Abstract
Once a liver offer has been refused locally and regionally, it is offered nationally. We characterized nationally (n = 1567) versus locally (n = 19 893) placed grafts from adult, nonfulminant, deceased donor liver transplants (LT) from 2/1/05 to 1/31/10. Donors of nationally versus locally placed livers differed by age (50 vs. 42 years), positive HCV antibody (11 vs. 2%) and death from stroke (51 vs. 42%) (p < 0.001 for all). Recipients of nationally versus locally placed livers differed by LT-MELD (20 vs. 24), rates of ascites (35 vs. 37%), encephalopathy (12 vs. 15%), hepatocellular (17 vs. 24%) and nonhepatocellular exceptions (6 vs. 11%) (p ≤ 0.03 for all). Six (5%) centers utilized 64% of the nationally placed grafts while 43 (38%) centers accepted zero during the 5-year period; all high volume centers used ≥1. Compared to local distribution, transplantation with a nationally placed liver was associated with a similar adjusted risk of graft (HR, 0.99; 95% CI, 0.86–1.14) and patient (HR, 0.98; 95% CI, 0.84–1.14; p = 0.77) survival. In conclusion, utilization of nationally placed livers is highly concentrated in very few centers, with no increased adjusted risk of graft loss. These findings provide the foundation for a more informed discussion about changing our current liver allocation and distribution policies.
Keywords: Distribution, extended criteria donor, geographic-disparity
Background
The decision to accept or refuse a liver graft offer depends upon the quality of the donor liver relative to the perceived need of the waitlist candidate and the probability that the patient will receive a subsequent, better liver offer. The balance between these factors varies by center, as each center differs with respect to the severity of candidate illness and the availability and quality of donor livers.
In the current liver distribution system in the United States, once a liver graft has been refused on both the local and the regional levels, it is then offered nationally. By virtue of having been refused by local and regional centers, grafts distributed nationally are of lower quality (1), but little else is known about these livers. We, therefore, embarked upon this study to characterize donor, recipient, center and geographic factors associated with nationally placed livers.
Methods
Study population
This study included all patients ≥18 years of age who underwent deceased donor liver transplantation in the United States from February 1, 2005 through January 31, 2010. This study period was selected to correspond to the implementation of the ‘share 15’ policy which stipulates that livers are first allocated locally and then regionally to waitlist candidates with Model for End-Stage Liver Disease (MELD) scores ≥15 before they are offered locally to candidates with MELD scores <15 (2). If a liver graft has been refused for all local and regional candidates, the graft is then offered nationally. Patients were excluded from the study if they were listed as status 1. They were also excluded if they received a split liver, as the liver is, in the majority of cases, first offered to a pediatric candidate such that distribution of the remaining portion to an adult candidate may not reflect standard allocation policy.
Data regarding recipient, donor and center characteristics were obtained via the Standard Transplant and Analysis Research (STAR) files from the United Network for Organ Sharing/Organ Procurement Transplantation Network (UNOS/OPTN) as of June 30, 2010.
Recipient characteristics
Baseline recipient demographic data included gender, race, age and height at the time of transplant. Etiologies of liver disease were grouped as follows: alcoholic, hepatitis C virus (HCV), hepatitis B virus (HBV), nonalcoholic fatty liver disease (NAFLD, including cryptogenic and nonalcoholic steatohepatitis), autoimmune (including autoimmune hepatitis, primary biliary cirrhosis and primary sclerosing cholangitis) and other (including alpha-1-antitrypsin deficiency, Budd–Chiari, hemochromatosis and others). Patients who were listed with HCV in addition to other diagnoses were included in the HCV cohort. A covariable was created for patients who received MELD exception points for hepatocellular carcinoma (HCC); this covariable was evaluated in our multivariable analyses separate from the primary etiology of liver disease. Patients were categorized as having “ascites” or “encephalopathy” if the ascites- or encephalopathy-related variables were coded in the STAR files as “moderate” or “severe”. Laboratory MELD scores at listing and at transplant were calculated using the standard formula (3) with a lower limit of 1.0 set for all variables. For patients on dialysis at the time of transplant, serum creatinine was set to 4.0 for laboratory MELD calculation. The MELD score at the time of transplantation (LT-MELD), which reflects exception points if granted, was obtained from the STAR files.
Donor characteristics
Donors were characterized by gender, race, age, height, HCV antibody status, Centers of Disease Control (CDC) high risk status, cause of death, donation after cardiac death and distance between the donor and the recipient hospitals. The donor risk index (DRI) was calculated using the formula established by Feng et al. (1), which included share region and cold ischemia time. Missing values for cold ischemia time were imputed with the median times for the recipient’s UNOS region and share region (e.g. local, regional or national share). Cut-offs for selected variables considered implausible for an adult recipient were as follows: recipient height <120 cm or >240 cm, recipient weight <30 kg or >180 kg, donor height <100 cm or >240 cm and donor weight <20 kg or >180 kg and cold ischemia time <1 h or >24 h and warm ischemia time <10 min or >120 min. Observations with implausible values were set as missing. Sensitivity analyses comparing the multivariable models using case-wise deletion for missing values confirmed that imputation did not substantially change the interpretation of the final results.
The share region (e.g. local, regional, and national) for each donor liver graft was determined using the designations by UNOS. Given that the livers are shared across all donation service areas (DSA) for the states of New York, Ohio and Tennessee, liver grafts that were coded as “regional” in the STAR file, were changed to “local”.
Center and geographic characteristics
All transplants performed at pediatric centers and centers that performed ≤5 total liver transplants over the 5-year study period were excluded from the analyses. Transplant center volume per year is the average number of transplants performed during the study period. Transplant centers were categorized as low (<62 transplants/year), mid (62–104 transplants/year), and high-volume (≥105 transplants/year) centers based on volume tertiles.
DSAs were characterized by tertiles of the median LT-MELD score over the 5-year study period (<22 = low MELD, 22–25 = mid MELD, ≥26 = high MELD). The 11 UNOS regions were categorized into low (regions 3, 6, 10 and 11), mid (regions 2, 4, 7 and 8), and high (1, 5 and 9) LT-MELD regions.
Statistical analysis
Descriptive continuous and dichotomous characteristics were compared using the Wilcoxon and chi-square tests, as appropriate. Patient and graft survival were obtained from the STAR files and supplemented with mortality information from the Social Security Administration Death Master File. Kaplan–Meier methods were used to estimate survival rates at 1 and 3 years. The primary predictor was national liver distribution, with local liver distribution as the reference group. The primary outcomes were patient and graft survival. Covariates included recipient, donor, center and geographic characteristics. Serum creatinine, rather than laboratory MELD score, was evaluated because the laboratory MELD score has not been associated with patient and graft survival. Adjusted Cox models were used to evaluate the independent association between nationally distributed livers and post-transplant outcomes. Covariates were selected using backward deletion with a liberal cut-off of p < 0.2 to reduce residual confounding. We used robust standard errors with clustering by center (4), and included DSA and region as fixed effects in all models. The proportional hazards assumption was assessed by fitting a separate relative hazard for the primary predictor in days 0–90, 90–180, 180–365, 365–730 and then after 730, then testing for heterogeneity in the resulting estimates; no evidence for violations were found (p for heterogeneity >0.51).
Analyses were performed using Stata® 11.0 statistical software (College Station, TX, USA). The institutional review board at the University of California-San Francisco approved this study.
Results
A total of 26 480 adult, deceased donor, whole liver transplants from 2/1/2005 through 1/31/2010 were included in this study. Nationally placed grafts accounted for 1567 (6%) of transplants. For the remaining transplants, 19 893 (75%) utilized locally placed livers for recipients with MELD scores ≥15, 4702 (18%) utilized regionally placed livers for recipients with MELD scores ≥15, and 318 (1%) utilized locally placed livers for recipients with MELD scores <15.
Donor characteristics
Nationally versus locally placed livers differed significantly (Table 1). Donors of nationally placed liver grafts were older (50 vs. 42 years), less likely to be male (58 vs. 61%), and more likely to be African-American (20 vs. 16%). They were more likely to die from cerebrovascular accident (51 vs. 42%) and less likely to die from trauma (25 vs. 39%). They were more likely to be categorized as CDC high risk (13 vs. 9%) and have positive HCV antibody (11 vs. 2%). Median cold ischemia time (9 vs. 7 hours) and distance between donor and recipient hospitals (528 vs. 26 miles) were significantly longer for nationally compared to locally placed livers.
Table 1.
Baseline characteristics of donors of nationally versus locally distributed livers
| Characteristics number (%) or median (IQR) |
National (n = 1 567) |
Local (n = 19 893) |
p-Value |
| Male gender | 867 (58) | 11 795 (61) | 0.04 |
| Age in years | 50 (36–62) | 42 (25–54) | <0.001 |
| African-American race | 293 (20) | 3 205 (16) | 0.002 |
| Height in centimeters | 170 (163–180) | 173 (165–180) | 0.02 |
| Positive HCV antibody | 165 (11) | 416 (2) | <0.001 |
| CDC high risk | 209 (13) | 1 706 (9) | <0.001 |
| Cause of death | |||
| Anoxia | 291 (19) | 3 279 (17) | <0.001 |
| Cerebrovascular accident | 765 (51) | 8 171 (42) | |
| Trauma | 379 (25) | 7 565 (39) | |
| Other | 66 (4) | 495 (3) | |
| Donation after cardiac death | 138 (9) | 893 (5) | <0.001 |
| Cold ischemia time in hours | 9 (7–11) | 7 (5–8) | <0.001 |
| Donor risk index | 2.1 (1.7–2.5) | 1.4 (1.1–1.7) | <0.001 |
| Distance between donor and recipient in miles | 528 (305–754) | 26 (4–89) | <0.001 |
IQR = interquartile range; MELD = Model for End-Stage Liver Disease.
Recipient characteristics
Baseline recipient characteristics are shown in Table 2. Although recipients of nationally versus locally placed livers were statistically significantly older (56 vs. 55 years), more likely to be Caucasian (77 vs. 71%) and have alcoholic liver disease (16 vs. 14%), less likely to be Hispanic (10 vs. 14%) and have HCV-related liver disease (40 vs. 43%), these differences were not clinically significant. Nor were differences in serum albumin (3.0 vs. 2.9 grams/dL), rates of prior transplant (5 vs. 7%), ascites (35 vs. 37%), or encephalopathy (12 vs. 15%) clinically relevant. Rates of hepatocellular carcinoma were lower among recipients of nationally compared to locally placed livers (17 vs. 24%) as were rates of nonhepatocellular exceptions (6 vs. 11%). Mean waitlist times were comparable in the two groups (71 vs. 79 days, p = 0.24).
Table 2.
Baseline characteristics of recipients of nationally versus locally distributed livers
| Characteristics number (%) or median (IQR) |
National (n = 1 567) |
Local (n = 19 893) |
p-Value |
|---|---|---|---|
| Male gender | 1 067 (68) | 13 934 (70) | 0.11 |
| Age, years | 56 (50–61) | 55 (49–60) | <0.001 |
| Race | |||
| Caucasian | 1 208 (77) | 14 165 (71) | <0.001 |
| Hispanic | 157 (10) | 2 696 (14) | <0.001 |
| African-American | 131 (8) | 1 898 (10) | 0.12 |
| Asian | 60 (4) | 907 (5) | 0.18 |
| Other | 11 (1) | 227 (1) | 0.11 |
| Private insurance | 880 (56) | 11 920 (60) | 0.003 |
| Height in centimeters | 173 (165–178) | 173 (165–180) | <0.001 |
| Etiology of liver disease | |||
| Hepatitis C | 622 (40) | 8 471 (43) | 0.03 |
| Alcoholic | 256 (16) | 2 835 (14) | 0.02 |
| Nonalcoholic fatty | 220 (14) | 2 529 (13) | 0.13 |
| Cholestatic liver disease | 140 (9) | 2 005 (10) | 0.15 |
| Hepatitis B | 39 (2) | 636 (3) | 0.12 |
| Other etiologies | 290 (19) | 3 417 (17) | 0.18 |
| Hepatocellular carcinoma | 260 (17) | 4 739 (24) | <0.001 |
| Ascites | 542 (35) | 7 435 (37) | 0.03 |
| Encephalopathy | 190 (12) | 2 904 (15) | 0.007 |
| Serum sodium at transplant | 137 (133–139) | 136 (133–139) | 0.51 |
| Serum albumin at transplant | 3.0 (2.5–3.5) | 2.9 (2.4–3.4) | <0.001 |
| Prior transplant | 76 (5) | 1 404 (7) | 0.001 |
| Nonhepatocellular carcinoma exception | 91 (6) | 2 179 (11) | <0.001 |
| LT-MELD at transplant | 20 (15–24) | 24 (22–29) | <0.001 |
| LT-MELD categories | |||
| <15 | 387 (25) | 0 (0) | <0.001 |
| 15–19.9 | 390 (25) | 3 137 (16) | |
| 20–24.9 | 427 (27) | 7 105 (36) | |
| 25–29.9 | 178 (11) | 4 722 (24) | |
| 30–34.9 | 77 y(5) | 2 191 (11) | |
| ≥35 | 108 (7) | 2 738 (14) | |
| Laboratory MELD at transplant | 15 (12–22) | 20 (14–27) | <0.001 |
| Waitlist time in days | 71 (22–213) | 79 (19–256) | 0.24 |
IQR = interquartile range; n = number; MELD = Model for End-Stage Liver Disease.
LT-MELD scores were significantly lower for recipients of nationally versus locally placed livers (20 vs. 24, p < 0.001), as were laboratory MELD scores (15 vs. 20, p < 0.001). Among national liver ecipients, 50% had a LT-MELD ≥20, 25% had a LT-MELD between 15–19, and the remaining 25% had a LT-MELD <15 (Table 2).
Center and geographic characteristics
Table 3 lists the proportion of nationally transplanted livers by center and geographic characteristics along with the proportion of all liver transplants for reference. Although the proportion of all livers transplanted was equal by center volume category (e.g. low, mid, high volume), a greater proportion of nationally distributed livers were transplanted by the high volume center category. Similarly, the proportion of nationally distributed livers increased with increasing center competition per DSA and with increasing region LT-MELD score (Table 3).
Table 3.
Proportion of total and nationally distributed livers by center and geographic characteristic
| Characteristics | % of total transplants (n = 26 480) |
% of nationally distributed livers (n = 1567) |
| Center volume, by tertile | ||
| Low (<62 transplants/year) | 34% | 11% |
| Mid (62–104 transplants/year) | 33% | 22% |
| High (≥105 transplants/year) | 33% | 67% |
| Center competition, by # centers per DSA | ||
| 1 | 17% | 12% |
| 2–3 | 47% | 30% |
| 4+ | 36% | 58% |
| Region, by median LT-MELD | ||
| Low (regions 3, 6, 10, 11) | 40% | 22% |
| Mid (regions 2, 4, 7, 8) | 36% | 31% |
| High (regions 1, 5, 9) | 24% | 47% |
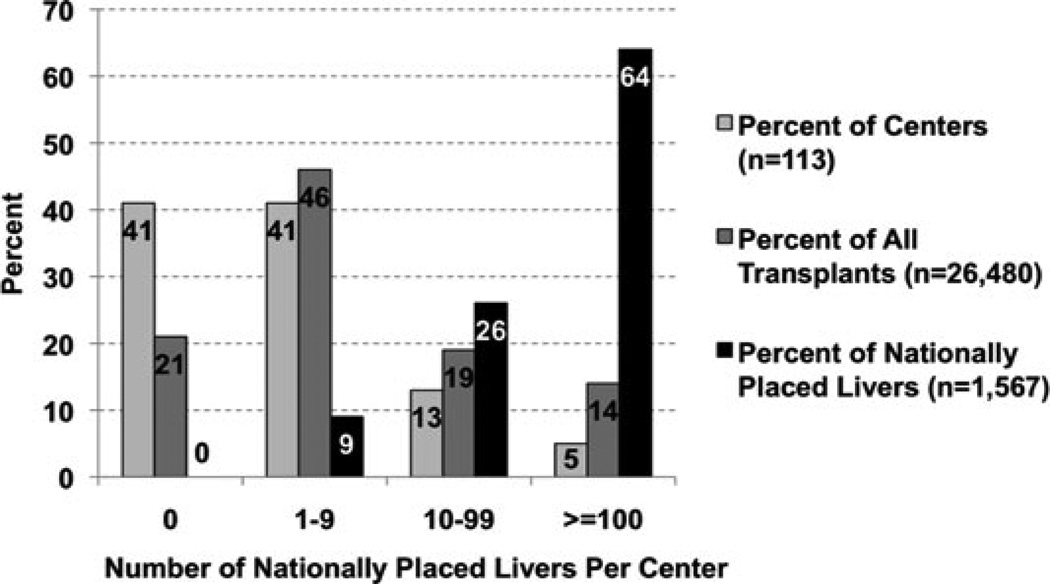
Notably, six of 113 (5 %) adult liver transplant centers utilized 1006 (64 %) of nationally placed grafts during the study period (Figure 1). As a point of reference, these six centers transplanted 14 % of the total number of transplants during this study period (Figure 1). Nationally placed livers accounted for 24 % of all adult deceased donor liver transplants performed by these six centers. Of these six centers, five were high volume centers while the remaining was a mid-volume transplant center. Three centers were located in high LT-MELD DSAs, one in amid-LT-MELD DSA, and two in low-LT MELD DSAs. These six centers were evenly split between low-, mid- and high-LT MELD UNOS regions.
Figure 1.
Center distribution of nationally placed livers.
In contrast, 43 (38 %) adult liver transplant centers did not utilize a single nationally placed graft. These centers accounted for 21% of the national transplant volume during the study period (Figure 1). Of these centers, 38 were low- and five were mid-volume centers. Therefore, all high volume transplant centers used at least one nationally placed liver.
Survival analyses
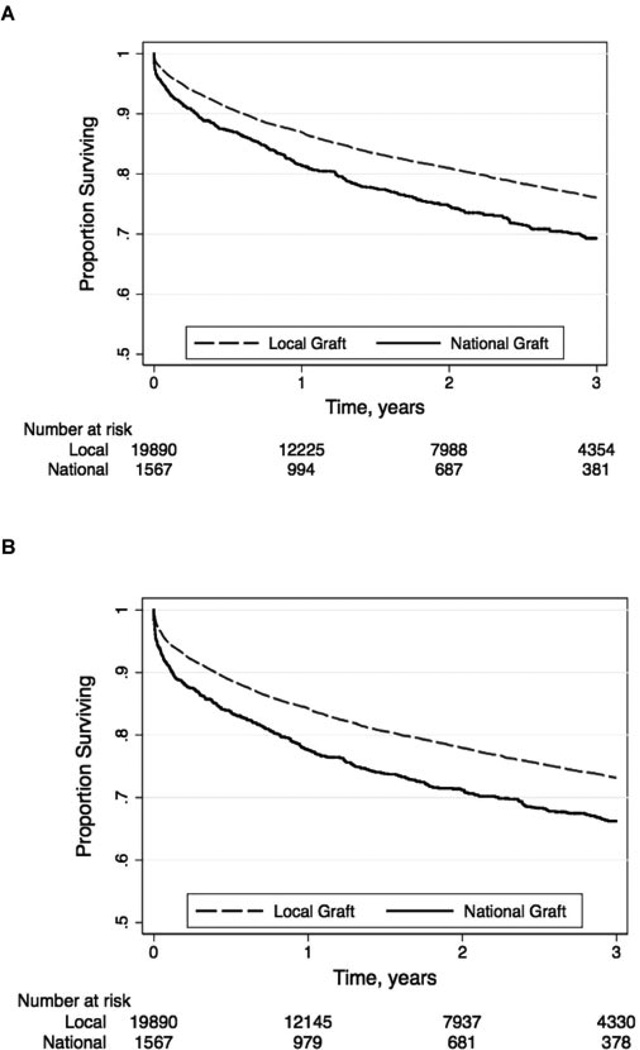
Graft loss occurred in 477 (33%) national liver recipients and 4412 (22%) local liver recipients (p < 0.001). Re-transplantation accounted for 113/477 (24%) and 865/4412 (20%) of these graft losses, respectively (p < 0.001). Unadjusted patient survival rates for nationally versus locally placed grafts were 81% versus 86% at 1 year, 74% versus 80% at 2 years, and 68% versus 75% at 3 years (p < 0.001; Figure 2). Unadjusted graft survival rates for nationally versus locally placed grafts were 77% versus 83% at 1 year, 70% versus 77% at 2 years and 65% versus 72% at 3 years (p < 0.001; Figure 2).
Figure 2.
Unadjusted (A) patient and (B) graft survival curves by national versus local distribution.
In Cox models adjusting for recipient (age, ethnicity, etiology of liver disease, hepatocellular carcinoma and creatinine at transplant) and donor (age, race, donation after cardiac death, height and cold ischemia time) characteristics, and accounting for center, DSA, and region, we found no evidence that recipients of nationally placed livers were at increased risk of graft loss (HR, 0.99; 95% CI, 0.86–1.14; p = 0.89) or death (HR, 0.98; 95% CI, 0.84–1.14; p = 0.77).
Discussion
Our study describes the U.S. experience with national sharing of livers for transplantation. We confirmed that donors of nationally compared to locally placed livers were of lower quality with respect to donor characteristics including age, African-American race, height, positive HCV-antibody, CDC high risk classification, cause of death and donation after cardiac death. However, after adjusting for these differences in donor quality—in addition to significant recipient characteristics and center/geographic effects-–we found that transplantation with a nationally placed liver was associated with no increased risk of patient and graft loss compared to transplantation with a locally placed liver.
Surprisingly, we found that utilization of nationally offered livers was highly concentrated among six centers in the United States, while 43 (38%) centers did not accept a single nationally offered liver. The majority of these high-utilizing centers were high-volume centers located in DSAs with greater center competition and high median LT-MELD scores. On average, nationally placed livers accounted for nearly one-quarter of the total adult transplant volume at each of these centers. This distribution pattern is certainly unexpected in the context of the current MELD allocation policy that is based on the disease severity of individual candidates. These data strongly suggest that factors other than pure donor and recipient variables influence the decision to accept a nationally offered liver. Moreover, the heavy concentration of nationally placed livers in a few centers suggests the existence of expedited placement pathways leading to a small number of transplant centers. Current OPTN policy does not include an overt mechanism of expedited organ placement so these placements are presumably being done on an ad hoc basis.
Our data also revealed that one quarter of national liver recipients had an LT-MELD <15, the threshold below which patients may not derive overall liver transplant survival benefit from deceased donors (6). This threshold for survival benefit was adjusted for average donor quality, so it is possible that this LT-MELD threshold would be even higher for patients accepting a lower quality, nationally distributed liver. Although these patients with a lower LT-MELD score may have fallen into the subgroup of patients who are underserved by the MELD estimation of waitlist mortality (7), we did not find evidence that these patients were sicker based on the available data related to encephalopathy, ascites, serum sodium or albumin, as collected in the UNOS/OPTN registry.
The reason why these patients with lower LT-MELD scores received these lower quality, nationally distributed livers rather than higher LT-MELD patients on the local, regional, or national waitlists is unknown. The accepting center may determine that the patient is at significant risk of death that is not reflected by his/her MELD score (such as an HCC patient beyond Milan). A less charitable scenario is that the center may transplant these low MELD patients with a national liver reserving their higher MELD patients to compete with patients at other local centers for local organs. This is supported by our data revealing that fewer recipients of nationally distributed livers were listed with HCC and non-HCC exceptions, as these patients otherwise have access to deceased donor livers at higher LT-MELD scores through predictable MELD increases.
One might have expected that nationally distributed livers—declined on both the local and regional levels, presumably for reasons of donor quality—would be associated with significantly higher rates of patient and graft loss. However, recent data have shown that centers transplanting a higher volume, as defined as >78 transplants per year, had improved graft survival with high-DRI livers compared to low volume centers (5). We speculate that the centers that utilize a high volume of nationally offered livers have greater experience with managing the immediate and long-term complications associated with high-DRI livers. A future study looking specifically at the association between the volume of nationally distributed livers and center-specific survival is planned to better understand our findings.
We acknowledge that there are limitations to our study. Administrative datasets are subject to entry error or inconsistencies with respect to subjective variables such as ascites or encephalopathy. By combining the categories of “moderate” and “severe” for these two variables, we attempted to capture simply the “presence” versus “absence” of these characteristics, which is less subject to error. In addition, some aspects of a recipient’s clinical status that may prompt a clinician to accept a national liver despite low prioritization by the LT-MELD, such as functional well-being and life-limiting symptoms related to liver disease, are not captured by the registry. Lastly, statistically significant differences between groups may, in fact, be an artifact of the large sample size, warranting caution when interpreting data especially with respect to the overall ‘health’ of national versus local liver recipients.
Despite these limitations, evaluation of national liver allocation and distribution could not have been performed without the use of the UNOS/OPTN registry, and our results have important implications for the U.S. liver distribution system. Currently, livers are allocated locally, regionally, then nationally, based on arbitrary geographic boundaries. Centers located in regions covering a small geographic area may better be able to utilize a nationally distributed liver with less cold ischemia time than centers in larger regions. As a purely hypothetical example, a center in Tennessee (UNOS region 11) can accept a nationally offered liver from centers in UNOS regions 2, 3, 4, 7, 8 and 10 with a transport distance shorter than that of a regionally offered liver travelling from Albuquerque, New Mexico, to San Francisco, California. Our data, demonstrating that nationally distributed livers have no increased risk of graft loss compared to a liver with similar donor characteristics, argue for the elimination of the arbitrary geographic DSA and UNOS region boundaries in favor of expanded sharing agreements over broader geographic areas. Alternatively, given the high concentration of nationally distributed livers within a few centers, expedited liver graft placement to centers that are experienced with utilizing these lower quality livers in a more transparent and formalized fashion may help to streamline the donor offer process.
In conclusion, our study is the first to shed light on the highly concentrated distribution of nationally placed livers. Let this be the foundation for an informed discussion about changing our current liver allocation and distribution policies.
Acknowledgments
Funding source: This project was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (T32 DK060414, JCL) and the University of California San Francisco Liver Center (JCL, NAT).
Abbreviations
- CDC
Centers for Disease Control and Prevention
- DRI
Donor risk index
- DSA
donation service area
- HBV
Hepatitis B Virus
- HCC
hepatocellular carcinoma
- HCV
Hepatitis C Virus
- LT-MELD
Model for End-Stage Liver Disease score at liver transplant
- MELD
Model for End-Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- STAR
Standard Transplant Analysis and Research
- UNOS/OPTN
United Network for Organ Sharing/Organ Procurement and Transplantation Network.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: The concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 2.Organ Procurement and Transplantation Network. Policy 3.6: Allocation of Livers. [Accessed July 8, 2011]; Available at: http://optntransplanthrsagov/PoliciesandBylaws2/policies/pdfs/policy_8pdf.
- 3.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 4.Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 5.Ozhathil DK, Li YF, Smith JK, et al. Impact of center volume on outcomes of increased-risk liver transplants. Liver Transplant. 2011;17:1191–1199. doi: 10.1002/lt.22343. [DOI] [PubMed] [Google Scholar]
- 6.Merion RM, Wolfe RA, Dykstra DM, Leichtman AB, Gillespie B, Held PJ. Longitudinal assessment of mortality risk among candidates for liver transplantation. Liver Transplant. 2003;9:12–18. doi: 10.1053/jlts.2003.50009. [DOI] [PubMed] [Google Scholar]
- 7.Biggins SW, Bambha K. MELD-based liver allocation: Who is underserved? Semin Liver Dis. 2006;26:211–220. doi: 10.1055/s-2006-947291. [DOI] [PubMed] [Google Scholar]