Abstract
Intrauterine growth restriction (IUGR) is an important fetal developmental problem resulting from 2 broad causes: maternal undernutrition and/or decreased fetal nutrient delivery to the fetus via placental insufficiency. IUGR is often accompanied by up-regulation of the hypothalamo-pituitary-adrenal axis (HPAA). Sheep studies show fetal HPAA autonomy in late gestation. We hypothesized that IUGR, resulting from poor fetal nutrient delivery, up-regulates the fetal baboon HPAA in late gestation, driven by hypothalamo-pituitary glucocorticoid receptor (GR) insensitivity and decreased fetal leptin in peripheral plasma. Maternal baboons were fed as ad libitum controls or nutrient restricted to produce IUGR (fed 70% of the control diet) from 0.16 to 0.9 gestation. Peripheral ACTH, cortisol, and leptin were measured by immunoassays. CRH, arginine vasopressin (AVP), GR, leptin receptor (ObRb), and pro-opiomelanocortin peptide expression were determined immunohistochemically. IUGR fetal peripheral cortisol and ACTH, but not leptin, were increased (P < .05). IUGR increased CRH peptide expression, but not AVP, in the fetal hypothalamic paraventricular nucleus (PVN) and median eminence (P < .05). PVN ObRb peptide expression, but not GR, was decreased (P < .05) with IUGR. ObRb and pro-opiomelanocortin were robustly expressed in the anterior pituitary gland, but ∼1% of cells showed colocalization. We conclude that (1) CRH, not AVP, is the major releasing hormone driving ACTH and cortisol secretion during primate IUGR, (2) fetal HPAA activation was aided by GR insensitivity and decreased ObRb expression in the PVN, and (3) the anterior pituitary is not a site for ObRb effects on the HPAA.
Intrauterine growth restriction (IUGR) is an important developmental problem arising from a number of causes, which fall into 2 broad categories: maternal undernutrition and decreased nutrient delivery to the fetus resulting from placental insufficiency. Several reviews have highlighted the negative offspring outcomes in a number of fetal and postnatal physiological systems that result from maternal undernutrition in pregnancy (1–3). Fetal nutrient deprivation is often accompanied by up-regulation of hypothalamo-pituitary-adrenal axis (HPAA) activity, resulting in fetal overexposure to glucocorticoid levels that are inappropriate for the current stage of fetal development at critical time windows with potential programming effects on neuroendocrine systems including the HPAA itself (for review, see Ref. 4). Fetal undernutrition is accompanied by dysfunctional development of fat metabolism (for review, see Ref. 5). In 1996, Ahima et al (6) demonstrated leptin inhibition of the gonadal axis, the thyroid axis, and, importantly for the present study, the HPAA; thus, we sought to evaluate changes induced in the fetal leptin regulatory system.
Most of our knowledge about the endocrinology of nutritional effects on the ontogeny of HPAA activity has been obtained in altricial species (7, 8). Studies in precocial species are restricted to the sheep, a species that allows fetal instrumentation including multiple fetal blood vessel catheterizations, surgical interventions, fetal blood sampling, and drug delivery to evaluate mechanisms (9–11). For example, an early study with this model found that the maternal contribution to the fetal cortisol pool decreases with increasing fetal age. Thus, Hennessy et al (12) found that at 0.7 gestation, the mother supplies 100% of the cortisol measured in fetal plasma, which decreases to 37% at 0.8 gestation. By 0.9 gestation, the fetal HPAA is quite autonomous with only 12% or less cortisol coming from the mother. In sharp contrast, information on the fetal primate HPAA is much more difficult to obtain because the fetal blood vessel catheterizations that allow long-term study are much more challenging in primates.
The results of maternal nutrient deprivation on the fetal HPAA have been published for rodents (7), but to our knowledge not for any fetal primate model, thus leaving a significant information gap as a barrier to translation to human development. In the present investigation, we studied 2 levels of nutrition (ad libitum vs 70% ad libitum global) in baboon pregnancy coupled with blood and tissue samples taken at 90% of gestation to determine the relationships between food availability, fetal growth, leptin, and HPAA function in late gestation. Fetuses of 30% global nutrient–restricted mothers were IUGR. We hypothesized that by the end of gestation, the challenge of IUGR, resulting from poor fetal nutrient delivery, would up-regulate the fetal and maternal baboon HPAA accompanied by down-regulation of the leptin system as fetal demand for nutrients peaks and the fetal HPAA matures toward term.
Materials and Methods
Animals
All animals were housed in 20 foot × 20 foot × 15 foot metal and concrete group cages at the Texas Biomedical Research Institute. Experimental animals were obtained from appropriate groups of 16 healthy female baboons (Papio sp.) of similar prestudy body weights (10–15 kg) and morphometric features (13). Females were randomly assigned to 1 of 2 feeding groups: (1) ad libitum fed control (CTR) (n = 10) or (2) nutrient-restricted mothers/fetuses (IUGR) (fed 70% of the global CTR diet; n = 6) and maintained with a single vasectomized male. After 30 days of acclimation, a fertile male was substituted into the harem cage. Pregnancy was dated initially by timing of ovulation and changes in sex skin color and confirmed at 30 days of gestation (dG) by ultrasonography. Details of housing, feeding, and environmental enrichment have been published elsewhere (13). All procedures were approved by the University of Texas Health Science Center and Texas Biomedical Research Institute internal animal care and use committees and performed in Association for Assessment and Accreditation of Laboratory Animal Care–approved facilities.
Feeding
Animals were individually fed to enable precise regulation of intake either between 7:00 am and 9:00 am or 11:00 am and 1:00 pm as described in detail elsewhere (13). At feeding time, the weight of each baboon was obtained via an electronic weighing system (GSE 665; GSE Scale Systems, Allen Park, Michigan) in a holding cage as the animal waited in line. Water was continuously available in each feeding cage (Lixit, Napa, California), and the animals were fed Purina Monkey Diet 5038 (Purina, St Louis, Missouri). From 30 to 165 dG (term ∼184 dG), IUGR group animals were fed 70% of the diet eaten by contemporaneous ad libitum fed CTR females on a per kilogram basis that was adjusted weekly. Food consumption of animals, body weights, and health status were recorded daily.
Cesarean sections
Cesarean sections/necropsies were conducted at 0.9 gestation (term ∼184 days) using a standard sterile surgical technique as described in detail previously. In brief, baboons were premedicated with ketamine hydrochloride (10 mg/kg i.m.), intubated, and maintained at a surgical plane of anesthesia with isoflurane (2%) throughout surgery. After hysterotomy, the umbilical cord was identified and used for fetal exsanguination with the baboon under general anesthesia as approved by the American Veterinary Medical Association Panel on Euthanasia. Placenta and fetus were removed from the uterus and immediately submitted for morphometric measurements, including fetal weight, pathological evaluation, and tissue sampling. Fetal brain tissue samples were fixed for 24 hours with 4% (wt/vol) paraformaldehyde solution in saline for immunohistochemistry and then moved through increasing percentages of alcohol to 100% and then blocked in paraffin.
Buprenorphine hydrochloride (buprenorphine HCl injection; Hospira, Inc, Lake Forest, Illinois), 0.015 mg/kg/d split as 2 doses for 3 days, was used for postoperative maternal analgesia as described in detail previously (14).
ACTH1–39 ELISA
A 2-site ELISA was used to determine plasma concentrations of ACTH1–39 at 0.9 gestation. This assay uses a biotinylated capture antibody generated against ACTH34–39 and a peroxidase-labeled reporter antibody against ACTH1–24. This assay yields ≥98% recovery of ACTH1–39 from fetal plasma and fetal plasma spiked with ACTH1–24 diluted in parallel with the standard curve. Recombinant ovine pro-opiomelanocortin (POMC) and recombinant ovine 22-kDa pro-ACTH exhibit <1% (POMC) and ∼5% (22-kDa pro-ACTH) cross-reactivity. The sensitivity of the assay is 2.5 pg/mL for ACTH1–39. The intra-assay coefficient of variation was 2.9%, and the interassay coefficient of variation was 4.2% (15).
Cortisol chemiluminescent immunoassay
Cortisol was measured using an Immulite 1000 analyzer with kits from Diagnostic Products Corporation (Los Angeles, California). Pooled serum was first tested to validate the performance of this system for baboon samples. Assay precision was determined by testing pooled samples using 5 replicates in each assay. These assays were repeated at 2 dilutions to assess linearity of the results. All test samples were run at dilutions estimated to achieve values in the middle of the assay calibration range. The intra-assay and interassay coefficients of variation for cortisol were 5.6 and 8.4, respectively. The correlation coefficient (r values) for dilution of cortisol was 0.90 for recovery with known standards (16).
Immunohistochemistry
Paraffin tissue sections, 5 μm in thickness, from the mid-anteroposterior region of the hypothalamic paraventricular nucleus (PVN) were deparaffinized in xylene, rehydrated in descending grades of ethanol (100%, 70%, and 45%) to water, immersed in citrate buffer (0.01 M citrate buffer, pH 6.0), and heated to boiling for 10 to 15 minutes for antigen retrieval. After cooling for 15 minutes, the sections were rinsed in potassium PBS (0.04 M K2HPO4, 0.01 M KH2PO4, and 0.154 M NaCl, pH 7.4) 7 times for 6 minutes each and for 10 minutes in a solution of 1.5% H2O2-methanol and then for 5 minutes in potassium PBS. Sections were placed in diluted (10%) normal serum for 20 minutes and covered with primary antibody overnight at 4°C. A standard immunohistochemical avidin-biotin-peroxidase complex technique (Elite ABC kits, catalog no. PK-6100; Vector Laboratories, Burlingame, California) was used to visualize protein expression using 0.02% 3,3′-diaminobenzidine tetrahydrochloride with 2.5% nickel sulfate as Chromagen (17).
Initial titrations were performed with 3 concentrations of primary antibody that bracketed the suggested dilution of the manufacturer. Final primary antibody concentration was adjusted to give the cleanest immunostaining achievable, ie, the strongest immunoreactivity in the compartment of interest, eg, cytoplasm, with the least extracellular immunogenicity and no nuclear immune product. Concentrations of antibodies that produced immune product in the “other” compartment were never used. Whenever the immunizing agent was available, the primary antibody in question was “preabsorbed,” ie, incubated with an excess of the antigen and then used in a staining run with the tissue of interest. The absence of immunostaining assured that an antibody had only a single antigen in the tissue of interest; antibodies failing this test were discarded. In the absence of availability of antigen for preabsorption, controls were run with normal serum from the species in which the antibody was generated in place of primary antibody to rule out nonspecific binding. Once the final dilution of the primary antibody was determined, all sections to be compared via the counting program were immunostained in the same assay to assure identical conditions. Three sections taken at 150-μm intervals were used for analysis from each animal. In addition, sections known to contain antigen expression were included as positive controls in each staining run.
Antibodies used
The following antibodies were used for immunohistochemistry. Information for each antibody is given in the following order—antigen, final dilution, catalog no., and manufacturer: glucocorticoid receptor (GR), 1:200, PA-512, Abcam (Cambridge, Massachusetts); phosphorylated GR (pGR), 1:100, SER-211, Abcam; 11β-hydroxysteroid dehydrogenase (HSD)-1, 1:500, sc-20175, Santa Cruz Biotechnology (Santa Cruz, California); 11β-HSD2, 1:500, sc-20176, Santa Cruz Biotechnology; ACTH, 1:3000, ES4008M, National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, Maryland); CRH-receptor 1 (CRH-R1), 1:5000, sc-1757, Santa Cruz Biotechnology; arginine vasopressin (AVP), 1:30, AB 1565, Millipore (Billerica, Massachusetts); CRH, 1:50, gift from Dr Ann-Judith Silverman, Columbia University (New York, New York); leptin receptor (ObRb), 1:100, sc-1833, Santa Cruz Biotechnology; signaling form of the ObRb, 1:400, sc-1832, Santa Cruz Biotechnology; and POMC, 1:500, H-029-30, Phoenix Pharmaceuticals, Burlingame, California).
Statistical analyses
An initial analysis was performed by t test to see whether male and female fetuses differed and, if not, their data were pooled. Differences between fetuses of control ad libitum fed mothers and fetuses of nutrient-restricted mothers were analyzed by Student's nonpaired t test with the α level set at .05. All data are expressed as mean ± SEM with CTR data presented first.
Results
Before pregnancy, maternal body weights were not different between groups (Table 1). At cesarean section, maternal, placental, and fetal weights were decreased (P < .05) in the IUGR group as was the ponderal index, a marker of leanness. Although there were no statistical differences in fetal weights between males and females in either group of animals in which histological analysis was conducted, we have studied a larger number of animals under exactly the same management but without the immunohistochemical evaluations in which, when divided by sex, control male fetuses (n = 10; 847 ± 36.4 g) were heavier than control female fetuses (n = 14; 747 ± 29.6 g) (P < .03). Male fetuses of nutrient-restricted mothers (n = 6; 729 ± 32.8 g) were lighter than control male fetuses (P < .02), and female fetuses of nutrient-restricted mothers (n = 5; 659 ± 28.4 g) were lighter than female control fetuses (P = .03). These data show that the degree of maternal nutritional challenge produced a similar degree of IUGR in males (14%) and females (12%). They also show that the weights of animals chosen at random for detailed immunohistochemical analysis fell within the weights for the larger groups. Fetal adrenal weights expressed as either milligrams or percentage of body weight were not significantly (P > .05) decreased in the IUGR group vs the CTR group (Table 1).
Table 1.
Maternal, Placental, and Fetal Data From Baboons Fed as CTR or IUGR (Fed 70% CTR Diet From 30 to 165 dG) With Cesarean Section or Necropsy at 165 dG
| Weights | CTR (n = 10) | IUGR (n = 6) |
|---|---|---|
| Mother prepregnancy, kg | 16.0 ± 0.8 | 14.9 ± 0.4 |
| Mother cesarean section, kg | 17.8 ± 0.8 | 14.1 ± 0.8a |
| Placenta, g | 202.1 ± 14.7 | 145.0 ± 7.2a |
| Fetus, g | 808.8 ± 40.5 | 668.5 ± 33.4a |
| Ponderal index, kg/m3 | 15.5 ± 0.8 | 12.8 ± 0.8a |
| Fetal adrenal, mg | 0.15 ± 0.1 | 0.14 ± 0.1 |
| Fetal adrenal, % body weight | 0.018 ± 0.001 | 0.018 ± 0.001 |
Data are expressed as means ± SEM.
P < .05 vs CTR.
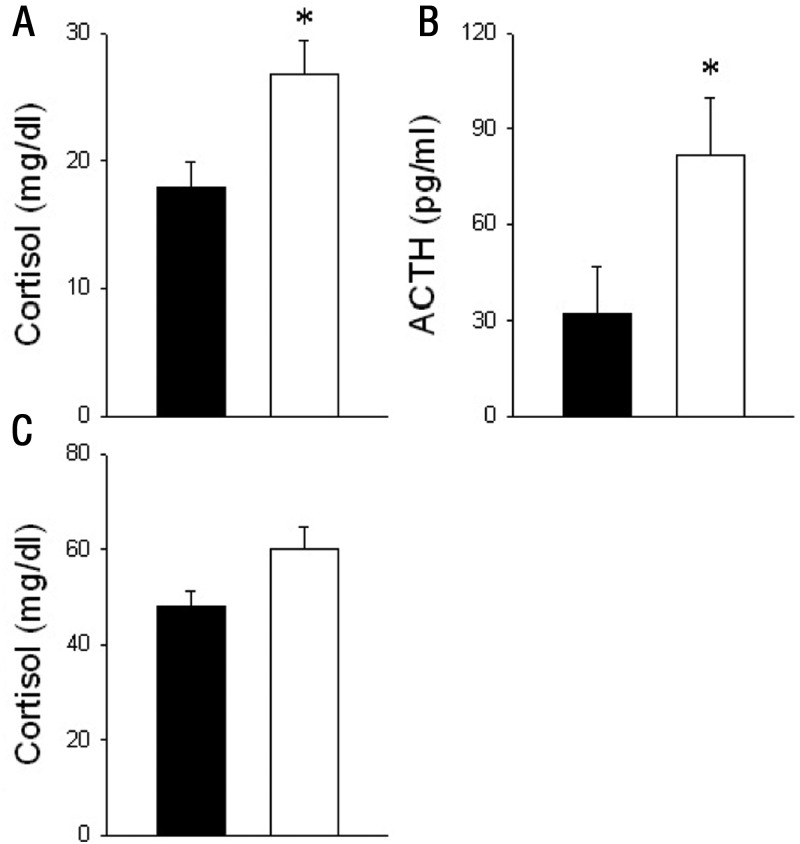
Fetal plasma ACTH was increased (P < .05) with IUGR at 0.9 gestation compared with CTR (Figure 1). Serum cortisol concentrations were not different in mothers (Figure 1), whereas fetal cortisol was increased (P < .05) with IUGR (Figure 1). No difference was found in fetal peripheral plasma leptin concentrations between groups (CTR 248.6 ± 4.97 pg/mL vs IUGR 239.5 ± 1.46 pg/mL, P > .05). Immunoreactive GR, pGR, ACTH, 11β-HSD1 and 11β-HSD2, and CRH-R1 were all detected by single immunostaining in the fetal anterior pituitary (AP) but did not differ in expression between CTR and IUGR fetuses (Table 2). Double immunostaining for POMC and the signaling form of the ObRb expression was robust in AP for each peptide, but only a small fraction (∼1.0%) of the corticotrophs presented with POMC/ObRb colocalization (data not shown).
Figure 1.
ACTH and cortisol concentrations in peripheral plasma of fetal baboons (A and B) and cortisol from their mothers (C) that were fed as ad libitum controls (filled bars; n = 7) or IUGR (open bars, mothers fed 70% CTR diet; n = 6) from 0.16 to 0.9 gestation with blood collected at cesarean section or necropsy at 0.9 gestation (165 dG). Data are expressed as means ± SEM. *, P < .05 vs CTR.
Table 2.
Summary of Results for Protein Expression of 11β-HSD1 and 11β-HSD2, GR, pGR, and CHR-R1 Where Appropriate in AP and PVN Neurons of Fetuses From Mothers Fed as CTR or IUGR (Fed 70% CTR Diet From 0.16 to 0.9 Gestation)
| Structure | Antigen | Treatment | Fraction | Density, × 106 |
|---|---|---|---|---|
| AP | GR | CTR | 3.5 ± 1.99 | 9.6 ± 5.46 |
| IUGR | 2.3 ± 0.34 | 6.3 ± 0.93 | ||
| AP | pGR | CTR | 6.0 ± 1.29 | 14.2 ± 3.02 |
| IUGR | 3.6 ± 0.44 | 8.5 ± 1.11 | ||
| AP | 11β-HSD1 | CTR | 3.9 ± 1.78 | 9.2 ± 4.07 |
| IUGR | 2.1 ± 0.76 | 5.0 ± 1.73 | ||
| AP | 11β-HSD2 | CTR | 11.4 ± 4.73 | 26.9 ± 10.9 |
| IUGR | 6.4 ± 0.73 | 15.8 ± 1.7 | ||
| AP | ACTH | CTR | 4.1 ± 0.61 | 9.6 ± 1.35 |
| IUGR | 4.1 ± 0.31 | 9.7 ± 0.80 | ||
| AP | CRH-R1 | CTR | 2.0 ± 0.7 | 6.1 ± 2.18 |
| IUGR | 1.0 ± 0.2 | 3.0 ± 0.67 | ||
| PVN | pGR | CTR | 10.1 ± 2.2 | 30.7 ± 6.95 |
| IUGR | 11.2 ± 2.5 | 33.8 ± 7.72 | ||
| PVN | GR | CTR | 5.0 ± 1.10 | 17.1 ± 3.50 |
| IUGR | 8.5 ± 1.51 | 29.1 ± 5.25 |
Protein expression for 11β-HSD1 and 11β-HSD2 was not observed in PVN of either group. All data are expressed as means ± SEM. No differences were found between any means.
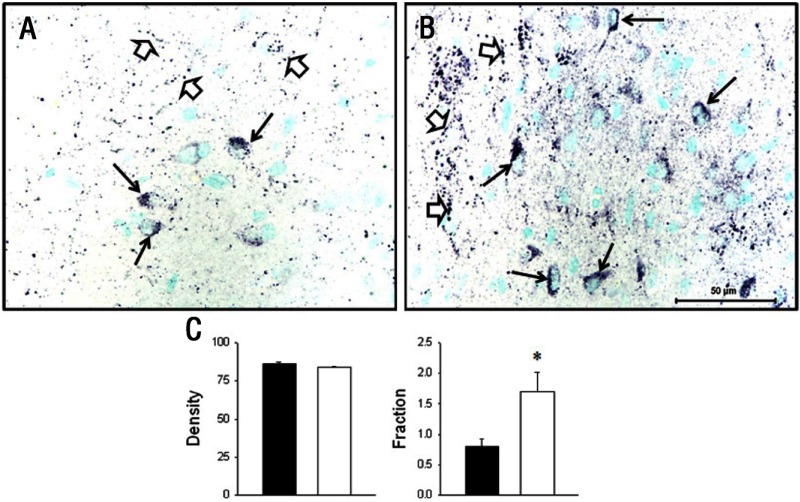
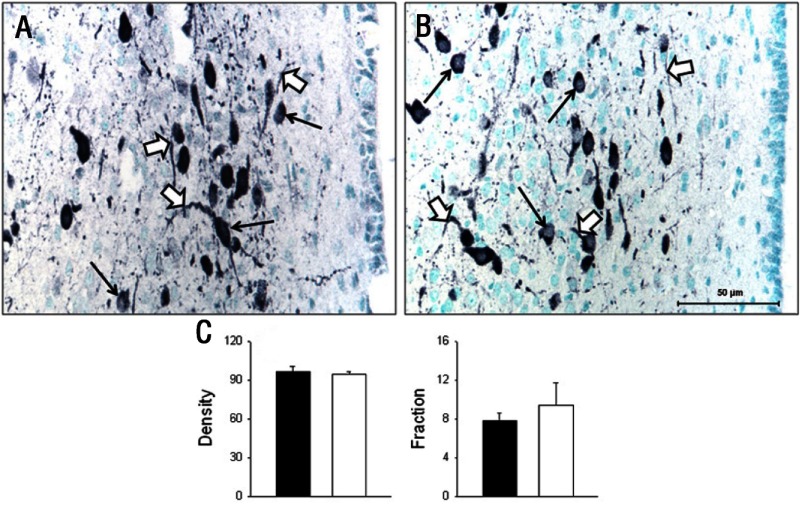
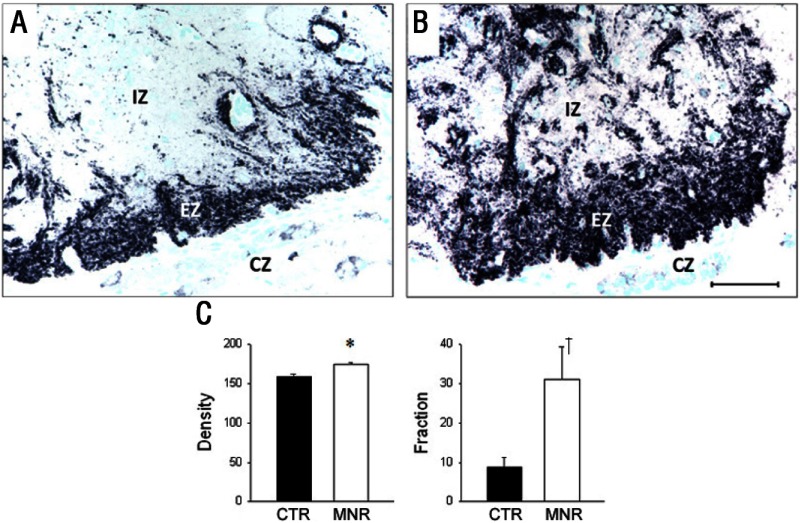
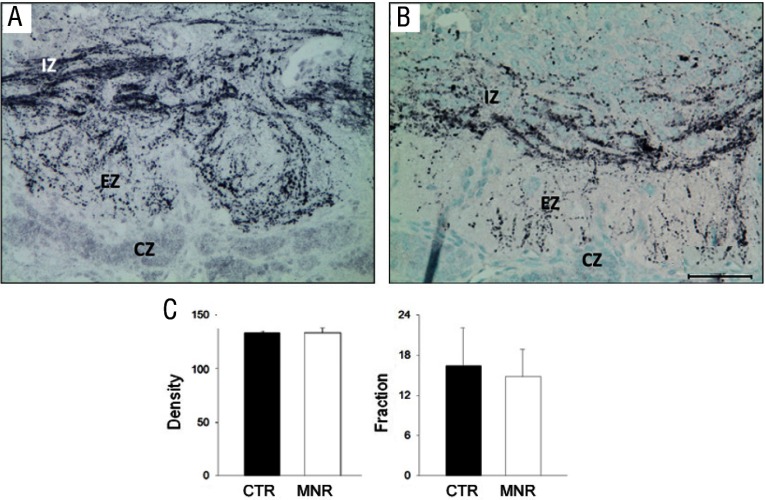
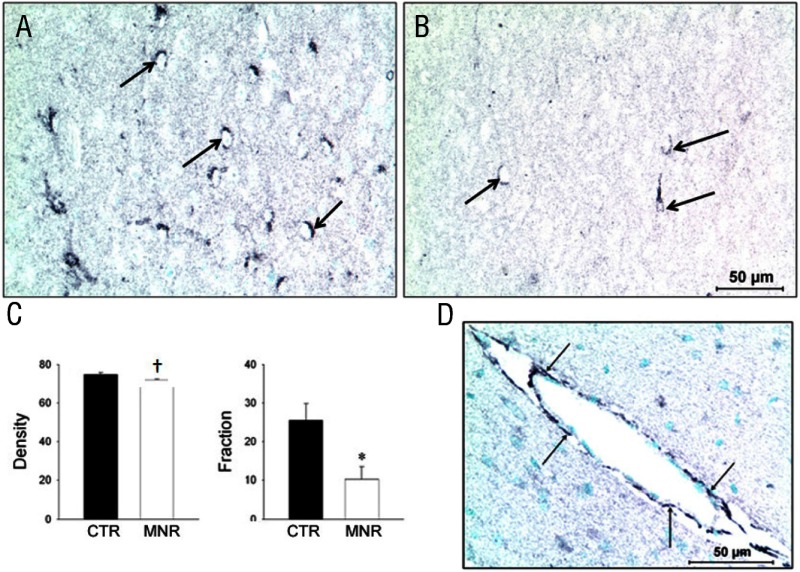
Both GR and pGR immunoreactivities were observed in the PVN but were not different between groups (Table 2), whereas 11β-HSD1 and 11βHSD2 were not found in the PVN of either CTR or IUGR fetuses. In the mid-anteroposterior region of the PVN, ie, that portion that projects axons to the median eminence (18), CRH was found in neuronal perikarya and in beaded fibers of the PVN, and expression was increased (P < .05) in IUGR group fetuses compared with CTR fetuses (Figure 2). In contrast, AVP also was robustly expressed in neuronal perikarya and fibers of the PVN but showed no difference (P > .05) for CTR vs IUGR (Figure 3). Immunoreactive CRH and AVP were also quantified in the median eminence with CRH showing a robust difference (P < .05) between groups in axons of the external zone (Figure 4), whereas AVP peptide expression was too highly varied within groups to show a difference in axons in the median eminence external zone (Figure 5). ObRb peptide expression was also found in neurons of the mid-anteroposterior region of the PVN, was decreased (P < .05) with IUGR (Figure 6), and also was localized to hypothalamic small blood vessel endothelia (Figure 6D) but could not be separately quantified.
Figure 2.
Photomicrographs (A and B) and data summary (C) of CRH peptide expression (black product) in neuronal perikarya (black arrows) and beaded neuronal fibers (open arrows) in the hypothalamic PVN of baboon fetuses from mothers fed as ad libitum fed controls (A, CTR; C, CTR, filled bars; n = 7) or IUGR (B, mothers fed 70% CTR diet; C, IUGR, open bars; n = 6) from 0.16 to 0.9 gestation with cesarean section/tissue collection at 0.9 gestation (165 dG). Data are expressed as means ± SEM. *, P < .05. Scale bar applies to both micrographs.
Figure 3.
Photomicrographs (A and B) and summary (C) of AVP peptide expression (black product) in neuronal perikarya (thin arrows) and neuronal fibers (black arrows) in the hypothalamic PVN of baboon fetuses from mothers fed as ad libitum controls (A, CTR; C, CTR, filled bars; n = 7) or IUGR (B, mothers fed 70% CTR diet; C, IUGR, open bars; n = 6) from 0.16 to 0.9 gestation with cesarean section/tissue collection at 0.9 gestation (165 dG). Data are expressed as means ± SEM. No difference (P ≫ .05) was seen between groups in AVP expression. Scale bar applies to both micrographs.
Figure 4.
Photomicrographs (A and B) and summary (C) of CRH peptide expression (black product) in terminal axons of the median eminence external zone (EZ) from baboon fetuses of mothers fed as ad libitum controls (A, CTR; C, CTR, filled bars; n = 7) or IUGR (B, mothers fed 70% CTR diet; C, IUGR [MNR], open bars; n = 6) from 0.16 to 0.9 gestation with cesarean section/tissue collection at 0.9 gestation (165 dG). IZ, internal zone of median eminence; EZ, external zone; CZ, capsule. Data are expressed as means ± SEM. *, P < .05; †, P = .05. Scale bar applies to both micrographs.
Figure 5.
Photomicrographs (A and B) and summary (C) of AVP peptide expression (black product) in terminal axons of the median eminence external zone (EZ) from baboon fetuses of mothers fed as ad libitum controls (A, CTR, filled bars; n = 7) or IUGR (B, mothers fed 70% CTR diet; C, IUGR [MNR], open bars; n = 6) from 0.16 to 0.9 gestation with cesarean section/tissue collection at 0.9 gestation (165 dG). IZ, internal zone of median eminence; EZ, external zone; CZ, capsule. Data are expressed as means ± SEM. *, P < .05; †, P = .05. Scale bar applies to both micrographs.
Figure 6.
Photomicrographs (A and B) and summary (C) of leptin long form receptor peptide expression (black product) in neurons of the hypothalamic PVN from baboon fetuses of mothers fed as ad libitum controls (A, CTR, filled bars; n = 7) or IUGR (B, mothers fed 70% CTR diet; C, IUGR [MNR], open bars; n = 6) from 0.16 to 0.9 gestation with cesarean section/tissue collection at 0.9 gestation (165 dG). Data are expressed as means ± SEM. *, P < .05; †, P = .05. Scale bar applies to both micrographs. D, Photomicrograph of leptin long form receptor peptide expression (black product) in endothelial cells (arrows) of a small hypothalamic blood vessel from a baboon fetus with cesarean section/tissue collection at 0.9 gestation (165 dG).
Discussion
Before diet assignment, prospective baboon mothers were selected to form a homogeneous group with no sibships or differences in morphometrics or maternal body weights. However, at necropsy at the end of gestation, IUGR group maternal, placental, and fetal weights were decreased compared with those for the CTR group. Because IUGR fetuses were exposed to decreased nutrient availability for nearly the full duration of development, decreased body weight was not surprising and could be attributed to several mechanisms. First, IUGR decreases circulating amino acids at midgestation vs those for CTR fetuses, and fetal demand on maternal resources would be greater at 90% of gestation than at 50% (19). Second, IUGR clearly increased activity of the fetal HPAA. Local peripheral cortisol production is increased in perirenal fat in IUGR female fetuses and in liver in male fetuses (1). Glucocorticoids have both antianabolic and procatabolic effects, leading to decreased growth rates in muscle and other tissues (for review, see ref. 20). For example, the action of glucocorticoids is normally opposed by IGF-I (20), and circulating IGF-I is decreased at 0.5 gestation in the IUGR fetuses (21). This finding is confirmed by a large body of literature showing that nutrient restriction down-regulates IGF-I in both pre- and postnatal animals (22, 23). We speculate that, given the increasing nutrient demands of the late-gestation fetus, IGF-I availability would decrease even further with increasing gestational age.
In a study similar to the present report, but using a rodent model, Lesage et al (7) showed that 50% global maternal nutrient restriction resulted in IUGR and increased circulating corticosterone in near-term rat fetuses and up to 2 hours after delivery in neonates. However, in neonates older than 2 hours, there was a reversal in the level of activity of the HPAA, ie, (1) decreased neonatal plasma ACTH, (2) decreased plasma corticosterone, and (3) decreased PVN CRH mRNA. These observations show the profound differences between the regulation and activity of the pre- and postnatal HPAA, ie, the prenatal insensitivity of CRH and ACTH to glucocorticoid negative feedback in the late gestation altricial rodent fetus also seen in the precocious sheep fetus by the simultaneous significant rises in ACTH and cortisol over the last 20 days of gestation (24). Such insensitivity allows for the relatively long-term increases in cortisol that prepare the fetus for birth (25). Likewise, the lack of a difference in GR peptide expression in hypothalamus and pituitary of our 0.9 gestation IUGR baboon fetuses vs CTR fetuses suggests an insensitivity of these receptors to glucocorticoid negative feedback and offers an explanation for the observed increase in fetal ACTH and cortisol as term approaches.
The lack of a difference between CTR and IUGR fetuses at 0.9 gestation in any of the parameters that were measured in the fetal AP in the present study suggests that the observed increases in circulating IUGR group ACTH and cortisol compared with those in the CTR group did not arise from differences in fetal pituitary drive between groups. The ACTH literature shows a more complicated story. For example, it is possible that ACTH was exported too quickly to the peripheral circulation to be seen as increased AP content. Another possibility arises from the observation that in starved young male rats there is uncoupling of the expected ACTH-corticosterone relationship (26). To complicate matters further, Reimsnider and Wood (27) presented evidence that prostaglandins produced within the central nervous system can stimulate ACTH secretion.
Leptin modulates activity of several neuroendocrine systems; eg, in a mouse model, leptin blocks the fasting-induced changes normally observed in the adrenal, gonadal, and thyroid axes (6). Leptin is present in human fetuses (28), and human placental levels are similar to or greater than those found in adipose tissue (29). Leptin concentrations in umbilical cord blood range widely both between (30) and within species (0.7–39.0 ng/mL) and are highly positively correlated with placental weight (31). This result is in sharp contrast to the very low leptin levels that we found in our CTR and IUGR fetal baboons, which did not differ between groups. However, unlike human fetuses, healthy baboon fetuses are normally quite lean near term and at birth. In addition, although our IUGR baboon fetuses weighed less than CTR fetuses, and the ponderal index, a measure of leanness, was less than that of CTR group fetuses, in the last trimester of gestation fetal leptin is highly correlated with body adiposity (32), suggesting that adiposity was not different between groups.
The results of our immunohistochemical examinations of the fetal hypothalamus in the present study indicate that CRH, but not AVP, from the fetal PVN is responsible for the increased plasma ACTH and cortisol of IUGR fetuses. In agreement with the present study, Dallman et al (26) used in situ hybridization to show in their young male rat model of starvation that mRNA for CRH, but not for AVP, was increased in PVN neurons. In fetal sheep, leptin infusion to the peripheral circulation in late gestation inhibits the HPAA, probably by a central effect (33). Moreover, the effects of leptin on CRH mRNA production and peripheral glucocorticoid concentrations are influenced by factors such as current nutritional status and genetic background of the study subjects (34). In addition, as opposed to the effects of peripheral leptin, intracerebroventricularly administered leptin given to nonstressed rodents produces (1) increased plasma levels of AVP and increased mRNA for AVP in the supraoptic nucleus and PVN (35) and (2) an activated HPAA via V1a AVP receptors in the PVN, which, in turn, activate CRH neurons to drive ACTH and corticosterone secretion in concert with AVP (36). Thus, leptin/AVP effects on the CRH/AP/adrenal cortex can result from complex interactions, which need to be considered for a thorough understanding of outcomes.
In rodents, strong peptide expression of ObRb (37) is present in the arcuate, dorsomedial, and ventromedial nuclei and also at a lower level in the PVN. To our knowledge there is little information available on the control of ObRb expression in the PVN of postnatal animals and none in the fetus. One study of brains of obese and lean humans found higher ObRb levels in cerebellum than in the hypothalamus and concluded that obesity and hyperleptinemia have no effect on brain ObRb expression (38). In contrast, ObRb peptide is increased in the ventromedial hypothalamus and arcuate nucleus of postnatal mice after fasting and decreased by refeeding (39). However, our data showed an opposite effect, ie, IUGR reduced ObRb peptide expression in the PVN. However, it is not unusual for fetal physiology to differ from that of postnatal animals (see the discussion of pre- and postnatal HPAA above), and acute effects may differ from the chronic exposures in our IUGR fetuses that have occurred for several weeks. Clearly more research is needed in this important area.
Besides being localized to the PVN, leptin receptor mRNA is present in moderate amounts in the rat AP (40) and the fetal human AP where human leptin stimulates GH, but not ACTH, in short-term pituitary cultures (41). Interestingly, whereas leptin receptor is expressed in ∼97% of somatotrophs of normal rat AP, <1% of corticotrophs show leptin receptor coexpression, suggesting that leptin effects on peripheral ACTH concentrations do not occur in the AP (42). Our similar finding in the fetal baboon AP with respect to POMC/ObRb colocalization would seem to effectively rule out the AP as a site for ObRb effects on ACTH expression in the fetal baboon AP.
In summary, we have shown that IUGR up-regulates the fetal baboon HPAA in late gestation because both ACTH and cortisol were increased in peripheral plasma of IUGR fetuses compared with those in CTR fetuses. Our data suggests the mechanisms for the observed up-regulation of the fetal HPAA include (1) a lack of cortisol negative feedback in the PVN and AP as shown by the lack of down-regulation of GR peptide expression in both areas despite the increased peripheral cortisol levels in the IUGR fetuses and (2) decreased ObRb expression in the fetal PVN effectively reducing leptin inhibitory effects on the fetal HPAA. Our original hypothesis of down-regulated leptin in the peripheral circulation allowing up-regulation of the fetal HPAA was not correct; however, the actual finding, ie, decreased PVN ObRb expression in late gestation, would have the same effect in that it allows the indispensable preparturient cortisol rise to occur regardless of the degree of fetal adiposity. Future studies will center on characterizing the phenotype(s) of those PVN neurons that express ObRb in late gestation and searching for the mechanism(s) that decrease ObRb with IUGR in fetal baboons. The increased activity of the HPAA demonstrated in the IUGR fetus will accelerate terminal differentiation of key fetal organs such as the lung, conferring a benefit by increasing the likelihood of neonatal survival. However, exposure of fetal tissues to cortisol levels in excess of those appropriate for the current stage of maturation can have lasting effects on offspring phenotype as a result of developmental programming.
Acknowledgments
We thank Greg Langone and Michelle Zavala for their technical expertise and Sue Jenkins for her statistical and graphics assistance.
This work was supported by the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant HD021350).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 2257
- AP
- anterior pituitary
- AVP
- arginine vasopressin
- CRH-R1
- CRH-receptor 1
- CTR
- control
- dG
- days of gestation
- GR
- glucocorticoid receptor
- HPAA
- hypothalamo-pituitary-adrenal axis
- HSD
- hydroxysteroid dehydrogenase
- IUGR
- intrauterine growth restriction
- ObRb
- leptin receptor
- pGR
- phosphorylated glucocorticoid receptor
- POMC
- pro-opiomelanocortin
- PVN
- paraventricular nucleus.
References
- 1. Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62:1175–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(suppl 1):4–26 [DOI] [PubMed] [Google Scholar]
- 3. Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72:257–265 [DOI] [PubMed] [Google Scholar]
- 4. Viltart O, Vanbesien-Mailliot CC. Impact of prenatal stress on neuroendocrine programming. ScientificWorldJournal. 2007;7:1493–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Symonds ME, Pope M, Sharkey D, Budge H. Adipose tissue and fetal programming. Diabetologia. 2012;55:1597–1606 [DOI] [PubMed] [Google Scholar]
- 6. Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252 [DOI] [PubMed] [Google Scholar]
- 7. Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–1702 [DOI] [PubMed] [Google Scholar]
- 8. Vieau D, Sebaai N, Léonhardt M, et al. HPA axis programming by maternal undernutrition in the male rat offspring. Psychoneuroendocrinology. 2017;32(suppl 1):S16–S20 [DOI] [PubMed] [Google Scholar]
- 9. McDonald TJ, Li C, Vincent SE, Nijland MJ. Fetal fornix transection and gestation length in sheep. Exp Neurol. 2006;200:532–537 [DOI] [PubMed] [Google Scholar]
- 10. Wood CE, Keller-Wood M. Influence of estradiol and fetal stress on luteinizing hormone, follicle-stimulating hormone, and prolactin in late-gestation fetal sheep. Neonatology. 2011;100:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giussani DA, Fletcher AJ, Gardner DS. Sex differences in the ovine fetal cortisol response to stress. Pediatr Res. 2011;69:118–122 [DOI] [PubMed] [Google Scholar]
- 12. Hennessy DP, Coghlan JP, Hardy KJ, Scoggins BA, Wintour EM. The origin of cortisol in the blood of fetal sheep. J Endocrinol. 1982;95:71–79 [DOI] [PubMed] [Google Scholar]
- 13. Schlabritz-Loutsevitch NE, Howell K, Rice K, et al. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol. 2004;33:117–126 [DOI] [PubMed] [Google Scholar]
- 14. Schlabritz-Loutsevitch NE, Hubbard GB, Dammann MJ, et al. Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (Papio species). J Med Primatol. 2004;33:152–162 [DOI] [PubMed] [Google Scholar]
- 15. Bell ME, McDonald TJ, Myers DA. Proopiomelanocortin processing in the anterior pituitary of the ovine fetus after lesion of the hypothalamic paraventricular nucleus. Endocrinology. 2005;146:2665–2673 [DOI] [PubMed] [Google Scholar]
- 16. Schlabritz-Loutsevitch NE, Lopez-Alvarenga JC, Comuzzie AG, et al. The prolonged effect of repeated maternal glucocorticoid exposure on the maternal and fetal leptin/insulin-like growth factor axis in Papio species. Reprod Sci. 2009;16:308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C, Schlabritz-Loutsevitch NE, Hubbard GB, et al. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150:4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550 [DOI] [PubMed] [Google Scholar]
- 19. McDonald TJ, Wu G, Nijland MJ, Jenkins SL, Nathanielsz PW, Jansson T. Effect of 30% nutrient restriction in the first half of gestation on maternal and fetal baboon serum amino acid concentrations. Br J Nutr. 2013;109:1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10 [DOI] [PubMed] [Google Scholar]
- 21. Li C, Levitz M, Hubbard GB, et al. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta. 2007;28:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15:80–101 [DOI] [PubMed] [Google Scholar]
- 23. Martin-Gronert MS, Ozanne SE. Programming of appetite and type 2 diabetes. Early Hum Dev. 2005;81:981–988 [DOI] [PubMed] [Google Scholar]
- 24. McDonald TJ, Nathanielsz PW. Bilateral destruction of the fetal paraventricular nuclei prolongs gestation in sheep. Am J Obstet Gynecol. 1991;165:764–770 [DOI] [PubMed] [Google Scholar]
- 25. Liggins GC. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev. 1994;6:141–150 [DOI] [PubMed] [Google Scholar]
- 26. Dallman MF, Akana SF, Bhatnagar S, et al. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023 [DOI] [PubMed] [Google Scholar]
- 27. Reimsnider S, Wood CE. Differential modulation of ovine fetal ACTH secretion by PGHS-1 and PGHS-2. Neuroendocrinology. 2006;83:4–11 [DOI] [PubMed] [Google Scholar]
- 28. Schubring C, Kiess W, Englaro P, Rascher W, Blum W. Leptin concentrations in amniotic fluid, venous and arterial cord blood and maternal serum: high leptin synthesis in the fetus and inverse correlation with placental weight. Eur J Pediatr. 1996;155:830. [DOI] [PubMed] [Google Scholar]
- 29. Hassink SG, de Lancey E, Sheslow DV, et al. Placental leptin: an important new growth factor in intrauterine and neonatal development?, Pediatrics. 1997;100:E1. [DOI] [PubMed] [Google Scholar]
- 30. Castracane VD, Hendrickx AG, Henson MC. Serum leptin in nonpregnant and pregnant women and in old and new world nonhuman primates. Exp Biol Med (Maywood). 2005;230:251–254 [DOI] [PubMed] [Google Scholar]
- 31. Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83:1243–1246 [DOI] [PubMed] [Google Scholar]
- 32. Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194:1537–1545 [DOI] [PubMed] [Google Scholar]
- 33. Howe DC, Gertler A, Challis JR. The late gestation increase in circulating ACTH and cortisol in the fetal sheep is suppressed by intracerebroventricular infusion of recombinant ovine leptin. J Endocrinol. 2002;174:259–266 [DOI] [PubMed] [Google Scholar]
- 34. Huang Q, Rivest R, Richard D. Effects of leptin on corticotropin-releasing factor (CRF) synthesis and CRF neuron activation in the paraventricular hypothalamic nucleus of obese (ob/ob) mice. Endocrinology. 1998;139:1524–1532 [DOI] [PubMed] [Google Scholar]
- 35. Yamamoto S, Morimoto I, Kai K, et al. Centrally administered murine leptin stimulates plasma arginine-vasopressin secretion and increases the level of mRNA expression in the supraoptic nucleus of conscious rats. Neuroendocrinology. 1999;70:207–212 [DOI] [PubMed] [Google Scholar]
- 36. Morimoto I, Yamamoto S, Kai K, Fujihira T, Morita E, Eto S. Centrally administered murine-leptin stimulates the hypothalamus-pituitary- adrenal axis through arginine-vasopressin. Neuroendocrinology. 2000;71:366–374 [DOI] [PubMed] [Google Scholar]
- 37. Patterson CM, Leshan RL, Jones JC, Myers MG. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res. 2011;1378:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burguera B, Couce ME, Long J, et al. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71:187–195 [DOI] [PubMed] [Google Scholar]
- 39. Mitchell SE, Nogueiras R, Morris A, et al. Leptin receptor gene expression and number in the brain are regulated by leptin level and nutritional status. J Physiol. 2009;587:3573–3585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology. 1997;65:223–228 [DOI] [PubMed] [Google Scholar]
- 41. Shimon I, Yan X, Magoffin DA, Friedman TC, Melmed S. Intact leptin receptor is selectively expressed in human fetal pituitary and pituitary adenomas and signals human fetal pituitary growth hormone secretion. J Clin Endocrinol Metab. 1998;83:4059–4064 [DOI] [PubMed] [Google Scholar]
- 42. Sone M, Nagata H, Takekoshi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res. 2001;305:351–356 [DOI] [PubMed] [Google Scholar]