Summary
Cytotoxic T lymphocytes (CTL) respond to antigenic peptides presented on MHC class I molecules. On most cells, these peptides are exclusively of endogenous, cytosolic origin. Bone marrow-derived antigen-presenting cells, however, harbor a unique pathway for MHC I presentation of exogenous antigens. This mechanism permits cross-presentation of pathogen-infected cells and the priming of CTL responses against intracellular microbial infections. Here, we report a novel diphtheria toxin-based system that allows the inducible, short-term ablation of dendritic cells (DC) in vivo. We show that in vivo DC are required to cross-prime CTL precursors. Our results thus define a unique in vivo role of DC, i.e., the sensitization of the immune system for cell-associated antigens. DC-depleted mice fail to mount CTL responses to infection with the intracellular bacterium Listeria monocytogenes and the rodent malaria parasite Plasmodium yoelii.
Introduction
The initiation of a protective adaptive immune response depends on the coordinated interaction of antigen-specific lymphocytes and so-called “professional” antigen-presenting cells (APC) of the myeloid lineage. During the last decade, there have been major advances in our understanding of the physiological roles of B and T lymphocytes, due in large part to the successful application of gene targeting strategies. Moreover, mice rendered deficient for lymphocyte subsets by ablation of the respective antigen receptors have become standard tools in immunological studies. In contrast, the accessory arm of the adaptive immune system has remained largely refractory to genetic ablation approaches. Thus, the developmental interrelations and differential in vivo functions of mononuclear phagocytes, i.e., macrophages and dendritic cells (DC), remain poorly understood.
DC are specialized migratory APC found as sentinels in periphery tissues and lymphoid organs. DC are potent immunostimulators, and antigen-pulsed DC are currently being tested in vaccinations against infectious agents and tumors in numerous clinical studies (Banchereau and Steinman, 1998). Our knowledge of this particular versatile cell type is mainly based on in vitro assays and in vivo transfer studies involving bone marrow (BM) and cord-blood culture-derived DC. A mouse model lacking DC would be a useful tool to determine the in vivo role of DC in T cell priming and tolerance establishment. However, there is a profound interdependence of the steady-state lymphocyte and DC compartments, with the involvement of DC in thymic negative selection and T cells in the control of DC maturation and half-life. Genetic approaches aiming at long-term DC depletion are therefore likely to have major impacts on immune homeostasis.
T lymphocytes respond to antigens that are processed and presented in the form of peptides bound to major histocompatibility complex (MHC) molecules. CD4-positive T helper cells recognize peptides displayed on MHC class II molecules while cytotoxic CD8-positive T lymphocytes (CTL) respond to antigenic peptides presented on MHC class I. Class I molecules of most cells are loaded exclusively with peptides of endogenous, cytosolic origin. These peptides generally reflect the peptidic self. After microbial infection, the spectrum of presented peptides can include pathogen-encoded proteins.
Infected nonhematopoietic cells are, however, incompetent to initiate a MHC class I-restricted CTL response (Lenz et al., 2000; Sigal et al., 1999). Immunosurveillance for intracellular pathogens hence requires sensitivity to cell-associated antigens. To meet this need, bone marrow (BM)-derived APC are equipped with a unique pathway that allows the presentation of exogenous particulate antigens in the context of MHC class I. Exogenous MHC class I presentation is generally TAP transporter dependent (Huang et al., 1996; Regnault et al., 1999) and thought to rely on a specialized phagosome-to-cytosol pathway in BM-derived APC (Kovacsovics-Bankowski and Rock, 1995; Rodriguez et al., 1999).
The existence of an exogenous MHC class I presentation pathway was first indicated by the fact that minor histocompatibility antigens could be transferred between cells for presentation on APC (Bevan, 1976). The observed resulting T cell stimulation was termed “cross-priming” (Bevan, 1976). Transfer of cell-associated antigen resulting in “cross-presentation” on APC was subsequently demonstrated for viral proteins, tumor antigens, and protein-coated spleen cells (for references see Heath and Carbone, 2001).
All three BM-derived APC, i.e., macrophages, DC, and B lymphocytes, have been reported to be competent to present class I-restricted exogenously derived antigens in vitro (for references see Heath and Carbone, 2001). In vitro presentation of exogenous cellular material, i.e., cross-presentation, has been demonstrated for macrophages and DC (Albert et al., 1998; Bellone et al., 1997). Macrophages are, however, poor stimulators of T cells in vitro (Steinman and Cohn, 1973).
In vivo, CTL-cross-priming persists in the absence of B cells (Schoenberger et al., 1998) but is abrogated by depletion of phagocytes (Debrick et al., 1991). This finding led originally to the interpretation that macrophages are the cross-priming APC (Debrick et al., 1991). However, “immature” DC are also highly effective in uptake of exogenous antigen (for references see Steinman and Swanson, 1995). The identity of the phagocyte responsible for in vivo cross-priming has therefore remained unclear. More recently, Kurts et al. reported that transgenic DC-restricted MHC class I expression restores in vivo cross-presentation in MHC class I-deficient mice (Kurts et al., 2001). While this finding establishes that DC are sufficient for in vivo cross-priming, it remains unknown whether the two mononuclear phagocyte subsets, i.e., macrophages and DC, are functionally redundant with respect to CTL priming.
Here, we report a novel diphtheria toxin-based system that allows the inducible in vivo ablation of dendritic cells. We demonstrate an essential in vivo role for CD11c+ DC, as the depletion of these cells abrogates the priming of CTL precursors in immune responses to cell-associated antigens (cross-priming). The physiological relevance of this finding is underlined by the fact that in vivo DC depletion impairs the establishment of CTL responses to infection with the intracellular bacterium Listeria monocytogenes and the murine Malaria parasite Plasmodium yoelii.
Results
A Mouse Model for Inducible In Vivo DC Ablation
To probe for essential DC functions in the intact animal while avoiding the complicating issue of the developmental interdependence of the DC and T cell pools, we have generated a mouse model that allows conditional ablation of DC. Our strategy is based on the fact that murine cells, unlike primate cells, are insensitive to killing by diphtheria toxin (DT) (Pappenheimer et al., 1982). DT is one of the best studied of all bacterial exotoxins (Holmes, 2000). The cytotoxicity of the heterodimeric DT is strictly dependent on receptor-mediated endocytosis. DT enters the cell via interaction of its DT B subunit with the cellular DT receptor (DTR), the heparin binding EGF-like growth factor (hbEGF) (Naglich et al., 1992). Upon endocytosis, the DT A subunit is released and catalyzes ADP-ribosylation of elongation factor 2, resulting in inhibition of protein synthesis. As a consequence, DT induces rapid apoptosis in both mitotic and terminally differentiated cells. DT resistance of murine cells results from the low affinity of the toxin for the rodent hbEGF. Transfer of a primate DTR into mice via transgenesis hence confers DT sensitivity to murine cells. Specificity and timing of cell ablation can be determined by cell type-restricted promoter/enhancer elements and by the regimen of the toxin administration, respectively. A similar conditional ablation strategy was recently used to study hepatocyte function (Saito et al., 2001).
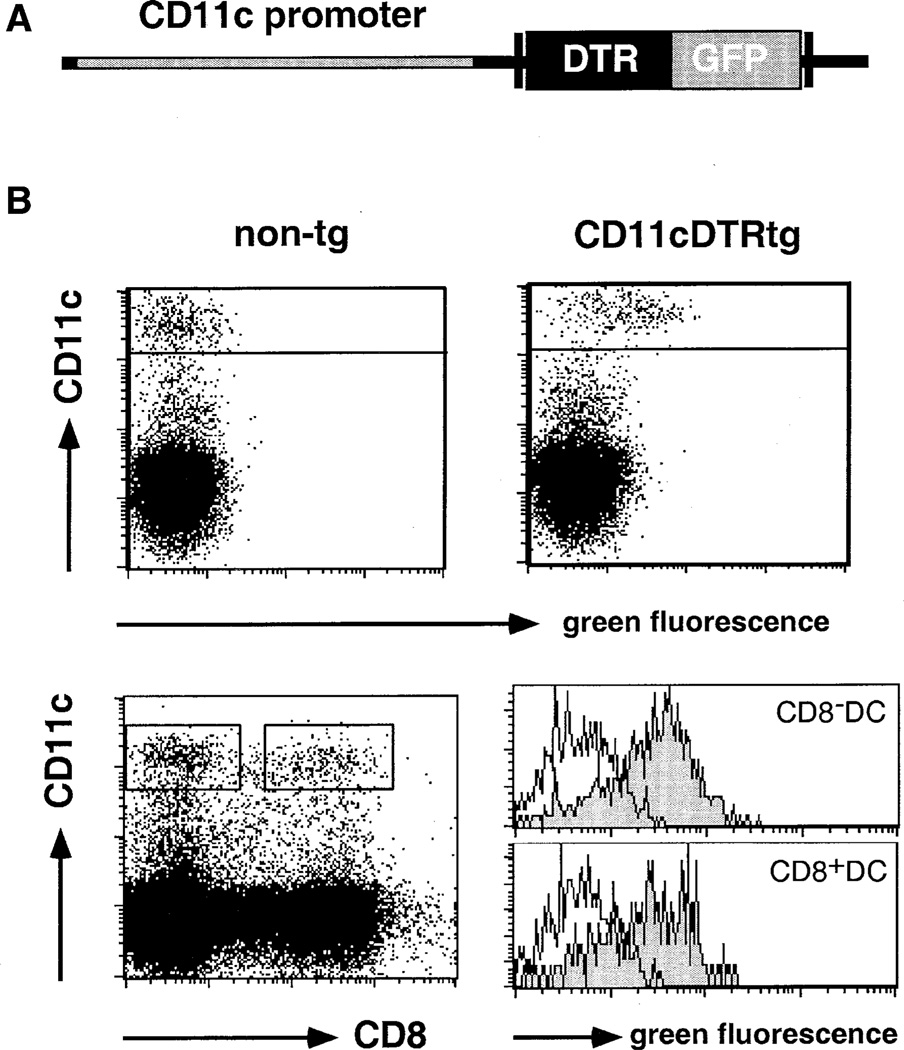
To target DT sensitivity to DC, we generated mice that carry a transgene encoding a simian DTR-GFP (green fluorescent protein) fusion protein under control of the murine CD11c promoter (Brocker et al., 1997) (Figure 1A). CD11c encodes a subunit of the CD11c/CD18 β integrin. All murine DC subsets express CD11c except for CD11cnegative-low epidermal Langerhans’ (LH) cells that upregulate CD11c expression upon maturation. Murine CD11c expression is largely restricted to the DC compartment. However, CD11c expression has also been reported for activated intraepithelial lymphocytes (IEL) and CD8+ LN T cells (Huleatt and Lefrancois, 1995).
Figure 1. Phenotypic Characterization of DTR Transgenic Mice.
(A) Schematic representation of the DTR/GFP transgene.
(B) Flow cytometric analysis of spleen cells of DTR transgenic (line 57) and nontransgenic FVB/N mice indicating GFP expression in CD11c+ DC. Histograms show GFP expression in CD8+ and CD8− DC subsets. Histogram gates are indicated in the dot plot. Open histograms, wild-type cells; filled histograms, DTR transgenic cells. Dead cells were excluded by propidium iodide (PI) staining.
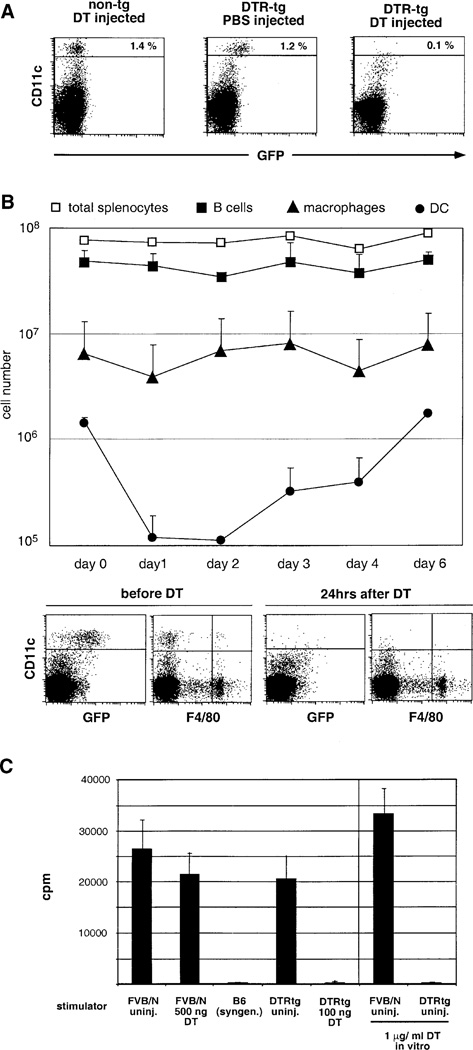
All splenic DC of CD11c-DTR transgenic mice were fluorescently labeled, with DTR-GFP expression on both the CD8− and the CD8+ DC subset (Figure 1B). To investigate toxin sensitivity, we injected CD11c-DTR transgenic mice intraperitoneally (i.p.) with 100 ng of DT (about 4 ng DT/g body weight) and analyzed the splenic DC compartment 1 day later. DT injection led to rapid depletion of CD11c+ DC (Figure 2A). To address whether other splenic APC populations are affected by the DT treatment, we determined the splenic APC counts of CD11c-DTR transgenic mice that had received a single DT injection. The toxin-induced DC depletion persisted for 2 days, after which DC numbers were gradually restored (Figure 2B). B cells and F4/80+ macrophages, however, remained unaffected (Figure 2B). DT injection had no effect on DC populations in nontransgenic littermates (Figure 2A, data not shown). Specificity of the splenic DC depletion was also confirmed by comparative immunohistochemical analysis of cryosections of DT-treated nontransgenic and DTR transgenic BALB/c spleens (see Supplemental Figure S1 at http://www.immunity.com/cgi/content/full/17/2/■■■/DC1). Transient DC depletion was not associated with sign of illness or long-term defects. Repetitive systemic DT application resulted, however, in lethality in DTR-transgenic mice (data not shown).
Figure 2. DT Sensitivity of the DC Compartment in DTR Transgenic Mice.
(A) Flow cytometric analysis of spleen cells of wt and DTR-transgenic (line 57) FVB/N mice 24 hr after injection of 100 ng DT or control vehicle.
(B) Analysis of splenic APC populations of homozygous CD11c-DTR transgenic mice (line 57) after i.p. injection of 100 ng DT(day 0). Data represent mean of two mice per time point and are representative of two independent experiments. B cells are represented by squares and defined as being CD19+; macrophages are represented by triangles and defined as being F4/80+; DC are presented by circles and defined as being CD11c+. Open squares represent total splenocyte counts. Dot blots below the graph show a representative flow cytometric analysis of two time points.
(C) Mixed leukocyte reaction with 8 × 105 stimulator splenocytes (FVB/N; H2q) and 105 responder T cells (C57BL/6; H2b). Data are representative of three experiments with similar results. Note that the MLR was also abrogated by addition of DT during the culture resulting in in vitro DC depletion.
In vitro DC depletion experiments using antibody-mediated complement lysis have shown that splenic DC are of critical importance as stimulators in a primary mixed leukocyte reaction (MLR) (Steinman et al., 1983). We were able to reproduce this observation by in vivo depletion of splenic DC, providing a demonstration of the effectiveness of DT-induced DC ablation. We injected DTR transgenic and nontransgenic FVB/N mice with DT, isolated their spleen cells at 24 hr, and mixed them with allogeneic C57BL/6 T cells. Splenocytes from DT-treated nontransgenic mice provided a strong stimulation in the MLR. However, depletion of DC from the spleen of the CD11c-DTR mice resulted in the complete loss of in vitro stimulation of alloreactive T cells (Figure 2C).
DT-induced DC depletion allows us to investigate the role of DC in in vivo T cell activation. However, in agreement with earlier studies (Huleatt and Lefrancois, 1995), when activated in vitro or in vivo (via graft-versus host reaction) a substantial proportion of CD11c-DTR transgenic CD8+ T cells displayed CD11c promoter activity, i.e., GFP expression (data not shown). These GFP-positive CD8+ T cells were also DT sensitive (data not shown). To circumvent this problem, we have used adoptive transfer of non-DTR transgenic T cells into CD11c-DTR transgenic mice prior to DT treatment. This approach has the added advantage of permitting the use of CFSE-labeled T cell grafts, which allows the monitoring of T cell priming as the fluorescent dye is diluted with cell proliferation. We have used the adoptive transfer approach to study the effect of DC ablation on T cell activation in the systems described below.
Requirement of DC for In Vivo Cross-Priming of CD8+ T Cells
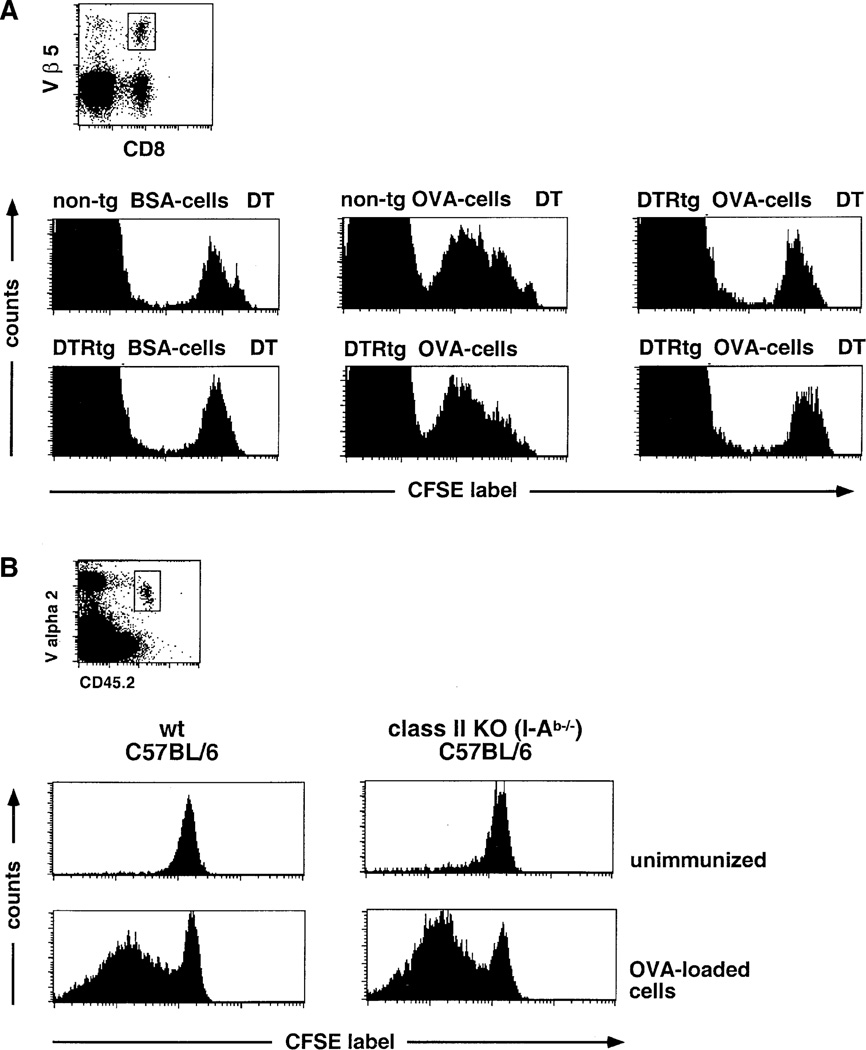
Presentation of exogenous cell-associated antigens (cross-presentation) requires the uptake of the antigen by phagocytosis. Cross-priming of T cells thus depends on the presence of BM-derived mononuclear phagocytes. To investigate the possibility of distinct roles of macrophages and DC in this process, we employed a model system involving the intravenous injection of mice with syngeneic, MHC class I-deficient spleen cells. When the splenocytes are loaded with ovalbumin (Ova), this protocol results in the expansion of Ova-specific CTL in response to antigen presented by host MHC class I molecules (den Haan et al., 2000; Moore et al., 1988). To determine whether DC are required for this in vivo cross-priming, we analyzed the priming of Ova-specific CTL precursors in a DC-depleted spleen. To this end, we adoptively transferred Ova-specific OT-I TCR transgenic T cells (Hogquist et al., 1994) into CD11c-DTR transgenic and nontransgenic C57BL/6 littermates. Two days after transfer of the CFSE-labeled OT-I cells, the animals were treated with DT to deplete DC. Eight hours later, the mice were immunized by i.v. injection with Ova-loaded MHC-class I-deficient (b2m−/−) splenocytes. Spleens were isolated 2 days later, and CFSE-labeled Ova-specific OT-I T cells in the wt and DC-depleted spleens were analyzed for the dilution of CFSE-label as an indicator of in vivo proliferation. As shown in Figure 3A, depletion of DC completely abrogated the priming of the Ova-specific CTL precursors.
Figure 3. DC Dependence of In Vivo CTL Cross-Priming with Cell-Associated Antigen.
(A) Flow cytometric analysis of CFSE-labeled OT-I CD8+ T cell graft (106 cells) in DT-treated nontransgenic and DTR transgenic (line 11) C57Bl/6 mice 2 days after immunization with OVA-loaded β2m−/− splenocytes or control splenocytes loaded with BSA. Histograms represent cells gated according to scatter, CD8, and Vβ5 expression as indicated in dot plots. Note that the large CFSE-negative peak represents host Vβ 5+ CD8 T cells.
(B) Analysis of CFSE-labeled OT-I T cells (CD45.2, 106 cells) 3 days after immunization of wt C57Bl/6 (B6.SJL, CD45.1) and MHC class II-deficient mice (I-Ab −/−, CD45.1) with OVA-loaded β2m−/− splenocytes. Histograms represent cells gated according to scatter, expression of CD8, and the allotypic marker CD45.2 as indicated in dot plots. Dead cells were excluded by PI staining.
In some experimental systems, CTL cross-priming has been reported to be dependent on “licensing” of the APC by cognate CD4+ T cell help (Bennett et al., 1998; Ridge et al., 1998; Schoenberger et al., 1998). However, with the protocol that we used, priming of OT-I CD8+ T cells was unimpaired in CD4 T cell-deficient mice (I-Ab−/−) (Figure 3B). The high CTL-precursor frequencies used in the adoptive transfers thus override the requirement for CD4+ T cell help, as previously described (Wang et al., 2001). The results are therefore consistent with direct impairment of CD8+ T cell priming with cell-associated antigen in DC-depleted mice.
Requirement of DC to Elicit Anti-Listeria CTL Responses
To extend the analysis of CD8+ T cell priming from responses against a model antigen to those specific for a microbial pathogen, we chose to examine priming of T cells by Listeria monocytogenes. This gram-positive microorganism is a facultative intracellular pathogen that infects macrophages and a wide range of nonprofessional phagocytes (Sheehan et al., 1994). After entering the eukaryotic cell, L. monocytogenes escapes into the cytosol, where it secretes proteins that are processed by the MHC class I presentation pathway. Upon infection with sublethal doses of bacteria, mice rapidly clear L. monocytogenes from spleen and liver, resulting in life-long, predominantly CTL-mediated immunity. Protective host CTL responses are directed against listerial antigens presented by both classical MHC class Ia and nonclassical MHC class Ib molecules.
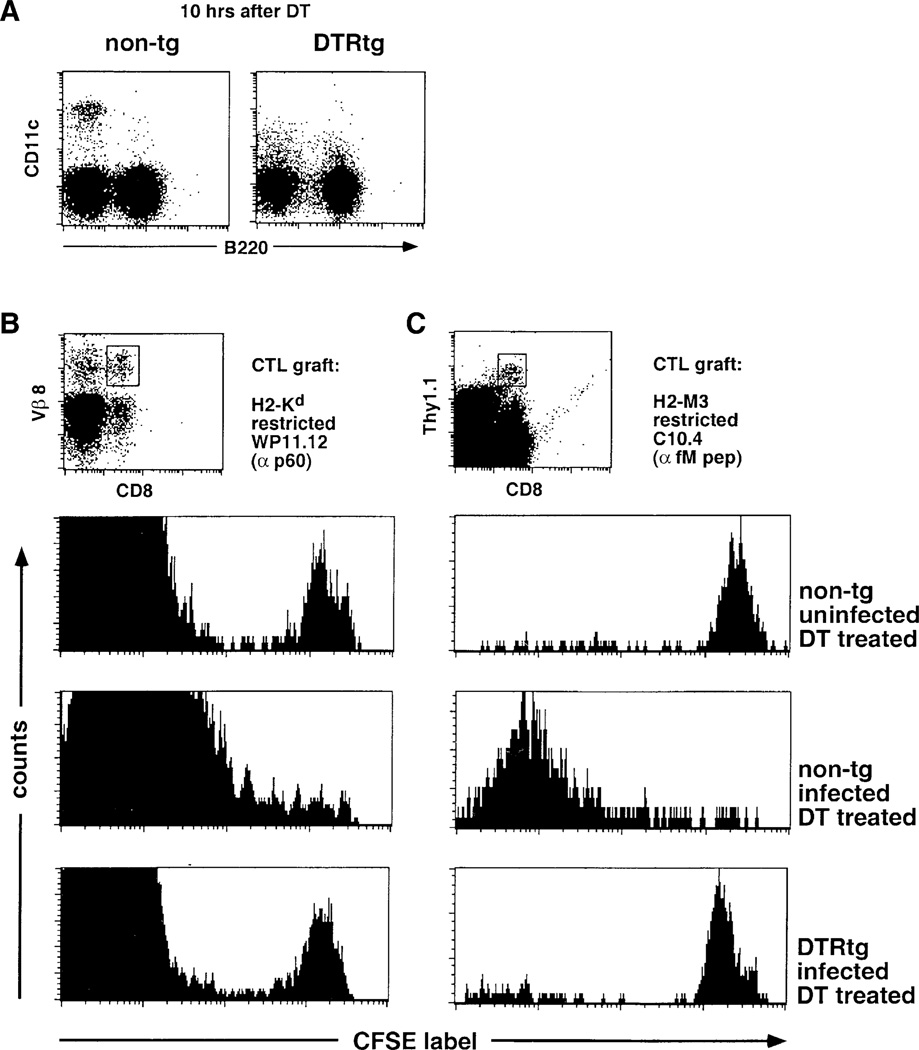
To investigate the role of DC in an immune response toward the intracellular bacterium, CD11c-DTR transgenic and control BALB/c mice received Listeria-specific CFSE-labeled CTL precursor grafts isolated from WP11.12 TCR transgenic mice. This TCR is directed against a peptide derived from the p60 protein secreted by L. monocytogenes into the cytosol of infected cells (Mercado et al., 2000). One day after CD8+ T cell transfer, the recipient mice were treated with DT, which resulted in depletion of splenic DC at 10 hr in the DTR transgenic mice (Figure 4A). Eight hours after DT treatment, the mice were infected intravenously with 2000 live L. monocytogenes. Four days after the infection, we analyzed the in vivo proliferative response of CFSE-labeled TCR transgenic T cells isolated from the spleen. Whereas infection with L. monocytogenes resulted in extensive proliferation of the TCR transgenic T cells in DT-treated control recipient mice, there was no proliferation observed in DT-treated DTR transgenic animals (Figure 4B). The anti-Listeria CTL response was recently shown to be independent of CD4+ T cell help (Lauvau et al., 2001; Hamilton et al., 2001). DC depletion therefore appears to have a direct effect on the priming of CTL precursors.
Figure 4. DC Dependence of Classical and Nonclassical MHC-Restricted Anti-Listeria CTL Responses.
(A) Flow cytometric analysis of splenocyte of nontransgenic and DTR transgenic (line 57) littermate BALB/c mice 10 hr after DT injection (4 ng/g body weight).
(B) Flow cytometric analysis of CFSE-labeled WP11.12 CD8+ T cell graft (106 cells) in DT-treated nontransgenic and DTR transgenic (line 57) BALB/c mice 4 days after i.v. injection of 2000 live L. monocytogenes. Histograms represent cells gated according to scatter, CD8, and Vβ 8 expression as indicated in dot plots. Note that the large CFSE− peak represents host Vβ 8+ CD8 T cells. Dead cells were excluded by PI staining.
(C) Flow cytometric analysis of CFSE-labeled C10.4 CD8+ T cell graft (Thy1.1, 106 cells) in spleens of DT-treated DTR transgenic (line 57) and nontransgenic littermate CB6F1 mice (Thy1.2) 4 days after i.v. injection of 2000 live L. monocytogenes. Histograms represent cells gated according to scatter, CD8, and Thy1.1 expression as indicated in dot plots. Dead cells were excluded by PI staining.
One of the nonclassical MHC-restricted anti-Listeria responses is directed against N-formylated peptides presented in the context of H2-M3 (Pamer et al., 1992). This MHC class Ib-restricted CTL response is sufficient for Listeria clearance (Seaman et al., 1999) and peaks 2 days prior to MHC class Ia-restricted CTL responses (Kerksiek et al., 1999). To investigate whether DC are also essential for the priming of CTL specific for the nonclassical H2-M3 molecule, CFSE-labeled CTL precursors isolated from C10.4 TCR transgenic mice were transferred into recipient mice (Berg et al., 1999). C10.4 mice express a TCR positively selected on a H2-M3 presented fM peptide derived from a mitochondrial NADH dehydrogenase subunit (Berg et al., 1999). CD11c-DTR transgenic and nontransgenic control recipient mice were treated with DT and inoculated with 2000 live L. monocytogenes. Anti-Listeria responses were assessed 4 days after infection. As shown in Figure 4C, DC depletion completely abrogated the in vivo proliferative response of H2-M3-restricted CTL precursors.
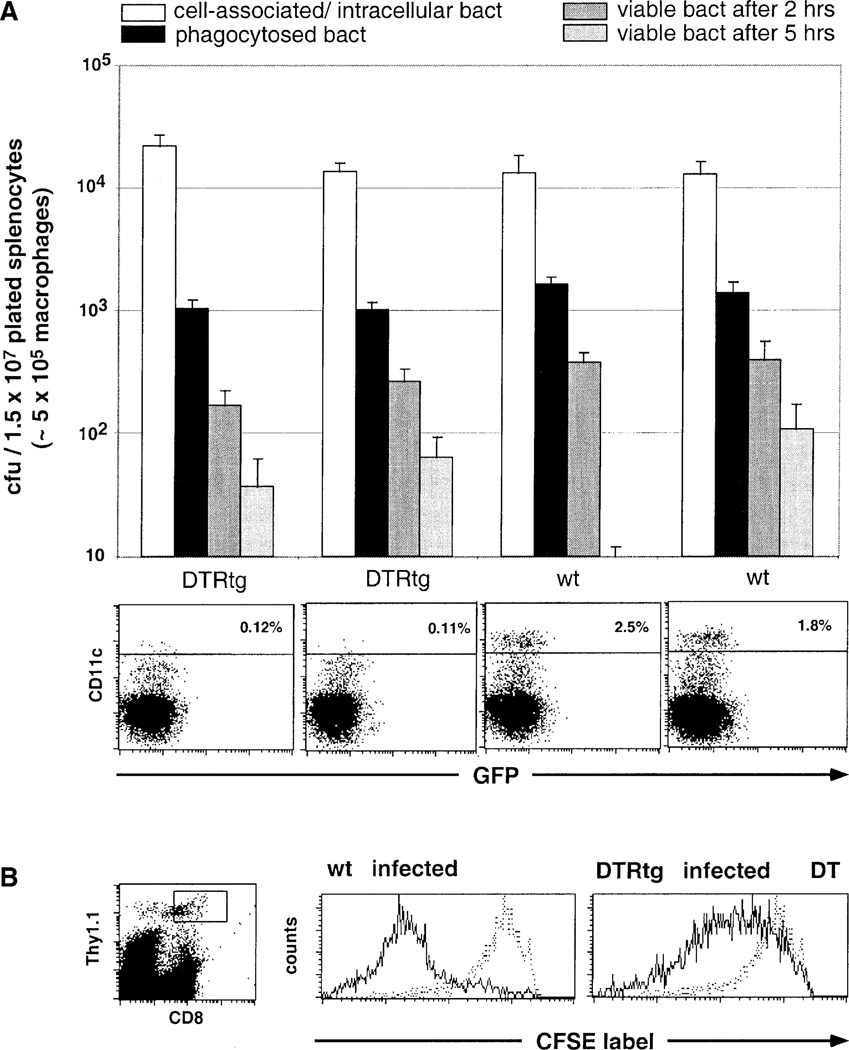
Macrophages, the primary targets of L. monocytogenes, are considered “professional” BM-derived APC. Their failure to initiate anti-Listeria CTL responses in the absence of DC is therefore surprising. DT treatment does not lead to depletion of splenic macrophages (see Figure 2B). To rule out the possibility that DC depletion may cause a functional impairment of splenic macrophages, we examined two hallmarks of macrophage function, i.e., their phagocytic and microbiocidal activity. We isolated macrophages from untreated and DC-depleted spleens 10 hr after DT injection, incubated them in vitro with an attenuated (noninvasive) Shigella mutant (BS 176) (Zychlinsky et al., 1992), and determined uptake and killing of the bacteria over time. As shown in Figure 5A, DT-induced in vivo DC depletion affected neither the efficacy of the bacterial uptake nor the kinetics of the killing by macrophages.
Figure 5. Functionality of Macrophage Compartment in DC-Depleted Spleens.
(A) In vitro analysis of phagocytic and bacteriocidal activity of macrophages isolated from spleens of two DT-treated DTR transgenic (line 57) and two nontransgenic littermate CB6F1 mice. Macrophages isolated by plastic adherence were incubated with the noninvasive Shigella mutant BS176 (at an moi of 1:10). Open bars indicate counts of cell-associated and intracellular BS176; black bars indicate counts of phagocytosed BS176; and dark and light gray bars indicate counts of BS176 surviving 2 hr and 5 hr inside the macrophages, respectively. Standard deviations are based on triplicate cultures. FACS blots below bar diagram show respective splenocyte analysis. Data are representative of two experiments with similar results.
(B) Flow cytometric analysis of a CFSE-labeled WP11.12 CD8+ T cell line graft (Thy1.1, 106 cells) in DT-treated nontransgenic and DTR transgenic (line 57) BALB/c mice (Thy1.2) 4 days after i.v. injection of 2000 live L. monocytogenes. Histograms represent cells gated according to scatter, CD8, and Thy1.1 expression, as indicated in dot plot. Stippled histograms represent CFSE-profile of uninfected wt control. Dead cells were excluded by PI staining.
Finally, we investigated whether the failure to mount a CTL response is due to a defect in presentation of Listeria-derived peptides in infected DC-depleted spleens. As a read-out for in vivo Listeria-peptide presentation, we adoptively transferred a prestimulated CFSE-labeled cytotoxic T cell line generated from anti-p60 TCR transgenic mice (WP11.12). Depletion of DC only partially impaired the in vivo restimulation of the T cell line (Figure 5B). These data suggest that infected phagocytes present Listeria-derived peptides infected DC-depleted spleens, yet are unable to prime naive CTL precursors.
Requirement of DC to Elicit a CTL Response against Malaria Liver Stages
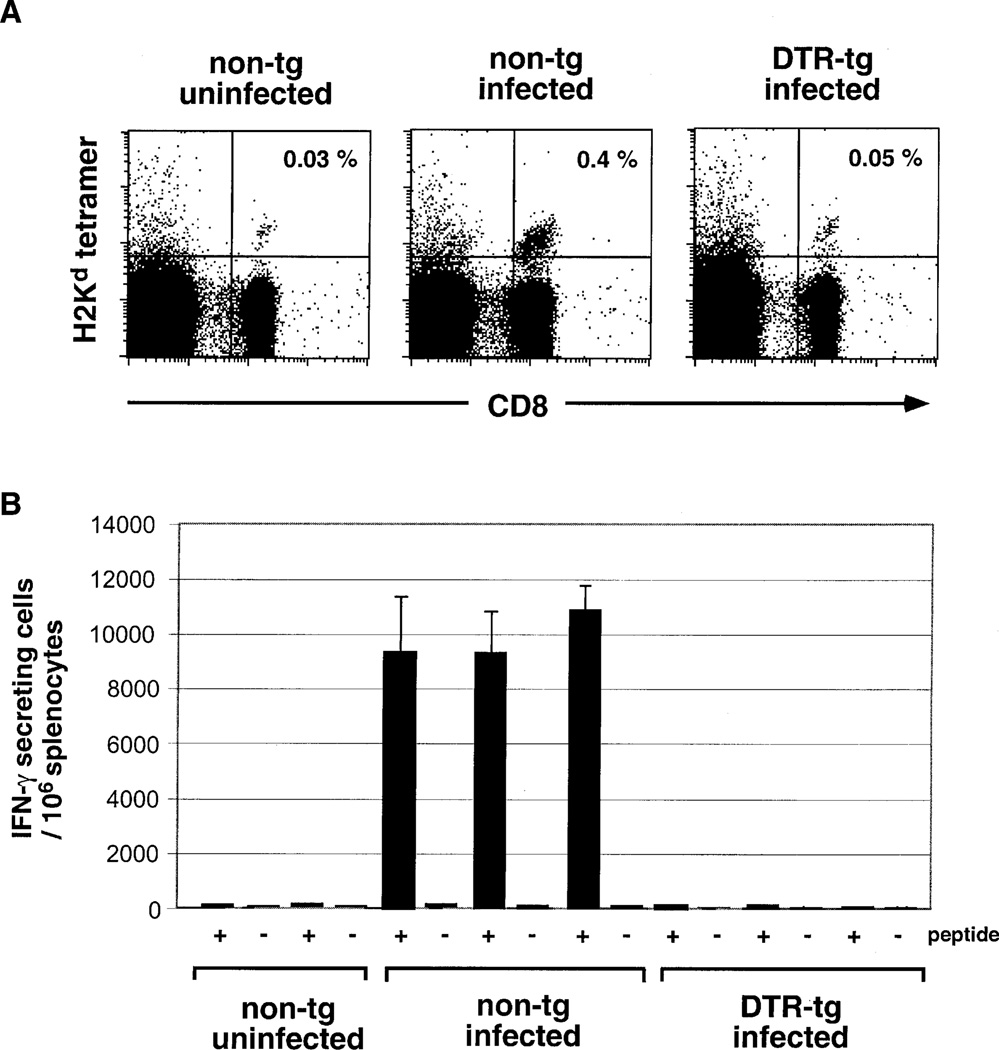
The above results provide evidence that DC play a crucial role in the priming of CTL against cell-associated proteins. To investigate whether the DC requirement extends to the immune response toward a eukaryotic pathogen, we decided to study the CTL response to the rodent parasite Plasmodium yoelii. Malaria sporozoites are introduced into mammalian hosts via mosquito bites, enter the bloodstream, and rapidly reach the liver, where they invade and proliferate exclusively in hepatocytes. Immunization with viable or attenuated malaria sporozoites has been shown to prime CTL capable of inhibiting the further parasite development in the liver (Romero et al., 1989). To study the involvement of DC in the anti-sporozoite CTL response, we adoptively transferred CTL precursors isolated from mice expressing a TCR transgene specific for a malaria circumsporozoite protein-derived peptide (SYVPSAEQI) (Sano et al., 2001). DTR transgenic and nontransgenic control recipient mice were treated with DT and inoculated 10 hr later by i.v. injection of 1 × 105 irradiated P. yoelii sporozoites. Four days after immunization, we analyzed the frequency of anti-sporozoite CTL in the spleens of the infected mice by staining with a H2-Kd/SYVPSAEQI peptide tetramer. Depletion of DC completely abrogated the expansion of the CTL-precursor graft (Figure 6A). To investigate effector functions of the primed CTL precursors, we determined the frequencies of IFNγ-producing peptide-specific cells in the spleens of the wt and DTRtg mice by an ELISPOT assay. Circumsporozoite-specific IFNγ-producing CTL were readily detected in infected wt spleens but absent in DT-treated DTRtg mice (Figure 6B). We have previously shown that a sustained CTL response to Malaria sporozoites depends on the presence of CD4+ T cells. However, in those studies, CD4+ T cell depletion did not affect the initial phase of the CD8+ response (up to day 4), which is being examined here (Carvalho et al., 2002). We therefore conclude that the impaired CTL response is directly caused by DC depletion and not by a lack of CD4+ T cell help.
Figure 6. DC Dependence of the CTL Response to Malaria Sporozoites.
(A) Flow cytometric analysis of spleens wt and DTRtg recipients of anti-sporozoite CTL precursor grafts 4 days after infection with Plasmodium yoelii sporozoites. Data are representative of three mice per group. All mice received a single DT injection (4 ng/g body weight) 10 hr prior to infection. Cells are gated according to scatter.
(B) ELISPOT assay of splenocytes of non-transgenic and DTR transgenic (line 57) BALB/c CTL-precursor recipients 4 days after infection with Plasmodium yoelii sporozoites. Black bars, number of sporozoite-peptide (SYVPSAEQI)-specific IFNγ-producing cells; open bars, number of IFNγ producers in absence of peptide.
Discussion
It is widely believed that dendritic cells are critical for initiating primary immune responses (Banchereau and Steinman, 1998). However, while numerous studies have demonstrated that antigen-pulsed DC are highly potent immunostimulators, experimental evidence for a unique in vivo function of DC has been lacking. In this study, we describe the use of a DTR-based transgenic system that allows the inducible in vivo ablation of DC. As such, the CD11c-DTR system allowed us for the first time to probe for essential in vivo DC functions. In the series of experiments reported in this study, we establish that DC play an essential role in the in vivo priming of CD8+ T cells. Furthermore, our finding that DC depletion abrogates CTL responses to intracellular pathogens, such as L. monocytogenes and the malaria parasite Plasmodium yoelii, emphasizes the crucial role of DC for the adaptive immune defense.
Cross-Presentation in the Anti-Listeria CTL Response
We showed in this study that DT-induced DC depletion in CD11c-DTR transgenic mice abrogated classical and nonclassical MHC-restricted anti-Listeria CTL responses. The question remains as to why the anti-Listeria CTL response is so critically dependent on DC. This could be due to a crucial direct uptake of Listeria by DC. However, DC are, at best, poorly permissive for Listeria infection (Pron et al., 2001). We therefore favor the interpretation that the DC requirement results from the dependence of the anti-Listeria CTL response on cross-presentation. In an elegant study, Shen et al. had previously demonstrated that nonsecreted Listeria antigens efficiently primed CTL responses but, as opposed to secreted Listeria proteins, failed to confer protection (Shen et al., 1998). Nonsecreted Listeria proteins have no access to the cytoplasm of infected cells and are hence excluded from MHC class I presentation in nonhematopoietic cells, which therefore fail to be recognized by CTL. The observed CTL response against these proteins thus needed to be elicited by BM-derived APC. Proteins that are secreted by Listeria into the cytoplasm of infected cells, such as p60, have ready access to their MHC class I molecules (Shen et al., 1998). Yet, the capacity to prime CTL against these proteins was also shown to be restricted to a BM-derived APC (Lenz et al., 2000). This APC had to acquire the antigen either directly via infection or indirectly via cross-presentation of infected apoptotic cells. Here, we show that presentation of “endogenous” peptides by Listeria-infected macrophages fails to initiate a CTL response from naive CD8 T cell precursors. Our results therefore extend the previous reports by suggesting that cross-presentation is essential for the anti-Listeria CTL response. Together with our experiments with the OVA-loaded cells, these studies identify the cross-priming APC as a CD11c+ DC. We therefore interpret the failure to mount anti-Listeria CTL responses as a consequence of the abrogation of an essential cross-presentation step in the priming of antibacterial CTL.
Cross-Presentation in the CTL Response to Malaria Parasites
DC depletion also abrogated the anti-sporozoite CTL response against a rodent Malaria parasite. Hepatocytes are the only cells infected by Plasmodium yoelii sporozoites. Moreover, since only live sporozoites lead to malaria protection, intrahepatocytic parasites are thought to be responsible for parasite immunity. The DC dependence of the anti-circumsporozoite CTL response therefore strongly suggests that the CTL are primed via cross-presentation of infected hepatocytes. Alternatively, the CTL priming we observe could be due to direct cross-presentation of the injected sporozoites in the spleen. In either case, the capacity for productive cross-presentation is restricted to CD11c+ DC.
Unique Capacity of CD11c+ DC to Prime CD8+ T Cells
Taken together, our data establish that DC are essential for in vivo CTL cross-priming, i.e., the priming with cell-associated antigens. The immune system is particularly sensitive for responding to cell-associated antigens, presumably because CTL immunity required to monitor intracellular pathogens is directed against such antigens (Li et al., 2001). Our findings establish that the ability to sense cell-associated antigens is dependent on the presence of CD11c+ DC. Moreover, our results suggest that the ability to prime naive CD8+ T cells is restricted to CD11c+ DC. This notion is supported by a recent report demonstrating that only vaccinia virus-infected DC but not infected macrophages present antigen to naive CD8+ T cells in a tissue-draining lymph node (Norbury et al., 2002). Interestingly, CD8+ T cell clusters were also observed surrounding noninfected DC, suggesting cross-presentation of virus-infected cells by DC.
Results of in vitro experiments had previously indicated that DC might be superior to macrophages with respect to the presentation of exogenous antigens (Rodriguez et al., 1999). Furthermore, it was shown that while i.v.-injected cell-associated antigens are rapidly taken up by all splenic mononuclear phagocytes, upon isolation only CD11b− (CD8+) DC but neither CD11b+ (CD8−) DC nor macrophages were able to cross-prime CTL in vitro (den Haan et al., 2000). This result could indicate that the ability for cross-priming is further restricted. However, additional studies will be required to define the DC subset harboring the in vivo cross-priming potential.
In light of in vitro results indicating that macrophages are capable of uptake, processing, and presentation of cell-associated proteins (Heath and Carbone, 2001), it remains unclear why in the absence of DC they fail to cross-prime CTL in vivo. Only CD11c+ DC appear able to present cell-associated antigen in immunogenic context to CTL precursors. Alternatively, only CD11c+ DC might be able to translocate after antigen uptake to a microenvironment in lymphoid tissue that promotes T cell activation. These interpretations assume that the triggering antigen is presented on the DC, a notion supported by the fact that it is the antigen-presenting capacity of DC that is crucial for in vivo cross-priming (Kurts et al., 2001). However, our results do not exclude the possibility that naive T cells encounter cross-presented antigen on a non-DC APC, but DC are essential to support a subsequent sustained CTL response.
In vivo depletion of DC results in the loss of the priming potential in a primary mixed leukocyte reaction. It has been previously noted that allogeneic DC present in organ transplants (albeit at low numbers) could trigger allograft rejection (Lechler et al., 2001). DT-treated CD11c-DTR transgenic mice could therefore be a valuable source of DC-depleted allografts to further investigate the role of DC in transplant survival.
The present study focuses on the potential of DC to induce protective CD8+ T cell responses, i.e., cross-priming. However, cross-presentation can also result in cross-tolerization via deletion or anergy (Heath and Carbone, 2001). Immature DC are particularly efficient in endocytosis and presentation of apoptotic cells (Albert et al., 1998) and were shown to continuously sample dying cells in peripheral tissues in vivo (Huang et al., 2000). Based on these and other findings (Hawiger et al., 2001), DC are currently considered prime candidates for the induction and maintenance of peripheral tolerance. The ability to induce transient depletion of DC might permit the examination of the role of these cells in the establishment of peripheral tolerance.
Experimental Procedures
Generation of CD11c-DTR Transgenic Mice
The transgene encoding the simian diphtheria toxin receptor (DTR)-GFP fusion protein was generated by cloning the PCR-amplified hbEGF cDNA (Genbank accession #M93012, base pair 56–682) as an EcoRI/ BamHI fragment into pEGFP-N1 (Stratagene). The fused gene was then PCR amplified and cloned as an EcoRI fragment into CD11c-pDOI-5 (kindly provided by K. Karjalainen [Brocker et al., 1997]). The transgene fragment was isolated following Not1 digestion and injected into the pronuclei of fertilized FVB/N oocytes. Transgenic offspring was screened by Southern blotting. Two transgenic lines with homogeneous DC-specific DTR/GFP expression were established (line DTR#11 and line DTR#57 with transgene copy numbers of 1–2 and ~20, respectively). CD11c-DTR mice were subsequently screened by PCR employing the following primers (DTR1, 5′-GCCACCATGAAGCTGCTGCCG-3′; DTR2, 5′-TCAGTGGGAATTAGTCATGCC-3′). To allow for adoptive transfer experiments, CD11c-DTR transgenes were crossed five generations onto the BALB/c (line #57) and C57Bl/6 (line #11) background. For systemic DC depletion, CD11c-DTR transgenic mice were injected intraperitoneally with 4 ng/g body weight DT (in PBS; Sigma D-0564, formerly D-2918).
Adoptive Transfers
Ovalbumin-specific CTL precursors carrying a Vα2/Vβ5 TCR specific for the SIINFEKL peptide presented in the context of MHC class I Kb were isolated from OT-1 mice (Hogquist et al., 1994). MHC class Ia-restricted Listeria-specific CTL precursors carrying a Vα8/Vβ8 TCR directed against p60449–457 presented in the context of MHC H2-Kd were isolated from WP11.12 mice (Mercado et al., 2000). H2-M3-restricted CTL precursors were isolated from C10.4 mice (Berg et al., 1999). Malaria sporozoite-specific CTL precursors were isolated from TCR transgenic mice expressing a TCR directed against the SYVPSAEQI peptide presented in the context of MHC H2-Kd (Sano et al., 2001). T cell grafts were isolated from spleens and LN and enriched by magnetic depletion of I-Ab+ B220+ non-T cells or positive enrichment of CD8+ T cells (Miltenyi Biotech). In some experiments, cells were labeled with the intracellular fluorescent dye car-boxyfluorescein diacetate succimidyl ester (CFSE; Molecular Probes, C-1157) by incubating them in the absence of serum for 8 min at RT at 107 cells/ml in 5 µM CFSE. CFSE loading was stopped by addition of an equal volume cold FCS. Cells were washed twice in complete RPMI medium. 106 clonotype-positive CD8-positive cells were injected in 200 µI of PBS into the tail veins of the recipient mice.
FACS Analysis
The staining reagents used in this study included the PE-coupled antibodies anti-Vβ5 and anti-CD11c; the biotinylated antibodies anti-F4/80 (Caltag), anti-CD11b, anti-CD11c, and anti Vβ8.1/2; and the APC-coupled antibodies anti-B220, anti-CD11b, and anti-CD8. Unless indicated otherwise, the reagents were obtained from Pharmingen. Cells were analyzed on a FACS Calibur cytometer or LSR cytometer (Becton-Dickinson, Mountain View, CA) using CellQuest software (Becton-Dickinson).
Mixed Leukocyte Reaction
Primary mixed leukocyte reactions were set up by titrating MHC-haplotype disparate leukocytes in 96-well plates. Stimulator cells were irradiated splenocytes from wt or DTR transgenic (line #57) FVB/N mice (MHC H2q) injected 20 hr prior to isolation i.p. with indicated amount of DT or vehicle control. Responder T cells were isolated from spleens and LN of C57BL/6 mice (MHC H2b) and enriched by magnetic depletion of I-Ab+ B220+ non-T cells. Cultures were pulsed after 72 hr with 1 µCi of [H3] Thymidine, and incorporation was measured 8 hr later.
Immunization with OVA-Loaded Splenocytes
MHC-class I-deficient (β2m−/−) splenocytes were used to ensure that CTL were primed via cross-presentation (den Haan et al., 2000). Splenocytes were loaded by osmotic shock as described (Moore et al., 1988). In brief, cells were loaded in hypertonic medium (0.5 M sucrose, 10% wt/vol polyethylene glycol 1000, and 10 mM HEPES in RPMI 1640) containing 10 mg/ ml OVA (Sigma) for 10 min at 37 C. 13 ml of prewarmed hypotonic medium (40% H2O, 60% RPMI 1640) was added, and cells were incubated for an additional 2 min at 37° C. Cells were washed twice in cold PBS and irradiated (2000 rads). Mice were immunized by intravenous injection of 2.5–3 × 107 OVA-loaded β2m−/− splenocytes.
Listeria and Plasmodium yoelii Infection
L. monocytogenes strain 10403S was grown in brain-heart infusion broth to an OD600 of approximately 0.1, diluted in PBS, and injected i.v. in 0.2 ml per animal. Plasmodium yoelii sporozoites (17× NL strain) were isolated from salivary glands of malaria-infected Anopheles mosquitoes. Sporozoites were γ irradiated (20 K rad) and i.v. injected into mice (1 × 105/mouse).
Macrophage Function Assay
Macrophages were isolated from wt and DC-depleted, collagenase-digested splenocyte suspensions via their adherence to plastic. After extensive washes with PBS, mutant noninvasive, noncytotoxic Shigella (BS176) (Zychlinsky et al., 1992) were added (at an estimated moi of 1:10). The plates were then centrifuged at 2000 rpm for 10 min followed by incubation for 30 min at 37 C. After 30 min, the cells were rinsed twice, trypsinized for 5 min, and then lysed in sterile water. The number of viable bacteria (intracellular and cell-associated) was determined by plating on molten Luria Broth agar. The number of phagocytosed BS176 was determined by counting the viable bacteria following treatment of the plated macrophages with gentamycin (100 µg/ml) for 30 min. Intracellular killing capacity of the macrophages was assessed by counting the number of viable bacteria following gentamycin treatment for an additional 90 and 270 min.
ELISPOT Assays
ELISPOT assays were done in Millipore HA plates (Millipore, Bedford, MA) coated with a mouse antibody against IFNγl (R4, ATCC, Rockville, MD). Serial dilutions of the freshly isolated spleen cells were plated in wells containing SYVPSAEQI-pulsed or control A20 (ATCC). Cytokine production was detected after 24 hr using biotinylated antibody against IFNγl (XMG 1.2, BD Pharmingen) followed by peroxidase-labeled streptavidin (Kirkegaard and Perry Laboratories, Gaithersburg, MD). Spots were developed after addition of peroxidase substrate.
Supplementary Material
Acknowledgments
We would like to thank Frederic Geissman and Yael Pewzner-Jung for helpful discussions and critical reading of the manuscript, Yvette Weinrauch for advice and expertise, and Farah Hatam, Kanchan Mirchandani, and MaryJean Sunshine for technical assistance. S.J. is supported by a Special Fellowship of the Leukemia & Lymphoma Society. D.R.L. is an investigator of the Howard Hughes Medical Institute. This work has been supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute and an award from the Searle Scholars Program to R.A.L.
References
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bellone M, Iezzi G, Rovere P, Galati G, Ronchetti A, Protti MP, Davoust J, Rugarli C, Manfredi AA. Processing of engulfed apoptotic bodies yields T cell epitopes. J. Immunol. 1997;159:5391–5399. [PubMed] [Google Scholar]
- Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Berg RE, Princiotta MF, Irion S, Moticka JA, Dahl KR, Staerz UD. Positive selection of an H2–M3 restricted T cell receptor. Immunity. 1999;11:33–43. doi: 10.1016/s1074-7613(00)80079-5. [DOI] [PubMed] [Google Scholar]
- Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LH, Sano Gi G, Hafalla JC, Morrot A, de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat. Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- Debrick JE, Campbell PA, Staerz UD. Macrophages as accessory cells for class I MHC-restricted immune responses. J. Immunol. 1991;147:2846–2851. [PubMed] [Google Scholar]
- den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Tvinnereim AR, Harty JT. Listeria monocytogenes infection overcomes the requirement for CD40 ligand in exogenous antigen presentation to CD8(+) T cells. J. Immunol. 2001;167:5603–5609. doi: 10.4049/jimmunol.167.10.5603. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Holmes RK. Biology and molecular epidemiology of diptheria toxin and the tox gene. J. Infect. Dis. 2000;181:156–167. doi: 10.1086/315554. [DOI] [PubMed] [Google Scholar]
- Huang AY, Bruce AT, Pardoll DM, Levitsky HI. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity. 1996;4:349–355. doi: 10.1016/s1074-7613(00)80248-4. [DOI] [PubMed] [Google Scholar]
- Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huleatt JW, Lefrancois L. Antigen-driven induction of CD11c on intestinal intraepithelial lymphocytes and CD8+ T cells in vivo. J. Immunol. 1995;154:5684–5693. [PubMed] [Google Scholar]
- Kerksiek KM, Busch DH, Pilip IM, Allen SE, Pamer EG. H2–M3-restricted T cells in bacterial infection: rapid primary but diminished memory responses. J. Exp. Med. 1999;190:195–204. doi: 10.1084/jem.190.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Kurts C, Cannarile M, Klebba I, Brocker T. Dendritic cells are sufficient to cross-present self-antigens to CD8 T cells in vivo. J. Immunol. 2001;166:1439–1442. doi: 10.4049/jimmunol.166.3.1439. [DOI] [PubMed] [Google Scholar]
- Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–1739. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation—friend or foe? Immunity. 2001;14:357–368. doi: 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- Lenz LL, Butz EA, Bevan MJ. Requirements for bone marrow-derived antigen-presenting cells in priming cytotoxic T cell responses to intracellular pathogens. J. Exp. Med. 2000;192:1135–1142. doi: 10.1084/jem.192.8.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Davey GM, Sutherland RM, Kurts C, Lew AM, Hirst C, Carbone FR, Heath WR. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J. Immunol. 2001;166:6099–6103. doi: 10.4049/jimmunol.166.10.6099. [DOI] [PubMed] [Google Scholar]
- Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J. Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Naglich JG, Metherall JE, Russell DW, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- Norbury CC, Malide D, Gibbs JS, Bennink JR, Yewdell JW. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 2002;3:265–271. doi: 10.1038/ni762. [DOI] [PubMed] [Google Scholar]
- Pamer EG, Wang CR, Flaherty L, Lindahl KF, Bevan MJ. H-2M3 presents a Listeria monocytogenes peptide to cytotoxic T lymphocytes. Cell. 1992;70:215–223. doi: 10.1016/0092-8674(92)90097-v. [DOI] [PubMed] [Google Scholar]
- Pappenheimer AM, Jr, Harper AA, Moynihan M, Brockes JP. Diphtheria toxin and related proteins: effect of route of injection on toxicity and the determination of cytotoxicity for various cultured cells. J. Infect. Dis. 1982;145:94–102. doi: 10.1093/infdis/145.1.94. [DOI] [PubMed] [Google Scholar]
- Pron B, Boumaila C, Jaubert F, Berche P, Milon G, Geissmann F, Gaillard JL. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol. 2001;3:331–340. doi: 10.1046/j.1462-5822.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Regnault A, Lankar D, Lacabanne V, Rodriguez A, Thery C, Rescigno M, Saito T, Verbeek S, Bonnerot C, Ricciardi-Castagnoli P, Amigorena S. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 1999;189:371–380. doi: 10.1084/jem.189.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Regnault A, Kleijmeer M, Ricciardi-Castagnoli P, Amigorena S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1999;1:362–368. doi: 10.1038/14058. [DOI] [PubMed] [Google Scholar]
- Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, Kohno K. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat. Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- Sano G, Hafalla JC, Morrot A, Abe R, Lafaille JJ, Zavala F. Swift development of protective effector functions in naive CD8(+) T cells against malaria liver stages. J. Exp. Med. 2001;194:173–180. doi: 10.1084/jem.194.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Seaman MS, Perarnau B, Lindahl KF, Lemonnier FA, Forman J. Response to Listeria monocytogenes in mice lacking MHC class Ia molecules. J. Immunol. 1999;162:5429–5436. [PubMed] [Google Scholar]
- Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld AD, Mengaud J, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr. Top. Microbiol. Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Swanson J. The endocytic activity of dendritic cells. J. Exp. Med. 1995;182:283–288. doi: 10.1084/jem.182.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J. Exp. Med. 1983;157:613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Norbury CC, Greenwood R, Bennink JR, Yewdell JW, Frelinger JA. Multiple paths for activation of naive CD8+ T cells: CD4-independent help. J. Immunol. 2001;167:1283–1289. doi: 10.4049/jimmunol.167.3.1283. [DOI] [PubMed] [Google Scholar]
- Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.