Summary
The Wnt-signaling pathway is necessary in a variety of developmental processes and has been implicated in numerous pathologies. Wntless (Wls) binds to Wnt proteins and facilitates Wnt sorting and secretion. Conventional deletion of Wls results in early fetal lethality due to defects in body axis establishment. To gain insight into the function of Wls in later stages of development, we have generated a conditional null allele. Homozygous germline deletion of Wls confirmed prenatal lethality and failure of embryonic axis formation. Deletion of Wls using Wnt1-cre phenocopied Wnt1 null abnormalities in the midbrain and hindbrain. In addition, conditional deletion of Wls in pancreatic precursor cells resulted in pancreatic hypoplasia similar to that previously observed after conditional β-catenin deletion. This Wls conditional null allele will be valuable in detecting novel Wnt functions in development and disease.
Keywords: Wnt, Evi, Gpr177, Sprinter, Wnt transporter
INTRODUCTION
The vertebrate Wnt family consists of 19 secreted, cysteine-rich glycoproteins essential for embryogenesis and homeostasis. The ability of Wnts to reach their target cell is dependent on proteins responsible for processing and secretion of Wnt proteins. One of these Wntless (Wls) (also known as Evenness Interrupted or Sprinter) encodes a seven-pass transmembrane protein and was orginally described in Drosophila and C. elegans. Wls is evolutionarily conserved in vertebrates and invertebrates with a Blast search revealing only 1 homologue in C. elegans, mice, and humans (Banziger et al., 2006; Bartscherer et al., 2006; Goodman et al., 2006). Wls mutant Drosophila larvae exhibit higher levels of Wg antigen than wild-type cells, suggesting that it is still expressed, but not secreted (Banziger et al., 2006; Bartscherer et al., 2006). Although it is possible that Wls might also be involved in lipid modifications, insights into Wls function have confirmed the role of Wls as a Wnt cargo receptor and thereby essential for Wnt secretion (Belenkaya et al., 2008; Franch-Marro et al., 2008; Pan et al., 2008; Port et al., 2008; Yang et al., 2008).
Previous studies have often relied on β-catenin deletion to understand the impact of canonical Wnt signaling in different processes. However, due to the dual function of β-catenin in both canonical Wnt signaling and cell adhesion, it has sometimes been difficult to separate canonical pathway-dependent effects. Because Wls appears to control the release of all Wnts, the utilization of Wls conditional mutants would be a powerful tool allowing spatial and temporal control of Wnt secretion both during development and in disease models. Deletion of Gpr177, the mouse orthologue of Wls, results in embryonic lethality due to impairment of the developing body axis (Fu et al., 2009). Therefore, we generated a Gpr177, or Wls, conditional null allele and affirmed its use as a means to study the loss of multiple Wnts by pheno-copying previously characterized Wnt-dependent development of the body axis, brain, and pancreas.
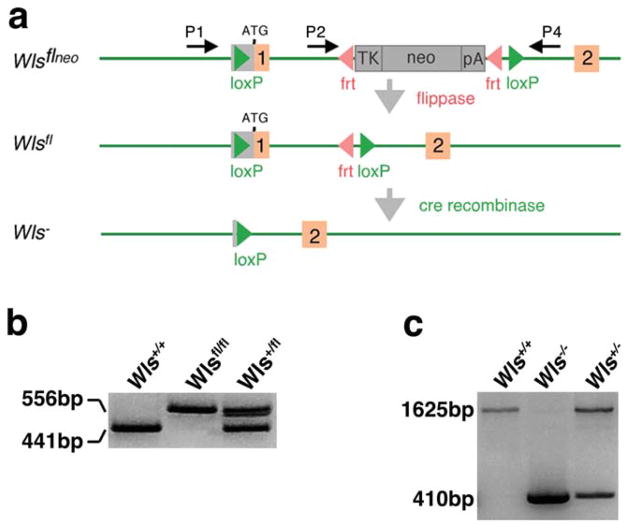
To test the role of the Wls gene in controlling Wnt signaling in mouse, we generated a conditional null allele using the Cre-LoxP and Flp-Frt systems. The targeting strategy is shown in Figure 1a. The Frt site-flanked neo cassette was placed in intron 1. One LoxP site was placed in front of the ATG start site in the 5′ untranslated region, and the other was placed after the Frt site-flanked neo cassette. Successful removal of the Neo cassette by crossing offspring that inherited the targeted allele with the flippase mouse was assessed by PCR using primers 2 and 4 (P2 and P4) (Fig. 1b).
FIG. 1.
Generation of the Wls conditional allele. (a) Orange boxes correspond to exons. Red arrowheads are Frt sites. Green arrowheads denote LoxP sites. Recombination of the Frt sites flanking the Neo results in the floxed Wls allele. Recombination of the LoxP sites removes the ATG start site in exon 1 resulting in the Wls null (Wls−) allele. P1, P2, and P4 represent the location of the primers used for Wls genotyping. (b) Successful Flp recombination was confirmed using PCR analysis to detect a 441-bp WT band and a 556 bp Wlsfl Neo deleted band (using primers P2 and P4). (c) Cre recombination was validated using PCR analysis to detect a 1,625-bp WT band and a 410-bp null band (using primers P1 and P4).
The germline null allele was generated by crossing Wlsfl/fl with the EIIa-cre transgenic line (Lakso et al., 1996). Recombination of the LoxP sites using EIIa-cre results in the removal of exon1. PCR genotyping using primers 1 and 4 (P1 and P4) on E7.5 embryos generated from crosses of heterozygotes gave the expected amplification products (Fig. 1c). Wls floxed mice and Wls heterozygotes (Wls+/fl; EIIa-cre and Wls−/fl) were viable and had no obvious phenotype (data not shown). No Wls homozygous null pups or embryos were recovered after E8.5. Morphological analysis of E7.5 Wls+/+ embryos confirmed that these embryos had undergone gastrulation (Fig. 2a). In addition, histological analysis demonstrated the presence of all three germ layers (Fig. 2c). At E8.5 Wls+/+ embryos displayed well-developed headfolds (Fig. 2e). In contrast, Wls germline homozygotes appeared arrested at E6 as they did not progress past the egg cylinder stage and by morphological criteria lacked mesoderm (Fig. 2b,d). At E8.5, 9 of 10 Wls−/− embryos were in the process of being reabsorbed. Wls−/− embryos that did survive until this stage maintained the morphological appearance of an egg cylinder (Fig. 2f). Perhaps, the earliest described Wnt phenotype comes from a loss of Wnt3 (Liu et al., 1999). Wnt3 null mice develop a normal egg cylinder, but fail to form a primitive streak or mesoderm. Our data confirms the recent finding of Fu et al. (2009) that germline Wls deletion results in the loss of primitive streak and mesoderm formation and is also very similar to Wnt3 deletion and β-catenin loss of function homozygotes (Fu et al., 2009; Huelsken et al., 2000; Liu et al., 1999).
FIG. 2.
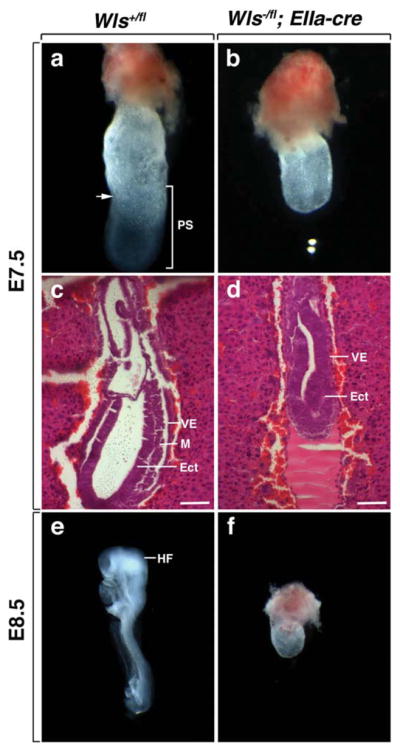
Deletion of germline Wls inhibits mouse embryogenesis. (a, b) Morphological analysis of embryos dissected at E7.5. The wild-type embryo has the characteristics of an early neural plate stage embryo with evidence of primitive streak formation. Wls−/− mutants resemble egg cylinders. The arrow denotes the embryonic-extra-embryonic junction at the anterior side. (c, d) Histological analysis of embryos dissected at E7.5. Wild-type embryos exhibit endoderm, mesoderm, and ectoderm. Wls−/− mutants display endoderm and ectoderm, but no mesoderm. Scale bars represent 100 μm. (e) Control embryo at E8.5 with a normal embryonic axis. (f) At E8.5 Wls−/− mutants still resemble egg cylinders. VE, visceral endoderm; M, mesoderm; Ect, ectoderm, PS, primitive streak; HF, head-fold.
Mice deficient in Wnt1 display loss of a significant portion of the developing brain (Echelard et al., 1994; McMahon and Bradley, 1990). A similar but more severe phenotype has been observed in mice that exhibit conditional deletion of β-catenin in neural crest cells (Brault et al., 2001). To assess the consequences of Wls loss-of-function in the dorsal midline of the diencephalon, we conditionally deleted Wls using Wnt1-cre. Heterozygotes (Wls+/fl; Wnt1-cre and Wls−/fl) did not exhibit any obvious phenotype (data not shown). Mutant embryos (Wls−/fl; Wnt1-cre) were recovered at Mendelian ratios, but mutant mice were not recovered postnatally. The mutant phenotype was obvious as early as E9.5 due to the presence of a shortened neural tube (Fig. 3j). Nonneuronal tissues appeared normal. At E10.5 and E11.5, Wls wild-type littermates (Wls+/fl) had a discernible forebrain (telencephalon and diencephalon), midbrain, and hind-brain (metencephalon and myelencephalon) (Fig. 3a,c). The isthmus was prominent. In contrast, mutant mice exhibited a shortened anteroposterior axis and no apparent isthmus, suggesting loss of midbrain and anterior hindbrain, that is similar to that observed in Wnt1 mutants (Fig. 3b). Additionally, a malformed forebrain was observed in whole-mount embryos as early as E11.5 (Fig. 3d). Histological sections at E10.5 verified deletion of much of the midbrain and of regions of metencephalon (Fig. 3f). Furthermore, E12.5 sections exhibited the obvious loss of midbrain and hindbrain structures including the cerebellum and choroid plexus and a severely truncated forebrain (Fig. 3h). These results parallel those observed in mice with Wnt1-cre mediated deletion of β-catenin (Brault et al., 2001). In addition, although no fore-brain phenotypes have been described in either Wnt1 or Wnt3a mutants, combined deletion has been shown to result in a loss of forebrain structures (Ikeya et al., 1997). These findings thus validate the use of the Wls conditional allele in studying Wnt-signaling pathways and argue that it is a means to overcome ligand redundancy.
FIG. 3.
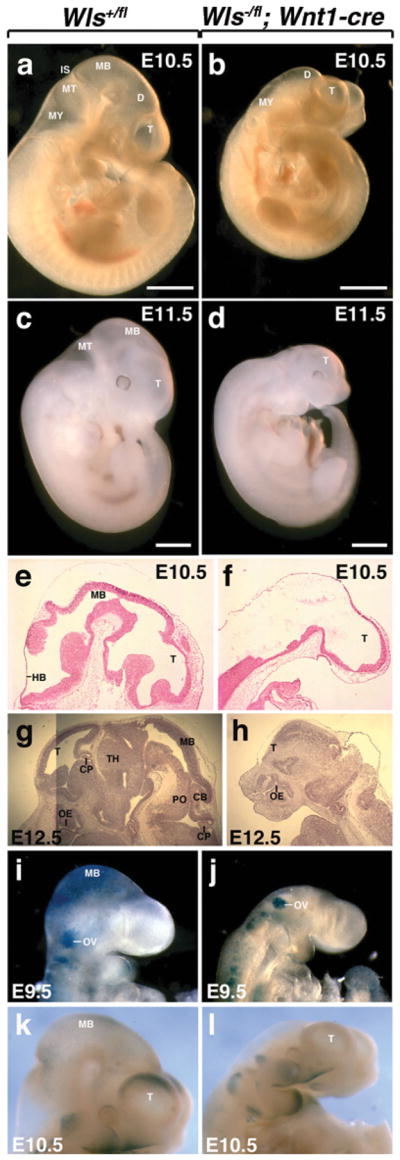
Deletion of Wls in the dorsal midline of the diencephalon using Wnt1-cre. (a, b) Morphological analysis of embryos dissected at E10.5. Wls+/fl embryos exhibit a forebrain, midbrain and hindbrain. Wls−/fl; Wnt1-cre embryos fail to develop a midbrain. (c) Wls+/fl embryos dissected at E11.5. (d) Wls−/fl; Wnt1-cre embryos at E11.5 show defective development of midbrain, hindbrain and forebrain. Histologic comparison of WT (e, g) and mutant embryos (f, h). Mutant embryos display loss of midbrain and hindbrain formation at E10.5 (f). At E11.5, mutant embryos additionally lack a forebrain choroid plexus and display a modified forebrain (h). β-catenin/Wnt activity in the brain of Wls+/fl (i and k) and Wls−/fl; Wnt1-cre (j and l) embryos stained for X-gal. Scale bars represent 1 mm. T, telencephalon; D, diencephalon; MB, midbrain; MT, metencephalon; MY, myencephalon; IS, isthmus; HB, hindbrain; CP, choroid plexus; OE, olfactory epithelium; TH, thalamus; PO, pons; CB, cerebellum; OV, otic vessicle.
To determine whether canonical Wnt function was in fact impaired in Wls mutant mice, we took advantage of the TOPGAL reporter line that expresses lacZ in response to activation of the canonical Wnt pathway (DasGupta and Fuchs, 1999). At E9.5, intense X-gal staining was present in regions of the midbrain and hindbrain (Fig. 3i). In mutant embryos, X-gal staining was absent in the head region with the exception of the otic vesicle (Fig. 3j). At E10.5, X-gal staining was more prominent in the dorsal forebrain (Fig. 3k), a region that has not been shown to express Wnt1, but does express other Wnt ligands (Parr et al. 1993). E10.5 mutants did not exhibit any central nervous system (CNS) X-gal staining (Fig. 3l). This suggests that the loss of Wls in the dorsal midline of the diencephalon, where Wnt1-cre is expressed, may have a secondary effect on Wnt ligand expression in the telencephalon.
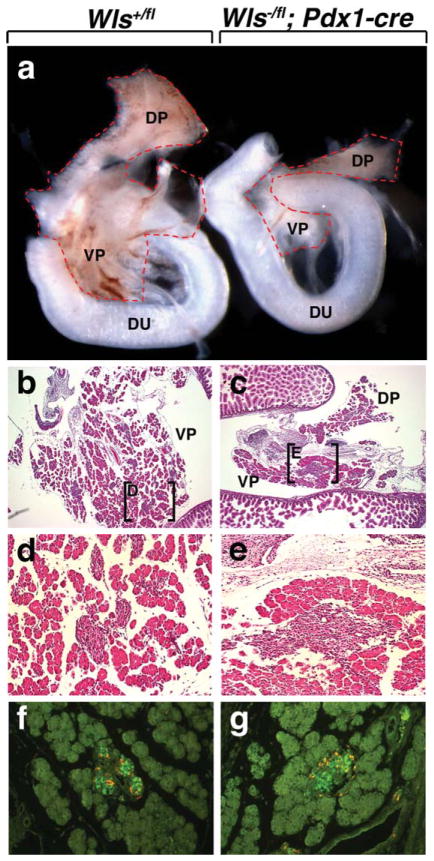
A number of Wnts are expressed during pancreas development including Wnt2b, Wnt4, Wnt5a, and Wnt7b (Heller et al., 2002). With the exception of the Wnt5a mutant, which exhibits defects in islet formation, none of the aforementioned Wnt gene mutants display a pancreas phenotype (Kim et al., 2005). Although there is some debate on the effects of conditional Pdx1-cre-mediated deletion of β-catenin in pancretic precursors due to the Pdx1-cre line used, all studies showed degrees of reduction in pancreas size (Dessimoz et al., 2005; Murtaugh et al., 2005; Wells et al., 2007). To determine whether Wls might be required for pancreas development, we conditionally deleted Wls in pancreatic precursor cells using a Pdx1-cre previously described (Wells et al., 2007). Early Pdx1-cre lineage tracing using Rosa26-Lacz reporter mice showed robust expression throughout the developing pancreas at E10.5 (Spence et al. 2009). Heterozygotes (Wls+/fl; Pdx1-cre and Wls−/fl) did not display any obvious pancreas abnormalities (data not shown). However, mice with conditional deletion of Wls were recovered postnatally, but the mutant pancreas was extremely hypoplastic (data not shown). At E15.5, the dorsal and ventral pancreatic lobes had formed in the mutant, although, overall, the pancreas was severely hypoplastic relative to wild-type control littermates (Fig. 4a). Histological analysis of E15.5 embryos revealed developing acinar, endocrine, and ductlike tissue in wild-type and mutant embryos (Fig. 4b,c). All three tissues appeared to be reduced in the mutant pancreas (Fig. 4e). To investigate the impact of deletion of Wls on endocrine pancreas development, we stained the E15.5 pancreas for glucagon and insulin. Similar to the phenotype observed with the Pdx1-cre β-catenin deletion, endocrine cell specification appeared unaffected by loss of Wls (Fig. 4f,g) (Wells et al., 2007). These findings further validate the use of the Wls allele to study the effects of Wnt-signaling pathways.
FIG. 4.
Deletion of Wls in pancreatic precursor cells using Pdx1-cre. (a, b) Morphological analysis of pancreata dissected at E15.5. Both Wls+/fl and Wls−/fl; Pdx1-cre pancreata exhibit a dorsal and ventral pancreas. Wls−/fl; Pdx1 pancreata are severely hypoplastic (b). Histologic comparison of WT (b, d, f) and mutant pancreata (c, e, g). Low-power magnification of both Wls+/fl and Wls−/fl; Pdx1-cre pancreata reveal the presence of acinar cells and Islets of Langerhans (b and c). Higher power magnification of the boxed regions in b and c (d and e, respectively). (f, g) E15.5 islets immunostained for insulin (green) and glucagon (orange). DP, dorsal pancreas; VP, ventral pancreas; Du, duodenum.
Taken together, these data demonstrate that the Wls allele is a functional conditional null. We anticipate it to be a valuable tool for use where precise spatial and temporal control of Wnt activity loss-of-function is advantageous. In addition, it will be useful to uncover Wnt ligand redundancy.
MATERIALS AND METHODS
Animal Maintenance and Use
Animals were housed in a pathogen-free vivarium in accordance with institutional policies. Gestational age was determined through detection of a vaginal plug. At specific gestational ages, dams were anesthetized with isoflurane and embryos removed by hysterectomy.
Generation of Wlsflox Allele
The Wlsfl allele was generated using conventional gene targeting methods. The Frt site-flanked neo gene of the targeting vector was excised in vivo using a flippase expressing mouse line (Rodriguez et al., 2000). Primers for genotyping of the Wlsfl allele were as follows: P1, CTTCCCTGCTTCTTTAAGCGTC; P2, AGGCTTCGAACG TAACTGACC; P4, CTCAGAACTCCCTTCTTGAAGC. The PCR protocol used was 94°C for 4 min followed by 30 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 40 s and a final extension of 72°C for 7 min. The use of primers P2 and P4 produce bands of 411 bp for the wild-type allele and 556 bp for the Wlsfl Neo deleted allele. The use of primers P1 and P4 produce bands of 1,625 bp for the wild-type allele and 410 bp for the Wls− allele. The stock number for this mouse that will soon be available at Jackson Laboratory is 012888.
Mouse Lines
The following transgenic mice used in this study were generated previously: EIIa-cre (Lakso et al., 1996), Wnt1-cre (Danielian et al., 1998), TOPGAL (DasGupta and Fuchs, 1999), and Pdx1-cre (Wells et al., 2007). The Wls floxed mice were crossed to the EIIa-cre, Wnt1-cre, and Pdx1-cre mice to generate conditional mice where Wls would be deleted in the germline, neural crest cells, or pancreatic progenitor cells, respectively. All mouse lines used in this study were genotyped by PCR using primers and protocols described previously. Yolk sacs from staged embryos or tail tips were digested overnight at 55°C in lysis buffer and genomic DNA extracted using a Kingfisher 96 Magnetic Particle Processor.
Immunofluorescence Staining
Pancreata were dissected in PBS and fixed in formalin. Pancreata were then processed in paraffin wax and sectioned at 4 μm. Primary antibody incubation was performed overnight at 4° followed by secondary antibody incubation for 1.5 h at room temperature. Antibody identities and dilutions are as follows: chicken anti-insulin (Abcam), guinea pig anti-glucagon (ABR) both used at 1:1,000, goat anti-chicken 488 (Jackson ImmunoResearch), and donkey anti-guinea-pig Cy5 (Jackson ImmunoResearch). Figures in this work were prepared digitally using Canvas 8.0 and Creative Suite 3.0 software.
X-gal Staining
Staged embryos expressing lacZ reporter genes were fixed for 30 min using X-gal fixative (1% formaldehyde, 0.2% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA, and 0.01% NP-40) and washed twice with 1× PBS/0.02% NP-40 for 15 min. Embryos were then stained with X-gal solution (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 M MgCl2, 0.01% NP-40, 1 mg/ml X-gal) overnight, postfixed with 4% paraformaldehyde for 1 h, cryoprotected, and 10 μM cryosections prepared.
Hematoxylin and Eosin Staining
For CNS staining, whole fetal samples were fixed in 4% paraformaldehyde overnight, cryoprotected in 15% then 30% sucrose before embedding and freezing in OCT. E7.5 and E8.5 embryos and pancreata were dissected in PBS and fixed in formalin and then processed in paraffin wax. Eight micromolar sections were cut and stained with hematoxylin and eosin.
Acknowledgments
Contract grant sponsor: NIH, Contract grant numbers: 5T32HD07463, NEI RO1 EY16241, NEI RO1 CA131270
We thank Paul Speeg for excellent technical assistance and Yinhuai Chen and Tina Grisham at the University of Cincinnati Gene Targeting Mouse Service for generation of the Wls allele.
LITERATURE CITED
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Wu Y, Tang X, Zhou B, Cheng L, Sharma YV, Yan D, Selva EM, Lin X. The retromer complex influences Wnt secretion by recycling Wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dessimoz J, Bonnard C, Huelsken J, Grapin-Botton A. Pancreas-specific deletion of β-catenin reveals Wnt-dependent and Wnt-independent functions during development. Curr Biol. 2005;15:1677–1683. doi: 10.1016/j.cub.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Franch-Marro X, Wendler F, Guidato S, Griffith J, Baena-Lopez A, Itasaki N, Maurice MM, Vincent JP. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat Cell Biol. 2008;10:170–177. doi: 10.1038/ncb1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci USA. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM. Sprinter: A novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Heller RS, Dichmann DS, Jensen J, Miller C, Wong G, Madsen OD, Serup P. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev Dyn. 2002;225:260–270. doi: 10.1002/dvdy.10157. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Schleiffarth JR, Jessurun J, Sumanas S, Petryk A, Lin S, Ekker SC. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 2005;3:23. doi: 10.1186/1741-7007-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Murtaugh LC, Law AC, Dor Y, Melton DA. β-catenin is essential for pancreatic acinar but not islet development. Development. 2005;132:4663–74. doi: 10.1242/dev.02063. [DOI] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993;119:247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Port F, Kuster M, Herr P, Furger E, Banziger C, Hausmann G, Basler K. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat Cell Biol. 2008;10:178–185. doi: 10.1038/ncb1687. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JM, Esni F, Boivin GP, Aronow BJ, Stuart W, Combs C, Sklenka A, Leach SD, Lowy AM. Wnt/β-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol. 2007;7:4. doi: 10.1186/1471-213X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]