Abstract
Lung cancer has traditionally been considered relatively resistant to immunotherapies. However, recent advances in the understanding of tumor-associated antigens, anti-tumor immune responses, and tumor immunosuppression mechanisms have resulted in a number of promising immunomodulatory therapies such as vaccines and checkpoint inhibitors. Locally advanced non-small cell lung cancer (NSCLC) is an optimal setting for these treatments because standard therapies such as surgery, radiation, and chemotherapy may enhance anti-tumor immune effects by debulking the tumor, increasing tumor antigen presentation, and promoting T-cell response and trafficking. Clinical trials incorporating immunomodulatory agents into combined modality therapy of locally advanced NSCLC have shown promising results. Future challenges include identifying biomarkers to predict those patients most likely to benefit from this approach, radiographic assessment of treatment effects, the timing and dosing of combined modality treatment including immunotherapies, and avoidance of potentially overlapping toxicities.
Keywords: Lung cancer, locally advanced, stage 3, radiation therapy, chemotherapy, immunotherapy, vaccines, abscopal effect, cytotoxic T-lymphocyte associated antigen 4 (CTLA4), programmed death 1 (PD1)
There is a clear rationale for immunotherapy in the treatment of cancer. It has long been observed that immunosuppressed individuals, such as solid organ transplant recipients or human immunodeficiency virus (HIV)-positive patients are more likely to develop malignancies.1,2 In contrast, small cell lung cancer patients with antibody-mediated paraneoplastic syndromes (which involve onconeural antibodies that cross react between tumor and normal tissues) have better clinical outcomes than similar patients without these conditions.3 Additionally, for some solid tumors, intratumoral immune cell infiltration has been associated with improved outcomes.4 Efforts to harness anti-tumor immune responses for cancer treatment date back decades. Immunotherapy in the form of allogeneic stem cell transplantation and donor T lymphocyte infusion are routinely employed in the treatment of certain hematologic malignancies. Intravesical bacillus Camille Guerin (BCG) administration, which generates a local inflammatory response, has become a mainstay of therapy for early stage bladder cancer. Immune-stimulating cytokines such as interferons and interleukins are established treatments for advanced melanoma and renal cell carcinoma. In 2012 the first dendritic cell vaccine (Sipuleucel-T for metastatic castrate-resistant prostate cancer) was approved for cancer therapy. Recent insights into immune cell-intrinsic checkpoints have led to the development of monoclonal antibodies targeting the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and the programmed death 1 (PD-1) receptor.
Historically, lung cancer has been considered resistant to immunotherapeutic strategies. It is less antigenically active than many other malignancies. There is also evidence of suppression of host immune responses; immunosuppressive T regulatory cells and cytokines have been identified in tumor tissue, pleural effusions, and peripheral blood.5,6 Because most patients present with advanced disease and existing treatments have limited efficacy in that context, it is difficult to debulk the tumor sufficiently to optimize the effects of immunotherapies. In advanced stages, bulky tumors may generate a systemic tolerogenic or immunodepressed that renders ineffective anti-tumor immune responses. Furthermore, it may be difficult for immune effector cells to gain access to large, highly necrotic areas of bulky tumors.
In contrast to treatment paradigms for metastatic disease, the multimodality approach to locally advanced lung cancer offers several advantageous features for immunotherapy. Tumor debulking may be achieved frequently through surgery and/or radiation therapy. Surgery, radiation therapy, and chemotherapy may enhance anti-tumor immunity by increasing tumor antigen presentation, inducing immunogenic cell death, and promoting T-cell response and trafficking.7–11 Clinical trials of vaccines and immunotherapies in lung cancer support these observations, as patients with locally advanced disease appear to derive greatest benefit in some cases. This review will cover the rationale for and outcomes from immunotherapy in locally advanced non-small cell lung cancer (NSCLC), with sections on the components of the general immune response; mechanisms and regulation of anti-tumor immunity ; interactions between anti-tumor immunity and cancer therapies (chemotherapy, radiation); and clinical experience with vaccines and other immunotherapies in locally advanced and other stages of NSCLC. A glossary of relevant terms is included in Table 1.
Table 1.
Glossary of terms
| Abscopal effect | Phenomenon in which distant, non-irradiated sites of disease demonstrate regression after focal radiation treatment. Thought to reflect enhancement of anti-tumor immune responses by radiation therapy. |
| Acquired (adaptive) immune system | Component of immune system that includes antigen presentation, generation of responses, and development of immunologic memory. In contrast to the innate immune system, it is host-specific. |
| Adjuvant | Agent designed to stimulate immune response to a vaccine, without having any specificintrinstic antigenic effect. Examples include inorganic substances, viral or bacterial components, or other cell types. |
| Antigen | Foreign substance that evokes an immune response either alone or after forming complexes with other molecules via interaction with antibodies or T cells. Well known tumor antigens include MUC1 and MAGE. |
| Antigen presenting cell (APC) | Cell that displays foreign antigen on cell surface complexed with major histocompatibility (MHC) proteins to elicit T cell recognition. Most commonly refers to macrophages, dendritic cells, and certain epithelial cells, but can include any cell recognizable for displaying antigen |
| B cell (B lymphocyte) | Lymphocyte that has antigen-binding antibody molecules on the surface. Principal functions include antibody production, antigen- presentation, and immunologic memory. |
| Cell-mediated immunity | Component of immune response that involves activation of phagocytes and antigen-specific cytotoxic T-lymphocytes in response to antigen. |
| Cluster of differentiation 28 (CD28) | T cell surface receptor that provides co-stimulatory signal required for T cell activation. Binds to CD80 (B7.1) and CD86 (B7.2) on antigen- presenting cells. |
| Complement system | Component of innate immune system that consists of circulating proteins that, when activated, initiate biochemical cascade resulting in the cell-killing membrane attack complex. |
| Cytokine | Small cell-signaling protein molecules involved in intercellular communication. Examples of immune-related cytokines include various interleukins (ILs), interferons (IFNs), tumor necrosis factor (TNF), and transforming growth factor (TGF). |
| Cytotoxic T-lymphocyte antigen 4 (CTLA-4) | T cell receptor that downregualtes T cell activation by inducing inhibitory signal transduction after activation. Functions to limit autoimmunity and allow self-tolerance. Targeted by anti-CTLA4 antibodies such as ipilimumab and tremelimumab. |
| Dendritic cell | Immune cell with key role in antigen presentation andlinking innate and adaptive immunity. Found predominantly in tissues that contact external environmentssuch as skin and mucosa. Targeted by talactoferrin and vaccines for activation as a potential means of promoting anti-tumor response |
| Effector T cell | Subset of antigen-specific memory T cells that persists long-term after resolution of initial infection/inflammatory process. Able to expand rapidly to large numbers of T cells upon re-exposure to the cognate antigen, thus providing the immune system with “memory” against past events. Also promotescytolytic events in antigen presenting cells. Targeted by PD1 and PD-L1 modulators |
| Granulocyte macrophage – colony stimulating factor (GM-CSF) | Protein secreted by mast cells, T cells, macrophages, and other immune cells that functions as a cytokine in signaling various inflammatory/immune events. Employed as a myeloid growth factor and as a vaccine adjuvant to increase anti-tumor immune responses |
| Helper T cell | T cell that participates in immune response by recognizing foreign antigen and secreting cytokines to activate T cell and B cell proliferation. Generally CD4+. |
| Histiocytes | A specialized macrophage or dendritic cell involved in phagocytosis and antigen presentation. |
| Human Leukocyte antigen (HLA) | Name of the major histocompatibility complex (MHC) in humans. Protein expressed on antigen presenting cells involved in accurate identification of foreign molecules by T cells; also relevant to organ transplant rejection and autoimmune processes. |
| Humoral immunity | B cell-driven immune component mediated by antibodies, complement, and antimicrobial peptides. Involved in generation of immunologic memory. |
| Immunologic memory | Aspect of adaptive immune response that remembers specific pathogens encountered, allowing strong response if pathogen detected again. |
| Innate immunity | Non-specific first line of defense of the immune system, involving mast cells, phagocytes, macrophages, neutrophils. Drives early inflammatory, cytokine, and complement response to pathogens. Activated by pattern recognition receptor-ligand, toll-like receptor TLR/TLR ligand interactions, and other surface molecules associated with antigen. |
| Kupffer cell | Resident macrophage of the liver that destroys antigens, bacteria, and older immune/hematopoietic cells; part of the innate immune response that respond to antigen components including toll-like receptor (TLR) ligands by releasing molecules spurring inflammation and activation of other immune cells. |
| Major histocompatibility complex (MHC) | Cell surface molecule on antigen presenting cells (APCs)that is required for recognition of antigen by T cell costimulatory molecules. |
| Macrophage | Monocyte-derived phagocytic tissue cell that may be fixed or freely motile. Functions in the destruction of foreign antigens (i.e. tumor, microbes) and serves as an antigen-presenting cell. Plays roles in both innate and acquired immunity. |
| Melanoma antigen (MAGE) | Tumor antigen expressed by melanoma, lung cancer, bladder cancer, liver cancer, and normal testis. Targeted by vaccine approaches. |
| Mucin 1 (mucinous glycoprotein 1) (MUC1) | Cell surface antigen overexpressed in colon, breast, ovarian, lung, and pancreatic cancers. Targeted by vaccine approaches. |
| Nucleotide-binding oligomerizationdomain (NOD) | Pattern recognition receptors critical to innate immune system in regulatingphagocytic and inflammatory activity. Involved in innate immune cellrecognition of foreign or pathogenic antigens. |
| Opsonization | Process by which a pathogen is marked for ingestion and destruction by a phagocyte. Involves the binding of an opsonin (e.g., antibody) to a receptor on pathogen cell membrane. Subsequently phagocytes (including macrophages)are attracted to the pathogen and ultimately engulf and destroy it. |
| Phagocytosis | Physical and mechanical process by which pathogens and antigen presenting cells are eliminated by immune cells including macrophages as part of the final steps of the immune response to antigen. |
| Programmed cell death 1 (PD1) | Cell surface protein expressed on T cells, B cells, and macrophages which negatively regulates T cell receptor signaling, thereby suppressing immune function; PD1 binds to its ligand, PD-L1, on tumor or stromal cells, leading to activation and a cascade of signaling that ultimately downregulates immune cell function. Critical for ensuring that self-tolerance limits autoimmune action. Antibodies targeting PD1 and PD-L1 reverse T cell suppression, promoting anti-tumor activity in clinical trials. |
| Programmed cell death ligand 1 (PD-L1) | Transmembrane protein involved in suppression of immune system via binding of PD1. Found on tumor cells and stromal cells. Blocking PD- L1 function may promote T cell anti-tumor response. |
| T cell (T lymphocyte) | Any of several lymphocytes that differentiate in the thymus and possess highly specific cell-surface antigen receptors. May be involved in initiation or suppression of cell-mediated and humoral immunity (as by the regulation of T and B cell maturation and proliferation) or destruction of antigen-bearing cells. |
| Toll like receptor (TLR) | Single membrane spanning receptors on immune cells including macrophages and dendritic cells that play key role in regulating the innate immune system. TLRs recognize conserved antigens from pathogens and/or tumors that stimulate innate immune responses. TLR agonists have been explored as an anti-tumor immune approach in clinical trials for lung cancer. |
| Tolerance | Complex process by which the immune system avoids attackingunique antigens, particularly to self. Akey consideration when attempting to promote anti-tumor immune responses. |
| Transforming growth factor beta (TGFβ) | Cytokine/growth factor involved in inflammation and immunity; thought to suppress anti-tumor immunity along with IL-10. Tumors induce the production of T cells that express IL-10 and TGFβ, thereby limiting production of adequate effector T cell anti-tumor responses. |
| T regulatory cell (Treg) | Suppressor T cell that maintains self-tolerance, modulates immune system, and abrogates autoimmune disease. Thought to limit T-cell immune action against tumor-associated antigens. |
| Vaccine | Biological preparation that improves immune responses to a particular disease. Tumor vaccines typically containa formulation of a malignancy-specific antigen/antigens or whole cell componentsthat when administered, elicits a stronger immune cell-mediated response. |
Components of the general immune response
Apart from the nervous system, perhaps no other set of physiologic functions is as evolved or complex as the mammalian immune system. The ability to ward off infection in regulating invasion by microbial pathogens, mount responses to foreign substances, and suppress tumor development is a result of a finely tuned and regulated network of immune derived cells and molecules. The immune system must work in a rigid balance or lead to significant sequelae, including autoimmune diseases such as lupus and scleroderma.
It is the critical task of the immune system to detect even the smallest quantities of disease-causing agents that enter the body before clinically significant harm occurs. However, in many instances, human pathogens and neoplasms have developed to elude and neutralize aspects of this immune response. In response, the human immune system has evolved to include multiple cell types and cytokines that work in concert—innate and acquired immunity.
Innate Immune System
The innate immune system provides an immediate maximal response to pathogens but—in contrast to acquired immunity—does not result in lasting or repeatable protection. It includes components of both cell-mediated and humoral immunity.12 The innate immune response is initiated in response to the release of inflammatory proteins by affected cells or by activation of specific pattern recognition receptors. The latter include (1) toll-like receptors (TLRs), membrane-bound receptor kinases that cause cells to secrete cytokines that induce a host defense response; (2) carbohydrate specific surface receptors including lectins and mannose receptors; (3) cytoplasmic nucleotide-binding oligomerization receptors (NODs); and (4) nucleic acid related signalers. Activation of these receptors results in an inflammatory cascade that may include prostaglandins, histamines, bradykinins, and cytokines. Depending on the involved, the process is then amplified by macrophages, dendritic cells, histiocytes, Kupffer cells, mastocytes, and neutrophils.12–15
The complement cascade serves to link this innate immune response with acquired immunity. This system includes serum proteins produced by hepatocytes that recruit inflammatory cells, coat pathogens for opsonization, perforate the plasma membranes of the pathogens resulting in cell lysis, and remove the resulting neutralized immune complex.16
Acquired Immune System
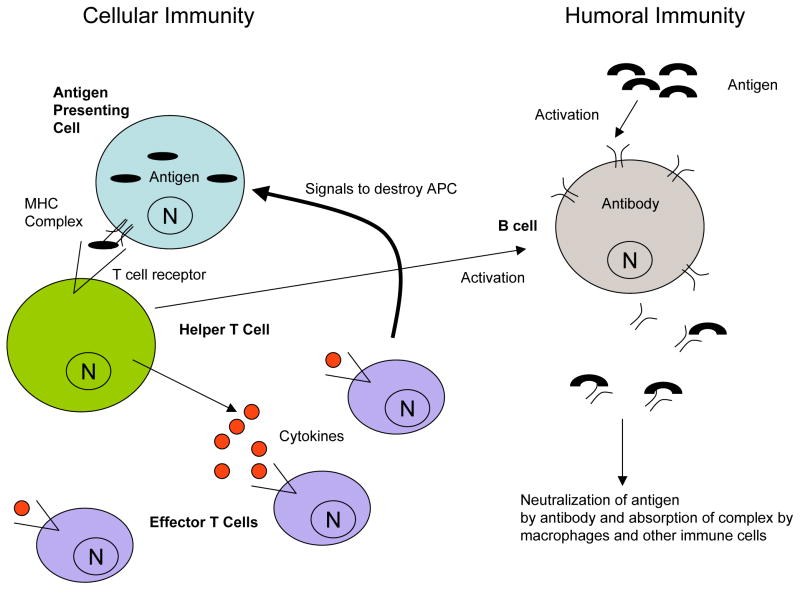
The acquired or adaptive immune response is more highly evolved than its innate counterpart, partly due to the genetics necessary to preserve knowledge of early pathogenic attack for future pathogenic protection. This type of immunologic memory demonstrates a specificity of response to unique antigens that usually lasts for the lifetime of the host. As with the innate immune system, acquired immunity includes both cellular (mediated by B lymphocytes) and humoral (mediated by T lymphocytes) components. When an antibody on a B cell recognizes an antigen, this receptor-antigen complex is endocytosed by the B cell, the antigen is cleaved into peptides, and these peptides are presented by major histocompatibility complex (MHC) class II molecules on the surface of the B cell. In turn, these antigen – MHC complexes are recognized by helper T cells, triggering B cell activation and the production of more B cells producing antigen-targeting antibody (Figure 1).12,17
Figure 1. Components of cellular and humoral immunity.
Cellular immunity is primarily driven by a T cell response to foreign pathogens. Subunits of pathogens or tumor are processed by specific antigen presenting cells (APCs) and represented on these cell surfaces with unique Major Histocompatibility Complex (MHC) proteins. These complexes are then recognized by T cell receptor complexes on helper T cells, leading to release of signaling cytokines. Effector T cells are activated by the cytokine-based secretory molecules, resulting in amplification of events that promotes destruction of antigen-bearing cells. In humoral immunity, antigens are recognized by B cell receptor complexes that ultimately results in the generation of “memory”-driven antibody responses to any future invasion by the same or similar pathogens. Helper T cells can be integral to B cell activation, demonstrating the crosstalk between cellular and humoral immune responses. N= cell nucleus
In general, this antibody response results in lifelong memory—that is, the ability to mount a similar response if exposed again to the same antigen. This concept provides the basis for developing vaccine strategies for combating disease. Vaccination indicates the administration of an antigen for the express purpose of generating a humoral response that can be kept in the immune system’s memory. Strategies for vaccine use to spur immune responses to tumor will be discussed in subsequent sections.
T cells recognize pathogens presented as antigens by a variety of immune antigen presenting cells (APCs). The process also requires complexing with another class of immune cell surface markers, the major histocompatibility complex (MHC). Killer T cells recognize MHC class I – antigen complexes, resulting in lysis of the targeted cell. Helper T cells identify MHC class II – antigen complexes, resulting in cytokine release that leads to recruitment of other immune cells. Other T cells, such as gamma-delta (γδ)T cells, have more specific and unique functions.18–20
A key characteristic of physiologic immunity is the ability to recognize self from non-self, thereby limiting autoimmune phenomena. This feature also occurs in pathologic processes that escape immune surveillance, such as developing and progressing malignancies. One mechanism of achieving this escape is altering or suppressing HLA expression.21 Another is activation of inhibitory pathways that suppress T cell function, such as those mediated by cytotoxic T-Lymphocyte Antigen 4 (CTLA4) and programmed death 1 (PD1) (Figure 2).22,23 Clinical trials are investigating the targeting of these pathways to spur anti-tumor immune responses.23–25
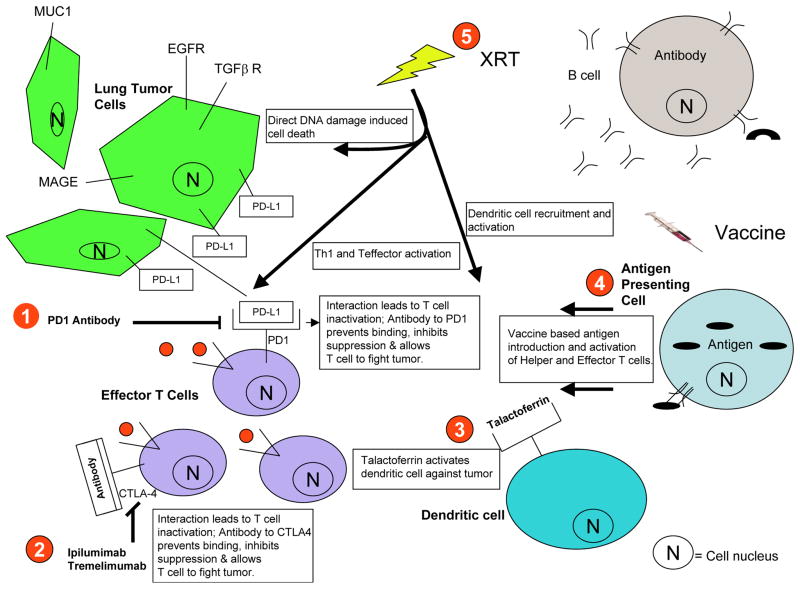
Figure 2. Role of immunotherapies and other cancer treatments in modulating anti-tumor immune response.
(1) Interaction between Programmed Death 1 (PD1) on T cells Programmed Death-Ligand 1 (PD-L1) expressed by tumor cells and/or stromal cells. Ligand-receptor leads to suppression of T cell activation and function and represents a key means of limiting autoimmunity. Anti-PD1 and PD-L1 antibodies block this interaction and enhance T cell action anti-tumor effects. (2) Another immune checkpoint, Cytotoxic T Lymphocyte Antigen 4 (CTLA-4) is a T cell surface receptor that binds to specific proteins on antigen presenting cells to suppress T cell function. The anti-CTLA4 antibodiesipilimumab and tremelimumab inhibit this binding, thereby stimulating T cell activity. (3) Recruitment and activation of dendritic cells. Talactoferrin is a recombinant protein that spurs recruitment of immature dendritic cells as a means of limiting tumor growth. (4) Vaccines enhance anti-tumor cellular immunity via introduction of tumor antigens such as mucin 1 (MUC1) and melanoma antigen A1 (MAGE-A1). (5)Ionizing radiation may contribute to anti-tumor immune responses via several mechanisms: promotion of immunogenic cell death; enhancement of antigen presentation; indirect activation of helper and effector T cells; recruitment and activation of dendritic cells.
Interplay between anti-tumor immunity and cancer therapies
The standard approach to locally advanced NSCLC entails multimodality therapy—combinations of surgery, radiation therapy, and chemotherapy. Through various mechanisms, these treatment modalities may augment anti-tumor immunity (see Table 2). Conversely, anti-tumor immunity may enhance the effects of these treatment modalities. Thus, there is strong rationale to incorporate vaccines and other immunotherapies into these regimens.
Table 2.
Effects of radiation therapy and chemotherapy on anti-tumor immunity
| Component of immune response | Potential effects of RT | Potential effects of chemotherapy |
|---|---|---|
| Antigen presentation | Translocation of calreticulin to cell surface activates dendritic and other antigen presenting cells7,106 Enhanced MHC class I expression30 Release of danger-associated molecular patterns (DAMPs) recruits and stimulates dendritic cells. 29 |
Increased antigen cross-presentation and dendritic cell activation107 Induction of immunogenic cell death44 |
| T-cell response, trafficking | Results in systemic inflammatory repsonse39 Release of alarmin and binding to TLR4 activates antitumor immunity108 CXCL16 release attracts effector T cells109 Tumor vascular endothelial changes promote immune cell extravasation8,110 |
Release of alarmin and binding to TLR4 activates antitumor immunity108 Increased intratumoral T cell accumulation111,112 |
| Target destruction | Tumor debulking | Tumor debulking |
| Generation of memory | Unknown | Increasing memory against tumor antigen47 |
MHC, major histocompatibility complex; TLR, toll-like receptor
Surgery
There has been historical data to suggest that debulking or removal of tumor spurs a heightened inflammatory and immune state, resulting in effects against residual microscopic disease. Such concepts have been described predominantly preclinically, with only limited clinical data—generally small series—demonstrating these findings.11,26
Radiation therapy
For more than 50 years, an association between the anti-tumor efficacy of ionizing radiation and the local and systemic immune milieu has been recognized.27,28 In preclinical studies, irradiating tumor in immunodeficient animals does not yield the same degree of cell kill as in immunocompetent systems, suggesting a role for immune cells and molecules in enhancing radiation response.27,28 Ionizing radiation, in turn, spurs anti-tumor immunity. Specifically, ionizing radiation initiates an immunogenic cell death cascade. Treated tumor cells release danger-associated molecular patterns (DAMPs)—heat shock proteins and high mobility group proteins—which recruit and stimulate dendritic cell function within the tumor microenvironment.29 Ionizing radiation also results in inflammatory responses and increased expression of cellular adhesion molecules and selectins, which leads to increased vascular permeability and delivery of immune cells. Radiation therapy has also been shown to enhance MHC class I expression on several tumor lines, leading to T cell anti-tumor activity.30
Based on these observations, vaccines and other immune modulators have been combined with radiation in numerous preclinical tumor models.31 In vaccine studies, the addition of radiation augments the generation of antigen-specific cytotoxic T lymphocytes.32 Because radiation also activates Toll-like receptors (TLRs), it has been combined with TLR agonists.33,34 When both radiation and the TLR agonist are delivered locally to a focal tumor site, a significant response has been observed at distant sites as well, presumably due to TLR effects on innate immune activity.33,34 Various immune-related cytokines (including tumor necrosis factor alpha [TNFα], interleukin-1 [IL-1], and IL6) have also been combined with radiation in mouse models.35 As noted with other immune modulators, anti-tumor responses have been augmented at all sites of disease, irradiated or not. Efforts to combine these systemic immunoregulators with radiation therapy for lung cancer in the clinical setting have been limited, with concern regarding the potential for excessive normal tissue toxicity to lung parenchyma.
It has also been shown that early dendritic cell migration to tumors may enhance radiation effects locally and distantly.27,36 Early phase clinical trials have combined radiation with intratumoral dendritic cell injection. In a sarcoma study, this approach resulted in 70% 1 year disease free survival, and 52% of patients mounted tumor-specific immune responses.37 In a trial of single fraction high-dose radiation plus intratumoral dendritic cell injection, there was limited toxicity and nearly 50% of patients achieved a clinically significant response.38
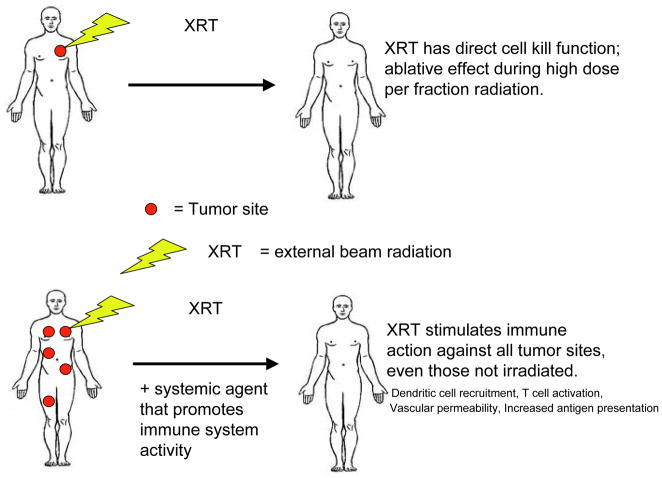
The observation that distant, non-irradiated tumor sites may respond after focal radiotherapy is known as the abscopal effect (Figure 3). Systemic inflammatory and immune responses to radiation appear to underlie this phenomenon.29,39 A recent clinical phase I clinical trial evaluated patients with widespread melanoma and renal cell carcinoma treated with high dose radiation to limited sites of gross disease followed by systemic IL-2 therapy. In most cases, significant responses were noted at non-radiated as well as radiated sites. More than half of patients had a complete metabolic response at all sites of disease. Among those patients with demonstrating the greatest benefit, there were higher levels of circulating MHC class II T cells.40 Similarly, a recently published case report described a patient with progressive metastatic melanoma (featuring hilar, liver, and paraspinous disease) while receiving the anti-CLTA4 antibody ipilimumab.41 For symptom control, the patient underwent short-course palliative radiation to the paraspinous mass, which was radiographically unchanged more than one month later. However, after re-initiation of ipilimumab, all sites of disease regressed considerably. Although the patient had received ipilimumab both before and after radiation, the timing of events suggests that the apparent abscopal effect was driven by post-radiation doses.
Figure 3. The abscopal effect.
Generally, the effect of radiation on tumor cell death is most apparent at the irradiated sites. In the abscopal effect, distant, non-irradiated sites of disease also demonstrate reduction in tumor volume. Proposed mechanisms include radiation-associated dendritic cell recruitment, T cell activation, increased vascular permeability, and increased antigen presentation. Abscopal effects appear to be more common when radiation is combined with immunotherapies.
Chemotherapy
Aside from cytotoxic properties, certain chemotherapeutic agents also alter anti-tumor immune responses. The degree to which this phenomenon occurs may depend on the type of cell death effected by a given chemotherapy drug. Apoptosis, an intrinsic mechanism of cell death, has traditionally been considered non-immunogenic or possibly immune suppressive. Apoptotic cells express phosphatidylserine (PS) on the outer membrane leaflet as the plasma membrane loses its integrity. PS functions as an immune downregulator; it suppresses release of the pro-inflammatory cytokine IL-12 and stimulates production of the anti-inflammatory cytokines transforming growth factor beta (TGFβ), IL-10, and prostaglandins. Anticancer drugs trigger apoptosis by death receptor (eg, FAS, TNF)-dependent and –independent pathways.42,43 By contrast, non-apoptotic mechanisms, which include necrosis, autophagy, and mitotic catastrophe, are considered immunogenic. The alkylating agent temozolomide appears to induce G2/M arrest and autophagy but not apoptosis.44 More recently, apoptosis has been acknowledged as potentially immunogenic, as the FAS and TNF pathways may promote CD8+ T cell direct lysis of tumor cell targets. In the setting of massive apoptosis, secondary necrosis may cause release of pro-inflammatory mediators including heat shock proteins, which in turn may stimulate dendritic cells.45–49
Through the initiation of cytotoxic tumor cell death, systemic chemotherapy may result in a plethora of immune effects: (1) deliver a larger range of diverse tumor antigens; (2) increase antigen cross presentation, stimulate dendritic cells, and prime antigen presenting cells; (3) suppress immune tolerance for tumor; (4) increase T cell access to tumor; (5) increase local tumor-antigen T cell activation; (6) promote memory-based humoral effects against tumor; (7) suppress excessive immune negative regulatory events.45–49
Development of and clinical experience with vaccines and immunotherapies in lung cancer
The characteristics of selected vaccines and other immunotherapies are listed in Tables 3 and 4. Selected clinical trials employing these agents for lung cancer are listed in Table 5. Many but not all of these agents have been studied in locally advanced disease. Certain agents found not to be effective in other disease contexts, such as metastatic disease, have been included. While drug development traditionally brings forward to earlier stages only those agents with efficacy in the metastatic setting, this paradigm may not hold true for vaccines and other immunotherapies, given the favorable conditions of locally advanced lung cancer for these treatment strategies. In general, this section focuses on phase 2 and 3 clinical trials, rather than case series.
Table 3.
Overview of selected vaccines studied for NSCLC therapy
| Vaccine | Target | Component | Adjuvant |
|---|---|---|---|
| L-BLP25 (Stimuvax) | MUC1 | MUC1 peptide | Monophosphoryl lipid A |
| TG4010 | MUC1 | MUC1 peptide | Recombinant viral vector expressing IL-2 |
| MAGE-A3 | MAGE-A3 | MAGE-A3 full protein | AS15 or AS02B adjuvant system |
| Belagenpumatucel-1 (Lucanix) | NSCLC antigens | Allogeneic tumor cells from 4 irradiated NSCLC cell lines (H460, H520, SKLU-1, RH2) | TGF-β2 antisense gene modification |
| rHuEGF (CimaVax) | EGF | EGF full protein | Oil emulsion ISA51 |
Table 4.
Overview of selected immunotherapies studied for NSCLC therapy
| Target | Agent (s) | Characteristics and Mechanism | Toxicities |
|---|---|---|---|
| CTLA4 | Ipilimumab (Yervoy) Tremelimumab |
Fully human IgGMAb blocks inhibitory interaction of CTLA-4 and B7 in priming phase resulting in T cell activation | Autoimmunedermatitis, colitis, hepatitis, thyroiditis, hypophysitis |
| PD-1 | Nivolumab (BMS-936558; MDX-1106) MK-3475 |
Fully human IgG4 MAb blocks inhibitory interaction of PD-1 with PD-L1 and PD-L2 in effector phase, resulting in preferential activation of cancer-targeting T cells | Pneumonitis, dermatitis, colitis, hepatitis, thyroiditis, hypophysitis |
| PD-L1 | BMS-936559 (MDX- 1105) | Fully human IgG4 MAb blocks inhibitory interaction of PD-L1 with PD-1 and B7 in effector phase, resulting in preferential activation of cancer-targeting T cells | Rash, diarrhea, infusion reactions, hypothyroidism, adrenal insufficiency |
| TLR9 | PF-3512676 (formerly CpG 7909) | Synthetic TLR9-activating oligodeoxynucleotide; upon binding to TLR9, results in induction of proinflammatory cytokines | Hematologic; flu-like symptoms |
| Gut-associated lymphoid tissue dendritic cells | Talctoferrin | Recombinant human lactoferrin is transported to gut-associated lymphoid tissue, resulting in recruitment and maturation of circulating immature dendritic cells bearing tumor antigens | No clearly associated toxicities |
CTLA, cytotoxic T-lymphocyte-associated antigen; MAb, monoclonal antibody; PD, programmed death; PD-L, programmed death ligand; TLR, toll-like receptor
Table 5.
Selected clinical trials of vaccines and immunotherapies for lung cancer
| Trial (Phase) | Target | Number, stage of patients | Treatment | Results | Predictive biomarkers |
|---|---|---|---|---|---|
| Post-resection of early stage or locally advanced NSCLC | |||||
| Vansteenkiste et al70 (II) | MAGE A3 | 182; resected stage IB/II MAGE A3- positive NSCLC | MAGE A3 vaccine 300 μg IM every 3 weeks × 5 doses (induction) then every 3 months × 8 doses (maintenance) vs placebo [no other adjuvant therapy allowed] | At median 44 mos F/U: recurrence rate 30% vs 42%; disease free interval HR 0.75 (95% CI, 0.46–1.23); OS HR 0.81 (95% CI, 0.47–1.4) | Gene signature (immune-related gene associated with pre- treatment tumor microenvironment): 43% reduction in recurrence if had signature vs 25% in overall population |
| MAGRIT (III) | MAGE A3 | 2315; resected stage IB-IIIA MAGE A3- positive NSCLC | MAGE A3 vaccine 300 μg IM every 3 weeks × 5 doses (induction) then every 3 months × 8 doses (maintenance) vs placebo [adjuvant chemotherapy allowed] | Enrollment completed 8/2012; results awaited | Awaited |
| Post-chemoradiotherapy for unresectable stage III NSCLC | |||||
| Butts et al76 (IIB) | MUC1 | 171; IIIB/IV with disease control after chemoradiation or chemotherapy | Cyclophosphamide 300–600 mg/m2 × 1 dose 3 days before L-BLP25 1000 μg SC weekly × 8 doses then every 6 weeks or placebo | Median OS 17.2 mos vs 13.0 mos (HR 0.75; 95% CI, 0.53–1.04; P=0.11). In stage III patients: OS 30.6 mosvs 13.3 mos (HR 0.55; 95% CI, 0.30–1.00) | |
| START (III) | MUC1 | 1476; stage III NSCLC with responding or stable disease after chemoradiation | Cyclophosphamide 300–600 mg/m2 × 1 dose 3 days before L-BLP25 1000 μg SC weekly × 8 doses then every 6 weeks or placebo | OS not significantly improved; further data awaited | Awaited |
| Advanced NSCLC | |||||
| Nemunaitis et al82 (II) | NSCLC antigens | 75; stage II (2), III (27), IV (46) NSCLC with total estimated tumor burden volume ≤ 125 mL | Belagenpumatucel-L (1.25, 2.5, or 5.0 × 107 transfected allogeneic tumor cells) monthly or every other month × up to 16 months | RR 15% in stage IIIB/IV Higher dose groups: 68% 1-yr survival; 52% 2-yr survival Low dose group: 39% 1-yr survival; 20% 2-yr survival |
Week 12 cytokine (IFN- γ, IL-6, IL-4) production and antibody mediated response to vaccine HLAs increased among clinical responders compared to patients with progressive disease |
| Nemunaitis et al83 (II) | NSCLC antigens | 21; IV | Belagenpumatucel-L (2.5 × 107 transfected allogeneic tumor cells) monthly × up to 16 months | Median OS 562 days | Baseline CTCs: <2: median OS 660 days ≥2: median OS 150 days (P=0.025) |
| Quoix et al56 (IIb) | MUC1 | 148; IIIB/IV MUC1- positive NSCLC (defined by expression in 25% of tumor cells) | Cisplatin-gemcitabine × up to 6 cycles ± TG4010 (108 plaque forming units SC) weekly × 6 wks then every 3 wks until progression | 6-month PFS 43% vs 35% (P=0.31) | Baseline activated NK cells (CD16+/CD56+/CD69+): High* (27% of pts): 6- month PFS 19% vs 31%; P=0.42. Normal (73% of pts): 6-month PFS 56% vs 38%; P=0.07. |
| NeningerVinageras et al89 (II) | EGF | 80; IIIB/IV after completion of first- line chemotherapy | Cyclophosphamide 200 mg/m2 × 1 dose 3 days before rHuEGF (CimaAvax) on days 1, 7, 14, 28, then monthly or best supportive care | Overall: Median OS 12.7 mos vs 8.5 mos (P=NS) Age ≤60y: median OS 11.5 mos vs 5.3 mos (P=0.01) |
Antibody response**: Good (51% of vaccinated patients): median OS 11.7 mos Bad (49% of vaccinated patients): median OS 3.6 mos. Minimal EGF concentration: <168 pg/mL: median OS 13 mos ≥168 pg/mL: median OS 5.6 mos |
| Lynch et al92 (II) | CTLA-4 | 204 previously untreated advanced NSCLC | Carboplatin-paclitaxel × up to 6 cycles + ipilimumab 10 mg/kg every 3 weeks × 4 cycles starting Cycle 1 (concurrent) or ipilimumab 10 mg/kg × 4 cycles every 3 weeks starting Cycle 3 (Phased) or placebo (Control) | Median PFS 4.2 mos (control) vs 5.1 mos (phased) vs 4.1 mos (concurrent) (HR 0.88; P=0.25 for phased vs control). Median irPFS 4.6 mos (control) vs 5.7 mos (phased) vs 5.5 mos (concurrent) (HR 0.72; P=0.05 for phased vs control). Median OS 8.3 mos (control) vs 12.2 mos (phased) vs 9.7 mos (control) (HR 0.87; P=0.23 for phased vs control). Greatest benefit in squamous tumors (irPFS HR 0.55 for phased vs control). |
None |
| Zatloukal et al93 (II) | CTLA-4 | 87; stage IIIB or IV NSCLC non- progressing after ≥4 cycles platinum- based therapy | Tremelimumab 15 mg/kg every 90 days or best supportive care until disease progression | PFS at 3 months 21% vs 14% (P=NS) | None |
| Topalian et al94 (I) | PD-1 | 296; 122 with previously treated advanced NSCLC | BMS-936558 in escalating doses up to 10 mg/kg every 2 weeks × up to 12 8-week cycles | RR 18% in NSCLC; 7% with SD × ≥ 24 wks | PD-L1 expression: RR 36% in PD-L1 positive tumors (60% of evaluable cases) vs 0% in PD-L1 negative tumors |
| Brahmer et al95 (I) | PD-L1 | 207; 75 with previously treated advanced NSCLC | BMS-936559 in escalating doses up to 10 mg/kg on Days 1, 15, and 29 every 6 weeks × up to 16 cycles | RR 10% (16% at 10 mg/kg dose) in NSCLC | None |
| Manegold et al99 (II) | TLR9 | 111; previously untreated stage IIIB/IV | Taxane/platinum chemotherapy ± PF- 3512676 0.2 mg/kg SC on Days 8, 15 of each 21- day cycle × up to 6 cycles | RR 38% vs 19% (P=0.04); median OS 12.3 mos vs 6.8 mos (HR 0.75; 95% CI, 0.48–1.15; P=0.19) | None |
| Hirsh et al101 (III) | TLR9 | 820; previously untreated stage IIIB/IV | Carboplatin-paclitaxel ± PF-3512676 0.2 mg/kg SC on Days 8, 15 of each 21-day cycle | Median PFS 4.8 mos vs 4.7 mos (P=0.79); median OS 10.0 mos versus 9.9 mos (P=0.56) | None |
| Manegold et al100 (III) | TLR9 | 839; previously untreated stage IIIB/IV | Cisplatin-gemcitabine ± PF-3512676 0.2 mg/kg SC on Days 8, 15 of each 21-day cycle × up to 6 cycles then weekly PF- 3512676 0.2 mg/kg SC | Median PFS 5.1 mos vs 5.1 mos). Median OS 11.0 mos vs 10.7 mos (P=0.98) | None |
| Parikh et al102 (II) | Talactoferrin | 100; previously treated stage IIIB/IV | Talactoferrin 1.5 g orally BID for 12 of every 14 weeks × up to 3 cycles vs placebo | Median OS 6.1 vs 3.7 mos (HR 0.68; 90% CI, 0.47–0.98; P=0.04) | None |
| Digumarti et al103 (II) | Talactoferrin | 110; previously untreated stage IIIB/IV | Carboplatin-paclitaxel ± talactoferrin 1.5 g orally BID × up to 6 cycles | RR 47% vs 29% (P=0.05); median PFS 7 vs 4.2 mos (HR 0.85; P=0.24); median OS 10.4 vs 8.5 mos (HR 0.87; P=0.26) | None |
| FORTIS-M (III) | Talactoferrin | 720; previously treated stage IIIB/IV | Talactoferrin 1.5 g orally BID for 12 of every 14 weeks × up to 3 cycles vs placebo | Median OS 7.5 vs 7.7 mos (HR 1.04; P=0.66) | None |
BMS, Bristol-Myers Squibb; CI, confidence interval; CTC, circulating tumor cell; CTLA4, cytotoxic T-lymphocyte-associated antigen 4; EGF, epidermal growth factor; HLA, human leukocyte antigen; HR, hazard ratio; IFN, interferon; IL, interleukin; irPFS, immune-related progression-free survival; MAGE A3, melanoma antigen A3; MAGRIT, MAGE A3 as Adjuvant, NSCLC Immunotherapy; MUC1, mucinous glycoprotein 1; NK, natural killer; NS, not significant; NSCLC, non-small cell lung cancer; OS, overall survival; PD-1, programmed death 1; PD-L1, programmed death ligand 1; PF, Pfizer; PFS, progression-free survival; RR, response rate; SC, subcutaneous; SD, stable disease; START, Stimulating Targeted Antigenic Responses to NSCLC; TLR, toll-like receptor
Defined as having a higher percentage than found in two independent sets of healthy donors.
Patients were classified as having good antibody response if anti-EGF antibody titers reached ≥1:4,000 and at least 4 times preimmunization values, and as having poor antibody response otherwise.
Vaccines
For cancer treatment, vaccines are composed of one or more tumor antigens and an adjuvant. The antigen may be recombinant proteins, specific peptides, whole tumor lysates, or irradiated tumor cells.50 The adjuvant stimulates the immune response to the vaccine without having any specific intrinsic antigenic effect. Molecules employed for this purpose include aluminum salts, oil suspensions, virosomes, toll-like receptor (TLR) ligands, and immune cells such as dendritic cells. Additionally, vaccination can be coordinated with microenvironments that render a tumor more immunogenic. Granulocyte-macrophage colony stimulating factor (GM-CSF) is a cytokine that evokes a powerful immune response. Bystander cell lines producing GM-CSF have been added to autologous patient tumor cell-based vaccines.51–53 Several vaccine strategies incorporate pre-treatment with low-dose cyclophosphamide, with reduces number and activity of immune tolerant T regulatory cells. Vaccines are generally well tolerated as lung cancer therapy, with local injection site reactions and flu-like symptoms the principal toxicities.54–56
Key to the development of tumor vaccines is the identification of tumor-specific antigen targets. The principal established antigen targets in NSCLC are melanoma associated antigen A3 (MAGE-A3) and mucin 1 (or mucinous glycoprotein 1) (MUC1). Although not tumor-specific, the epidermal growth factor (EGF) ligand has also been targeted, based on the critical role of EGF ligand and receptor signaling in NSCLC. Although not covered in detail in this review, antigens identified as potential vaccine targets in small cell lung cancer include p53 and glycosphingolipid GD3.57–59 To limit the possibility that vaccine efficacy will diminish over time due to down-regulation of expression of a single antigen, an alternate approach is to vaccinate against entire tumor cells (as can be achieved using tumor cell lines).60,61 Dendritic cell vaccine protocols are also being investigated.62,63 With this approach (exemplified by the approved Sipuleucel-T vaccine for prostate cancer), host dendritic cells are exposed to antigen in vitro and then re-injected into patients.62,63 From dendritic and non-dendritic vaccine efforts, toxicities have not been considered excessively extreme to date.
Melanoma Antigen A3 (MAGE A3)
MAGE is a tumor-specific shared antigen with normal expression limited to placental trophoblasts and testicular germ cells. However, in those tissues there is no antigen presentation due to lack of class I presenting molecules expressing the gene, making MAGE a highly tumor-specific target. MAGE is expressed in a variety of tumors, including melanoma, NSCLC, bladder, head and neck, esophageal, and liver. Its physiologic role is unknown.64 MAGE is expressed in approximately 35% of NSCLC. MAGE expression is associated with histology (approximately 50% in squamous cases versus 25% in non-squamous cases), stage (ranging from 16% in stage I to 35% in stage III to 50% in stage IV disease), and worse tumor-specific survival.65–68
The MAGE A3 vaccine consists of purified MAGE-A3 recombinant protein in combination with a liposomal formulation containing the AS15 adjuvant system, which consists of protein D (a lipoprotein on the surface of Haemophilus influenza B) and a polyhistidine tail. The importance of the vaccine adjuvant is evident in a phase 2 trial of 17 patients with resected early stage NSCLC. Of 9 patients vaccinated with MAGE A3 protein, 3 (33%) had an increase in anti-MAGE A3 antibody titers as determined by enzyme-linked immunosorbent assay (ELISA). In contrast, of 8 patients vaccinated with MAGE A3 protein plus AS15 adjuvant, 7 (88%) had a substantial increase in anti-MAGE A3 antibody titers.69
The MAGE A-3 vaccine was evaluated in a double blind phase 2 trial in completely resected stage IB/II NSCLC expressing MAGE A3 (assessed by quantitative polymerase chain reaction [qPCR]). Vaccine was administered every 3 weeks for 5 doses (induction) then every 3 months for 8 doses (maintenance). No other adjuvant therapy was permitted. There was a trend toward benefit in disease-free interval (primary endpoint), disease-free survival, and overall survival.70 A gene signature consisting of chemokines and T cell markers was predictive of clinical benefit; in the overall population there was a 25% reduction in risk of cancer recurrence, versus 43% in patients with a positive gene signature. The gene profile had substantial overlap with one found to correlate with outcomes in an earlier melanoma clinical trial.71
While the phase II study did not include patients with locally advanced disease, the phase 3 MAGRIT (MAGE A3 as Adjuvant, NSCLC Immunotherapy) trial (NCT00480025) includes resected stage IB-IIIA A3-positive NSCLC. The trial, the largest adjuvant study ever conducted in lung cancer, screened 13,915 patients, randomized 2315 to vaccine or placebo, and completed enrollment in 2012.
MUC-1
MUC-1 (Mucin-1 or Mucinous glycoprotein-1) is a membrane-associated glycoprotein expressed on secretory epithelial cells. In cancer, it is overexpressed and aberrantly glycosylated. This version is antigenically distinct from that in normal tissue and functions as an oncogene, rendering it a suitable target for immunotherapy.72 MUC-1 expression is associated with cell transformation, immunosuppression, and resistance to chemotherapy.73–75
Liposomal BLP25 (L-BLP25)
L-BLP25 is a peptide vaccine targeting the exposed core peptide of MUC-1. It incorporates a synthetic MUC-1 lipopeptide and the immunoadjuvant monophosphoryl lipid A in a liposomal delivery system. L-BLP25 is generally administered with low-dose cyclophosphamide to reduce T regulatory cell activity and thereby enhance the elicited immune response. In lung cancer, the clinical development of L-BLP25 has been centered on locally advanced disease.76–78 In a randomized phase 2B study, 171 patients with stage IIIB or IV NSCLC with disease control after first-line chemotherapy or chemoradiation were assigned to L-BLP 25 or best supportive care. A single dose of cyclophosphamide (300 mg/m2 up to 600 mg/m2) was administered 3 days before the first dose of vaccine, which was given as 8 weekly vaccinations (1000 mcg) then maintenance vaccinations every 6 weeks starting Week 13. In the overall population, survival favored the vaccine arm, with the greatest benefit noted in the subset of patients with stage IIIB disease (approximately 40% of the study population). In this group, median overall survival was 30.6 months versus 13.3 months (HR 0.55; 95% CI, 0.30–0.99).76
Based on these promising results, two phase 3 trials in patients with unresectable stage III NSCLC with responding or stable disease after primary chemoradiation have been conducted. The START (Stimulating Targeted Antigenic Responses to NSCLC) trial (NCT01015443) was placed on clinical hold in 2010 when a patient with melanoma on an exploratory trial of L-LBP25 developed fatal encephalitis, which was ultimately deemed unrelated to study drug. Patients on the START study went an average of 5 months without therapy; those within 6 months of randomization (the period of more intensive therapy) were replaced, yielding a total enrollment of 1476 patients. In a December 2012 press release, it was reported that the START trial did not meet the primary endpoint of a statistically significant improvement in overall survival, but “notable treatment effects were seen for L-BLP25 in certain subgroups.” Further data are anticipated in 2013. The INSPIRE (Stimuvax trial in Asian NSCLC Patients: Stimulating Immune Response) (NCT00409188) has enrolled over 400 patients in 5 Asian countries. Results have not yet been reported.
TG4010
The TG4010 vaccine comprises a suspension of recombinant vaccinia vector (Modified Vaccinia virus Ankara, MVA) containing the coding sequence for human MUC1 antigen and human IL-2 (Mva-Muc1-Il2). It has been studied in conjunction with chemotherapy in advanced MUC1-positive NSCLC, where the combination was noted to be feasible and well tolerated, and did not prevent induction of a cellular response against MUC1.79 In a randomized 2b trial, the primary endpoint of 6-month PFS rate was 43% with TG4010 versus 35% for chemotherapy alone.56 The subset of patients with normal levels of activated natural killer (NK) cells at baseline (73% of the evaluable population) derived greatest benefit, with median survival 17.1 months in the experimental arm versus 11.3 months in the control arm. A phase 3 trial is underway.
Belagenpumatucel-L
Belagenpumatucel-L is an allogeneic tumor-cell vaccine cocktail that incorporates 4 irradiated NSCLC cell lines modified with transforming growth factor beta (TGF-β) antisense plasmid. The rationale for this therapeutic approach is that NSCLC tumor cell lines appear to share immunogenic epitopes with primary tumors, as evidenced by cross-reactivity between cytotoxic T lymphocytes generated against human lung adenocarcinoma cell lines and other lung cancer cell lines. By using 4 cell lines (H460, H520, SKLU-1, RH2), the total number of tumor antigens in the vaccine is increased. The TGF-β antisense plasmid augments the immune response because TGF-β exerts has a role in allowing tumors to escape host immunosurveillance by inhibiting cytotoxic T-cell activation and converting naïve T cells to T regulatory cells.80 In NSCLC, there exists an inverse correlation between TGF-β concentration and survival.81
In a dose-variable phase 2 trial, 75 patients with stage II-IV NSCLC with a total estimated tumor burden volume 125 mL or less were randomized to one of 3 doses of belagenpumatucel-L administered monthly or on alternate months for up to 16 doses.82 In the highest dose cohort (5.0 × 107 cell per injection intradermally) the 2-year survival rate in advanced disease was 47%. Responding patients were noted to have increased cytokine production (IFN-gamma and IL-6) and increased antibody-mediated response to vaccine human leukocyte antigens. In a separate phase 2 trial in advanced disease, median survival was approximately 19 months.83 The phase 3 STOP (Survival, Tumor-free, Overall, and Progression-free) trial (NCT00676507) is ongoing. Patients in this trial have locally advanced or advanced NSCLC without progression after first-line chemotherapy or chemoradiation.
Other vaccines in development for lung cancer therapy
The antigen PRAME (preferentially expressed antigen of melanoma) is expressed at low levels in only a limited set of normal adult cell types, among them testis, ovary, endometrium, kidney, and adrenal medulla.84 It is also expressed in a variety of tumors, including melanoma and NSCLC.85,86 The precise function of PRAME is not well defined, but it appears to be associated with repression of rertinoic acid receptor signaling and is thus implicated in regulation of cell cycle and cell death.87 As the case for other tumor antigens, including MAGE-A3, its expression appears to be regulated by promoter region methylation. A dose escalation study of GSK2302032, which comprises a purified PRAME recombinant protein combined in a liposomal formulation containing the AS15 Adjuvant System, recruited patients with resected stage IB-IIIA NSCLC.
A vaccine targeting epidermal growth factor (EGF) ligand incorporating low-dose cyclophosphamide as an immunoadjuvant agent (CIMAvax) has been studied in advanced NSCLC after 4–6 cycles of first-line platinum-based chemotherapy. While the improvement in overall survival did not reach statistical significance in the overall population, it did so among patients who were less than age 60 years, had a robust immunologic response, or had a strong decrease in circulating EGF levels.88,89 The vaccine has received approval for clinical use in Cuba.
Vaccines consisting of autologous tumor cells engineered to express granulocyte-macrophage colony stimulating factor (GM-CSF) (which functions as an adjuvant to enhance production and migration of granulocytes) have been studied in advanced NSCLC. Production of these individualized therapies required surgical resection of a metastatic site, processing the resected tissue to single-cell suspension, infecting with replication-defective adenoviral vector encoding GM-CSF, irradiation, and cryopreservation. Lesions resected after vaccination revealed T cell and plasma cell infiltrates and tumor necrosis in some patients, with prolonged disease control in a subset of cases.90,91
Immunotherapies
Checkpoint inhibitors
In recent years, checkpoint inhibitors have emerged as the principal immunotherapies in development for cancer therapy (see Figure 2). These monoclonal antibodies interfere with inhibitory pathways in the immune response, thereby augmenting anti-tumor immunity. The principal concern of this approach is the non-specific nature of the response, which may result in considerable autoimmune effects. Furthermore, with immune based therapy approaches, there can be a clear clinical benefit despite an initial apparent radiographic disease progression. As a result of this flare phenomenon, the concept of “immune-related” response criteria has been promoted in parallel to standard imaging criteria for traditional cytotoxic agents. Thus, the addition of immunotherapies to existing treatments of locally advanced NSCLC may further increase the high rates of treatment-related radiographic abnormalities that occur following conventional multimodality therapy. Clinically, the key pathways targeted, CTLA-4 and PD1, differ by toxicity and efficacy profiles.
Cytotoxic T-lymphocyte-associated antigen 4 (CLTA-4)
In addition to binding of the T cell receptor on the surface of T cells to cancer cell antigens presented by the major histocompatibility complex (MHC) on dendritic cells, T cell activation requires additional signaling between costimulatory molecules. In the priming phase, which occurs in regional lymph nodes, dendritic cell costimulatory molecules B7-1 (or CD80) and B7-2 (or CD86) bind to CD28 on T cells to generate activation signals. When dendritic cells bind to CTLA-4 on T cells the interaction results in inhibitory signals. Via competitive binding, anti- CTLA-4 antibodies block this inhibitory pathway.
Ipilimumab (Yervoy) and tremelimumab are both fully human anti-CTLA4 antibodies. As monotherapy, they have activity against melanoma, and ipilimumab has been approved for this indication. Principal toxicities include autoimmune dermatitis, colitis, hepatitis, thyroiditis, and hypophysitis (ie, hypopituitarism), requiring clinicians to recognition and treat these adverse events rapidly. In lung cancer, both agents have been combined with chemotherapy for the treatment of advanced disease. Ipilimumab was investigated in a three-arm trial combined in a concurrent (starting cycle 1) or phased (starting cycle 3) approach with carboplatin-paclitaxel. The greatest benefit was seen in squamous tumors and with the phased approach, suggesting that pre-treatment with chemotherapy augmented anti-tumor immune effects, either through tumor debulking or other mechanisms.92 A phase 3 trial employing this strategy is underway. A phase 2 open label trial of maintenance tremelimumab after first-line chemotherapy for advanced NSCLC resulted in a numerical improvement in progression-free survival, but this did not reach statistical significance.93
Programmed death 1 (PD-1)
In contrast to CTLA-4, the negative regulatory effects of the PD-1 pathway occur in the priming phase of the immune response in peripheral tissues. The PD-1 inhibitory receptor, which is expressed by T cells during prolonged antigen exposure, binds to PD-L1 and PD-L2 expressed in the tumor microenvironment and within inflamed tissues, resulting in blockade of negative regulatory signals. Anti-PD1 and anti-PDL1 antibodies have slightly different mechanistic effects. PD1 blockade interferes with binding to PDL1 and PDL2, but does not have effects on B7. PDL1 blockade interferes with binding to PD1 and B7, but does not impact PDL2 binding, a distinction that may impact toxicity profiles.
Early clinical trials of PD1- and PDL1-directed therapies suggest single-agent activity in lung cancer. In a dose-escalation trial of the anti-PD1 antibody BMS-936558, nearly 20% of patients with NSCLC achieved radiographic response. These responses were durable in many cases. Clinical benefit appeared to be limited to patients with PD-L1 positive tumors. Immune-related adverse effects appeared less common and less severe than those seen with anti-CLTA4 antibodies. Nevertheless, grade 3–4 toxicity occurred in approximately 10% of patients, including three cases of fatal pneumonitis.94 In a trial of the anti-PDL1 antibody BMS-936559, radiographic response was achieved in approximately 10% of NSCLC cases, with similar rates of grade 3–4 toxicities.95 In a number of cases, prolonged responses lasting months were noted. As these drugs arguably demonstrate the strongest anti-cancer activity in NSCLC of any immunotherapy to date, they are currently being compared to single-agent chemotherapy in phase 2 trials and studied in combination with multiple chemotherapy regimens and molecularly targeted agents in the phase 1 setting. These drugs have not been evaluated in locally advanced disease, where potential overlapping pulmonary toxicity with thoracic radiation therapy would be a theoretical concern.
Toll-like receptor (TLR) ligands
TLRs are membrane glycoproteins involved in the recognition of broad classes of microorganism-associated molecular structures. They have a role in both innate and adaptive immune responses.96 Ligand binding to TLRs results in induction of proinflammatory cytokines and other antimicrobial compounds. Functionally active TLR9 is overexpressed in lung cancer compared with normal lung tissue.97 PF-3512676 (formerly termed CpG 7909) is a synthetic TLR9-activating oligodeoxynucleotide that mimics the natural ligand of TLR9 (unmethylated CpG motifs) that induces immune responses, potentially promoting anti-tumor effects.98 In a randomized phase 2 study, the addition of PF-3512676 to first-line taxane-based chemotherapy resulted in a significant increase in response rate and a trend toward improved survival.99 However, two phase 3 trials were closed at interim analysis due to crossing the predefined futility threshold for overall survival.100,101 The addition of PF-3512676 to chemotherapy resulted in increased hematologic adverse events, transfusions, infections, myeloid growth factor use, and chemotherapy dose delays and reductions.
Talactoferrin
Talactoferrin is a recombinant human lactoferrin purified from Aspergillusniger. Lactoferrin is an iron-binding glycoprotein with immunomodulatory properties. When administered orally, talactoferrin binds gut epithelium and is subsequently transported to gut-associated lymphoid tissue, where it recruits and induces maturation of circulating dendritic cells. In turn, these dendritic cells may have anti-tumor activity. Talactoferrin is not systemically absorbed; there is no increase in serum lactoferrin concentrations and no resulting talactoferrin-specific antibodies. In phase 2 trials, talactoferrin improved survival as monotherapy compared to placebo and in combination with chemotherapy.102,103 Talactoferrin was very well tolerated, with fewer adverse events than seen in the placebo arms. However, the international phase 3 FORTIS-M trial, which enrolled 720 patients with previously treated advanced NSCLC to talactoferrin or placebo, did not demonstrate a benefit for the primary endpoint of overall survival (median 7.5 months with talactoferrin versus 7.7 months with placebo; HR 1.04; P=0.66) or for progression-free survival.104 Following these negative results, the phase 3 FORTIS-C trial of carboplatin-paclitaxel ± talactoferrin was stopped, with 94 of the planned 1,100 patients enrolled. No difference in overall survival or progression-free survival was noted.105
CONCLUSION
Historically, vaccines and immunotherapies have had a limited role in the treatment of lung cancer. Lack of antigenic activity, immunosuppressive effects of the tumor, and difficulties debulking the disease in advanced stages all hinder the effects of these strategies. Recent years have seen several promising agents fail to improve clinical outcomes in phase 3 trials, including the L-BLP-25 vaccine, TLR-targeting drugs, and talactoferrin. However, newly emerging approaches, such as immune checkpoint inhibition, demonstrate considerable promise in this challenging disease. Nowhere are these therapies of greater potential interest than in locally advanced NSCLC, a setting where disease debulking is feasible and radiotherapy and chemotherapy may enhance the effects of immunotherapies. Critical to moving forward with such an approach will be mitigation of potentially overlapping toxicities, optimizing dosing and schedule when combining immunotherapy with other treatment modalities, standardizing the approach to interpreting radiographic abnormalities during and after treatment, and the development of biomarkers to predict which patients are most likely to derive benefit.
Acknowledgments
Funding:
Funded in part by National Cancer Institute Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement) (to D.E.G.).
Footnotes
Disclosures:
The authors have no relevant disclosures.
References
- 1.Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors, and management. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al] 2012;38:1622–30. doi: 10.1111/j.1524-4725.2012.02520.x. [DOI] [PubMed] [Google Scholar]
- 2.Tessari G, Naldi L, Boschiero L, et al. Incidence of primary and second cancers in renal transplant recipients: a multicenter cohort study. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:214–21. doi: 10.1111/j.1600-6143.2012.04294.x. [DOI] [PubMed] [Google Scholar]
- 3.Graus F, Dalmou J, Rene R, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 4.Senovilla L, Vacchelli E, Galon J, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1:1323–43. doi: 10.4161/onci.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meloni F, Morosini M, Solari N, et al. Foxp3 expressing CD4+ CD25+ and CD8+CD28- T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Human immunology. 2006;67:1–12. doi: 10.1016/j.humimm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.DeLong P, Carroll RG, Henry AC, et al. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer biology & therapy. 2005;4:342–6. doi: 10.4161/cbt.4.3.1644. [DOI] [PubMed] [Google Scholar]
- 7.Formenti SC, Demaria S. Effects of chemoradiation on tumor-host interactions: the immunologic side. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1562–3. doi: 10.1200/JCO.2007.15.5499. author reply 3. [DOI] [PubMed] [Google Scholar]
- 8.Ryschich E, Harms W, Loeffler T, Eble M, Klar E, Schmidt J. Radiation-induced leukocyte adhesion to endothelium in normal pancreas and in pancreatic carcinoma of the rat. International journal of cancer Journal international du cancer. 2003;105:506–11. doi: 10.1002/ijc.11073. [DOI] [PubMed] [Google Scholar]
- 9.Lesterhuis WJ, de Vries IJ, Aarntzen EA, et al. A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients. British journal of cancer. 2010;103:1415–21. doi: 10.1038/sj.bjc.6605935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3100–4. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 11.Brown MD, van der Most R, Vivian JB, et al. Loss of antigen cross-presentation after complete tumor resection is associated with the generation of protective tumor-specific CD8(+) T-cell immunity. Oncoimmunology. 2012;1:1084–94. doi: 10.4161/onci.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy K. Janeway’s Immunobiology. 8. New York: Garland Science; 2012. [Google Scholar]
- 13.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Current biology : CB. 2011;21:R488–93. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 14.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clinical & developmental immunology. 2012;2012:836485. doi: 10.1155/2012/836485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Said-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomedical journal. 2012;35:437–49. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricklin D, Lambris JD. Progress and trends in complement therapeutics. Advances in experimental medicine and biology. 2013;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith KA. Toward a molecular understanding of adaptive immunity: a chronology, part I. Frontiers in immunology. 2012;3:369. doi: 10.3389/fimmu.2012.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum JS, Wearsch PA, Cresswell P. Annual review of immunology. 2013. Pathways of Antigen Processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurtulus S, Tripathi P, Hildeman DA. Protecting and rescuing the effectors: roles of differentiation and survival in the control of memory T cell development. Frontiers in immunology. 2012;3:404. doi: 10.3389/fimmu.2012.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thibodeau J, Bourgeois-Daigneault MC, Lapointe R. Targeting the MHC Class II antigen presentation pathway in cancer immunotherapy. Oncoimmunology. 2012;1:908–16. doi: 10.4161/onci.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bukur J, Jasinski S, Seliger B. The role of classical and non-classical HLA class I antigens in human tumors. Seminars in cancer biology. 2012;22:350–8. doi: 10.1016/j.semcancer.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer immunity. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 23.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Current opinion in investigational drugs. 2010;11:1354–9. [PubMed] [Google Scholar]
- 25.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Seminars in oncology. 2010;37:508–16. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Tucker ZC, Laguna BA, Moon E, Singhal S. Adjuvant immunotherapy for non-small cell lung cancer. Cancer treatment reviews. 2012;38:650–61. doi: 10.1016/j.ctrv.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Manda K, Glasow A, Paape D, Hildebrandt G. Effects of ionizing radiation on the immune system with special emphasis on the interaction of dendritic and T cells. Frontiers in oncology. 2012;2:102. doi: 10.3389/fonc.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Frontiers in oncology. 2012;2:191. doi: 10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludgate CM. Optimizing cancer treatments to induce an acute immune response: radiation Abscopal effects, PAMPs, and DAMPs. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:4522–5. doi: 10.1158/1078-0432.CCR-12-1175. [DOI] [PubMed] [Google Scholar]
- 30.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. The Journal of experimental medicine. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannan R, Zhang H, Wallecha A, et al. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer immunology, immunotherapy : CII. 2012;61:2227–38. doi: 10.1007/s00262-012-1257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newcomb EW, Demaria S, Lukyanov Y, et al. The combination of ionizing radiation and peripheral vaccination produces long-term survival of mice bearing established invasive GL261 gliomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4730–7. doi: 10.1158/1078-0432.CCR-06-0593. [DOI] [PubMed] [Google Scholar]
- 33.Dewan MZ, Vanpouille-Box C, Kawashima N, et al. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6668–78. doi: 10.1158/1078-0432.CCR-12-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Liu L, Yu D, et al. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PloS one. 2012;7:e38111. doi: 10.1371/journal.pone.0038111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiation research. 2012;178:505–23. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finkelstein SE, Fishman M. Clinical opportunities in combining immunotherapy with radiation therapy. Frontiers in oncology. 2012;2:169. doi: 10.3389/fonc.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein SE, Iclozan C, Bui MM, et al. Combination of external beam radiotherapy (EBRT) with intratumoral injection of dendritic cells as neo-adjuvant treatment of high-risk soft tissue sarcoma patients. International journal of radiation oncology, biology, physics. 2012;82:924–32. doi: 10.1016/j.ijrobp.2010.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. Journal of immunotherapy. 2005;28:129–35. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 39.Frey B, Rubner Y, Wunderlich R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Current medicinal chemistry. 2012;19:1751–64. doi: 10.2174/092986712800099811. [DOI] [PubMed] [Google Scholar]
- 40.Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Science translational medicine. 2012;4:137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 41.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. The New England journal of medicine. 2012;366:925–31. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Seminars in cancer biology. 2003;13:135–47. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 43.Mesner PW, Jr, Budihardjo, Kaufmann SH. Chemotherapy-induced apoptosis. Advances in pharmacology. 1997;41:461–99. doi: 10.1016/s1054-3589(08)61069-8. [DOI] [PubMed] [Google Scholar]
- 44.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell death and differentiation. 2004;11:448–57. doi: 10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
- 45.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Current opinion in immunology. 2008;20:545–57. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 46.van der Most RG, Currie AJ, Robinson BW, Lake RA. Decoding dangerous death: how cytotoxic chemotherapy invokes inflammation, immunity or nothing at all. Cell death and differentiation. 2008;15:13–20. doi: 10.1038/sj.cdd.4402255. [DOI] [PubMed] [Google Scholar]
- 47.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nature reviews Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 48.McDonnell AM, Nowak AK, Lake RA. Contribution of the immune system to the chemotherapeutic response. Seminars in immunopathology. 2011;33:353–67. doi: 10.1007/s00281-011-0246-z. [DOI] [PubMed] [Google Scholar]
- 49.McCoy MJ, Lake RA, van der Most RG, Dick IM, Nowak AK. Post-chemotherapy T-cell recovery is a marker of improved survival in patients with advanced thoracic malignancies. British journal of cancer. 2012;107:1107–15. doi: 10.1038/bjc.2012.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Pas T, Giovannini M, Rescigno M, et al. Vaccines in non-small cell lung cancer: rationale, combination strategies and update on clinical trials. Critical reviews in oncology/hematology. 2012;83:432–43. doi: 10.1016/j.critrevonc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Kelly RJ, Giaccone G. Lung cancer vaccines. Cancer journal. 2011;17:302–8. doi: 10.1097/PPO.0b013e318233e6b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiang CL, Kandalaft LE, Coukos G. Adjuvants for enhancing the immunogenicity of whole tumor cell vaccines. International reviews of immunology. 2011;30:150–82. doi: 10.3109/08830185.2011.572210. [DOI] [PubMed] [Google Scholar]
- 53.Gupta R, Emens LA. GM-CSF-secreting vaccines for solid tumors: moving forward. Discovery medicine. 2010;10:52–60. [PMC free article] [PubMed] [Google Scholar]
- 54.Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer control : journal of the Moffitt Cancer Center. 2013;20:22–31. doi: 10.1177/107327481302000105. [DOI] [PubMed] [Google Scholar]
- 55.Reck M. What future opportunities may immuno-oncology provide for improving the treatment of patients with lung cancer? Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 8):viii28–34. doi: 10.1093/annonc/mds260. [DOI] [PubMed] [Google Scholar]
- 56.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. The lancet oncology. 2011;12:1125–33. doi: 10.1016/S1470-2045(11)70259-5. [DOI] [PubMed] [Google Scholar]
- 57.Farkas AM, Finn OJ. Vaccines based on abnormal self-antigens as tumor-associated antigens: immune regulation. Seminars in immunology. 2010;22:125–31. doi: 10.1016/j.smim.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Decoster L, Wauters I, Vansteenkiste JF. Vaccination therapy for non-small-cell lung cancer: review of agents in phase III development. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:1387–93. doi: 10.1093/annonc/mdr564. [DOI] [PubMed] [Google Scholar]
- 59.Finn OJ. Cancer immunology. The New England journal of medicine. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 60.Meek DW, Marcar L. MAGE-A antigens as targets in tumour therapy. Cancer letters. 2012;324:126–32. doi: 10.1016/j.canlet.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Dasanu CA, Sethi N, Ahmed N. Immune alterations and emerging immunotherapeutic approaches in lung cancer. Expert opinion on biological therapy. 2012;12:923–37. doi: 10.1517/14712598.2012.685715. [DOI] [PubMed] [Google Scholar]
- 62.Hespel C, Moser M. Role of inflammatory dendritic cells in innate and adaptive immunity. European journal of immunology. 2012;42:2535–43. doi: 10.1002/eji.201242480. [DOI] [PubMed] [Google Scholar]
- 63.Drake CG. Combination immunotherapy approaches. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23(Suppl 8):viii41–6. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer science. 2009;100:2014–21. doi: 10.1111/j.1349-7006.2009.01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolli M, Kocher T, Adamina M, et al. Tissue microarray evaluation of Melanoma antigen E (MAGE) tumor-associated antigen expression: potential indications for specific immunotherapy and prognostic relevance in squamous cell lung carcinoma. Annals of surgery. 2002;236:785–93. doi: 10.1097/01.SLA.0000036266.09823.6C. discussion 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:8055–62. doi: 10.1158/1078-0432.CCR-05-1203. [DOI] [PubMed] [Google Scholar]
- 67.Sienel W, Varwerk C, Linder A, et al. Melanoma associated antigen (MAGE)-A3 expression in Stages I and II non-small cell lung cancer: results of a multi-center study. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2004;25:131–4. doi: 10.1016/j.ejcts.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 68.Kim JH, Zo J, Nakayama H. Patient and tumor characteristics impacting on MAGE-A3 expression: screening data from the MAGRIT Phase III trial. J Thorac Oncol. 2011;6:S662. (abstract M021.08) [Google Scholar]
- 69.Atanackovic D, Altorki NK, Stockert E, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. Journal of immunology. 2004;172:3289–96. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- 70.Vansteenkiste J, Zielinski M, Linder A. Phase II randomized study of MAGE-A3 immunotherapy as adjuvant therapy in stage IB/II Non-Small Cell Lung Cancer (NSCLC): 44 month follow-up, humoral and cellular immune response data. J Thorac Oncol. 2008;3:S55–6. (abstract 1480) [Google Scholar]
- 71.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer journal. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 72.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, et al. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. The Journal of biological chemistry. 1990;265:15286–93. [PubMed] [Google Scholar]
- 73.Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:s4652–4. doi: 10.1158/1078-0432.CCR-07-0213. [DOI] [PubMed] [Google Scholar]
- 74.Ren J, Agata N, Chen D, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer cell. 2004;5:163–75. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddish MA, MacLean GD, Poppema S, Berg A, Longenecker BM. Pre-immunotherapy serum CA27. 29 (MUC-1) mucin level and CD69+ lymphocytes correlate with effects of Theratope sialyl-Tn-KLH cancer vaccine in active specific immunotherapy. Cancer immunology, immunotherapy : CII. 1996;42:303–9. doi: 10.1007/s002620050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. Journal of cancer research and clinical oncology. 2011;137:1337–42. doi: 10.1007/s00432-011-1003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palmer M, Parker J, Modi S, et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clinical lung cancer. 2001;3:49–57. doi: 10.3816/clc.2001.n.018. discussion 8. [DOI] [PubMed] [Google Scholar]
- 78.Ohyanagi F, Horai T, Sekine I, et al. Safety of BLP25 liposome vaccine (L-BLP25) in Japanese patients with unresectable stage III NSCLC after primary chemoradiotherapy: preliminary results from a Phase I/II study. Japanese journal of clinical oncology. 2011;41:718–22. doi: 10.1093/jjco/hyr021. [DOI] [PubMed] [Google Scholar]
- 79.Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3:735–44. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 80.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nature reviews Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 81.Kong F, Jirtle RL, Huang DH, Clough RW, Anscher MS. Plasma transforming growth factor-beta1 level before radiotherapy correlates with long term outcome of patients with lung carcinoma. Cancer. 1999;86:1712–9. [PubMed] [Google Scholar]
- 82.Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:4721–30. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 83.Nemunaitis J, Nemunaitis M, Senzer N, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer gene therapy. 2009;16:620–4. doi: 10.1038/cgt.2009.15. [DOI] [PubMed] [Google Scholar]
- 84.Kessler JH, Beekman NJ, Bres-Vloemans SA, et al. Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. The Journal of experimental medicine. 2001;193:73–88. doi: 10.1084/jem.193.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikeda H, Lethe B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 86.Bankovic J, Stojsic J, Jovanovic D, et al. Identification of genes associated with non-small-cell lung cancer promotion and progression. Lung cancer. 2010;67:151–9. doi: 10.1016/j.lungcan.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–47. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 88.Gonzalez G, Crombet T, Catala M, et al. A novel cancer vaccine composed of human-recombinant epidermal growth factor linked to a carrier protein: report of a pilot clinical trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1998;9:431–5. doi: 10.1023/a:1008261031034. [DOI] [PubMed] [Google Scholar]
- 89.Neninger Vinageras E, de la Torre A, Osorio Rodriguez M, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:1452–8. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 90.Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:624–30. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 91.Nemunaitis J, Sterman D, Jablons D, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. Journal of the National Cancer Institute. 2004;96:326–31. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 92.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 93.Zatloukal P, Heo DS, Park K, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(suppl):abstr 8071. [Google Scholar]
- 94.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akira S, Takeda K. Toll-like receptor signalling. Nature reviews Immunology. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 97.Droemann D, Albrecht D, Gerdes J, et al. Human lung cancer cells express functionally active Toll-like receptor 9. Respiratory research. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nature reviews Drug discovery. 2006;5:471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 99.Manegold C, Gravenor D, Woytowitz D, et al. Randomized phase II trial of a toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3979–86. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 100.Manegold C, van Zandwijk N, Szczesna A, et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:72–7. doi: 10.1093/annonc/mdr030. [DOI] [PubMed] [Google Scholar]
- 101.Hirsh V, Paz-Ares L, Boyer M, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2667–74. doi: 10.1200/JCO.2010.32.8971. [DOI] [PubMed] [Google Scholar]
- 102.Parikh PM, Vaid A, Advani SH, et al. Randomized, double-blind, placebo-controlled phase II study of single-agent oral talactoferrin in patients with locally advanced or metastatic non-small-cell lung cancer that progressed after chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4129–36. doi: 10.1200/JCO.2010.34.4127. [DOI] [PubMed] [Google Scholar]
- 103.Digumarti R, Wang Y, Raman G, et al. A randomized, double-blind, placebo-controlled, phase II study of oral talactoferrin in combination with carboplatin and paclitaxel in previously untreated locally advanced or metastatic non-small cell lung cancer. J Thorac Oncol. 2011;6:1098–103. doi: 10.1097/JTO.0b013e3182156250. [DOI] [PubMed] [Google Scholar]
- 104. [Accessed March 9, 2013];Agennix Reports Results of FORTIS-M Phase III Trial With Talactoferrin Alfa in Non-Small Cell Lung Cancer. 2012 at http://www.marketwire.com/press-release/agennix-reports-results-fortis-m-phase-iii-trial-with-talactoferrin-alfa-non-small-cell-frankfurt-agx-1687453.htm.
- 105. [Accessed March 9, 2013];FORTIS-C Phase III trial with talactoferrin in first-line non-small cell lung cancer closed early and unblinded; top-line results announced. 2012 at http://agennix.com/index.php?option=com_content&view=article&id=232%3Aagennix-ag-reports-additional-talactoferrin-alfa-data&catid=23%3Apress-releases-2012&Itemid=6&lang=en.
- 106.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nature medicine. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 107.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. Journal of immunology. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 108.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nature medicine. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 109.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. Journal of immunology. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer research. 1996;56:5150–5. [PubMed] [Google Scholar]
- 111.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer research. 2003;63:4490–6. [PubMed] [Google Scholar]
- 112.Demaria S, Volm MD, Shapiro RL, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:3025–30. [PubMed] [Google Scholar]