method was collected at the same time. The reaction was measured at 546 nm using a Hitachi Chemistry 911 analyzer (Hitachi, Tokyo, Japan), and specific bile acid species were measured using electrospray ionization liquid chromatography or gas chromatography mass spectrometry as previously described (18, 19).
Glucose tolerance tests
Rats were fasted overnight (8 h) and then administered a glucose load by oral gavage (1 g of 20% d-glucose). Samples were obtained from tail vein and assessed by ELISA kit (Crystal Chem, Inc, Downers Grove, Illinois). Glucose was determined at 0, 15, 30, 45, 60, and 120 minutes with a One Touch Glucometer (LifeScan, Milpitas, California).
Body composition analysis
Echo MRI Whole Body Composition Analyzer (Echo Medical Systems, Houston, Texas) (17) was performed on all rats before surgery and just before being humanely destroyed.
Oil red-O staining on frozen liver sections
Livers were fixed in Tissue-Tek O.C.T. medium (Electron Microscopy Sciences) over dry ice. Oil red-o staining was performed using Oil Red-O kit (American MasterTech, Lodi, California) adhering to manufacturers guidelines on frozen sections.
Triglyceride (TG), GLP-1, bilirubin, and alanine aminotransferase (ALT) quantification
Hepatic TG content was determined as previously described (15). Briefly, 100 mg of wet liver tissue were homogenized in a 20 mM Tris buffer. Triglycerides Reagent Set (Pointe Scientific, Inc, Canton, Mississippi) was used for the assay per instructions. GLP-1 was measured from serum utilizing Meso-scale Discoveries Immunoassay System (Gaithersburg, Maryland). ALT was measured from serum utilizing DiscretPak ALT Reagent Kit (Catachem, Inc, Oxford, Connecticut). Photometric kinetic absorbance was read over 5-minute intervals at 340 nm. Serum bilirubin was measured with manufacturer's instructions (Pointe Scientific, Inc.).
Plasma, serum, and fecal bile acid assays
Total serum bile acids were assayed using the 3α-hydroxysteroid dehydrogenase method. The reaction was measured at 546 nm using a Hitachi Chemistry 911 analyzer (Hitachi, Tokyo, Japan). Serum and fecal bile acid composition and quantification in later experiments were assayed using electrospray ionization liquid chromatograpy/gas chromatograpy/Mass Spectoscopy (ESI LC/GC MS) (Figure 3A) as previously described (18, 19).
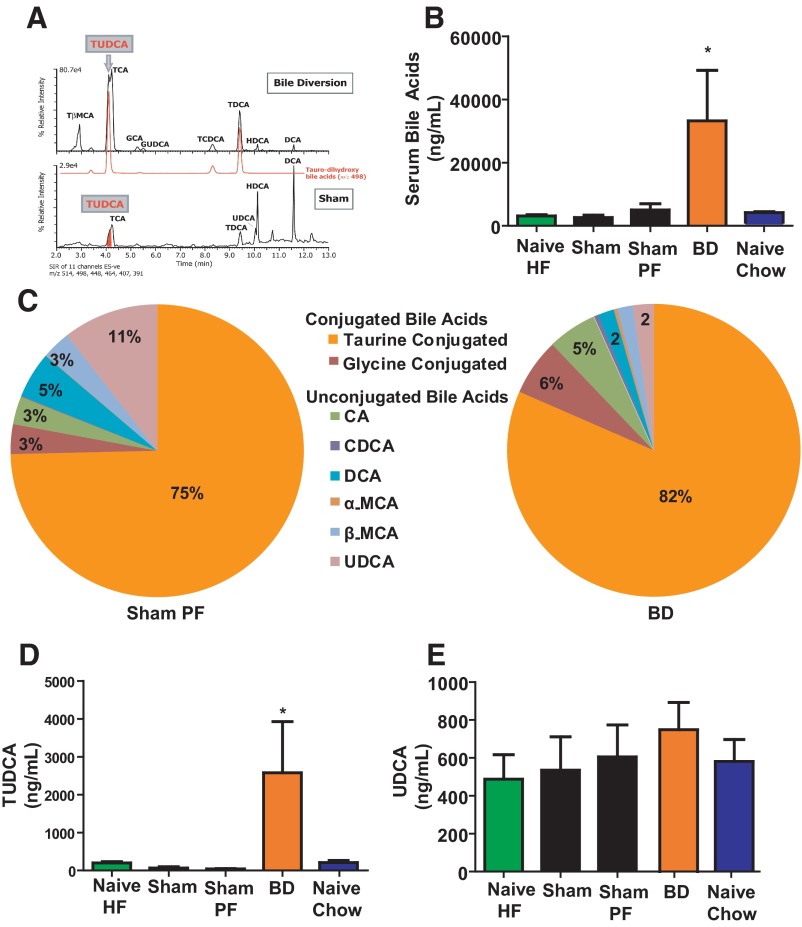
Figure 3.
A, Negative ion ESI-Liquid chromatography-mass spectroscopy summed ion chromatograms. Mass size 514, 498, 448, 464, 407, 391 specific to the major conjugated bile acids in extracts of bile of rats after BD (top chromatogram) or SH operated (bottom chromatogram). The ion chromatogram for m/z 498 is separately depicted to indicate the presence of taurine-conjugated dihydroxy-cholanoic acids, including TUDCA. The following peaks are indicated: taurocholate (TCA), taurodeoxycholate (TDCA), tauro-β-muricholic (TβMCA), tauro-chenodeoxycholate (TCDCA), glycocholate (GCA), glycoursodeoxycholate (GUDCA), uncnjugtaed hyodeoxycholate (HDCA), unconjugated UDCA, and unconjugated deoxycholate (DCA). These chromatograms are normalized to the most intense ion in the profile and note the difference (27-fold) in relative intensity of the ions between the BD and SH-operated bile samples. B, Serum bile acid levels after surgery. Postprandial total serum bile acid levels were higher in BD rats compared with all other groups including weight-matched Chow Naïve and food intake-matched SH-PF. N for groups: Naïve-HF = 8; SH = 9; SH-PF = 10; BD = 11; Naïve-Chow = 7 (ANOVA, post hoc Tukey's: *, P < .05). C, Serum bile acid composition analysis. Bile acid composition analyzed by liquid chromatography-mass spectroscopy in serum is shown with a significant proportion being conjugated (glycine or taurine) bile acids. There is a significant increase seen in the taurine-conjugated bile acids from a mean of 75% to that of 82% between these 2 rat groups. The following unconjugated bile acids are also indicated: cholic acid (CA), deoxycholate (DCA), α-muricholic acid (α-MCA) β-muricholic acid (β-MCA), chenodeoxycholate (CDCA), and UDCA. D, Taurine and unconjugated UDCA serum levels after surgery. Postprandial serum TUDCA levels were higher in BD rats compared with all other groups including weight-matched Chow Naïve and food-intake-matched SH-PF whereas no difference was observed in serum UDCA (E) levels between groups. N for groups: Naïve-HF = 8; SH = 9; SH-PF = 10; BD = 11; Naïve-Chow = 7 (ANOVA, post hoc Tukey's: *, P < .05].
Western blotting
Liver samples loaded onto sodium dodecyl sulfate-polyacrylamide gel (Invitrogen, Carlsbad, California) were probed for pJNK (catalog no. 9251), Total c-Jun-N-terminal kinase (JNK) (catalog no. 9252) (Cell Signaling Technology, Beverly, Massachusetts) and β-actin (Santa Cruz Biotechnology, Santa Cruz, California) using standard Western blotting methods.
qPCR gene expression
RNA was isolated using TRIzol reagent protocol (Molecular Research Center, Cincinnati, Ohio). cDNA was made using TaqMan (Applied Biosystems, Foster City, California) and Eppendorf Mastercycler (Eppendorf, Hamburg,Germany). mRNA expression was measured using Stratagene SYBR green real-time kinetic PCR (Stratagene, Agilent Technologies, Santa Clara, California). TaqMan primers for rat C/EBP homologous protein (CHOP) (Rn00492098_g1), and 18S rRNA (RN00821260_g1) were used (Applied Biosystems) using the standard curve method.
Statistical analysis
Results are expressed as mean ± SEM. Where indicated, the statistical significance between two groups was estimated by Student's t test or among three or more groups using ANOVA. *, **, and *** indicate statistical significance with P < .05, P < .01, and P < .001, respectively. Statistically nondifferent results were labeled ns where appropriate.
Results
BD in diet-induced obese rats produces weight loss, metabolic improvements, and elevated serum bile acids
Adult Long-Evans Rats were made obese (450–500 g) by providing ad libitum access to a diet with 40% of calories from fats (20). These rats were then randomized to receive either BD or SH surgery while a third group did not undergo any surgical procedure (Naïve). The BD group had weight loss over the first 2 weeks after surgery (458.07 ± 11.3 g; Figure 1B) whereas the SH group's weight (504.23 ± 13.7 g) was not statistically different from that of the Naïve group (520.04 ± 24.3 g) at the same time point. There was significantly lower plasma fasting insulin in the BD group (4.15 ± 1.8 ng/ml) compared with both the SH (5.91 ± 2.0 ng/mL) and Naïve groups (7.55 ± 1.3 ng/mL) at time of death 4 weeks after surgery (Figure 1C).
In a second cohort of diet-induced obese rats (550–600g), we compared 2 points of catheter insertion into the jejunum (15 and 30 cm from the ligament of Treitz; BD-15 and BD-30, respectively). There was no difference in weight loss 5 weeks after surgery by insertion point of the BD catheter (BD-15 545.27 ± 16.9 g, BD-30 550.79 ± 16.3 g; Figure 1D). Further, both BD-15 and BD-30 groups had a similar decrease in fat mass 5 weeks after surgery (BD-15 35.85 ± 7.1 g, BD-30 30.57 ± 4.4 g) which was not seen in the SH control group (Figure 1E). There was an increase in serum fasting bile acid levels in both BD groups (SH, 23.6 ± 5.3; BD-15, 64 ± 18.3; BD-30, 90.25 ± 17.8 μmol/L; Figure 1F). Plasma GLP-1 levels were higher in the BD group (Figure 1G).
We found that despite being on a HF diet after surgery there was only a subtle increase in the amount of fat by weight seen in feces between the BD (BD-15; 10.4 ± 0.5%: BD-30 9.9 ± 0.2%) and SH rats (6.2 ± 0.4%). No difference in serum ALT or plasma bilirubin was seen between groups at time of death (data not shown).
BD in diet-induced obese rats produces calorie intake-independent metabolic improvements
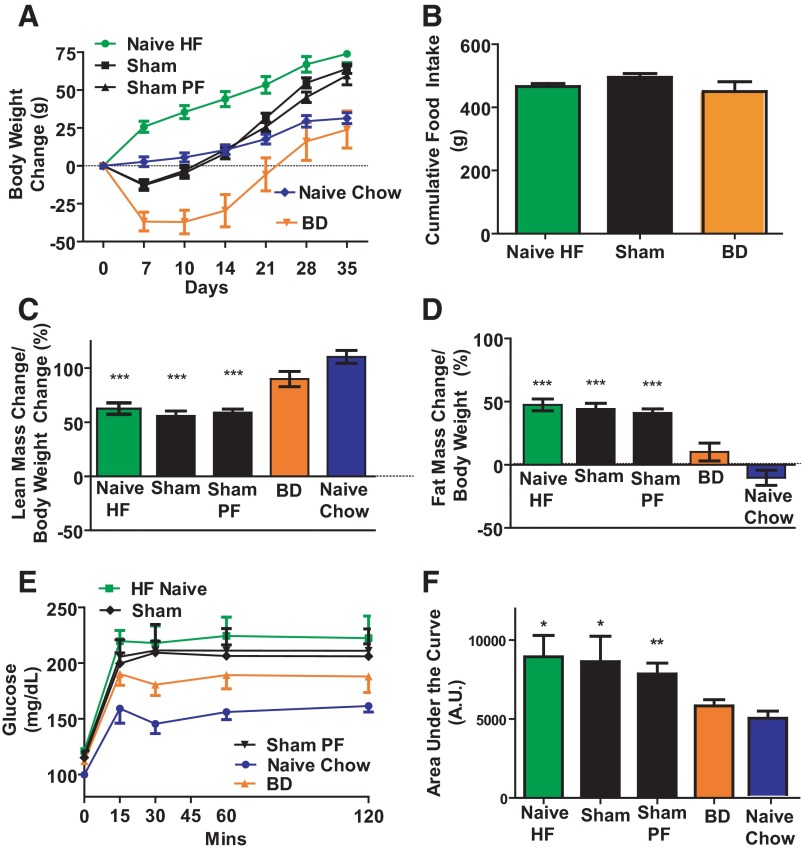
To determine possible caloric intake-independent effects of BD, we performed an experiment utilizing 5 groups of rats. 4 groups were made obese on a HF diet and then randomized to either surgically naïve (Naïve-HF), BD surgery with catheter insertion 15 cm from the ligament of Treitz (BD), SH surgery, or SH surgery wherein access to food after surgery was limited to that consumed by the BD rats (SH-PF). A fifth group was maintained on standard low-fat chow throughout the experiment (Naïve-Chow). Rats in the BD group lost the most weight (37.07 ± 7.7 g over the first 10 d), and their cumulative weight gain over 5 weeks was similar to that of the rats in the Naïve-Chow group (BD 23.95 ± 12.3 vs Naïve-Chow 31.4 ± 3.6 g; Figure 2A). The BD rats had reduced food intake immediately after surgery. At 14 days after surgery the food intake of BD rats was 9.4 ± 1.8 g of food/24 h compared with the 20.9 ± 0.5 g for the Naïve HF and 17.3 ± 0.7 g for the SH-operated rats. This decreased food intake in the BD rats reversed over time and beyond day 18 there was no difference between daily food consumption of BD, SH, and Naïve-HF rats. Thus the cumulative data for the total 35 days was not significantly different between the groups (Figure 2B). The BD rats gained lean mass (89.86 ± 7.0%; Figure 2C) and fat mass (10.14 ± 7%; Figure 2D) similarly to the Naïve-Chow (Lean, 110.3 ± 5.9%; Fat, −10.35 ± 5.9%). These were both different from the other groups (Naïve-HF, SH, and SH-PF; Figure 2, C and D). During the glucose tolerance test Naïve-Chow rats had the lowest glucose excursion curves and the Naïve-HF rats had the worst glucose tolerance (Figure 2E). The area under the curve (AUC) for BD rats (5835 ± 390.8 arbitrary units; A.U.) was statistically smaller than that of the Naïve-HF (8949 ± 1350 A.U.), SH (7860 ± 686.6 A.U.), and SH-PF rats(8631 ± 1616 A.U.), while not different than that of the Naïve-Chow controls (4908 ± 448.1 A.U.; Figure 2 F). The plasma 60 minutes postprandial GLP-1 levels were higher in the BD group (4.87 ± 2.4 pg/mL) compared with SH-PF (0.497 ± 0.38pg/mL; P = .04). GLP-1 levels were not significantly different from BD group rats in the other groups but trended to be lower in Naïve-HF (1.43 ± 0.92 pg/mL) and SH (2.24 ± 0.97 pg/mL) but higher in Naïve-Chow rats (10.13 ± 3.41 pg/mL).
Figure 2.
A, Body weight change after surgery. Rats in BD had catheter inserted 15 cm distal to ligament of Treitz. Rats in the BD surgery group lost more body weight compared with rats in the SH-PF surgery and Naïve HF surgery Naïve groups. At time of death there was no difference in body weight between Naïve-Chow-fed and BD rats. N: Naïve-HF = 8; SH = 9; SH-PF = 10; BD = 12; Naïve-Chow = 8. B, Food intake after surgery. There was no significant difference between cumulative food intake after surgery from days 14–35 between the 3 ad libitum HF-fed groups; Naïve-HF, SH, and BD groups. N for groups: Naïve-HF = 8; SH = 9; BD = 12. C, Body composition estimation of lean mass change by magnetic resonance. BD and Naïve Chow-fed rats had significantly higher gain in lean mass as a percentage of weight gained after surgery compared with Naïve-HF-fed and SH-PF rats. N for groups: Naïve-HF = 8; SH = 9; SH-PF = 10; BD = 11; Naïve-Chow = 8 (ANOVA, post hoc Tukey's: ***, P < .001). D, Body composition estimation of fat mass change by magnetic resonance. BD and Naïve-Chow-fed rats had significantly lower gain in fat mass as a percentage of weight gained after surgery compared with Naïve-HF-fed and SH-PF rats. N for groups: Naïve-HF = 8; SH = 9; HF-PF = 10; BD = 11; Naïve-Chow = 8 (ANOVA, post hoc Tukey's: ***, P < .001). E, Glucose tolerance after BD. BD rats had an improvement in their oral glucose tolerance compared with Naïve-HF and SH-PF rats and F, glucose tolerance test (GTT) AUC. AUC for 120 minutes was significantly less after BD surgery. N for groups: Naïve-HF = 8; SH = 9; SH-PF = 9; BD = 12; Naïve-Chow = 8 (ANOVA, post hoc Tukey's: *, P < .05; **, P < .01).
BD in diet-induced obese rats increases serum bile acid concentration and preferentially raises taurine-conjugated species (Figure 3A)
Total serum bile acids were significantly elevated in the BD group (33219 ± 16003 ng/mL) compared with all other groups (Figure 3B). Composition of the serum bile acids was different between SH-PF and BD rats (Figure 3C). There was a significant increase in taurine-conjugated bile acids (Figure 3C), specifically TUDCA, in BD (2575 ± 1352 ng/mL), compared with SH-PF (39.07 ± 9 ng/mL; Figure 3D). There was no significant increase in the concentration of unconjugated UDCA (Figure 3E) although there was a perceptible decrease of UDCA as a percentage of the bile acid composition in BD rat serum (Figure 3C).
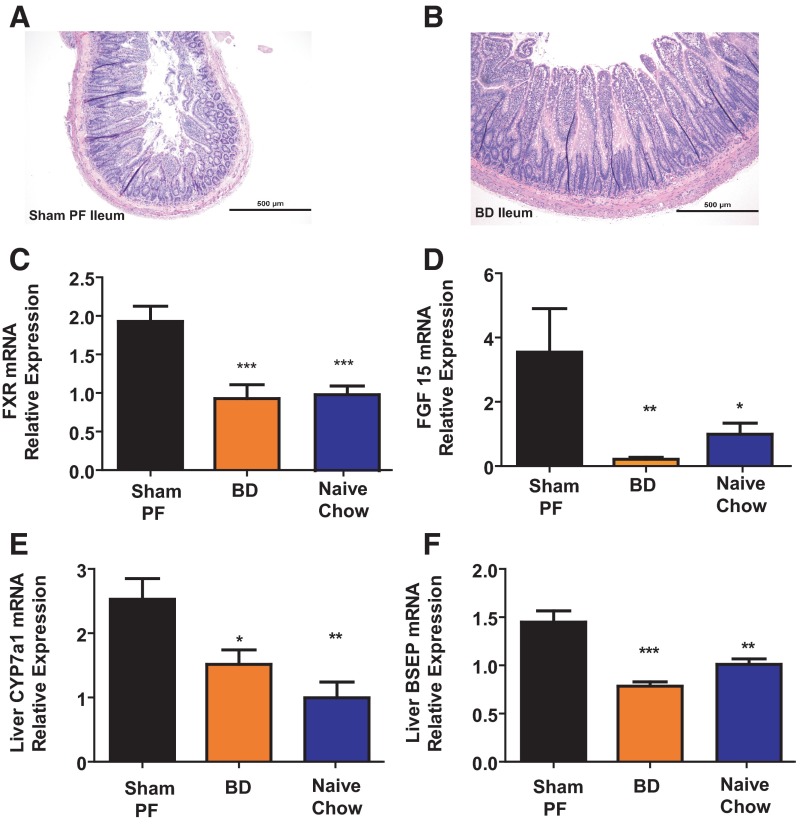
BD causes changes in intestinal histology and intestinal and liver bile acid physiology
There was ileal hypertrophy and increased villi length in BD rats compared with SH-PF (Figure 4, A and B). Ileal FXR mRNA expression was decreased in BD (0.92 ± 0.2 A.U; Figure 4C) and Naïve-Chow (1 ± 0.1 A.U) in comparison with SH-PF (1.93 ± 0.2 A.U) rats 5 weeks after surgery. There was no significant difference in mRNA expression levels between groups for the ileal apical sodium bile acid transporter (ASBT) or short-heterodimer partner (SHP; data not shown) although there was a decrease in ileal FGF15 expression (Figure 4D). Further there was no difference in fecal bile acid levels between groups (data not shown).
Figure 4.
A, Ileal histology after surgery. Rats humanely destroyed 5 weeks after surgery from the BD group showed histologic adaptation of the ileum. Hematoxylin and eosin-stained sections) (magnification, times]10)of the BD rat's ileum at time of death shows marked increase in length and number of villi compared with the SH-PF (B) animals ileal histology, which has remained typical of a rat ileal with short villi length. C, Response of ileal epithelial bile acid-responsive gene FXR. mRNA levels of bile acid-responsive nuclear receptor gene FXR were measured by RT-PCR and expressed in relative expression units. FXR mRNA was decreased in BD rats compared with SH-PF. N for groups: BD = 7; SH-PF = 5; Chow Naive = 5 (ANOVA, Post hoc Tukey's: ***, P < .001). D, Fecal bile acid quantification. Using a 3-day fecal collection, total fecal bile acid levels were measured and found to be similar in BD rats compared with all other groups including weight-matched Chow Naïve and food-intake-matched SH-PF. E, Hepatic bile acid production. mRNA levels of the gene coding for the rate-limiting bile acid production enzyme 7α-hydroxylase (CYP7A1) were measured by RT-PCR and expressed in relative expression units. CYP7A1 mRNA was decreased in BD rats compared with SH-PF. N for groups: BD = 6; SH-PF = 4; Naïve-Chow = 6 (ANOVA, post hoc Tukey's: *, P < .05; **, P < .01). F, Hepatic bile acid export. mRNA levels of the gene coding for the major BSEP and OATPs were measured by RT-PCR and expressed in relative expression units. BSEP and OATPs mRNA were decreased in BD rats compared with SH-PF. BD = 6, SH-PF = 4, Naïve-Chow = 6 (ANOVA, post hoc Tukey's: **, P < .01; ***, P < .001).
mRNA relative expression of bile acid rate-limiting enzyme gene cyp7A1 was lower in BD (1.52 ± 0.2) vs SH-PF (2.53 ± 0.3; Figure 4E). Bile acid import mechanism, sodium taurocholate-cotransporting polypeptide gene expression was decreased in BD (0.65 ± 0.1) vs SH-PF (1.02 ± 0.2, P = .04). Similarly the organic anion transporting polypeptides (OATP-A1; BD 0.53 ± 0.1 vs SH-PF 0.99 ± 0.1, P = .004; OATP-B2; BD 0.58 ± 0.1 vs SH-PF 1.16 ± 0.2, P = .02) and bile salt export pump (BSEP, BD 0.78 ± 0.0 vs SH-PF 1.45 ± 0.1; Figure 4F) were also reduced in the BD group. There was no difference for liver X receptor, SHP, or fibroblast growth factor receptor 4 (data not shown).
BD results in reduced hepatic steatosis and endoplasmic reticulum (ER) stress
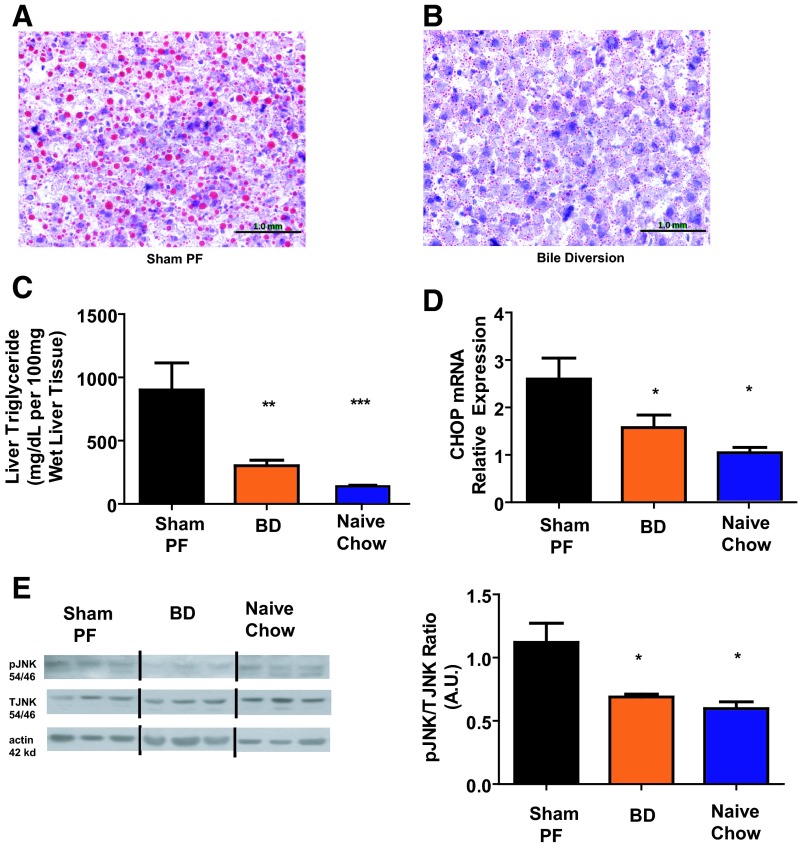
There was less visible lipid (Figure 5, A and B) and quantified TGs in BD (302.3 ± 43.1 mg/100 mg wet liver) rat livers compared with SH-PF (901 ± 213.9; Figure 5C). The plasma TG and cholesterol levels of SH-PF and BD animals were not different (data not shown). C/EBP-homologous protein (CHOP) mRNA relative expression was lower in BD (1.58 ± 0.3 A.U) and Naïve-Chow (1 ± 0.1) liver tissue at 5 weeks after surgery compared with SH-PF (2.6 ± 0.4; Figure 5D). There was a decrease in pJNK-2 in the Naïve-Chow (0.6 ± 0.1 A.U.) and BD (0.68 ± 0.02) rat livers compared with SH-PF (1.12 ± 0.2; Figure 5E). Together the reduction in JNK phosphorylation and mRNA CHOP expression suggest that reduced ER stress might be the underlying mechanism for protection against steatosis in the BD rats with elevated TUDCA serum levels.
Figure 5.
A, Hepatic histology postsurgery. Rats humanely destroyed at 5 weeks after surgery from the BD group showed decreased steatosis by oil-red O staining on frozen liver sections. Sections (×10) of the BD rat's liver at time of death show marked decrease in number and size of steatosis compared with the SH-PF (B) animals, which has remained typical of a HF-fed obese rat. C, Hepatic TG content. Liver TG levels were lower in BD rats compared with SH-PF and Naïve-HF groups. N for groups: BD = 9; SH-PF = 6; Chow Naive = 8 (ANOVA, Post hoc Tukey's: **, P < .01; ***, P < .001). D, Hepatic ER Stress gene expression. mRNA levels of the gene coding for the ER stress component CHOP were measured by RT-PCR and expressed in relative expression units. CHOP mRNA was decreased in BD rats compared witho SH-PF. N for groups: BD = 9; SH-PF = 6; Chow Naive = 8 (ANOVA, post hoc Tukey's: *, P < .05). E, Hepatic ER Stress-protein expression. pJNK2 by Western blot was decreased in BD liver tissue compared with SH-PF and Naïve-HF. Equal protein loading confirmed by β-actin levels. Representative blots shown. Representative blots shown. Quantification done by densitometry ratios of pJNK to total JNK. N for groups: BD = 9; SH-PF = 6; Chow Naive = 8 (ANOVA, post hoc Tukey's: *, P < .05).
Bile acid gavage in diet-induced obese rats results in reduced hepatic steatosis and ER stress with a concomitant increase in serum TUDCA
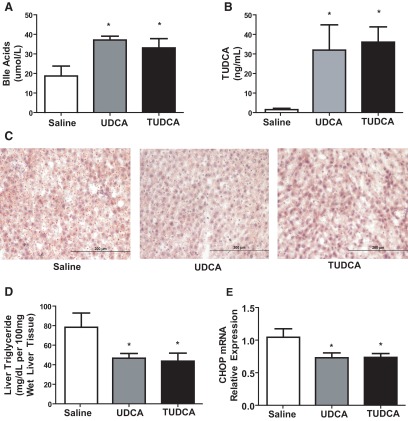
Diet-induced obese rats were gavaged with saline, UDCA, or TUDCA daily for 3 weeks. Body weight in three groups did not differ at any time (data not shown). Expectedly, total serum bile acids were increased in both bile acid-fed groups in comparison with the saline-gavaged group (Figure 6A). Interestingly, TUDCA serum levels were increased in both the UDCA (127.9 ± 51.6 ng/mL) and TUDCA (144.2 ± 31.2) gavaged groups compared with saline (5.95 ± 2.6; Figure 6B). There was reduced lipid accumulation in the UDCA and TUDCA groups compared with saline (Figure 6C). Liver TGs were decreased significantly in both the TUDCA (46.7 ± 4.9 mg/100 mg wet liver) and UDCA (43.73 ± 8.1) groups when compared with saline (78.18 ± 14.6; Figure 6D). CHOP mRNA relative expression was lower in the TUDCA (0.73 ± 0.1 A.U.) and UDCA (0.73 ± 0.1) gavaged groups when compared with saline (1.05 ± 0.1; Figure 6E).
Figure 6.
A, Serum bile acid levels after gavage for 3 weeks. Postprandial total serum bile acid levels were higher in UDCA and TUDCA gavage rats compared with saline gavage rats. N for groups: saline = 4; UDCA = 5; TUDCA = 5 (ANOVA, post hoc Tukey's: *, P < .05). B, Serum bile acid composition analysis. Serum bile acid composition analyzed by liquid chromatography-mass spectroscopy shows increased level of TUDCA in both UDCA- and TUDCA-gavaged rats after 3 weeks. N for groups: Saline = 4; UDCA = 5; TUDCA = 5 (ANOVA, post hoc Tukey's: *, P < .05). C, Hepatic histology after gavage for 3 weeks. Diet-induced obese rats gavaged for 3 weekd showed decreased steatosis by oil-red O staining on frozen liver sections for TUDCA- and UDCA-gavaged rats. Sections (×10)of the saline gavaged rat's liver at time of death show marked steatosis. D, Hepatic TG content. Liver TG levels were lower in UDCA- and TUDCA-gavaged rats compared with saline-gavaged group. N for groups: saline = 4; UDCA = 5; TUDCA = 5 (ANOVA, post hoc Tukey's: *, P < .05). E, Postgavage hepatic ER stress-gene expression. mRNA levels of the gene coding for the ER stress component CHOP were measured by RT-PCR and expressed in relative expression units. CHOP mRNA was decreased in UDCA- and TUDCA-gavaged rats compared with saline-gavaged group. N for groups: saline = 4; UDCA = 5; TUDCA = 5 (ANOVA, post hoc Tukey's: *, P < .05).
Discussion
The success of bariatric surgical procedures to produce weight loss and improved metabolic regulation makes it imperative to understand the mechanisms by which these procedures exert their powerful biologic effects. Whereas most hypotheses to explain the impact of bariatric surgery on body weight have focused on either gastric restriction or intestinal malabsorption, it is increasingly clear that these do not fully explain the complex physiologic changes caused by such procedures (21). A wide range of data suggest altered bile acid dynamics as a possible alternative hypothesis for the effects of bariatric surgical procedures such as RYGB (5, 6, 13, 15). Manfredini et al (16) identified that diverting bile directly to the jejunum in rats produces an initial weight loss and further a sustained calorie intake-independent improved glucose tolerance. We used a similar technique of BD to directly test the impact of altering the flow and serum level of bile acids on key metabolic parameters without gastric restriction or altering nutrient flow through the intestine. Further, we have extended this work to examine the mechanistic effects on bile acid physiology and liver outcomes.
BD resulted in a short circuiting of the enterohepatic circulation of bile acids, resulting in increased total and conjugated bile acids in the serum. BD in our hands also led to weight loss and improvements in body composition and glucose tolerance. Of particular interest were the effects on hepatic physiology. BD resulted in dramatically decreased levels of steatosis associated with reduced pJNK and reduced expression of CHOP, indicating that the reduced lipid content was accompanied by reduced markers of ER stress.
The BD procedure not only raised serum bile acid concentration but also deprived the normal mixing of bile acids with chyme in the duodenum. Bile acids such as UDCA are rapidly absorbed and on first-pass are efficiently conjugated with taurine in the rat, resulting in TUDCA. Taken together, these data are consistent with the hypothesis that taurine-conjugated bile acids such as TUDCA improve liver parameters consistent with the known ER chaperone role (22). Gavage of TUDCA to ob/ob mice decreased hepatic steatosis by reducing the expression of genes known to regulate de novo lipogenesis (23). Further, oral TUDCA administration has also been reported to improve human liver insulin sensitivity (24). However, similar to this report, despite improved liver parameters, we did not observe a difference in body weight after the 3-week treatment with either UDCA or TUDCA bile acids (data not shown). Because we did not specifically assess glucose and metabolic parameters including fat mass changes in this bile acid gavage experiment, we can only speculate that there might be weight loss-independent mechanisms that contribute to the improvement in hepatic steatosis in the bile acid-gavaged rats. One such mechanism could be improved hepatic insulin sensitivity secondary to reduced ER stress, and we will investigate these potential mechanisms in follow-up studies.
Further, presurgery weight appears to have an impact on BD outcomes overall. BD rats with presurgery weights < 500 g regain their baseline weight that, in large part, is driven by lean mass accrual compared with the weight regain being equally fat and lean mass in SH, SH-PF, and Naïve rats (Figure 2, C and D). Interestingly, the SH and BD rats that had a heavier presurgery weight (>550 g) not only lost more weight but also did not reach their presurgery weight despite an equal length of follow-up postsurgery (Figure 1, D and E). Thus, heavier presurgery weight animals have more perioperative morbidity similar to that seen in humans (25).
Our data suggest an important mechanistic role for altering the flow of bile acids to mimic metabolic improvements seen in established bariatric surgeries such as RYGB and others. Patti et al (6) reported that after RYGB, patients had significantly higher levels of glycine- and taurine-conjugated bile acids than their body mass index-matched controls. Adult humans preferentially conjugate bile acids with glycine and taurine (26) whereas in the fetal (27)and neonatal periods taurine conjugation predominates (28). Rodents however, preferentially conjugate bile acids with taurine throughout their lifespan (29). It was therefore not surprising to find that taurine-conjugated bile acid species including TUDCA made up most of the elevated level of serum bile acids in our BD rats.
Increased levels of serum bile acids provide negative feedback to bile acid synthesis and export into bile secretion. In BD rats we saw increased serum bile acid levels and also decreased mRNA levels of the rate-limiting enzyme for bile acid production, cholesterol 7α-hydroxylase (CYP7A1), as well as the major determinant of bile acid hepatocyte export, BSEP and OATPs. Bile acids are ligands for FXR in the intestine and especially in the ileum whereas SHP and FGF15 respond to FXR activation downstream. We do not have direct evidence, but presume that it is secondary to this decreased output of bile acids from the biliary tract that we observe decreased intestinal FXR, SHP, and FGF15 mRNA levels. It has been previously reported that intestinal FXR knock-out mice have an increased bile acid pool size (30) and that FGF15/19 can reduce hepatic bile acid production (31).
Human serum FGF19 levels are elevated after bariatric surgery such as RYGB, whereas we found that the expression of the orthologous gene, FGF-15, was decreased in BD rats (Figure 4D). This may seem contradictory to our hypothesis that BD is mimicking human bariatric surgery; however, it is critical to consider multiple potentially confounding issues. FGF15/19 released from the intestine relaxes the gall bladder and leads to its filling with bile in humans and mice. However, rats do not have gall bladders and it is not completely understood whether this contributes to differences in the actions of FGF15/19. Further, due to lack of necessary tools (32), we were unable to successfully measure FGF15 protein in either ileum or plasma. In the absence of data regarding ileal FGF19 mRNA expression in humans after RYGB, it is difficult to assess whether this discrepancy reflects a physiologic difference between the BD and RYGB surgeries, a species difference (because rats do not have a gallbladder) or simply a difference between the regulation of mRNA expression and protein levels. We therefore cannot conclude with conviction that the FXR-FGF15 pathway is suppressed in the BD rats.
BD produced calorie-intake-independent metabolic improvements in glucose tolerance and lean mass as a percentage of weight gained postsurgery. There was predominantly a loss of body fat after BD surgery and the gain in weight after BD surgery was predominantly in the form of lean mass (Supplemental Figure 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). We therefore speculate that this improved body composition may contribute to the improved glucose tolerance after BD surgery. We further speculate that these improvements maybe signaled through the receptor TGR5 (11). This G protein-coupled receptor, expressed in brown adipose tissue, gall bladder epithelium, and skeletal muscle is known to be stimulated by bile acids. This is similar to the intestinal GLP-1 release induced by bile acids This together may lead to improved liver and pancreatic function and enhanced glucose tolerance and weight loss (10). Recent work with apical sodium bile acid transporter inhibitors by Chen et al (33) showed a 2-fold increase in postprandial GLP-1 release. Similar to our data, they also observed decreased mRNA expression in FXR target genes in the small intestine. Further, another study showed that intestinal TGR5 was most abundantly expressed in the colon, and rectal administration of a bile acid binder/bile acid complex was sufficient to induce increased GLP-1 concentration but did not activate the nuclear bile acid receptor FXR. We do not have direct evidence of intestinal bile acid levels; however, in our prior work using ileal interposition surgery we found that there was a decrease in small intestinal bile acids just beyond the interposed ileal segment and an increase in bile acid content in the colon thereafter (15). We therefore speculate that our bile acid-driven effects after BD surgery may be Fxr independent but TGR5 dependent. This is consistent with the finding of increased GLP-1 levels observed in the BD rats.
Earlier versions of certain bariatric procedures that involved manipulations of the enterohepatic circulation, such as the jejuno-ileo bypass, resulted in metabolic improvements but were associated with significant hepatotoxicity (34, 35). The work by Manfredini et al (16) that used a BD similar to our experiments did not investigate the mechanistic role of bile acids or hepatotoxicity in much detail. Similar to Manfredini we did not see a change in bilirubin, but further we did not see any increase in serum ALT or histologic liver fibrosis either. We actually observed a remarkable decrease in hepatic lipid content accompanied by markers of reduced ER stress.
Consequently, we sought to directly test whether addition of specific bile acid species could recapitulate our results. In common with BD surgery, oral gavage of TUDCA decreased hepatic steatosis and reduced markers of ER stress. Furthermore, gavaging the unconjugated bile acid, UDCA, led to similar physiologic hepatic improvements to that of TUDCA gavage. UDCA is rapidly absorbed and on first pass is efficiently conjugated with taurine in the rat. It is therefore likely that UDCA gavage may have had similar effects to that of TUDCA but perhaps requiring further metabolism. This was evident from the finding that the serum bile acid composition of UDCA-treated rats revealed elevated levels of TUDCA similar to those of rats fed TUDCA directly. Taken together, these data are consistent with the hypothesis that taurine-conjugated bile acids such as TUDCA improve liver parameters consistent with the known ER chaperone role (22). Gavage of TUDCA to ob/ob mice decreased hepatic steatosis by reducing the expression of genes known to regulate de novo lipogenesis (23). Further, oral TUDCA administration has also been reported to improve human liver insulin sensitivity (24).
In summary, we have found that direct manipulation of bile flow can recapitulate a number of key benefits that occur after bariatric surgery, but without restriction or intestinal rerouting. Our data show that there is decreased steatosis in the liver of rats after BD surgery. These data raise some very important mechanistic issues regarding energy expenditure, hepatic lipid metabolism, and transport, which need to be addressed in future studies. Thus, the mechanism of improvement in hepatic steatosis needs to be further delineated and is probably multifactorial including energy expenditure. Together these results have two important implications. The first is that these data provide compelling evidence that manipulation of the bile acids is sufficient to recapitulate key effects of bariatric procedures including RYGB. The second is that it may be possible to develop less invasive procedures that focus on altering key aspects of bile acid physiology and enterohepatic cycling. Such procedures may provide novel ways to treat obesity and key obesity-related conditions. In particular, the current results indicate that such procedures may be especially beneficial to individuals with obesity-related liver dysfunction.
Acknowlegments
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) Grant K08 DK084310–03, U01 DK08505 (to R.K.), and Ethicon Endo-Surgery (to R.J.S. and R.K.) and NIH/NIDDK Grant P30 DK078392.
Disclosure Summary: R.J.S. receives research support from Ablaris, Johnson and Johnson, Novo Nordisk, and Pfizer, is a paid speaker for Johnson and Johnson, Merck, Novo Nordisk, and Pfizer, and serves as a consultant for Angiogen, Eli Lilly, Johnson and Johnson, Novartis, Novo Nordisk, Takeda, and Zafgen, and has equity in Zafgen. R.K. receives research support from Johnson and Johnson. The other coauthors have no disclosures.
For editorial see page 2255
- ALT
- alanine aminotransferase
- A.U.
- arbitrary units
- BSEP
- bile salt export pump
- CHOP
- C/EBP homologous protein
- ER
- endoplasmic reticulum
- GLP-1
- glucagon-like peptide 1
- HF
- high fat
- JNK
- c-Jun-N-terminal kinase
- OATP
- organic anion transporting polypeptide
- PF
- pair fed
- RYGB
- roux-en-Y gastric bypass
- SH
- sham
- SHP
- short-heterodimer partner
- TG
- triglyceride
- TGR
- triglyceride receptor
- TUDCA
- tauroursodeoxycholic acid
- UDCA
- ursodeoxycholic acid.
References
- 1. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;26:497–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xanthakos SA, Miles L, Bove K, Inge T. Outcome of nonalcoholic fatty liver disease (NAFLD) in adolescents after bariatric surgery. (Obesity Society Annual Meeting, New Orleans, Louisiana). Obesity (Silver Spring). 2007;15:A209 [Google Scholar]
- 3. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;26:497–504 [DOI] [PubMed] [Google Scholar]
- 4. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 5. Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism. 2009;58:1400–1407 [DOI] [PubMed] [Google Scholar]
- 6. Patti ME, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553 [DOI] [PubMed] [Google Scholar]
- 8. Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368 [DOI] [PubMed] [Google Scholar]
- 9. Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489 [DOI] [PubMed] [Google Scholar]
- 12. Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191 [DOI] [PubMed] [Google Scholar]
- 13. Jansen PL, van Werven J, Aarts E, et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis. 2011;29:48–51 [DOI] [PubMed] [Google Scholar]
- 14. Wu T, Bound MJ, Standfield SD, Jones KL, Horowitz M, Rayner CK. Effects of taurocholic acid on glycemic, glucagon-like peptide-1, and insulin responses to small intestinal glucose infusion in healthy humans. J Clin Endocrinol Metab. 2013;98:E718–E722 [DOI] [PubMed] [Google Scholar]
- 15. Kohli R, Kirby M, Setchell KD, et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652–G660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manfredini G, Ermini M, Scopsi L, Bonaguidi F, Ferrannini E. Internal biliary diversion improves glucose tolerance in the rat. Am J Physiol. 1985;249:G519–G527 [DOI] [PubMed] [Google Scholar]
- 17. Strader AD, Vahl TP, Jandacek RJ, Woods SC, D'Alessio DA, Seeley RJ. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453 [DOI] [PubMed] [Google Scholar]
- 18. Melgarejo T, Williams DA, O'Connell NC, Setchell KD. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig Dis Sci. 2000;45:407–414 [DOI] [PubMed] [Google Scholar]
- 19. Hagio M, Matsumoto M, Fukushima M, Hara H, Ishizuka S. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J Lipid Res. 2009;50:173–180 [DOI] [PubMed] [Google Scholar]
- 20. Woods SC, Seeley RJ, Rushing PA, D'Alessio D, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087 [DOI] [PubMed] [Google Scholar]
- 21. Stefater MA, Sandoval DA, Chambers AP, et al. Sleeve gastrectomy in rats improves postprandial lipid clearance by reducing intestinal triglyceride secretion. Gastroenterology. 2011;141:939–949 e1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen YJ, Inouye M. The intramolecular chaperone-mediated protein folding. Curr Opin Struct Biol. 2008;18:765–770 [DOI] [PubMed] [Google Scholar]
- 23. Yang JS, Kim JT, Jeon J, et al. Changes in hepatic gene expression upon oral administration of taurine-conjugated ursodeoxycholic acid in ob/ob mice. PLoS One. 2010;5:e13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kars M, Yang L, Gregor MF, et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perlow JH, Morgan MA. Massive maternal obesity and perioperative cesarean morbidity. Am J Obstet Gynecol. 1994;170:560–565 [DOI] [PubMed] [Google Scholar]
- 26. Sjovall J. Dietary glycine and taurine on bile acid conjugation in man; bile acids and steroids 75. Proc Soc Exp Biol Med. 1959;100:676–678 [DOI] [PubMed] [Google Scholar]
- 27. Setchell KD, Dumaswala R, Colombo C, Ronchi M. Hepatic bile acid metabolism during early development revealed from the analysis of human fetal gallbladder bile. J Biol Chem. 1988;263:16637–16644 [PubMed] [Google Scholar]
- 28. Encrantz JC, Sjovall J. On the bile acids in duodenal contents of infants and children. Bile acids and steroids 72. Clin Chim Acta. 1959;4:793–799 [DOI] [PubMed] [Google Scholar]
- 29. Shonsey EM, Wheeler J, Johnson M, et al. Synthesis of bile acid coenzyme a thioesters in the amino acid conjugation of bile acids. Methods Enzymol. 2005;400:360–373 [DOI] [PubMed] [Google Scholar]
- 30. Stroeve JH, Brufau G, Stellaard F, Gonzalez FJ, Staels B, Kuipers F. Intestinal FXR-mediated FGF15 production contributes to diurnal control of hepatic bile acid synthesis in mice. Lab Invest. 2010;90:1457–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sinha J, Chen F, Miloh T, Burns RC, Yu Z, Shneider BL. β-Klotho and FGF-15/19 inhibit the apical sodium-dependent bile acid transporter in enterocytes and cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2008;295:G996–G1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potthoff MJ, Boney-Montoya J, Choi M, et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen L, Yao X, Young A, et al. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am J Physiol Endocrinol Metab. 2012;302:E68–E76 [DOI] [PubMed] [Google Scholar]
- 34. Holzbach RT. Hepatic effects of jejunoileal bypass for morbid obesity. Am J Clin Nutr. 1977;30:43–52 [DOI] [PubMed] [Google Scholar]
- 35. Moxley RT, 3rd, Pozefsky T, Lockwood DH. Protein nutrition and liver disease after jejunoileal bypass for morbid obesity. N Engl J Med. 1974;290:921–926 [DOI] [PubMed] [Google Scholar]