Abstract
Previous studies indicate involvement of the multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) in vascular smooth muscle (VSM) cell migration. In the present study, molecular loss-of-function studies were used specifically to assess the role of the predominant CaMKIIδ2 isoform on VSM cell migration using a scratch wound healing assay. Targeted CaMKIIδ2 knockdown using siRNA or inhibition of activity by overexpressing a kinase-negative mutant resulted in attenuation of VSM cell migration. Temporal and spatial assessments of kinase autophosphorylation indicated rapid and transient activation in response to wounding, in addition to a sustained activation in the leading edge of migrating and spreading cells. Furthermore, siRNA mediated suppression of CaMKIIδ2 resulted in the inhibition of wound-induced Rac activation and Golgi reorganization, and disruption of leading edge morphology, indicating an important function for CaMKIIδ2 in regulating VSM cell polarization. Numerous previous reports link activation of CaMKII to ERK 1/2 signaling in VSM. Wound induced ERK1/2 activation was also found to be dependent upon CaMKII, however, ERK activity did not account for effects of CaMKII in regulating Golgi polarization, indicating alternative mechanisms by which CaMKII affects the complex events involved in cell migration. Wounding a VSM cell monolayer results in CaMKIIδ2 activation, which positively regulates VSM cell polarization and downstream signaling, including Rac and ERK1/2 activation, leading to cell migration.
Introduction
Vascular smooth muscle cells (VSM) found within the medial wall of the vasculature are quiescent and express a differentiated phenotype. Differentiated VSM cells function to maintain vascular tone, which is regulated primarily by increases in free intracellular Ca2+ and/or signaling pathways affecting the balance of myosin light chain kinase and myosin phosphatase activities (14; 37). In response to injury or disease, VSM cells may become proliferative and migrate across the internal elastic lamina resulting in neointima formation (46). Although this phenotypic transition correlates with changes in expression of the ion channels and mechanisms regulating Ca2+ signals (4; 52), several studies have demonstrated dependence of VSM cell proliferation and migration on Ca2+-dependent regulatory pathways (17; 22; 31; 44). In contrast to Ca2+-dependent regulation of differentiated VSM function, our understanding of the cellular mechanisms and intracellular targets of Ca2+ signals in the regulation of VSM cell proliferation and migration is still unclear.
Calcium/calmodulin-dependent protein kinase II (CaMKII) is a ubiquitous multifunctional serine/threonine protein kinase, with complex structural and autoregulatory properties (23). CaMKII has been implicated in the regulation of VSM cell migration (2; 38; 40). However, existing studies are not entirely consistent, with divergent results apparently dependent on the specific pharmacological or molecular approaches used to manipulate CaMKII activity. For example, attenuation of CaMKII activity with the pharmacological inhibitors KN-93 and KN-62 has been reported to block VSM cell migration in a transwell assay (38) and VSM cells stably over-expressing constitutively active CaMKII α-subunits demonstrated enhanced migration (2). On the other hand, transiently over-expressed constitutively-active CaMKIIδ2, the predominant endogenous CaMKII isoform in cultured VSM cells, was found to inhibit cell migration, and conversely, over-expression of kinase-negative CaMKIIδ2 enhanced VSM cell migration (40). Additional approaches, such as loss-of-function siRNA silencing, are needed to resolve the functional importance of endogenous CaMKII isoforms in regulating VSM cell migration.
Cell migration on a surface is a multifaceted process with an initial step of cell polarization and extension of a leading edge toward the direction of migration. New focal adhesions are formed and mature to stabilize the cell, followed by retraction of the cell body, resulting in net translocation (41). Interpretation of existing studies of CaMKII involvement in VSM migration are further complicated by the complexity of the modified Boyden chamber or transwell assays commonly used to model the process. Concerted regulation of cell attachment, spreading, matrix degradation and invasion through a pore involves processes in addition to those already complicated events involved in moving across as surface. It is noteworthy in this regard, that simple adherence and spreading of cultured VSM cells on extracellular matrices such as fibronectin or collagen also results in the activation of CaMKII and subsequent downstream signaling involving ERK1/2 (29). The migration process itself might be more directly assayed using a scratch wound healing model which also provides the opportunity for visualizing protein dynamics using fluorescence microscopy approaches.
Collectively, discrepancies using various pharmacological and molecular approaches and the complexity of potential underlying mechanisms have thwarted a definitive conclusion as to the role of CaMKII in VSM cell migration. In this study we used loss-of-function molecular approaches and a wound healing assay to evaluate the activation and function of the endogenous CaMKIIδ2 isoforms in VSM cell migration. CaMKII was found to be acutely activated at the wound edge and to contribute net positively to migration and wound closure. Moreover, we have for the first time demonstrated that CaMKII is activated in the leading edge of migrating VSM cells and promotes cellular polarization by regulating Golgi reorientation and leading edge dynamics, the latter inferred by changes in leading edge morphology. Both wound-induced ERK1/2 and rac activation were found to be dependent on CaMKII activation, suggesting potential mechanisms by which CaMKII could exert effects on VSM cell polarization and migration.
Material and Methods
Cell Culture
VSM cells were enzymatically dispersed from thoracic aortas of 200–300g male Sprague-Dawley rats as previously described (15; 47). Cells were cultured in DMEM/F-12 medium supplemented with 10% fetal bovine serum (FBS) at 37°C and 5% CO2. Confluent cultures from passages 3–9 were used for each experiment to minimize phenotypic variations.
Antibodies and Materials
Creation and specificity of the anti-peptide polyclonal antibody against the δ2 specific isoform of CaMKII and phosphorylated CaMKII (Thr287) was described previously (15; 51). Other antibodies used include anti-Rac (Upstate), anti-GM130 (BD Transduction), β-actin (Sigma), Phosphop44/42 MAP kinase (Cell Signaling), ERK2 (BD Biosciences) and FITC-conjugated phalloidin (Sigma). All cell culture media and supplies were from Fisher Scientific (Pittsburgh, PA) unless otherwise specified. Platelet derived growth factor and ERK inhibitor U0126 is from Calbiochem (La Jolla, CA).
siRNA Electroporation
Ambion Silencer® Pre-designed siRNA (ID 1998162, standard purity) and Dharmacon ON-TARGET plus SMARTpool (Catalog # L-099520-00-0010, Thermo Fisher Scientific., Lafayette, CO) siRNA specifically targeting rat CaMKIIδ protein were electroporated in VSM cells. Controls included Silencer® Negative Control #1 siRNA (Ambion) and ON-TARGETplus siCONTROL Non-targeting siRNA #1. Rac siRNA was a Dharmacon ON-TARGET plus SMARTpool (Catalog # L-080171-00-0010). Electroporation was performed as follows; subconfluent VSMC were removed from the culture dish by addition of trypsin and pelleted. The pellet was resuspended in siPORT™ siRNA Electroporation Buffer (Ambion) at a concentration of 2 × 105 cells per cuvette with 1.5 ug of the respective siRNA. Cells were electroporated with one 0.15 ms pulse of 300V (Gene Pulser II, BioRad). After an additional incubation for 10 min at 37°C, cells were plated on the appropriate culture dishes. Knock down was confirmed by western blot after 48 hours of exposure.
Adenoviral shRNA
We have developed successful CaMKIIδ2 knockdown in our cultured rat aortic smooth muscle cells using a GFP tagged adenoviral vector to deliver short hairpin RNA (shRNA) constructs (20; 29). Target sequences for suppressing rat and mouse CaMKIIδ2 were selected using the siRNA Target Finder and Design Tool (Ambion) online program. To confirm specificity, the potential small interfering RNAs were subjected to BLAST searches against expressed sequence tag libraries. The sequence targeting the translated region of the CaMKIIδ2 isoform is 5’-ATAAACCAATCCACACTAT-3’, nt 1543–1562. Control virus was constructed to target a region of the firefly luciferase with 5’-CGUACGCGGAAUACUUCGATT-3’ as previously described (11). The target sequences were subcloned into the AdTrackHP vector (generous gift from Dr. JL Zhang) and positive colonies were purified (Qiagen) and electroporated into electrocompetent AdEasy cells (Stratagene) as previously described (19).
Rac and Rho Activity Assays
Rac and Rho activity was determined from whole cell lysates and isolated leading edge of migrating VSM cells using a commercially available, enzyme-linked colorimetric assay (ELISA)-based Rac or Rho activity assay (G-LISA; Cytoskeleton, Denver, CO). Protein was isolated using lysis buffer provided, snap-frozen and processed according to the G-LISA protocol. The lysates were incubated in microwells to which the p21-activated kinase or Rhotekin binding domain peptide was bound to capture active Rac or Rho, respectively. Active protein was detected using indirect immunodetection followed by a colorimetric reaction measured by absorbance at 490 nm.
Leading edge Isolation
The leading edge and whole cell lysate was isolated using a modified Boyden chamber assay as previously described (6). Control cells or cells deficient in CaMKIIδ2 due to RNA interference, were plated onto a modified Boyden chamber (3uM pores, 6 well format; BD BioCoat) coated with 10ug/ml fibronectin (Sigma) at a density of 1.5 × 106 cells per well and allowed to adhere for 2 hours in 37°, 5% CO2 incubator in media containing 0.4% FBS. PDGF-BB (10ng/ml) is then added into the lower chamber to stimulate migration for 120 minutes. The tops of the membranes are swabbed to remove the cell body. The remaining cell is lysed and processed for immunoblotting or relative Rac or Rho activity.
Immunofluorescence
Cells plated on collagen coated glass coverslips or cell culture dishes were wounded using 10ul pipette tip, washed and placed back into a 37°C incubator in the presence of 10% FBS for the indicated time. Cells were fixed using 4% paraformaldehyde for 20 minutes and permeablized with 0.2% Triton X-100 in PBS for 5 minutes. Nonspecific binding was blocked with 5% fish gelatin in PBS plus 0.1% Triton X-100 followed by a 1 hour incubation at room temperature with the described anti-CaMKII, anti-P-CaMKIIThr287 or anti-GM130 antibodies diluted to 1:100–1:250 in blocking buffer. This was followed by washes and a 1 hour incubation at room temperature with the appropriate fluorochrome conjugated secondary antibodies. F-actin was labeled using rhodamine- or FITC-conjugated phalloidin diluted 1:250. Coverslips were mounted onto slides using Vectashield with Dapi (Vector Laboratories, Inc., Burlingame, CA). Cells were imaged on a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Inc., Thornwood, NY) or a Leica DM IRB (Leica Microsystems, Bannockburn, IL).
Cell Lysates and immunoblotting
Cells were maintained at 37°C in 5% CO2 during the pretreatment. Reactions were stopped by removal of HBSS, transferring the dishes to ice and lysed (0.5 ml/60 mm dish or 1 ml/100 mm dish) in a modified RIPA buffer composed of 10 mM Tris (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 2 mM Na3VO4, 1% Triton X-100, 0.1% SDS, 0.5% deoxycholate, 10% glycerol, 1 mM DTT, 0.1 mM PMSF, and 0.2 U/ml aprotinin. The lysates were collected into 1.5ml tubes and cleared by centrifugation at 14,000rpm at 4°C for 10min.
The multi-wound assay was performed by dragging a multi-toothed comb (0.5µM per tooth, 0.5 µM apart) through a VSM monolayer, placing the dish on a 37°C warming plate for the indicated time. Cells were lysed using appropriate buffer for the specific subsequent assay.
Lysates were resolved on an SDS-PAGE gel and transferred to nitrocellulose. The membranes were blocked in Tris-buffered saline containing 0.2% Tween 20 (TBST) and 5% nonfat dry milk. After blocking, the membranes were incubated in primary antibody for 1h at 22°C, washed 3X with TBST and incubated with horseradish peroxidase-conjugated secondary antibody (Amersham) for 1h at 22°C followed by washing 3X with TBST. Membranes were developed using chemiluminescence substrate (Amersham) and analysis of signal intensity was measure with a Fuji LAS4000 Imaging Station and band intensity compared utilizing Multi Gauge V3.1. All blots shown are representative of at least 3 experiments.
Migration assay and quantification
Two days post siRNA treatment an artificial wound was made in the monolayer by scraping a 10 µl pipette tip across the bottom of the dish. The wound was extensively washed and media containing 10% Fetal Bovine Serum was replaced and cells were allowed to migrate for the appropriate time in a 37°C incubation chamber with 5% CO2. Cells were subsequently fixed with 4% paraformaldahyde and stained with Coomassie Blue. Images were taken with a Leica DM IRB at 10x using brightfield microscopy. The remaining open area of the wound was measured using Image J as previously described with some modifications (9). Images were cropped to specified size and with the ‘area method’ in Image J (NIH) the total area absent of Coomassie Stained cells can be measured by setting a threshold using ImageJ's threshold tool for selection of this area open area only. From this setting the open area for an image can be calculated in arbitrary units.
Golgi reorientation analysis
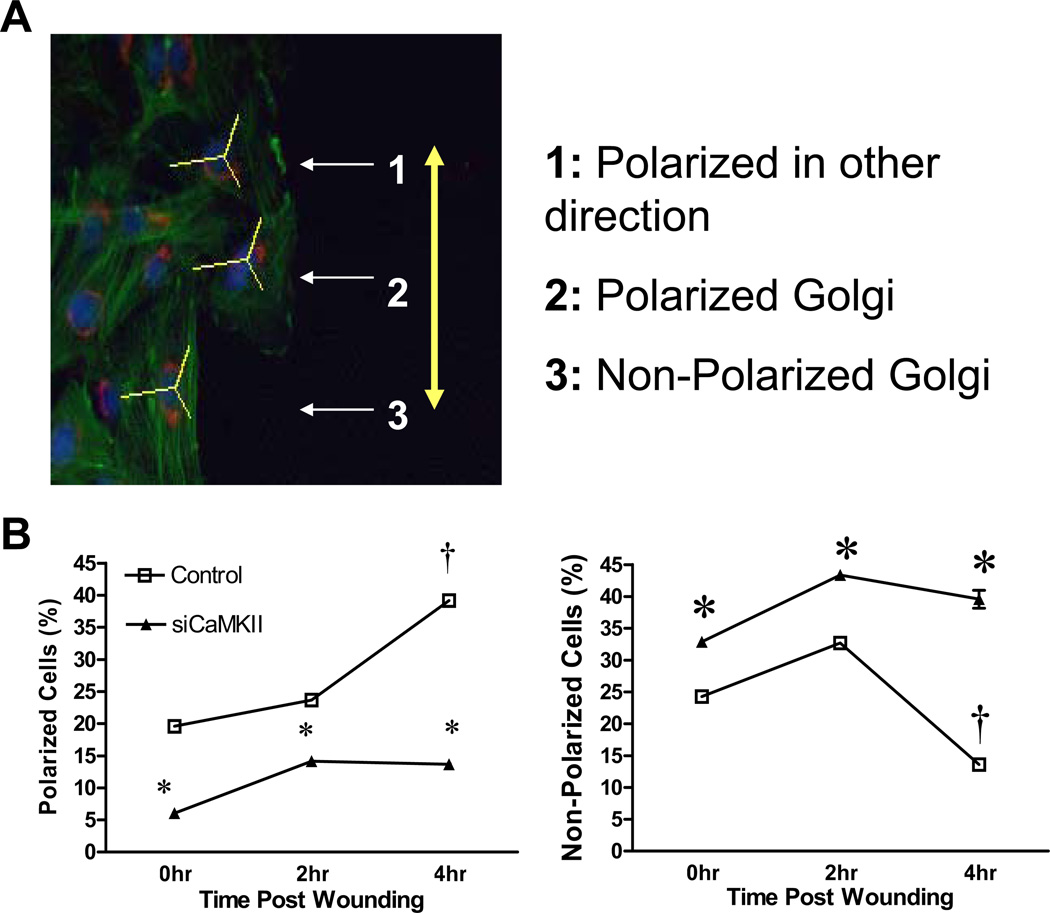
Analysis of Golgi reorientation was carried out, with some modifications, as described previously (36) . Cells were fixed at 0, 2, and 4 hours following wounding of the confluent monolayer with a pipette tip ,prior to VSM cell migration which initiates approximately 5–6 hours post-wounding. The Golgi was stained with an antibody directed against the Golgi-specific membrane protein GM130, actin stained with rhodamine-conjugated phalloidin and the nucleus stained with dapi. To measure Golgi orientation, 120 degree angles were drawn (ImageJ) from the center of the nucleus on cells that line the edge of the wound creating three sectors. The angles are drawn such that one of the 120 degree sectors faced the edge of the wound (sector A). If the Golgi encompassed a sector away from the wound edge or spanned any two sectors it was categorized as polarized in a different direction away from the wound edge. Golgi found within all three sectors were classified as non-polarized. All of the transduced cells at the wound edge in three fields were categorized (120–150 total cells) per siRNA treatment, per time point. Data are expressed as means ± SE. Mean values of groups were analyzed using GraphPad PRISM version 4.00 and compared by ANOVA with post hoc comparisons using the Bonferroni’s multiple comparison test. For all comparisons, P < 0.05 was considered statistically significant.
Results
CaMKII silencing inhibits VSM cell migration
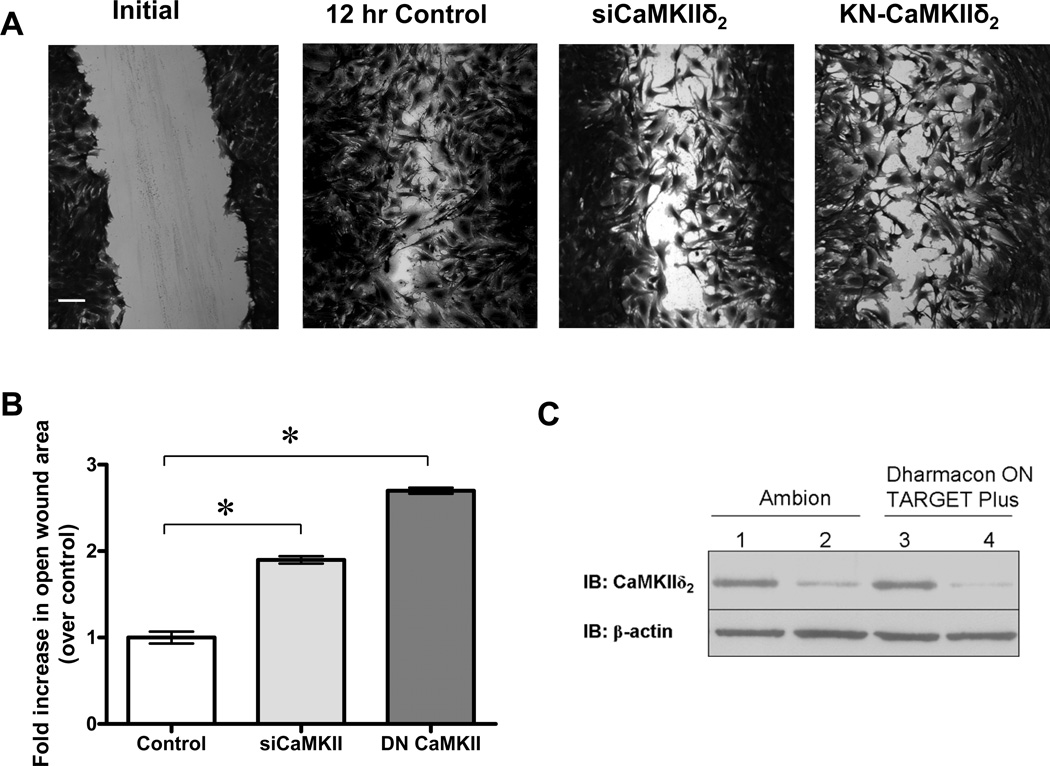
Molecular loss-of-function approaches were used to suppress endogenous CaMKIIδ2 expression (siRNA) or activity (kinase-negative CaMKIIδ2 mutant) and the effects on VSM cell migration assessed using a scratch wound assay (Figure 1). CaMKIIδ2 expression was consistently suppressed by at least 80% 48 hrs post-electroporation using two separate commercial pools of siRNA duplexes (Figure 1C). Confluent monolayers of either control siRNA-treated or CaMKIIδ2 siRNA-treated cells were scratch ‘wounded’ with a 10 µ1 pipette tip and followed in the presence of serum containing media (10% FBS) for 12 hr. Cell proliferation over this period is expected to be minimal, therefore wound closure under these conditions primarily reflects VSM cell spreading and migration. CaMKIIδ2 suppression using siRNA, inhibited wound closure (Figure 1A), quantified by threshold analysis of the open wound area (Figure 1B), as previously described (9) (see Methods). Adenoviral transduction of a kinase-negative CaMKIIδ2 has been shown by our laboratory to inhibit CaMKIIδ2 catalytic activity, both in vitro and in intact cells (40). Similar to siRNA mediated knockdown of CaMKII, over-expression of the kinase-negative CaMKIIδ2 mutant also suppressed VSM cell migration in the scratch wound (Figure 1A,B). Together, these data indicate a positive role for CaMKIIδ2 in regulating cell migration.
Figure 1. CaMKIIS2 promotes VSM cell migration.
A: VSM cells were electroporated with control siRNA, CaMKIIδ2 siRNA, or transduced with a kinase-negative CaMKIIδ2 adenoviral construct. Confluent cultures were scrape wounded and allowed to migrate for 12 hr. Photomicrographs are fixed and stained cells imaged at 10X magnification. The white bar equals 100 um. B: The open wound area at 12 hr was quantified using threshold analysis and Image J software. Values are means ± SE, n = 3. * P < 0.05 C: CaMKIIδ2 levels determined by immunoblot (IB: CaMKIIδ2) 48 hr after electroporation with control siRNAs (lane 1 and 3), a single siRNA oligo (Ambion; lane 2) or a pool of siRNA (Dharmacon; lane 4) that specifically targets rat CaMKIIδ.
In order to gain insight into potential mechanisms by which CaMKII exerts its effect on the complex process of cell migration, temporal and spatial activation of CaMKIIδ2 was first assessed using an antibody that specifically recognizes the thr287 auto-phosphorylated form of the kinase (Figure 2). Previous studies have validated the utility of this antibody using Western blotting approaches (16; 29; 40) and control experiments using Ca2+-depletion protocols and siRNA knockdown of CaMKII were used to validate usefulness of the antibody using immunofluorescence approaches (supplemental figure 1). Scratch wounding produced a rapid increase in CaMKII thr287 autophosphorylation, reflective of CaMKII activation, apparent in cells near the wound edge (Figure 2A). Staining in cells away from the wound edge serve as an internal control and reflect patterns in unwounded monolayers. As an alternative approach, Western blot analysis of lysates from VSM monolayers with multiple parallel scratch wounds also indicated transient activation of CaMKIIδ2 within 30 seconds post-wounding (Figure 2B). Ionomycin, a calcium ionophore, was used as a positive control to induce maximal CaMKII activation. Quantification of the phospho-thr287 signal normalized to total CaMKII expression indicated a peak 2.5 fold increase in active CaMKII under these conditions returning to levels not different from control by 45 minutes (Figure 2C). These data indicate acute activation of CaMKIIδ2 in response to wounding, consistent with a regulation of early events involved in the initiation of VSM cell migration.
Figure 2. Wound-induced activation of CaMKIIδ2.
A : VSM monolayers were scrape wounded for 30”, fixed and processed for immunofluorescence using antibody specific for CaMKII autophosphorylated on thr287 (IF: P-CaMKIITHR287) or total CaMKIIδ2 (IF:CaMKII). Representative 10x (left panel) and 20x (right panel) images are shown. B: VSM cell monolayers were wounded with multiple scratches and a time course of CaMKII activation determined by immunoblotting with the thr287 phospho-site specific antibody (IB: P-CaMKIITHR287). β-actin served as a loading control. C: Phosphorylated CaMKII signal was normalized to total CaMKII and expressed as a fold increase over that from a non-wounded monolayer. Values are means ± SE, n = 3.
CaMKII activation in the leading edge
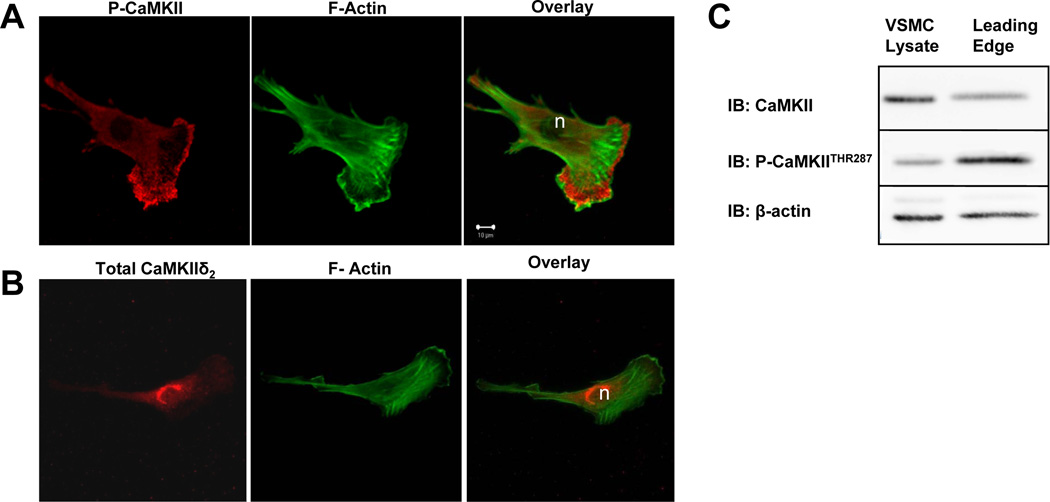
Effects of a multifunctional protein kinase like CaMKII may in part be dependent upon localized activation (23). Knowledge of the spatial distribution of active CaMKII could provide insight into potential cellular processes and proteins targeted by the kinase. To investigate the localization of active CaMKII in spreading VSM cells, indirect immunofluorescence of phospho-thr287 or total CaMKIIδ2 antibody distribution was visualized using confocal microscopy and distribution of FITC-conjugated phalloidin was used to localize filamentous actin. The results indicated a distinct distribution of activated CaMKII in lamellipodia with a pattern that was non-overlapping with either cortical or stress fiber filamentous actin (Figure 3A). This pattern contrasts with the distribution of total CaMKIIδ2 which localizes throughout the cell with a perinuclear concentration ((25); Figure 3B). Biochemical analysis of a leading edge fraction isolated using a 3 µm pore transwell apparatus confirmed activated CaMKII in this cellular fraction, judged by comparing the Western blot signals of thr287 phospho-CaMKII vs. total CaMKII (Figure 3C). Quantitation of immunoblots from three separate experiments indicates a phospho-CaMKII:total CaMKIIδ2 signal ratio of 1.22±0.26 in the leading edge versus 0.64±.06 for the cell lysate, reflecting an approximate two-fold increase in CaMKII activation in the leading edge.
Figure 3. Localization of activated CaMKIIδ2 in spreading VSM cells.
VSM cells plated on collagen-coated coverslips were fixed and imaged using confocal immunofluorescence microscopy with antibody specific for (A) CaMKII autophosphorylated on thr287 (red) and FITC-conjugated phalloidin for filamentous actin (green) or (B) total CaMKII (red) and FITC-conjugated phalloidin. C: Representative immunoblots of total (IB: CaMKII) and activated CaMKIIδ2 assessed with antibody specific for CaMKII autophosphorylated on thr287 (IB:P-CaMKII) in leading edge fractions of VSM isolated using 3um pore transwells (blots are representative of 3 experiments)
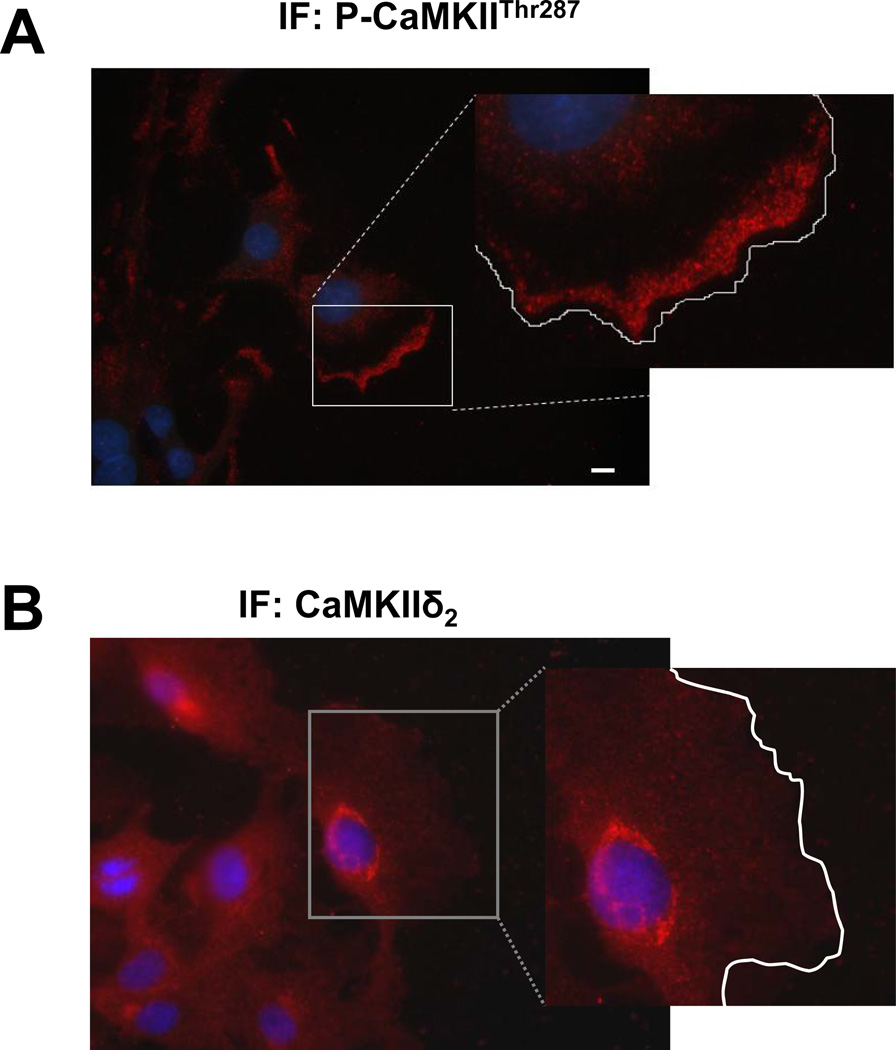
VSM cell polarization, with formation of a leading edge is apparent within 4–6 hr following scratch wounding. Figure 4A demonstrates localization of activated CaMKII in the leading edge of VSM cells at the wound edge 6 hr after wounding. Total CaMKII distribution in the migrating cells was concentrated in the perinuclear area similar to that observed in spreading (Fig. 3B) or stationary cells (25). These results indicate selective activation of a pool of CaMKIIδ2 in the leading edge of spreading or migrating VSM, and are consistent with previous biochemical results that demonstrate adhesion and spreading of VSM cells on multiple extracellular matrix coated substrates is a stimulus for CaMKII activation and downstream signaling (29). Repeated cytosolic calcium transients have been demonstrated to occur during migration of VSM cells (45) and may account for the increase in autophosphorylated CaMKII at this 6 hour time point.
Figure 4. Localization of activated CaMKII in migrating VSM cells.
Active CaMKII was localized in VSM cells at the wound edge 6 hr after wounding using confocal immunofluorescence microscopy with an antibody specific for phosphorylated Thr287 (P-CaMKIITHR287,Panel A) or total CaMKIIδ2 (IF: CaMKIIδ2, Panel B)). Nuclei are stained blue with dapi. Cell periphery is outlined in white in the enlarged insets. White bar is 10 µm.
CaMKIIδ2 regulates VSM polarization
Cells undergoing directional migration maintain a polar morphology with a leading lamella, driven by actin polymerization and focal adhesion dynamics, and a retracting trailing edge regulated by cytoskeletal contraction and dissolution of focal adhesions. In many cells, polarization of intracellular membrane traffic accompanies and promotes cellular polarity (27; 50). Polarized activation of CaMKII in the leading edge raised the possibility that it could act to regulate VSM cell polarity, accounting for positive function in regulating VSM migration. Figure 5 demonstrates the effect of CaMKII suppression using siRNA on VSM cell polarity in cells at the wound edge. Control cells typically exhibit a defined leading edge oriented towards the open area of the wound and polarization of the Golgi apparatus typically to a position between the nucleus and leading lamellipodia (Figure -5A,B). In CaMKIIδ2 deficient cells, the leading edge was characteristically irregular with multiple protrusions and the Golgi apparatus appeared poorly organized and or localized in directions away from the wound edge (Figure 5C,D). These single section confocal images suggest less total Golgi staining with CaMKII suppression, however Golgi content was not quantified in these experiments. Insets illustrate non-wounded VSM cells with the same staining regimen. This experiment suggests a role for CaMKIIδ2 in regulating cellular polarization as measured by both Golgi reorientation and leading edge formation.
Figure 5. Lamellipodia formation and Golgi orientation in cells treated with CaMKII siRNA.
Representative confocal immunofluorescence images of VSM cells treated with either control (A,B) or CaMKII siRNA (C,D). Cells at the wound edge 5.5 hours post-wounding were fixed and processed for immunofluorescence to detect phalloidin labeled f-actin (red), GM130 a Golgi marker (green) and nucleus (blue). Narrow arrows indicate typical smooth leading edge morphology in control cells. Large arrowheads denote aberrant leading edge morphology in CaMKIIδ2 siRNA treated cells. The insets show non-wounded VSM cells with the same staining regimen.
CaMKIIδ2 promotes Golgi polarization
Given the importance of Golgi polarization as an early step in acquiring and promoting cellular polarity necessary for directional migration (28), additional experiments were performed to quantify these effects and confirm the morphological observations. In these experiments, an adenoviral construct was used to transduce a short hairpin siRNA targeting CaMKIIδ gene products. GFP was also encoded by the construct under a separate promoter allowing for identification of transduced cells. This construct has been used in previous studies and was demonstrated to efficiently suppress CaMKIIδ2 protein expression 48–96 hr following infection (20; 29). After identification of transduced cells at the wound edge, Golgi polarization in those cells was characterized as shown in Figure 6A and as described in detail in the Methods section. Based on an increase in fraction of polarized cells relative to the wound edge and decrease in non-polarized cells, a significant percent of control cells reoriented their Golgi apparatus within 4 hr after wounding and prior to the initiation of migration (Figure 6B). In contrast, in CaMKIIδ2 deficient cells, there were fewer cells with a polarized morphology and more non-polarized cells at all time points, and Golgi reorientation towards the wound edge after 4 hr was not statistically different from 0 hr (Figure 6B). The rather strict criterion for polarization towards the wound edge may have resulted in drop out of cells with leading edges oriented obliquely to the wound edge from the analysis. However, the reciprocal changes in non-polarized vs. polarized towards the wound edge cells suggest that this was not a major factor in minimizing or maximizing effects. There were no statistically significant changes in the percentage of cells that were polarized in directions away from the wound edge between control and knockdown cells (data not shown). This analysis indicates that CaMKIIδ2 promotes Golgi polarization in VSM cells, a process known to be important for the establishment and reinforcement of leading edge dynamics and establishment of cellular polarity necessary for directed migration.
Figure 6. CaMKIIδ2 regulates Golgi polarization.
A: VSM cells treated with either control or CaMKIIδ-specific siRNA were subjected to a scratch wounding, stained for Golgi (GM130; red), nuclei (dapi; blue), and filamentous actin (phalloidin; green), and analyzed for Golgi orientation as described in Material and Methods. The yellow line indicates the direction of the wound. B: Quantification of class 2 polarized and class 3 non-polarized cells in control (open square) and CaMKIIδ2 suppressed (closed triangle) cells. Values are means ± SE, n = 3. * P < 0.05 siCaMKII vs. control; + P < 0.05 compared to 0 hr.
CaMKIIδ2 promotes rac activation
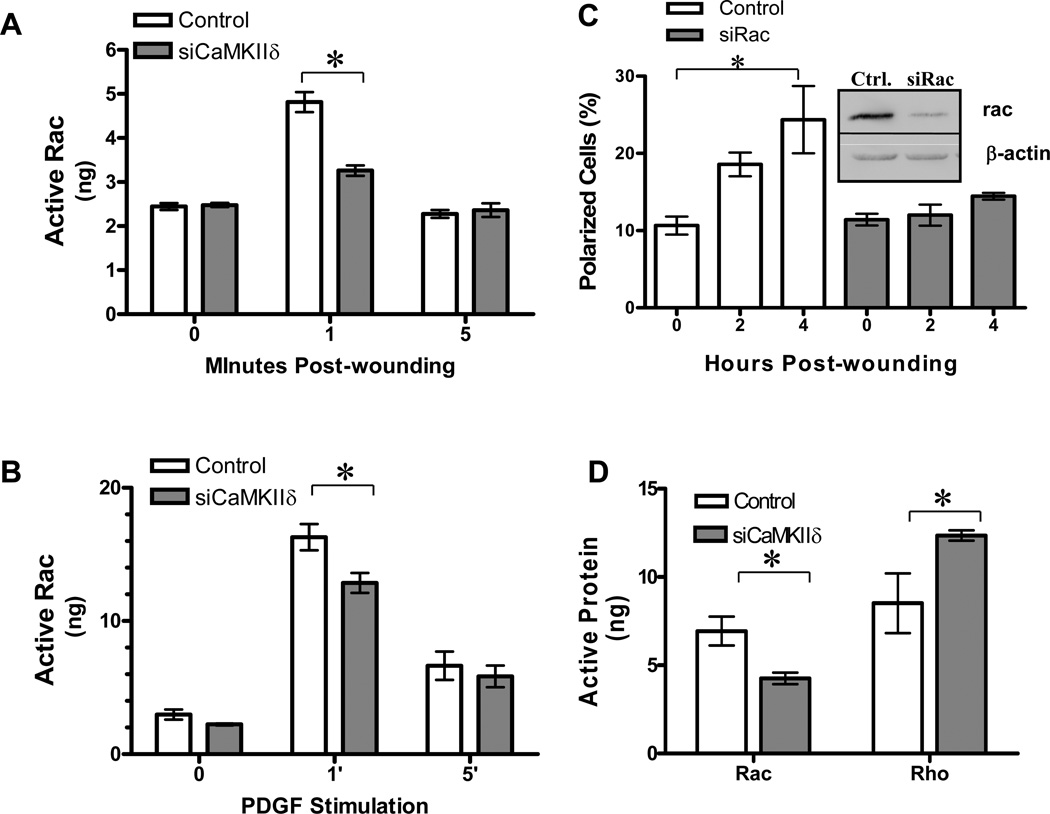
The Rho GTPase Rac is active at the leading edge of polarized cells and has been found to be a key factor in regulating diverse aspects of cell polarity and migration (41). In order to determine if the effects of CaMKII on regulation of VSM polarity and migration were proximal or distal to Rac activation we measured scratch wound-induced GTP-bound Rac in VSM cell lysates from control or CaMKIIδ suppressed cells (Figure 7A). Wounding resulted in a 2-fold increase in active Rac, a response that was inhibited approximately 70% in CaMKII depleted cells. As a control, stimulation of VSM cells with PDGF transiently induced a 10-fold increase in active Rac (Figure 7B), consistent with the literature (5). Suppression of CaMKIIδ2 protein significantly decreased active Rac in response to PDGF, demonstrating a partial dependence on CaMKIIδ2 activity. The lower level of Rac activation in response to wounding compared to PDGF stimulation is most likely explainable by a localized response in those cells at the wound edge.
Figure 7. CaMKII-dependent activation of Rac and Rac-dependent polarization of VSM cells.
Cells were treated with either control or CaMKIIδ specific siRNA 48 hours before scrape wounding (A) or addition of platelet derived growth factor (PDGF; 10ng/ml) (B). GTP-bound Rac was quantified by ELISA as described in Materials and Methods. C: VSM cells treated with either control or Rac-specific siRNA were subjected to a scratch wounding and analyzed for Golgi polarized towards the wound edge as described in Figure 6. Polarized cells are expressed as a percentage of total number of cells. Rac levels were determined by immunoblot (IB:Rac) 48 hr after Electroporation with control or Rac specific siRNA. D: Active Rac and Rho in VSM cell leading edge fractions isolated with 3 µm pore transwells. Values are means ± SE, n=3. *P < 0.05.
The Rho family GTPases are implicated in regulation of cytoskeletal dynamics leading to cell polarity (3; 12; 18). We therefore tested the hypothesis that Rac is necessary for Golgi polarization in cultured VSM cells. Cells electroporated with Rac-specific siRNA lost 68% +/− 3.3% of their Rac protein within 48 hours after introduction (Figure 7C inset). Following scratch wounding, control cells increased their proportion of polarized Golgi with increasing time post-wounding while Rac suppressed cells failed to reorient their Golgi (Figure 7C). These data directly implicate Rac in VSM cell Golgi polarization.
Reciprocal regulation of Rac and Rho activity in the leading edge has been reported to be an important mechanism in maintaining cell polarity during migration (41). These GTPases act in concert to correctly reorganize the cytoskeleton in response to migratory clues. Similar to the analysis of whole cell lysates, Rac activation was significantly suppressed in leading edge fractions from CaMKIIδ2 –depleted cells (Figure 7D). This decrease in active Rac protein was not due to a decrease in total Rac protein levels in the fraction (data not shown). A concomitant 50% increase in Rho activity was observed in leading edge fractions from CaMKIIδ-depleted cells, resulting in a 3-fold increase in the Rho/Rac activity ratio. Combined, these experiments indicate that CaMKIIδ2 promotes rac activation in VSM cells and may be a particularly important factor in regulating wound induced rac activation in leading edges affecting leading edge dynamics.
CaMKIIδ2 promotes wound-induced ERK1/2 activation
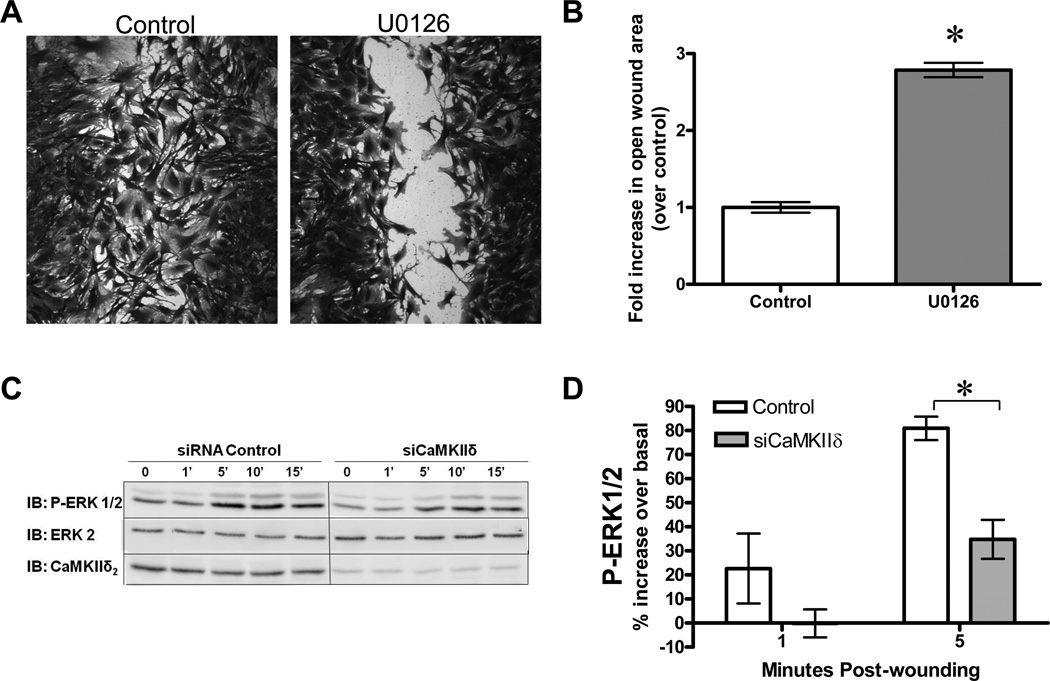
A positive role for CaMKII in ERK1/2 activation is well established in VSM cells (16; 29; 35) and ERK activation presents a potential mechanism by which CaMKII may exert functional effects on VSM polarity and migration. Inhibition of ERK activation with the MEK inhibitor U0126 resulted in significant inhibition of VSM cell migration (Figure 8A,B), supporting previous work that implicates ERK as a positive modulator of migration (26). Moreover, ERK1/2 activation was increased in response to monolayer wounding maximally within 5 min (Figure 8C), consistent with previous results in VSM cells (34). CaMKIIδ2 suppression significantly inhibited ERK activation at in response to monolayer wounding (Figure 8D), implicating a role for CaMKIIδ2 as a proximal regulator of ERK activation.
Figure 8. CaMKII mediates wound induced ERK1/2 activation in VSM cell monolayers.
A: VSM cell migration 12 hr following wounding of vehicle control or U0126 (10µM) treated cells to inhibit ERK1/2 activation. B: Monolayers from A were fixed, stained, imaged at 10x magnification and analyzed using ImageJ (NIH) to quantify the open wound area. Values are means ± SE, n = 3. *P < 0.05 C: ERK1/2 activation in cells treated with control siRNA and siRNA targeting CaMKIIδ. ERK activation was assessed by immunoblotting with an antibody that recognizes active phosphorylated form of Erk1/2 (IB: P-Erk1/2). Total ERK2 and CaMKIIδ2 was determined to control for loading and efficacy of CaMKIIδ2 suppression. D: Densitometric quantification of blots shown in C. Values are means ± SE, n=3. *P < 0.05.
ERK1/2 does not mediate CaMKII-dependent regulation of VSM cell polarization
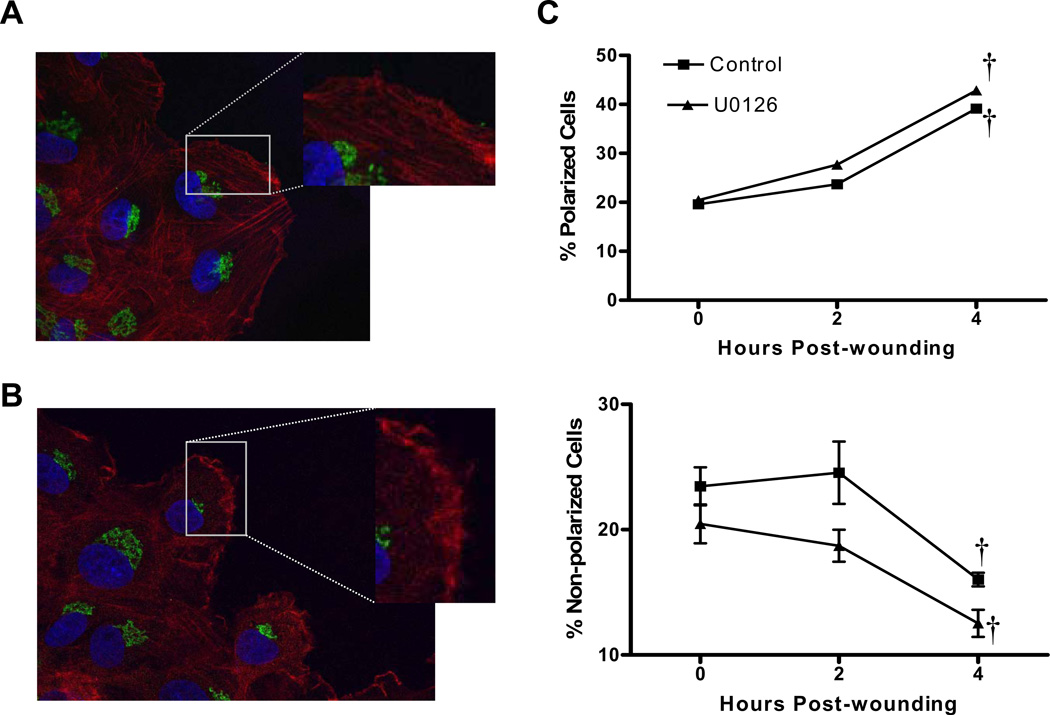
In order to test the function of ERK as a mediator of CaMKII effects on VSM polarization, leading edge morphology and Golgi polarizations was assessed in U0126-treated cells (Figure 9). Inhibition of ERK activation with U0126 resulted in changes in leading edge morphology (Figure 9B), however the phenotype characterized by increased membrane ruffling and filopodia formation was different than that observed in cells depleted of CaMKIIδ2 (Figure 5C,D). Additionally, pre-treatment of VSM with U0126 had no significant effects on Golgi organization or polarization toward the wound edge (Figure 9C), in contrast to results observed in CaMKIIδ-depleted cells (Figure 6B). These data implicate CaMKIIδ2 as a proximal mediator of wound-induced ERK1/2 activation, but this pathway fails to account for the effects of CaMKIIδ2 in promoting VSM polarization.
Figure 9. Effects of ERK1/2 inhibition on wound-induced VSM cell polarization.
Representative immunofluorescence images of wound edge VSM cells treated with vehicle control (A) or 10µM U0126 (B) 30 minutes prior to and after wounding. Cells were stained for actin (phalloidin; red), Golgi (GM130; green) and nucleus (Dapi; blue). C: Golgi polarization was quantified as described in Figure 5. Distribution of class 2 polarized and class 3 non-polarized cells were not significantly different between control and U0126 treated cells. Values are means ± SE, n=3. †P < 0.05 compared to 0 hr.
Discussion
The overall objective of this study was to understand how multifunctional CaMKII enables Ca2+−dependent control of VSM cell migration. In this study we used molecular loss-of-function approaches to assess regulation of VSM migration by endogenous CaMKII using a scratch wound healing assay. Suppression of CaMKIIδ2 protein expression in cultured VSM cells using siRNA technology, or inhibiting activity by over-expressing a kinase-negative mutant, inhibited cell migration in this model system. The wound healing assay also provided the opportunity for assessing CaMKII activation dynamics and it was determined that the kinase is both activated acutely upon monolayer wounding, as well as in a sustained manner localized in the leading edge of migrating cells. The results advance understanding of mechanisms underlying CaMKII regulation by demonstrating for the first time a positive function for the kinase in regulating VSM cell polarization assessed by Golgi reorganization, leading edge morphology, and Rac/Rho activity gradients. Although the specific CaMKII protein targets remain to be determined, our results indicate that downstream signaling to ERK1/2 does not account for the observed effects on cell polarization. Overall, these data indicate an important role for CaMKIIδ2 in directing VSM cell migration by regulating leading edge dynamics and cellular polarity.
Rapid activation of CaMKIIδ2 in response to VSM monolayer wounding confirms one previous report using this approach (53) and is also consistent with previously reported wound-induced stimulation of Ca2+ transients and ERK1/2 activation in VSM cells (34). Active ERK1/2 phosphorylates regulatory proteins in various cellular compartments including the nucleus, cytosol, membrane, and cytoskeleton (48) and has been reported to regulate aspects of cell migration in a number of systems, including VSM (30). This was confirmed in the present study by demonstrating that pharmacological inhibition of ERK1/2 activation results in suppression of VSM migration and disruption of leading edge morphology. In addition, the current results provide new evidence for a mechanistic link between CaMKII activation and ERK1/2 activation in this setting and are consistent with a number of previous reports linking activation of CaMKII to ERK1/2 signaling in both cultured and intact VSM (1; 2; 16). However, ERK activation did not account for effects of CaMKII in regulating Golgi polarization, indicating alternative mechanisms by which CaMKII affects the complex events involved in cell migration.
Rapid CaMKIIδ2 activation in response to wounding is consistent with a function in initiating early signaling pathways and events important in cell migration. To our knowledge, this study for the first time demonstrates involvement of the kinase in regulation of cell polarity as indicated by a dependence of wound-induced Rac activation, Golgi reorganization, and leading edge morphology on CaMKIIδ2 expression. Mechanistic relationships between these indices of cell polarization were not determined in the present study, and based on extensive literature using other cell systems it is expected that they will be complex. For example, Golgi polarization is tightly linked to lamellipodia formation and reinforcement by providing asymmetric delivery of membrane and membrane proteins to the dynamic leading edge (28).
Rho family GTPases are also known to be intimately involved in regulating multiple aspects of cell migration (18). Rac activity has been shown to be functionally important in growth factor-stimulated VSMC migration (10) and a number of studies have demonstrated localized Rac activation at the leading edge of migrating cells where it regulates cytoskeleton and focal adhesion dynamics (33). Rac has also been found to positively regulate Golgi polarization in migrating keratinocytes (7; 39), results now confirmed here in VSM. Thus, CaMKII-dependent activation of Rac in both isolated leading edge of migrating cells and in whole cell lysates could account for the observed effects of CaMKIIδ suppression on Golgi polarization and leading edge morphology. We also observed an increase in Rho activity concomitant with the CaMKIIδ2 dependent decrease in Rac activity in the leading edge. This is consistent with the literature in that Rac and Rho have an antagonistic relationship and must be tightly regulated to ensure proper signaling for directed cell migration (41). For example, in NIH 3T3 fibroblasts, Rac signaling was shown to antagonize Rho activity directly at the GTPase level, and the balance between Rac and Rho activities determined cellular morphology and migration (43).
In considering potential mechanisms by which CaMKII might activate Rac, Tiam1 ( a Rac GEF), has been shown to be phosphorylated on threonine residues upon treatment of fibroblasts with lysophophatidic acid (LPA) and PDGF (13). In vitro studies indicated that phosphorylation of Tiam1 by CaMKII, but not PKC, enhanced Rac nucleotide exchange rates, which was abrogated by pre-treatment with a protein phosphatase (13). Importantly, threonine phosphorylation of Tiam1 and associated membrane translocation was prevented by pharmacological inhibitors of CaMKII (5). Future experiments using molecular approaches to manipulate CaMKII expression of activity, such as those applied in the present study, could be used to test this specific mechanism in migrating VSM cells.
A key finding in the present study was asymmetric activation of CaMKII in the leading edge region, in a cellular domain that was non-overlapping with f-actin. The pattern of localization alone suggests a potential role for CaMKIIδ2 in regulating leading edge dynamics, perhaps by regulating actin filament polymerization, contractile events, or focal adhesion stability and turnover. In this regard CaMKII and/or ERK1/2 have been implicated in regulating cytoskeletal interactions in differentiated VSM (32; 42) and ERK1/2 has been implicated in regulating both actin/myosin interactions (26) and focal adhesion dynamics (8; 24; 49) in several cultured cell systems.
Conflicting data in the literature indicate either net positive (2; 38) or negative (40) functions for CaMKII in regulating VSM cell migration using a modified Boyden chamber assay. Some of the conflicting data may be explained by non-specific effects of pharmacological approaches, or even molecular approaches used to over-express a kinase which is multifunctional and may exhibit non-catalytic scaffolding activity (23). Regardless, the loss-of-function approaches used here have the advantage of targeting endogenous CaMKIIδ2 which has been found to be specifically upregulated in synthetic phenotype and cultured VSM (20; 21; 47) and to contribute to VSM migration in vivo in response to vascular injury (21). Based on the data reported here, we propose a model whereby monolayer wounding results in CaMKIIδ2 activation which positively regulates VSM cell polarization and downstream signaling events which promote cell migration, including rac and ERK1/2 activation. Once cell polarity and a leading edge is established, localized activation of CaMKII may further promote leading edge dynamics facilitating VSM cell migration. The current studies provide rationale and groundwork for more detailed studies to evaluate mechanisms of leading edge CaMKII activation and regulation of leading edge dynamics.
Supplementary Material
Reference List
- 1.Abraham ST, Benscoter HA, Schworer CM, Singer HA. A role for Ca2+/calmodulin-dependent protein kinase II in the mitogen-activated protein kinase signaling cascade of cultured rat aortic vascular smooth muscle cells. Circ Res. 1997;81:575–584. doi: 10.1161/01.res.81.4.575. [DOI] [PubMed] [Google Scholar]
- 2.Bilato C, Curto KA, Monticone RE, Pauly RR, White AJ, Crow MT. The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the alphavbeta3 integrin complex is reversed by activated calcium/calmodulin-dependent protein kinase II. J Clin Invest. 1997;100:693–704. doi: 10.1172/JCI119582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;2:241–255. 348 Pt. [PMC free article] [PubMed] [Google Scholar]
- 4.Block LH, Buhler FR. Atherosclerosis, cell motility, calcium, and calcium-channel blockers. J Cardiovasc Pharmacol. 1992;2(19 Suppl):S1–S3. doi: 10.1097/00005344-199219002-00002. [DOI] [PubMed] [Google Scholar]
- 5.Buchanan FG, Elliot CM, Gibbs M, Exton JH. Translocation of the Rac1 guanine nucleotide exchange factor Tiam1 induced by platelet-derived growth factor and lysophosphatidic acid. J Biol Chem. 2000;275:9742–9748. doi: 10.1074/jbc.275.13.9742. [DOI] [PubMed] [Google Scholar]
- 6.Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choma DP, Pumiglia K, DiPersio CM. Integrin {alpha}3{beta}1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117:3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- 8.Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, Ware M, Jones PL, Weed SA, DeBiasi RL, Oka Y, Tyler KL, Johnson GL. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. EMBO J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai L, Alt W, Schilling K, Retzlik J, Gieselmann V, Magin TM, Kappler J. A fast and robust quantitative time-lapse assay for cell migration. Exp Cell Res. 2005;311:272–280. doi: 10.1016/j.yexcr.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Doanes AM, Irani K, Goldschmidt-Clermont PJ, Finkel T. A requirement for rac1 in the PDGF-stimulated migration of fibroblasts and vascular smooth cells. Biochem Mol Biol Int. 1998;45:279–287. doi: 10.1080/15216549800202652. [DOI] [PubMed] [Google Scholar]
- 11.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 12.Evers EE, Zondag GC, Malliri A, Price LS, ten Klooster JP, van der Kammen RA, Collard JG. Rho family proteins in cell adhesion and cell migration. Eur J Cancer. 2000;36:1269–1274. doi: 10.1016/s0959-8049(00)00091-5. [DOI] [PubMed] [Google Scholar]
- 13.Fleming IN, Elliott CM, Buchanan FG, Downes CP, Exton JH. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem. 1999;274:12753–12758. doi: 10.1074/jbc.274.18.12753. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil. 1997;18:1–16. doi: 10.1023/a:1018616814417. [DOI] [PubMed] [Google Scholar]
- 15.Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988;62:749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- 16.Ginnan R, Singer HA. CaM kinase II-dependent activation of tyrosine kinases and ERK1/2 in vascular smooth muscle. Am J Physiol Cell Physiol. 2002;282:C754–C761. doi: 10.1152/ajpcell.00335.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hainaud P, Bonneau M, Pignaud G, Bal dit SC, Andre P, Hadjiisky P, Fieffe JP, Caen JP, Herbert JM, Dol F, Drouet LO. The calcium inhibitor SR33805 reduces intimal formation following injury of the porcine carotid artery. Atherosclerosis. 2001;154:301–308. doi: 10.1016/s0021-9150(00)00487-1. [DOI] [PubMed] [Google Scholar]
- 18.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 19.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.House SJ, Ginnan RG, Armstrong SE, Singer HA. Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C2276–C2287. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- 21.House SJ, Singer HA. CaMKII-δ Isoform Regulation of Neointima Formation Following Vascular Injury. Arteriosclerosis, thrombosis, and vascular biology. 2007 doi: 10.1161/ATVBAHA.107.156810. In Press. [DOI] [PubMed] [Google Scholar]
- 22.Huang P, Hawthorne WJ, Peng A, Angeli GL, Medbury HJ, Fletcher JP. Calcium channel antagonist verapamil inhibits neointimal formation and enhances apoptosis in a vascular graft model. Am J Surg. 2001;181:492–498. doi: 10.1016/s0002-9610(01)00615-8. [DOI] [PubMed] [Google Scholar]
- 23.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunger-Glaser I, Salazar EP, Sinnett-Smith J, Rozengurt E. Bombesin, lysophosphatidic acid, and epidermal growth factor rapidly stimulate focal adhesion kinase phosphorylation at Ser-910: requirement for ERK activation. J Biol Chem. 2003;278:22631–22643. doi: 10.1074/jbc.M210876200. [DOI] [PubMed] [Google Scholar]
- 25.Jones RJ, Jourd'heuil D, Salerno JC, Smith SM, Singer HA. iNOS regulation by calcium/calmodulin-dependent protein kinase II in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2634–H2642. doi: 10.1152/ajpheart.01247.2006. [DOI] [PubMed] [Google Scholar]
- 26.Klemke RL, Cai S, Giannini AL, Gallagher PJ, Lanerolle Pd, Cheresh DA. Regulation of Cell Motility by Mitogen-activated Protein Kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi Apparatus and the Microtubule-Organizing Center in Cultured Fibroblasts at the Edge of an Experimental Wound. PNAS. 1982;79:2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauffenburger DA, Horwitz AF. Cell Migration: A Physically Integrated Molecular Process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 29.Lu KK, Armstrong SE, Ginnan R, Singer HA. Adhesion-dependent activation of CaMKII and regulation of ERK activation in vascular smooth muscle. Am J Physiol Cell Physiol. 2005;289:C1343–C1350. doi: 10.1152/ajpcell.00064.2005. [DOI] [PubMed] [Google Scholar]
- 30.Lundberg MS, Curto KA, Bilato C, Monticone RE, Crow MT. Regulation of vascular smooth muscle migration by mitogen-activated protein kinase and calcium/calmodulin-dependent protein kinase II signaling pathways. J Mol Cell Cardiol. 1998;30:2377–2389. doi: 10.1006/jmcc.1998.0795. [DOI] [PubMed] [Google Scholar]
- 31.Mancini GB. Antiatherosclerotic effects of calcium channel blockers. Prog Cardiovasc Dis. 2002;45:1–20. doi: 10.1053/pcad.2002.122694. [DOI] [PubMed] [Google Scholar]
- 32.Marganski WA, Gangopadhyay SS, Je HD, Gallant C, Morgan KG. Targeting of a Novel Ca+2/Calmodulin-Dependent Protein Kinase II Is Essential for Extracellular Signal-Regulated Kinase-Mediated Signaling in Differentiated Smooth Muscle Cells. Circ Res. 2005;97:541–549. doi: 10.1161/01.RES.0000182630.29093.0d. [DOI] [PubMed] [Google Scholar]
- 33.Michiels F, Habets GGM, Stam JC, van der Kammen RA, Collard JG. A role for Rac in Tiaml-induced membrane ruffling and invasion. Nature. 1995;375:338–340. doi: 10.1038/375338a0. [DOI] [PubMed] [Google Scholar]
- 34.Moses S, Dreja K, Lindqvist A, Lovdahl C, Hellstrand P, Hultgardh-Nilsson A. Smooth Muscle Cell Response to Mechanical Injury Involves Intracellular Calcium Release and ERK1/ERK2 Phosphorylation. Experimental Cell Research. 2001;269:88–96. doi: 10.1006/excr.2001.5308. [DOI] [PubMed] [Google Scholar]
- 35.Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobes CD, Hall A. Rho GTPases Control Polarity, Protrusion, and Adhesion during Cell Movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 38.Pauly RR, Bilato C, Sollott SJ, Monticone R, Kelly PT, Lakatta EG, Crow MT. Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation. 1995;91:1107–1115. doi: 10.1161/01.cir.91.4.1107. [DOI] [PubMed] [Google Scholar]
- 39.Pegtel DM, Ellenbroek SIJ, Mertens AEE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 Complex Controls Persistent Migration by Stabilizing Microtubule-Dependent Front-Rear Polarity. Current Biology. 2007;17:1623–1634. doi: 10.1016/j.cub.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 40.Pfleiderer PJ, Lu KK, Crow MT, Keller RS, Singer HA. Modulation of vascular smooth muscle cell migration by calcium/ calmodulin-dependent protein kinase II-delta 2. Am J Physiol Cell Physiol. 2004;286:C1238–C1245. doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 41.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 42.Rokolya A, Singer HA. Inhibition of CaM kinase II activation and force maintenance by KN-93 in arterial smooth muscle. Am J Physiol Cell Physiol. 2000;278:C537–C545. doi: 10.1152/ajpcell.2000.278.3.C537. [DOI] [PubMed] [Google Scholar]
- 43.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac Downregulates Rho Activity: Reciprocal Balance between Both GTPases Determines Cellular Morphology and Migratory Behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schachter M. Vascular smooth muscle cell migration, atherosclerosis, and calcium channel blockers. Int J Cardiol. 1997;2(62 Suppl):S85–S90. doi: 10.1016/s0167-5273(97)00245-3. [DOI] [PubMed] [Google Scholar]
- 45.Scherberich A, Campos-Toimil M, Ronde P, Takeda K, Beretz A. Migration of human vascular smooth muscle cells involves serum-dependent repeated cytosolic calcium transients. J Cell Sci. 2000;113:653–662. doi: 10.1242/jcs.113.4.653. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–S89. [PubMed] [Google Scholar]
- 47.Schworer CM, Rothblum LI, Thekkumkara TJ, Singer HA. Identification of novel isoforms of the delta subunit of Ca2+/calmodulin-dependent protein kinase II. Differential expression in rat brain and aorta. J Biol Chem. 1993;268:14443–14449. [PubMed] [Google Scholar]
- 48.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 49.Subauste MC, Pertz O, Adamson ED, Turner CE, Junger S, Hahn KM. Vinculin modulation of paxillin-FAK interactions regulates ERK to control survival and motility. J Cell Biol. 2004;165:371–381. doi: 10.1083/jcb.200308011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilghman RW, Slack-Davis JK, Sergina N, Martin KH, Iwanicki M, Hershey ED, Beggs HE, Reichardt LF, Parsons JT. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J Cell Sci. 2005;118:2613–2623. doi: 10.1242/jcs.02380. [DOI] [PubMed] [Google Scholar]
- 51.Van Riper DA, Schworer CM, Singer HA. Ca2+-induced redistribution of Ca2+/calmodulin-dependent protein kinase II associated with an endoplasmic reticulum stress response in vascular smooth muscle. Mol Cell Biochem. 2000;213:83–92. doi: 10.1023/a:1007116231678. [DOI] [PubMed] [Google Scholar]
- 52.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res. 2006;98:868–878. doi: 10.1161/01.RES.0000216596.73005.3c. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Yang Y, Kone BC, Allen JC, Kahn AM. Insulin-stimulated cyclic guanosine monophosphate inhibits vascular smooth muscle cell migration by inhibiting Ca/calmodulin-dependent protein kinase II. Circulation. 107:1539–1544. doi: 10.1161/01.cir.0000056766.45109.c1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.