Abstract
Chinese herbal medicine has shown promise for heroin detoxification. This review extends a prior meta-analysis of Chinese herbal medicine for heroin detoxification, with particular attention to the time course of symptoms. Both English and Chinese databases were searched for randomized trials comparing Chinese herbal medicine to either α2-adrenergic agonists or opioid agonists for heroin detoxification. The methodological quality of each study was assessed with Jadad’s scale (1–2 = low; 3–5 = high). Meta-analysis was performed with fixed- or random-effect models in RevMan software; outcome measures assessed were withdrawal-symptoms score, anxiety, and adverse effects of treatment. Twenty-one studies (2,949 participants) were included. For withdrawal-symptoms score relieving during the 10-day observation, Chinese herbal medicine was superior to α2-adrenergic agonists in relieving opioid-withdrawal symptoms during 4–10 days (except D8) and no difference was found within the first 3 days. Compared with opioid agonists, Chinese herbal medicine was inferior during the first 3 days, but the difference became non-significant during days 4–9. Chinese herbal medicine has better effect on anxiety relieving at late stage of intervention than α2-adrenergic agonists, and no difference with opioid agonists. The incidence of some adverse effects (fatigue, dizziness) was significantly lower for Chinese herbal medicine than for α2-adrenergic agonists (sufficient data for comparison with opioid agonists were not available). Findings were robust to file-drawer effects. Our meta-analysis suggests that Chinese herbal medicine is an effective and safety treatment for heroin detoxification. And more work is needed to determine the specific effects of specific forms of Chinese herbal medicine.
Keywords: Chinese herbal medicine, Heroin, Detoxification, Meta-analysis
Introduction
Opioid abuse and addiction are major risk factors for disease, particularly in Asia, Europe, Oceania, and the Middle East (United Nations Office on Drugs and Crime 2007). Opioid addiction can be treated with a variety of pharmacological agents (including opioid agonists, partial opioid agonists, opioid antagonists, and α2-adrenergic agonists); the use of such agents during detoxification (managed withdrawal) and maintenance has recently been reviewed (Gonzalez et al. 2002).
Detoxification from opioids is not itself a sufficient treatment for opioid addiction (Sees et al. 2000), but for many individuals, it is the first instance of contact with treatment services and it may facilitate the transition into long-term care (Amato et al. 2004). In Western medicine, the pharmacological agents most commonly used for detoxification are either opioid agonists or α2-adrenergic agonists (or a combination of the two). Chinese herbal medicine (CHM) is also used for detoxification (Shi et al. 2006; Ghodse et al. 2006), but most of the controlled clinical trials of CHM have been published in Chinese journals and are not available to English readers. A preliminary meta-analysis of 11 such trials, by Min et al. (2005), suggested that CHM was more effective than α2-adrenergic agonists and possibly more effective than opioid agonists. Due to the limited number of trials available for review at the time, Min et al. called for further meta-analyses to be performed as more data become available.
Therefore, the aim of our study was to replicate and extend Min et al.’s earlier systematic review and meta-analysis of randomized controlled trials assessing the efficacy and safety of CHM on heroin detoxification. We identified additional trials and, unlike Min et al. (2005), we separately assessed outcomes for each of the first 10 days of withdrawal rather than reporting a time-averaged outcome measure for each trial.
Methods
Literature Search
We attempted to locate all reports of relevant clinical trials published from January 1990 to August 2007, and we also contacted with experts in the field to find ongoing and unpublished studies. The databases searched included three English databases (EMBASE, PUBMED, and the Cochrane Central Register of Controlled Trials) and four Chinese databases (Chinese Biomedical Literatures, China National Knowledge Infrastructure, Wan Fang, and VIP). The following search terms were used: opioid detoxification, opioid dependence, opioid withdrawal, opiate, heroin, and morphine, along with their Chinese counterparts. Further narrowing of the results was done by reading titles and abstracts rather than via search terms, because nearly 70 different types of CHM are used in drug-dependence treatment. In addition, the reference list from each relevant article was inspected to find other relevant studies.
Inclusion/Exclusion Criteria for Meta-analysis
Inclusion criteria: (1) randomized controlled trials for heroin detoxification comparing CHM as the experimental condition and α2-adrenergic agonists or opioid receptor agonists as a control condition; (2) patients diagnosed with opioid dependence or heroin dependence and in the acute stage of abstinence symptoms; (3) demographic and drug-use characteristics of patients reported in detail; (4) at least 15 patients in each group; (5) at least one of the following three outcome measures: total score on the opioid-withdrawal symptoms scale (WSS) (Wang et al. 2002), anxiety score (Hamilton Anxiety Scale, HAMA), and rate of adverse effects; (6) WSS were assessed day by day. Exclusion criteria: (1) CHM given only in combination with other medications; (2) results shown only in figures; (3) inconsistencies in reporting of the number of participants; (4) design features that could cause heterogeneity—for example, 3-day medication versus 10-day medication (Zhou et al. 2004b); (5) trials scoring low in quality (see below for quality-assessment method) that caused significant heterogeneity (Mo et al. 2003). If a study was reported in more than one publication, only the first version was included. No distinction was made between patients dependent on heroin alone or heroin plus other drugs (such as benzodiazepines).
Data Extraction and Quality Assessment
Two reviewers reviewed the search hits by reading the titles and abstracts and assessed the article for inclusion. Uncertainties were resolved through discussion. For each study, the following key information was extracted: first author, publication year, study design, patients’ demographic characteristics, sample size for each intervention, and outcomes.
The methodological quality of the included trials was assessed using the Jadad scale (Jadad et al. 1996) for: (1) randomization, (2) double-blinding, (3) description of withdrawal, (4) description of randomization, (5) description of blinding. Trials scoring 1 or 2 points are considered low quality, trials scoring 3–5 points are considered high quality.
Data Synthesis and Analysis
Demographic information was summarized for all patients in included studies. RevMan software (Cochrane Collaboration 2004) was used for meta-analysis. Continuous outcomes (such as scores on rating scales) were assessed in terms of the weighted mean difference (WMD) between CHM and the control intervention, and 95% confidence intervals (95% CIs) were calculated. Dichotomous outcomes (such as the presence or absence of an adverse effect) were assessed in terms of relative risk (RR) for CHM and the control intervention, and 95% CIs were calculated. Random-effects models were used to analyze pooled effects when heterogeneity was significant otherwise fixed-effects models were used. All pooled analysis was stratified by control interventions: α2-adrenergic agonists and opioid-receptor agonists. Differences were taken to be statistically significant when 95% CIs did not overlap (a more stringent criterion than a significance test at P <0.05; Wolfe and Hanley 2002). Sensitivity analysis was then carried out to assess the influence of study quality on effect estimates and to estimate fail-safe numbers of unpublished negative studies that could overturn the results.
Results
Characteristics of Studies
We found 48 reports of RCTs of Chinese herbal medicine alone versus western medication for heroin detoxification, of which 21 (enrolling 2,949 participants) were eligible for inclusion in the meta-analysis (for detailed information, see Table 1). The average age of patients analyzed ranged from 23 to 39 years, and most of them were males (76.8%). The average amount of daily heroin use was 0.09–1.26 g, with duration of use of 7–94 months. Intravenous injection and insufflation were the commonly reported routes of administration.
Table 1.
Characteristics of included studies
| Author Year | Jadad score | Participants
|
Intervention
|
Outcomes* | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (CHM/ Control) | CHM
|
Control
|
CHM/Control | Duration (days) | |||||
| Age | Male (%) | Age | Male (%) | ||||||
| Deng et al. (2006) | 3 | 70/70 | 32.6 | 92.9 | 31.2 | 85.7 | shifusheng/lofexidine | 10 | 1;2;4;6 |
| Guo et al. (1995) | 2 | 212/104 | 26.4 | 82.7 | 26.3 | 78.4 | fukangpian/clonidine | 10 | 1;2;3;4; 6 |
| Guo et al. (2001) | 5 | 103/69 | 28.5 | 76.6 | 29.2 | 77.1 | zhengtongning/clonidine | 10 | 1;3;4;7 |
| Hao and Zhao (2000) | 2 | 21/21 | 27.7 | 85.7 | 24.6 | 90.5 | weinicom/buprenorphine | 10 | 1;3;5 |
| Huang et al. (2005) | 2 | 52/53 | 30.8 | 75.0 | 29.4 | 71.7 | yianhuisheng/methadone | 10 | 1;4;7 |
| Li et al. (1999a) | 5 | 70/70 | 28.2 | 65.7 | 29.0 | 75.5 | lingyi/clonidine | 10 | 1;4;6;8;10 |
| Li et al. (1999b)** | 1 | 100/50/50 | 39.0 | 80.0 | 37.0/38.0 | 84.0/86.0 | Jiedukoufuye/methadone/clonidine | 10 | 1;7 |
| Li et al. (2002) | 5 | 33/30 | 30.2 | – | 30.0 | – | Shenfutuodu/clonidine | 10 | 1;4;7;10 |
| Liu et al. (2001) | 2 | 34/32 | 29.7 | 64.7 | 28.6 | 68.8 | wenyangyiqi/buprenorphine | 7 | 1 |
| Lu et al. (1997)** | 1 | 100/50/50 | 24.0 | 80.0 | 23.0/23.0 | 72.0/76.0 | Qingjunyin/methadone/clonidine | 10 | 1;3 |
| Lu et al. (2000) | 3 | 30/30 | 23.5 | 80.0 | 24.3 | 73.1 | baokangjiedu/clonidine | 10 | 1;6;11 |
| Sha et al. (2000)*** | 2 | 50/50 | Xinsheng/methadone | 9–11 | 1;3; | ||||
| Tu et al. (1999) | 3 | 48/49 | 29.2 | 70.8 | 29.5 | 69.4 | Jitai/Lofexidine | 10 | 1;6;8;9 |
| Wang et al. (2002) | 5 | 86/29 | 28.6 | 81.1 | 27.9 | 80.0 | shenfutuodu/clonidine | 10 | 1;6;8;10 |
| Wen et al. (2000) | 2 | 68/32 | 28.6 | 80.9 | 30.4 | 84.4 | jiaweishenfu/lofexidine | 10 | 1;4;8;9 |
| Xu et al. (2000) | 2 | 20/20 | 31.4 | 100 | 29.3 | 85.0 | qingdubuzheng/methadone | 8 | 1;4;6;11 |
| Xu et al. (2002) | 3 | 80/79 | 30.2 | 82.5 | 29.6 | 77.2 | fuzhengkang/lofexidine | 10 | 1;2;3;4;5;7 |
| Yang et al. (2006) | 4 | 302/278 | 23.8 | 64.9 | 22.9 | 66.6 | paiduyangsheng/methadone | 10 | 4 |
| Zhang et al. (2001) | 4 | 80/30 | 28.2 | 91.3 | 28.0 | 96.7 | shenfutuodu/clonidine | 10 | 1;3;6;7 |
| Zhang (1998) | 2 | 30/15 | 28.8 | 71.4 | 31.2 | 53.3 | ketongning/methadone | 10 | 1 |
| Zhou et al. (2004a) | 2 | 79/20 | 26.9 | 73.0 | 26.6 | 70.0 | yianhuisheng/clonidine | 10 | 1;6;7 |
| Two studies excluded due to heterogeneity | |||||||||
| Mo et al. (2003) | 2 | 110/76 | 32.7 | 62.73 | 30.12 | 61.84 | qingfeng/lofexidine | 10 | 1;4 |
| Zhou et al. (2004b) | 2 | 62/61 | 33.3 | 87.10 | 34.03 | 83.61 | Tuoduling/lofexidine | 3/10 | 1;2;3;4;5 |
Notes:
Outcomes: 1—total score on withdrawal-symptoms scale (WSS); 2—change score on WSS; 3—specific items on WSS; 4—HAMA score; 5—ratings of craving; 6—rate of adverse effects; 7—scoring of adverse effects; 8—blood pressure; 9—heart rate; 10—laboratory exam; 11—number of participants completing detoxification
Including 2 control groups
No detailed characteristics of treatment and control groups were listed, and only the merging information of total participants was listed in study of ‘Sha et al. (2000)’. And the average age was 28.0; the percent of male was 89.4%
Twelve studies used α2-adrenergic agonists as a control medication and seven studies used opioid agonists as a control medication; two studies used both (clonidine and methadone). Of the 21 studies, 10 were judged high in quality (scoring 3–5 points).
Eighteen herb formulas were included in the 21 studies; the most frequently used herbs were Radix Ginseng (Renshen) (eight studies), Rhizoma Corydalis (Yanhusuo) (seven studies), Radix Aconiti Lateralis Preparata (Fuzi) (five studies), Radix Glycyrrhizae (Gancao) (five studies), Flos Daturae (Yangjinhua) (four studies), and Radix Angelicae Sinensis (Danggui) (three studies). Sample sizes did not permit separate meta-analytic evaluation of the effect of herb type.
Efficacy of Chinese Medicine
Total score of withdrawal-symptom score (WSS)
CHM Versus α2-Adrenergic Agonists
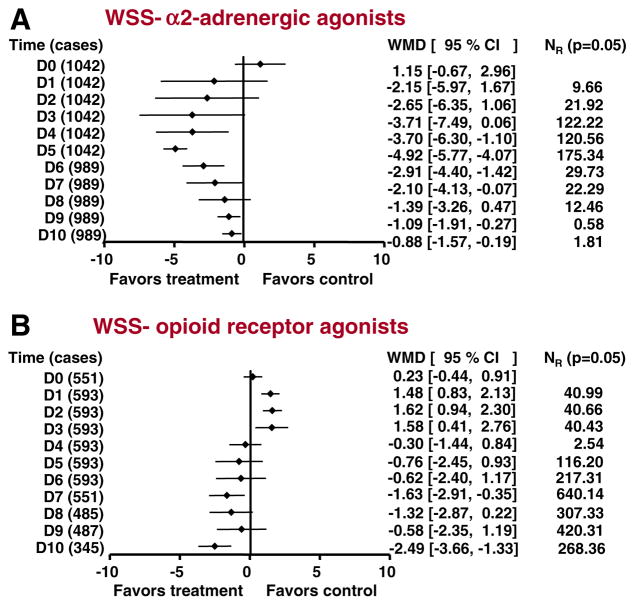
Fourteen studies assessed WSS day by day. Of these, nine studies (with 1,042 participants) scored 3–5 on the Jadad scale, and five studies (with 815 participants) scored 1–2. Only the results of the nine high-quality studies are shown in Fig. 1A. Pretreatment baseline scores (D0) were similar across treatments. For the first 3 days (D1–D3), there was no significant difference between CHM and α2-adrenergic agonists. From the fourth day onward (D4–D10), CHM was significantly more effective than α2-adrenergic agonists, except on D8.
Fig. 1.
Efficacy of Chinese herbal medicine (CHM) compared with α2-adrenergic agonists (A), and opioid agonists (B) in alleviating opioid-withdrawal symptoms. Summary estimates of the weighted mean differences (WMDs) and their 95% CIs are given day by day. D0 indicates pretreatment baseline. D0, D5, D6, D9, D10 in (A) and D0, D1, D2 in (B) were analyzed in fixed-effects models. The other time points were analyzed in random-effects models. NR indicates the fail-safe number, i.e. the number of unpublished negative studies that would be required to overturn each significant finding at an alpha level of 0.05
CHM Versus Opioid-Receptor Agonists
Seven studies involving 593 patients were analyzed (Fig. 1B); all were judged relatively low quality (1–2 on the Jadad scale). Again, pretreatment baseline scores (D0) were similar across treatments. For the first 3 days of treatment (D1–D3), CHM was significantly less effective than opioid-receptor agonists. From the fourth day onward, there were no statistically significant differences across treatments, except on D7 and D10, when differences favored CHM.
Anxiety
Most studies estimated anxiety at three time points: before treatment (D0), during treatment (D5), and after treatment (D10). Only studies with at least two time points were included in our analyses.
CHM Versus α2-Adrenergic Agonists
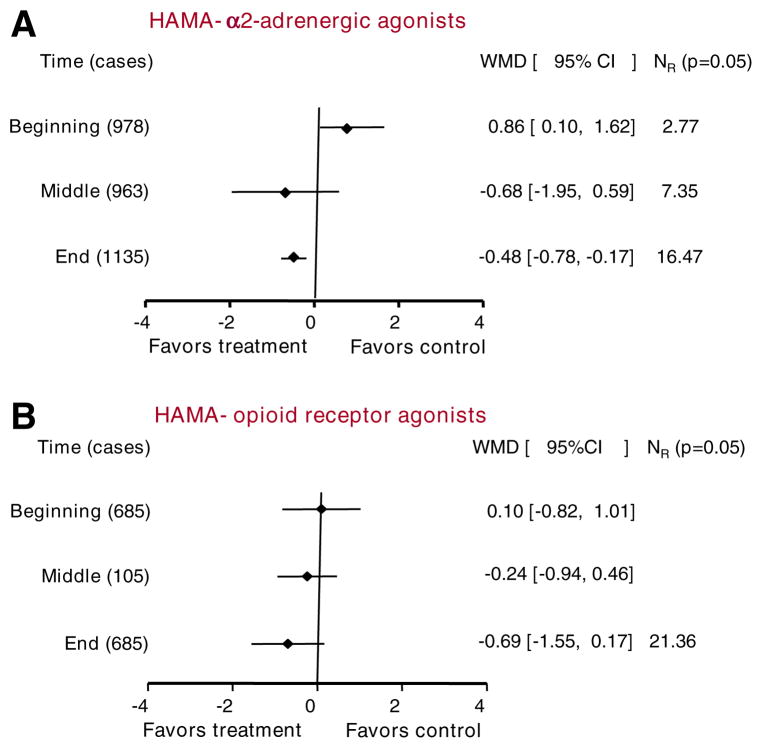
Seven studies assessed anxiety. Five studies were judged high quality, and the other two were judged low quality. Six studies (with 978 participants) included pretreatment data, six studies (with 963 participants) included during-treatment data, and seven studies (1135 cases) included end-of-treatment data (Fig. 2A). Results at the end of treatment favored CHM.
Fig. 2.
Efficacy of CHM compared with α2-adrenergic agonists (A), and opioid agonists (B) in relieving anxiety. Summary estimates of the weighted mean differences (WMDs) and their 95% CIs are given at three time points. “Beginning” and “End” in (A) and “Beginning,” “middle,” and “End” in (B) were analyzed in fixed-effects models. The other time points were analyzed in random-effects models. NR indicates the fail-safe number, i.e. the number of unpublished negative studies that would be required to overturn each significant finding at an alpha level of 0.05
CHM Versus Opioid-Receptor Agonists
Two studies (with 685 participants) included pretreatment data, one study (with 105 participants) included during-treatment data, and two studies (with 685 participants) included end-of-treatment data (Fig. 2B). There was no significant difference between groups at any time point.
Safety of Chinese Medicine: Adverse Effects
Rates of specific adverse effects were reported for nine studies, of which only one used opioid-receptor agonists; therefore, we included only the eight studies using α2-adrenergic agonists. Six studies were judged high quality (3–5 points); the other two were judged low quality (1–2 points). The main side effects reported in these studies were: dry mouth (eight studies, 1,036 patients), dizziness (eight studies, 1,036 patients), blurred vision (seven studies, 983 patients), fatigue (five studies, 723 patients), and somnolence (four studies, 387 patients).
Figure 3 shows the relative risks for specific adverse effects with CHM or α2-adrenergic agonists. CHM was less likely to produce reports of fatigue or dizziness; there were no significant differences across treatments for the other commonly reported effects (blurred vision, somnolence, and dry mouth).
Fig. 3.
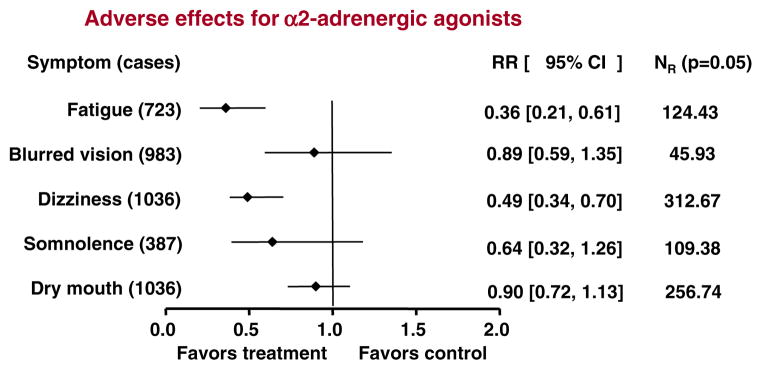
Safety of CHM compared with α2-adrenergic agonists. Summary estimates of the relative risks (RRs) and their 95% CIs are shown for each of the five most commonly reported adverse effects. All RRs were analyzed in random-effects models. NR indicates the fail-safe number, i.e. the number of unpublished negative studies that would be required to overturn each significant finding at an alpha level of 0.05
Few studies reported the other side effects, such as sagging, instability of gait, dysuria, headache, nausea, and inappetency. Thus, we did not analyze them. But the original data showed the side effects of Chinese herbal medicine were slight or moderate, and most of them disappeared spontaneously without treatment.
Sensitivity Analysis and the File-Drawer Effect
Inclusion or exclusion of the low-quality studies had no effect on the opioid-agonist results, but did affect some of the α2-agonist results, as follows.
Withdrawal-Symptom Scores (WSS)
With the five low-quality α2-agonist studies included (14 studies total), results favored CHM over α2-adrenergic agonists from the first day rather than from only the third day: D1, −3.52 [−5.81, −1.22], D2; −4.32 [−6.60, −2.04]. When only the five low-quality studies were included, results were similar to those for all 14 studies: D1, −5.30 [−8.00, −2.61], D2, −6.04 [−9.41, −2.66].
Anxiety
The five high-quality α2-agonist studies assessing anxiety showed that the difference in HAMA scores was no longer significant at any of the three time points: beginning, 0.36 [−0.68, 1.40]; middle, 0.30 [−0.37, 0.98]; end, −0.32 [−0.71, 0.08]. However, the two low-quality studies assessing anxiety favored CHM over α2-adrenergic agonists from the middle time point: beginning, 1.43 [0.31, 2.55]; middle, −1.74 [−2.56, −0.91]; end, −0.72 [−1.20, −0.24].
Adverse Effects
Results of the six high-quality studies assessing adverse effects were similar to those of all eight studies pooled. However, results of the two low-quality studies assessing adverse effects showed no significant difference between Chinese medicine and α2-agonist treatment except for somnolence (one study, 99 cases): 0.02 [0.00, 0.11].
To address the “file-drawer problem” (the possibility of unpublished negative studies that would negate the findings of the published studies), we calculated the fail-safe number NR (Rosenthal 1979), representing the number of studies with null results that would be necessary to render each of our findings non-significant at P = 0.05. NRs are shown in the rightmost portions of Figs. 1, 2, and 3. Note that NRs are calculated in terms of significant differences at P = 0.05, which sometimes emerge even in instances when 95% CIs overlap (Wolfe and Hanley 2002).
Discussion
Our meta-analysis shows that CHM was superior to α2-adrenergic agonists in relieving opioid-withdrawal symptoms during 4–10 days (except D8). Compared with opioid agonists, CHM was inferior during the first 3 days, but the difference became non-significant during days 4–9 (except D7). During the first 3 days of opioid abstinence that has been considered as the critical stage of withdrawal-symptom management, CHM has the same effectiveness with α2-adrenergic agonists but inferior to opioid agonists in relieving opioid-withdrawal symptoms. Anxiety may be of motivational relevance for the maintenance of addiction (Schulteis et al. 1998). CHM has better effect on anxiety relieving at late stage of intervention than α2-adrenergic agonists, and no difference with opioid agonists. The incidence of adverse effects (fatigue, dizziness) was significantly lower for CHM than for α2-adrenergic agonists (sufficient data for comparison with opioid agonists were not available).
Sensitivity analyses showed that when only high-quality studies were included in the meta-analyses, the advantage for CHM on one outcome measure (anxiety reduction, CHM, versus α2-adrenergic agonists) was lost. However, the bulk of the other findings remained the same. When differences were significant, fail-safe numbers were high, often in the hundreds, strongly suggesting that our findings did not merely reflect publication bias in favor of CHM.
Our findings differ somewhat from those of the earlier meta-analysis we had sought to replicate and extend (Min et al. 2005) in which CHM had appeared to be superior to methadone for relief of withdrawal symptoms. In the earlier meta-analysis, this comparison relied on only three trials (706 cases); our finding relied on nine randomized trials (1,278 cases) and thus seems more likely to be reliable.
CHM in the treatment of drug dependence has a long history and a complex doctrinal basis (Li et al. 2005). However, only in the last 20 years has it been assessed with well-designed clinical trials. On the basis of such trials, some forms of CHM have already been approved by the Chinese State Food and Drug Administration (SFDA) for use in addiction treatment, and clinical trials of another six forms are in progress (Shi et al. 2006). The forms of CHM included in this meta-analysis included both SFDA-approved and non-approved herbs. Traditional herbal medicine is also being systematically tested for addiction treatment in India, Thailand, Iran, and elsewhere (Alper et al. 1999, 2000; Akhondzadeh et al. 2001).
Of the six herbs most frequently used in the studies in our meta-analysis, five (all except Radix Aconiti Lateralis Preparata) are among the top ten most frequently used to treat addiction in China (Min et al. 2007). Preclinical work has demonstrated the actions of some of these herbs on the behavioral effects of abused drugs (e.g. Takahashi and Tokuyama 1998) and has begun to clarify the actions of their main active constituents (e.g. Mantsch et al. 2007). However, such work remains in its early stages.
Our meta-analysis had some limitations. Most important, interventions and outcome measures both had to be lumped together more than would be ideal. Different CHM preparations presumably have different mechanisms of action and may be differentially effective against specific symptoms of heroin withdrawal; we were unable to assess this. We were also unable to examine the influence of duration/severity of heroin dependence, though we suspect that CHM alone is probably not adequate to manage withdrawal in the most severely addicted patients (Shi et al. 2006). The clinical trials we assessed involved only detoxification; in future studies, CHM needs to be evaluated for relapse prevention (Shi et al. 2006). Some of the lower-quality trials included in our main analyses did not contain adequate details about blinding. All included trials were conducted in China, so results may not be generalizable to other regions. We were unable to pool all the reported adverse-effect data due to its heterogeneity (some studies reported data on blood pressure, heart rate, and liver and kidney function; no adverse effects of CHM appeared to emerge). Finally, not all forms of CHM thought to have potential for opioid-addiction treatment were included in this analysis.
However, this meta-analysis represents the first time that the results of some of these studies are readily available to an English-language readership. We found that CHM, while not as effective as opioid agonists, does compare favorably to α2-adrenergic agonists in relieving abstinence symptoms and perhaps in relieving anxiety during managed withdrawal from heroin, and that CHM’s side-effect profile appears more benign than that of α2-adrenergic agonists. More well-designed clinical trials are needed to enable conclusions about specific forms of CHM and to determine for which patients it is most likely to be appropriate.
Acknowledgments
This work was supported in part by the National Basic Research Program of China (973 Program, 2003CB515400), the National High Technology Research and Development Program of China (863 Program, 2006AA02Z4D1), and the China–Canada Joint Health Research Program (No: 30611120528)
Contributor Information
Ting-ting Liu, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, Hai Dian District, Beijing 100083, China.
Jie Shi, Email: shijie@bjmu.edu.cn, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, Hai Dian District, Beijing 100083, China.
David H. Epstein, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Baltimore, MD 21224, USA
Yan-Ping Bao, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, Hai Dian District, Beijing 100083, China.
Lin Lu, Email: linlu@bjmu.edu.cn, National Institute on Drug Dependence, Peking University, 38, Xue Yuan Road, Hai Dian District, Beijing 100083, China.
References
- Akhondzadeh S, Kashani L, Mobaseri M, Hosseini SH, Nikzad S, Khani M. Passionflower in the treatment of opiate withdrawal: a double-blind randomized controlled trial. J Clin Pharm Ther. 2001;26:369–373. doi: 10.1046/j.1365-2710.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J. Treatment of acute opioid withdrawal with ibogaine. Am J Addict. 1999;8:234–242. doi: 10.1080/105504999305848. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Frenken GM, Luciano DJ, Bastiaans J. Ibogaine in acute opioid withdrawal. An open label case series. Ann N Y Acad Sci. 2000;909:257–259. doi: 10.1111/j.1749-6632.2000.tb06687.x. [DOI] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M, Vecchi S, Ferri M, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2004:CD005031. doi: 10.1002/14651858.CD005031. [DOI] [PubMed] [Google Scholar]
- Cochrane Collaboration. [Accessed December 21, 2007];RevMan User Guide. 2004 http://www.cc-ims.net/download/revman/Documentation/User%20guide.pdf.
- Deng YP, Shen LY, Gao WY, An YQ, Liu LJ, Duan LX, et al. Multi-centered clinical trial of shifusheng capsule for the management of heroin withdrawal. Chin J Drug Depend. 2006;15:28–32. [Google Scholar]
- Ghodse H, Schifano F, Szendrei K, Carver S, Annan J. Herbal medicine in the treatment of addictions. St. George’s Hospital Medical School, Department of Addictive Behavior & Psychological Medicine; London: 2006. [Google Scholar]
- Gonzalez G, Oliveto A, Kosten TR. Treatment of heroin (diamorphine) addiction: current approaches and future prospects. Drugs. 2002;62:1331–1343. doi: 10.2165/00003495-200262090-00004. [DOI] [PubMed] [Google Scholar]
- Guo S, Jiang ZN, Wang YD, Hu GC, Wu YM, Huang MS. A comparative study of Chinese herbal medicine fukangpian with clonidine hydrochloride on opioid withdrawal symptoms. China Bull Drug Depend. 1995;4:210–216. [Google Scholar]
- Guo S, Jiang ZN, Sheng LX. A double-blind clinical trial of zheng tongning vs clonidine hydrochloride of heroin withdrawal symptoms. Chin J Drug Depend. 2001;10:111–115. [Google Scholar]
- Hao W, Zhao M. A comparative clinical study of the effect of WeiniCom, a Chinese herbal compound, on alleviation of withdrawal symptoms and craving for heroin in detoxification treatment. J Psychoactive Drugs. 2000;32:277–284. doi: 10.1080/02791072.2000.10400450. [DOI] [PubMed] [Google Scholar]
- Huang P, Wu G, Zhou PL, Wu HJ, Tang YM. Clinical observation on Yianhuisheng koufuye in the treatment of heroin dependence. Pract Clin J Integr Tradit Chin West Med. 2005;5:15–16. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Li J, Huang MS, Wan WP, Tian WC, Liu C, Chen XR, et al. Clinical trials of lingyi capsule in treatment of opioid withdrawal syndrome. W China Med J. 1999a;14:18–21. [Google Scholar]
- Li JS, Pei LJ, Zhang XF, Du YZ. Effect of detoxifying jiedu koufuye on heroin addicts. Med J Chin People’s Armed Police Forces. 1999b;10:502–504. [Google Scholar]
- Li J, Kang L, Zhang SS, Li GH, Chen YM, Li KM, et al. Double blind and randomized clinical trial on the effect of shenfutuodu capsule compared with clonidine on withdrawal syndrome of heroin addicts. Chin J Drug Depend. 2002;11:125–128. [Google Scholar]
- Li SC, Li B, Cheng DG, Li F. Advancement of traditional Chinese medicine in opioid dependence. J Beijing Univ Tradit Chin Med. 2005;28:84–88. [Google Scholar]
- Liu JY, Yang QH, Wu XW. A clinical study on the treatment of heroinism abstinence syndrome by compound yang—warming, qi-invigorating and blood-activating prescription. N J Tradit Chin Med. 2001;33:19–22. [Google Scholar]
- Lu HQ, Wang G, Lan SM, Yuan DF, Zhan CM. Clinial study of “qingjunyin” detoxification for the treatment of heroin addicts. J Chin Med Mater. 1997;20:319–321. [PubMed] [Google Scholar]
- Lu XJ, Tan GC, Liang F, Ban CT, Li HX. A randomized controlled trial of baokangjiedu decoction vs lofexidine in the treatment of heroin detoxification. Chin J Drug Abuse Prev Treat. 2000;6:37–40. [Google Scholar]
- Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA, et al. Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology (Berl) 2007;192:581–591. doi: 10.1007/s00213-007-0754-7. [DOI] [PubMed] [Google Scholar]
- Min X, Lee DTS, Wong W. [Accessed December 21, 2007];meta-analysis on Chinese herbal therapy for heroin withdrawal syndrome. 2005 http://www.nd.gov.hk/conference_proceedings/Drugs_proBK_Part5/Drugs_proBK_XuMin1.pdf.
- Min X, Lee DT, Jinhua X, Wenjun D, Li C, Bin D, et al. A database on treating drug addiction with traditional Chinese medicine. Addiction. 2007;102:282–288. doi: 10.1111/j.1360-0443.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- Mo ZX, Wang CY, Luo XY, Zhang XF. Clinical observation of qingfeng capsule on heroin detoxification. J Chin Med Mater. 2003;26:531–533. [Google Scholar]
- Rosenthal R. The “file drawer problem” and tolerance for null results. Psychol Bull. 1979;86:638–641. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol Biochem Behav. 1998;60:727–731. doi: 10.1016/S0091-3057(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Sees KL, Delucchi KL, Masson C, Rosen A, Clark HW, Robillard H, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283:1303–1310. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- Sha LJ, Zhang ZX, Cheng LX, Liu J, Zhang ZM. Clinical observation on heroinism (424 cases) treated with xinsheng decoction. Chin J Integr Tradit West Med. 2000;20:267–268. [Google Scholar]
- Shi J, Liu YL, Fang YX, Xu GZ, Zhai HF, Lu L. Traditional Chinese medicine in treatment of opiate addiction. Acta Pharmacol Sin. 2006;27:1303–1308. doi: 10.1111/j.1745-7254.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tokuyama S. Pharmacological and physiological effects of ginseng on actions induced by opioids and psychostimulants. Methods Find Exp Clin Pharmacol. 1998;20:77–84. doi: 10.1358/mf.1998.20.1.485635. [DOI] [PubMed] [Google Scholar]
- Tu QX, Zhao HG, Chen YP, Chen YM, Huang XP, Chen YM, et al. Comparison study on clinical efficacy of jitai capsule with lofexidine in the treatment of opioid addicts. Chin J Drug Depend. 1999;8:285–288. [Google Scholar]
- United Nations Office on Drugs and Crime . [Accessed December 21, 2007];World drug report. 2007 http://www.unodc.org/pdf/research/wdr07/WDR_2007.pdf.
- Wang XP, Liu TQ, Hao W. Efficacy comparison of shenfutuodu capsules and clonidine on treatment of heroin dependence. Chin J Drug Depend. 2002;11:120–124. [Google Scholar]
- Wen L, Zhen YS, Yu LZ, Mo ZX, Ou JW. A clinical study of modified shenfu decoction on 68 cases of heroin addicts. Pharmacol Clin Chin Materia Med. 2000;16:40–42. [Google Scholar]
- Wolfe R, Hanley J. If we’re so different, why do we keep overlapping? When 1 plus 1 doesn’t make 2. CMAJ. 2002;166:65–66. [PMC free article] [PubMed] [Google Scholar]
- Xu BS, Tie EG, Wang PX, Lv QL, Sun ZW, Jin J, et al. A comparative clinical study of qingdubuzheng decoction on heroin addiction. Chin J Drug Abuse Prev Treat. 2000;6:6–9. [Google Scholar]
- Xu GZ, Duan LX, Liu C, Gao WY, Wang ZF, Xu BS, et al. A randomized controlled clinical trial of fuzhengkang decoction in the treatment of heroin dependence. Chin J Drug Abuse Prev Treat. 2002;8:146–151. [Google Scholar]
- Yang L, Chen J, Zhang XL, Xu X, Hu ML, Li LJ. Controlled clinical study on paiduyangsheng capsule in detoxification of heroin abuse. Chin J Drug Abuse Prev Treat. 2006;12:86–88. [Google Scholar]
- Zhang RM, Li JX, Sun XH, Zheng L, Yang LP, Zhang J, et al. A double-blind clinical trial of shenfutuodu capsule in the treatment of heroin withdrawal symptoms. Chin J Drug Depend. 2001;10:197–201. [Google Scholar]
- Zhang XD, Gou XH, Zhao TS, Yang J, Zhao Q, Yin CB. Clinical study of fufangketonning in heroin dependence. Chin J Drug Abuse Prev Treat. 1998;4:45–47. [Google Scholar]
- Zhou C, Zhang DR, Wang LQ. Compared clinical researches on treating opium withdrawal syndrome by TCM complex, yi an decoction, and clonidine. Chin J Drug Abuse Prev Treat. 2004a;10:68–71. [Google Scholar]
- Zhou KC, Xie RQ, Liu JG, Zhan XM. An observation on clinical efficacy of tuoduling capsule in 3-day treatment of heroin dependence. Chin J Drug Abuse Prev Treat. 2004b;10:97–100. [Google Scholar]