Abstract
The activity of six hydrolytic enzymes-carboxyl esterase, acid phosphatase, alkaline phosphatase, β-galactosidase, β-glucosidase and β-hexosaminidase, were studied in different regions of the normal human brain tissue obtained at autopsy. Protein estimation and activities of the hydrolytic enzymes with respective substrates were assayed by spectrophotometric and spectroflourometric methods. Amongst the eight regions of the brain-frontal, parietal, occipital, temporal, thalamus, cerebellum and hippocampus, the pineal gland showed highest activity for all hydrolytic enzymes studied except for carboxyl esterase. Among six hydrolases studied, hexosaminidase exhibited highest activity in all regions of the human brain while alkaline phosphatase activity was the least amongst all regions studied. A majority of the enzymes studied showed higher activity in gray matter as compared to the white matter except acid phosphatase and β-glucosidase which exhibited higher activity in the white matter. The most significant finding in the present study was the high activity of all hydrolytic enzymes noted in the pineal gland as compared to all other regions of the human brain. Such a finding has not been hitherto reported earlier in human brain tissue samples. If the specific activities of these enzymes are to be considered as any functional index, then pineal gland may be more metabolically active tissue with respect to the hydrolytic function as compared to the other regions of the brain.
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-012-0273-0) contains supplementary material, which is available to authorized users.
Keywords: Acid phosphatase, Alkaline phosphatase, Carboxyl esterase, β-Galactosidase, β-Glucosidase, β-Hexosaminidase, Hydrolytic enzymes, Pineal gland
Introduction
Hydrolytic enzymes alternatively referred to as hydrolase’s, split different groups of biomolecules such as esters, peptides and glycosides. Hydrolytic enzymes break down protein, lipids, nucleic acids, carbohydrate and fat molecules into their simplest units. Lysosomal hydrolase’s comprising phosphatases, carboxylesterases, glucuronidases and β-galactosidase, ribonuclease and acid proteinase are believed to play an important role in brain tumor [1].
Several studies have earlier highlighted the importance of studying hydrolytic enzymes in a variety of pathological conditions such as brain tumors [2, 3], neurodegenerative diseases such as Alzheimer’s disease [4] and inherited metabolic disorders [5]. Among all the hydrolase’s, two phosphomonoesterases viz., alkaline and acid phosphatase(s) have been extensively studied in tumors [6] then esterase’s were studied [7].
In particular, alkaline phosphatase activity was reported to be higher in meningiomas as compared to gliomas [8] where as acid phosphatase activity, localized in the form of coarse granules in the lysosomes, was found to be increased in reactive glial cells of gemistocytic and microglial origin. Carboxyl esterase was reported in endothelial cells and blood brain barrier of human brain [9].
There are few studies of the lysosomal hydrolase’s in developing human brain indicate brain growth occurs in alternate phases of structural plasticity and trophic acquisition [10]. The localization of β-galactosidase isoenzymes determined separately and hexosaminidase B was higher in granular layer than the molecular layer of cerebellum [11]. However there are not many studies that have elucidated the role of these enzymes in the normal human brain.
In the absence of precise quantitative and topographic distribution of the hydrolytic enzymes in the normal human brain, interpretation of results obtained in pathological conditions can either be fallacious or incomplete. Even amongst the few studies reported in literature, all the six hydrolytic enzymes have not been quantified in the same set of brain tissues. Further, the relative expression of these enzymes in the different regions of the human brain has also not been studied. Therefore, the present study was undertaken to quantify the six important hydrolytic enzymes (carboxyl esterase (E.C.3.1.1.1.)—Carboxylic ester hydrolase, acid phosphatase (E.C 3.1.3.2)—Orthophosphoric monoester phosphohydrolase (acid medium), alkaline phosphatase (E.C.3.1.3.1) Orthophosphoric monoester phosphohydrolase (alkaline optimum), β-galactosidase (E.C.3.2.1.23.) β-d-galactoside galactohydrolase, β-glucosidase (E.C. 3.2.1.21) β-d-glucoside glucohydrolase and β-hexosaminidase (E.C.3.2.1.52) β-N-acetyl-d-hexosaminide N-acetylhexosaminohydrolase) in various regions of the normal human brain.
Materials and Methods
Normal Brain Tissue
The Department of Neuropathology, NIMHANS has a National brain tissue repository wherein fresh brain tissue samples obtained at autopsy after informed consent is available for research purposes. A total of 27 fresh brain tissue samples were obtained from this repository for the study after obtaining institutional ethics committee approval. Amongst them 14 were males (13–50 years) and 13 (7–51 years) were female subjects. All these subjects had died of head injury following road traffic accidents. The salient socio-demographic features of these subjects are provided in Table 1 (25 no’s). The brain tissue samples were collected and processed between 3 and 17.5 h after death.
Table 1.
Specific activity of six hydrolytic enzymes in normal human brain white and gray matter regions frontal, parietal, temporal and occipital regions
| Brain | White matter (WM) | ||||
|---|---|---|---|---|---|
| Enz | Frontal | Parietal | Temporal | Occipital | Mean WM |
| CE (n) | (15) | (11) | (11) | (7) | (44) |
| Mean ± SD range | 38.37 ± 5.62 (26.45–52.51) | 33.95 ± 7.95 (20.23–51.55) | 44.39 ± 10.23 (30.57–58.76) | 42.53 ± 11.7 (30.22–61.95) | 39.81 ± 4.64 (33.95–44.39) |
| Acd P (n) | (17) | (13) | (12) | (9) | (51) |
| Mean ± SD range | 8.20 ± 2.24 (5.65–13.45) | 8.04 ± 1.61 (6.19–10.94) | 8.87 ± 1.85 (6.45–12.02) | 7.62 ± 1.73 (5.66–10.94) | 8.18 ± 0.51 (7.62–8.87) |
| Alk P (n) | (16) | (13) | (11) | (9) | (49) |
| Mean ± SD range | 1.198 ± 0.501 (0.407–1.86) | 1.24 ± 0.5 (0.5–2.06) | 1.68 ± 0.731 (0.704–2.52) | 1.52 ± 0.43 (0.90–2.28) | 1.40 ± 0.23 (1.19–1.68) |
| β-Gal (n) | (17) | (11) | (13) | (7) | (48) |
| Mean ± SD range | 120.28 ± 4.02 (72.61–185 .75) | 129.16 ± 26.27 (92.46–158. 48) | 140.7 ± 38.24 (92.6–192.9) | 134.27 ± 15.93 (110.11–149 .65) | 131.10 ± 8.62 (120.28–14 0.7) |
| β-Glu (n) | (16) | (12) | (10) | (7) | (45) |
| Mean ± SD range | 188.22 ± 46.41(125.58–259 .02) | 198.28 ± 39.93 (141.4–279 .75) | 164.97 ± 41.12 (108.09–231 .21) | 163.86 ± 32.9 (126.52–211 .81) | 178.83 ± 17.15 (9163.86–198 .28) |
| Hex (n) | (13) | (12) | (10) | (10) | (45) |
| Mean ± SD range | 2195.43 ± 382 .67 (1755.66–2796 .4) | 2503.83 ± 398 .88 (1876.15–3065.93) | 2616.79 ± 366.98 (2296.49–3360.82) | 2666.56 ± 511.02 (2282.095–3270.16) | 2495.65 ± 211.41 (2503.83–2666.56) |
| Brain | Gray matter (GM) | ||||
|---|---|---|---|---|---|
| Enz | Frontal | Parietal | Temporal | Occipital | Mean GM |
| CE (n) | (13) | (11) | (10) | (7) | (41) |
| Mean ± SD range | 62.47 ± 19.51 (25.58–90.72) | 57.28 ± 16.19 (34.23–83.52) | 62.40 ± 17.88 (38.66–89.24) | 50.33 ± 15.37 (29.57–66.03) | 58.12 ± 5.73 (50.33–62.47) |
| Acd P (n) | (15) | (13) | (11) | (8) | (47) |
| Mean ± SD range | 5.63 ± 1.65 (2.69–8.58) | 6.06 ± 1.961 (3.66–8.75) | 5.61 ± 2.06 (2.74–8.46) | 4.95 ± 0.937 (3.73–6.51) | 5.56 ± 0.458 (4.95–6.06) |
| Alk P (n) | (14) | (13) | (11) | (8) | (46) |
| Mean ± SD range | 3.29 ± 1.411 (1.38–5.74) | 3.87 ± 1.87 (0.924–6.75) | 3.78 ± 1.24 (2.47–6.25) | 3.43 ± 1.17 (2.02–5.51) | 3.59 ± 0.27 (3.29–3.87) |
| β-Gal (n) | (14) | (12) | (12) | (7) | (45) |
| Mean ± SD range | 182.83 ± 48.36 (132.67–273 .77) | 188.85 ± 41.5 (142.39–250 .93) | 171.83 ± 35.82 (136.28–252 .14) | 250.21 ± 40.69 (207.71–315 .77) | 198.43 ± 35.23 (171.83–250 .21) |
| β-Glu (n) | (15) | (13) | (9) | (7) | (44) |
| Mean ± SD range | 134.48 ± 28.63 (87.71–166 .61) | 151 ± 30.815 (89.74–197 .04) | 136.14 ± 34.51 (81.83–187 .72) | 151.4 ± 41.67 (96.08–208 .79) | 143.25 ± 9.2 (134.48–151 .4) |
| Hex (n) | (16) | (12) | (9) | (7) | (44) |
| Mean ± SD range | 2767.95 ± 500.21 (2012.23–3991.82) | 2976.99 ± 440.3 (2116.96–3866.14) | 2637.12 ± 310.84 (2080.16–2972.31) | 3333.92 ± 356.67 (2625.67–3685.55) | 2928.99 ± 304.08 (2637.12–3333.92) |
CE carboxyl esterase, Acd P acid phosphatase, Alk P alkaline phosphatase, β-Gal β-galactosidase, β-Glu β-glucosidase, Hex hexosaminidase. Specific activity nmoles/min/mg of protein. ± ≥ Standard deviation, n number of samples
Brain Tissue Processing
Fresh frozen tissues from frontal lobe (n = 10) parietal lobe (n = 13) temporal (n = 13) occipital (n = 10) regions of brain were dissected to obtain white matter (WM) and gray matter (GM) rich areas and processed for biochemical studies. In addition, WM and GM areas of thalamus (n = 12) hippocampus (n = 10) cerebellum (n = 7) and pineal gland (n = 6) were also obtained for biochemical studies. Briefly, the normal postmortem brain slices were cleared of the blood clots, if any and rinsed in normal saline and the net weight of the brain was noted. Subsequently, the tissue specimens were subjected to homogenization in tissue extraction buffer Tris Buffered saline (TBS containing 1 % (v/v) triton-X 100) using a in a glass homogenizer. The tissue to buffer ratio was maintained at of 1:31 w/v (ice) for homogenization. Homogenates were centrifuged (16,000 rpm/30 min) at 4 °C. The supernatants were collected for biochemical analysis and stored at −20 °C then at 4°C until the enzyme activity was assessed.
Protein Estimation
Protein estimation was carried out according to Lowry’s method [12, 13] using Bovine serum Albumin solution (BSA) as a standard.
Estimation of Hydrolytic Enzymes
Activities of the hydrolytic enzymes in supernatant tissue extracts were assayed by spectrophotometric (modified total assay volume was 1.5 ml) and spectroflourometric (modified total assay volume was 2.05 ml) methods as described below.
Carboxyl Esterase
Assay was carried out by Gomori [14] and later modified by Van Asperen [15]. Enzyme reaction was initiated by adding 900 μl of 5 mM α-naphthyl acetate in Phosphate assay buffer (pH 7.0) to pre incubated 100 μl tissue extract and was incubated for 15 min at 27 °C. Subsequently, the reaction was stopped by the addition of 500 μl DBLS reagent and enzyme activity was measured at 600 nm.
Acid Phosphatase
Assay was carried out according to the method described earlier [16]. Briefly, the enzyme reaction was initiated by allowing 100 μl of the supernatant obtained following tissue homogenization to react with 900 μl of 5 mM pNPP (SIGMA) in 0.1 M-citrate buffer (pH 5.0) and incubated for 15 min at 37 °C in a water bath. The reaction was stopped by the addition of 500 μl 1.6 N NaoH and enzyme activity was measured at 410 nm.
Alkaline Phosphatase
Assay was carried out using the method described earlier [17, 18]. Briefly, the enzyme reaction was initiated by allowing 100 μl of the supernatant obtained following tissue homogenization to react with 900 μl of 5 mM pNPP (SIGMA) in 0.1 M-citrate buffer (pH 5.0) and incubated for 15 min at 37 °C in a water bath. The reaction was stopped by the addition of 500 μl 1.6 N NaoH and enzyme activity was measured at 410 nm.
Glycosidase(s)
The following estimation of enzyme assays each of 2.05 ml total volume in which Enzyme action was stopped by adding 2.0 ml of Gly/CO3 buffer. Then the Fluorescence intensity was measured at 450 nm after exciting 360 nm in a spectrophotoflourimeter.
β-Galactosidase
Assay was carried out using the method described earlier [19] and subsequently modified with 0.29 mmol/L 4-methyl umbelliferyl β-d-galactoside (SIGMA) substrate. The supernatant tissue extract of 20 μl was pre incubated at 37 °C for 5 min and 40 μl substrate was added to initiate the enzyme action and allowed the reaction to continue for 60 min at 37 °C.
β-Glucosidase
Assay was carried out using the method described earlier [20] subsequently modified by using 2.5 mM 4-methyl umbelliferyl β-d-glucoside (SIGMA) substrate. The supernatant tissue extract of 20 μl was pre incubated at 37 °C for 5 min and 40 μl substrate was added to initiate the enzyme action and allowed the reaction to continue for 60 min at 37 °C.
β-Hexosaminidase
Assay was carried out using the method described earlier [21, 22] subsequently modified with 0.52 mmol/L 4-methyl umbelliferyl N-acetyl-β-d-Glucosaminide (SIGMA). The supernatant tissue extract of 20 μl was pre incubated at 37 °C for 5 min and 40 μl substrate was added to initiate the enzyme action and readings taken after 60 min at 37 °C.
Electrophoresis [23]
The brain tissue supernatant sample of 20 μl (20 μg to higher protein content) was electrophoresed in a native polyacrylamide gel (10 %) at a constant voltage of 50 Volts for 10 min, followed by 150 Volts for 1 h (approximately). The run was stopped when the marker dye reached 1–2 mm above the lower edge of the plate. The gel was carefully transferred to the staining solution with Coomassie Brilliant Blue R-250 at room temperature for 1 h and de-stained with glacial acetic acid till the background became clear.
Results
Specific Activity of Hydrolytic Enzymes in Normal Human Brain
The summary of the six hydrolytic enzymes studied in the frontal, parietal, temporal and occipital regions of brain is presented in Table 2 [30]. The specific activity of enzymes was expressed in nanomoles of product formed/min/mg protein in white and gray matter rich tissues representative of these four regions of the brain. By and large, the activities of different enzyme(s) studied were found to differ in white and gray matter rich tissues (Table 2). Further, the activity of a given enzyme was also found to differ between white and gray matter rich tissues of frontal, parietal, temporal and occipital lobes. In these four regions of the brain, the enzyme activities of tissue extracts from gray matter rich tissues (G), were found be relatively high as compared to the white matter rich fraction (W) for the four enzymes-Carboxyl esterase (G58.12 ± 5.73 vs. W39.81 ± 4.64, Fig. 1a), Alkaline phosphatase (G3.59 ± 0.27 vs. W1.40 ± 0.23, Fig. 1c), β-Galactosidase (G198.43 ± 35.23 vs. W131.10 ± 8.62, Fig. 1d).
Table 2.
Specific activity of six hydrolytic enzymes in normal human brain thalamus, hippocampus, cerebellum and pineal gland
| Enz | Thalamus | Hippocampus | Cerebellum | Pineal gland |
|---|---|---|---|---|
| CE (n) | (7) | (8) | (6) | (5) |
| Mean ± SD range | 65.46 ± 9.69 (52.46–78.45) | 54.41 ± 10.01 (39.93–67.91) | 64.32 ± 25.34 (32.91–96.205) | 51.63 ± 14.6 (28.4–62.35) |
| Acd P (n) | (8) | (8) | (6) | (5) |
| Mean ± SD range | 8.22 ± 2.40 (5.21–11.91) | 8.09 ± 3.0 (4.06–12.63) | 6.86 ± 1.63 (5.66–9.91) | 22.92 ± 5.75 (18.42–29.7) |
| Alk P (n) | (7) | (10) | (5) | (5) |
| Mean ± SD range | 2.32 ± 0.87 (1.4–4.04) | 2.74 ± 0.839 (1.8–4.01) | 2.03 ± 0.67 (1.07–2.915) | 12.39 ± 2.77 (10–15.7) |
| β-Gal (n) | (7) | (9) | (6) | (4) |
| Mean ± SD range | 166.82 ± 19.99 (135.59–202.74) | 192.74 ± 38.53 (150.95–264.205) | 227.08 ± 26.37 (193.11–257.76) | 1536.48 ± 372.068 (1278.14–2068.63) |
| β-Glu | (9) | (8) | (5) | (4) |
| (n) Mean ± SD range | 180.28 ± 41.54 (137.76–263.75) | 146.67 ± 18.24 (133.405–173.1) | 234.62 ± 77.072 (158.84–362.75) | 1072.71 ± 434.48 (515.305–1546.33) |
| Hex (n) | (7) | (7) | (5) | (6) |
| Mean ± SD range | 3082. ± 492.76 (2422.7–4008.55) | 2962.40 ± 477.37 (2366.73–3742.9) | 3818.09 ± 509.22 (3078.9–4422.55) | 12601.53 ± 3679.30 (8763.48–18663.58) |
CE carboxyl esterase, Acd P acid phosphatase, Alk P alkaline phosphatase, β-Gal β-galactosidase, β-Glu β-glucosidase, Hex hexosaminidase. Specific activity = nmoles/min/mg of protein. ± ≤ Standard deviation, n number of samples
Fig. 1.
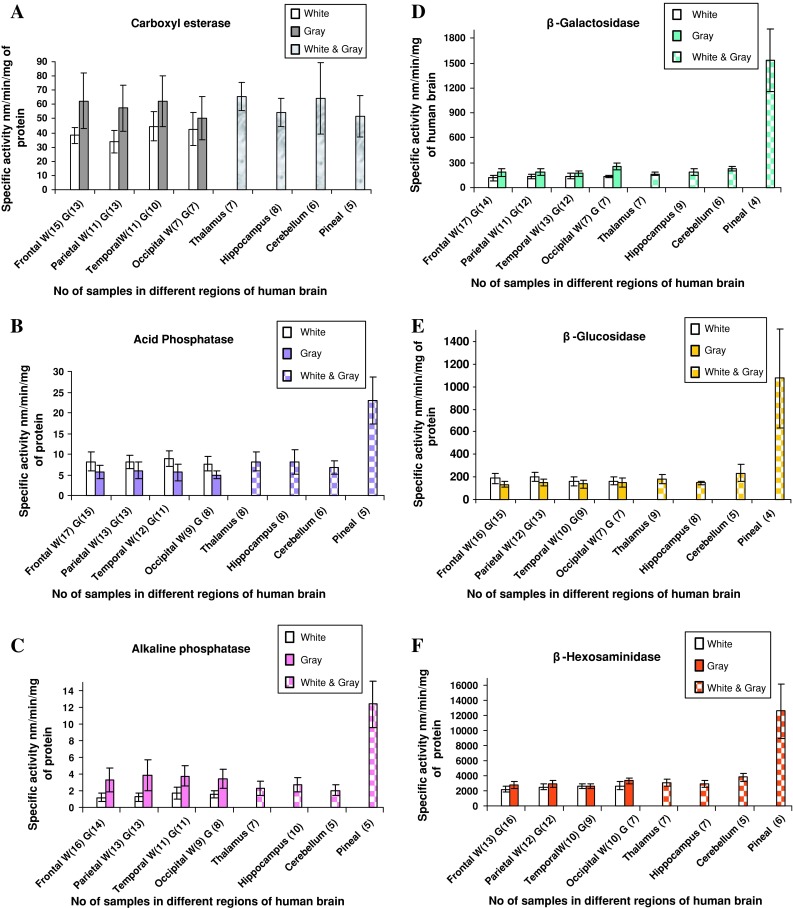
Specific activity of six hydrolases in different regions of normal (postmortem) human brain a carboxyl esterase, b acid phosphatase, c alkaline phosphatase, d β-galacatosidase, e β-glucosidase and f β-hexosamindase. (SA specific activity)
β-Hexosaminidase (G2928.99 ± 304.8 vs. W2495.65 ± 211.411, Fig. 1f). On the contrary, Acid phosphatase (G5.56 ± 0.458 vs. W8.18 ± 0.519, Fig. 1b), and β-Glucosidase activity (G143.25 ± 9.2 vs. W178.83 ± 17.15, Fig. 1e), was higher in white matter as compared to gray matter.
In the other regions of the brain such as thalamus, hippocampus, cerebellum and pineal gland, distinct gray and white matter regions could not be dissected out. Hence the enzyme activities were measured in the mixture of both gray and white matter was estimated. The results were similar to those obtained in the frontal, parietal, temporal and occipital regions of the brain excepting for pineal gland (Tables 3, 4). In the pineal gland, however, significantly higher enzyme activities were noted for all enzymes except for carboxyl esterase (Tables 3, 4). The cerebellum showed higher glycosidase activities compared to hippocampus and thalamus although it was much lower than the activity noted in the pineal gland (Tables 3, 4). Further, the mean specific activities of enzymes in tissue extracts from thalamus, hippocampus and cerebellum were 59.36 (±7.0) nm/min/mg of protein for carboxyl esterase, 7.10 (±0.86) for Acid phosphatase, 2.38 (±0.502) for Alkaline phosphatase, 209.91 (±24.28) for β-galactosidase, 190.645 (±62.19) for β-glucosidase and β-hexosaminidase showed 3390.245 (±605.06) nm/min/mg of protein.
Table 3.
Mean specific activity of six hydrolytic enzymes in eight regions of normal human brain and pineal gland
| Mean sp act of enz | CE | Acd P | Alk P | β-Gal | β-Glu | Hex |
|---|---|---|---|---|---|---|
| W & G (n) of F, P, Te & Oc | (85) | (98) | (95) | (93) | (89) | (89) |
| Mean ± SD range | 48.965 ± 10.91 (33.95–62.47) | 6.858 ± 1.54 (4.95–8.87) | 2.58 ± 1.22 (1.198–3.87) | 169.17 ± 46.024 (120.28–250.21) | 160.94 ± 21.08 (134.48–198.28) | 2789.201 ± 310.26 (2503.83–3333.92) |
| W & G (n) of T, H & C | (21) | (22) | (22) | (22) | (22) | (19) |
| Mean ± SD range | 59.36 ± 7.0 (54.41–65.46) | 7.47 ± 0.869 (6.86–8.22) | 2.38 ± 0.502 (2.03–2.74) | 209.91 ± 24.28 (166.82–227.08) | 190.645 ± 62.19 (146.67–234.62) | 3390.245 ± 605.064 (2962.4–3818.09) |
| Mean sp act of 7 regions (n) | (106) | (120) | (117) | (115) | (111) | (108) |
| Mean ± SD range | 52.355 ± 11.15 (33.95–65.46) | 7.104 ± 1.33 (4.95–8.87) | 2.463 ± 1.01 (1.198–3.87) | 173.16 ± 41.11 (120.28–250.21) | 168.17 ± 30.16 (134.48–234.62) | 2869.189 ± 440.26 (2195.43–3818.09) |
| Pineal gland (Pi) (n) | (5) | (5) | (5) | (4) | (4) | (6) |
| Mean ± SD range | 51.63 ± 14.6 (28.4–62.35) | 22.92 ± 5.75 (18.42–29.7) | 12.39 ± 2.77 (10–15.7) | 1536.48 ± 372.068 (1278.14–2068.63) | 1072.71 ± 434.48 (515.305–1546.33) | 12601.53 ± 3679.30 (8763.48–18663.58) |
F frontal, P parietal, Te temporal, O occipital, WM white matter, GM gray matter, T thalamus, O occipital, H hippocampus, Pi Pineal gland,
CE carboxyl esterase, Acd P acid phosphatase, Alk P alkaline phosphatase, β-Gal β-galactosidase, β-Glu β-glucosidase, Hex hexosaminidase. Specific activity = nmoles/min/mg of protein. ± ≥ Standard deviation, n number of samples
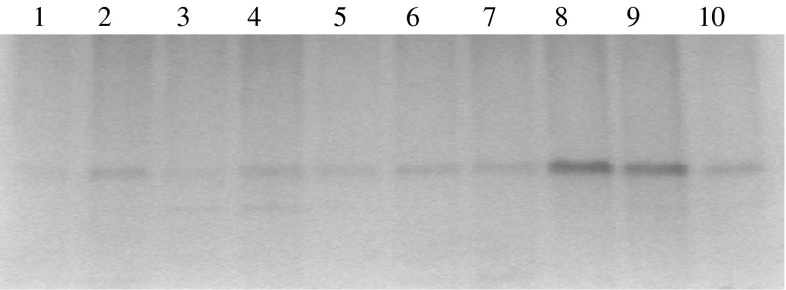
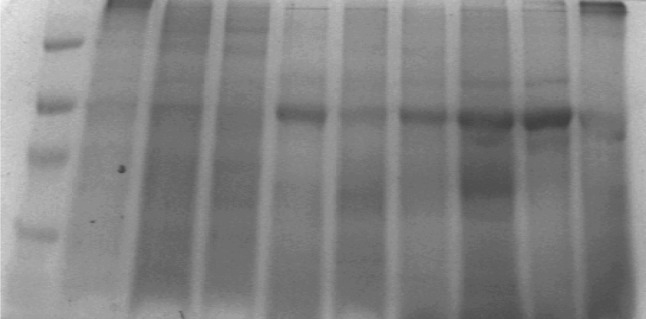
Native PAGE for protein in brain tissue extracts showed thin band (lane # 2 and 4) (Fig. 2) for grey matter of frontal and occipital regions. While their white matter (lane # 1 and 3) have not shown clear bands as compared to grey matter. However the 20 μl of pineal tissue extract of the brain (n = 3) showed an intense band (Fig. 2, lane # 8 and 9, Fig. 3, lane # 5 and 7). Other lanes showed thick band patterns for protein content in brain tumors (Fig. 3, lane # 8, 9 and 10).
Fig. 2.
Native PAGE profile of protein in different regions of normal (postmortem) human brain (Protein content of ~20 μg were loaded on to each lane): 1 Frontal (white); 2 Frontal (gray) 42 years/F; 3 Occipital (white) 40/M; 4 Occipital (gray) 40/F; 5 Parietal (white) 7/F; 6 Parietal (gray) 7/F; 7 Cerebellum 42/F; 8 Pineal gland 40/M; 9 Pineal gland 15/M; and 10 Pineal gland 51/M
Fig. 3.
1. Marker; 2. Frontal (white); 3. Frontal (gray); 4. Cerebellum 40/M; 5. Pineal gland 40/M; 6. Pineal Gland 51/M, 7. Pineal gland 15/M; 8. Meningothelial meningioma G-I olfactory groove; 9. Anaplastic meningioma G-III recurrent right frontal 75/M; and 10. Glioblastoma multiforme (G-IV) frontal tumor shows florid micro vascular proliferation 40/M
Discussion
The study of hydrolytic enzymes in the normal human brain has been attempted earlier by some investigators [10, 11, 24, 25, 31]. However, none of these studies have addressed the topographical distribution of hydrolytic enzymes in the various regions of the brain. Therefore, the present study was carried out with a view to analyze the distribution of these enzymes in the various anatomical regions of ‘normal’ human brain. The main objective of the present study was to describe the distribution of these enzymes in various discrete areas of brain, in various white matter fiber tracts, and gray matter and to describe the sub cellular distribution of these six enzymes in the central nervous system, utilizing Tris buffered saline (homogenization buffer) as a suspending medium. The use of an autopsy brain offers an opportunity to establish the normal range for the study of hydrolytic enzymes. Indeed, a number of metabolic and functional parameters have already been shown to be well retained in p.m. human preparations and these compare quite favorably with the corresponding data from laboratory animals [25]. One of the main limitations of studying the biochemical profiles of postmortem brain specimens is the postmortem effect on the parameters of interest. Enzymes, unlike some of the biologically labile metabolites, are relatively more stable, provided the postmortem removal of brain tissue is effected within the reasonable time of couple of hours. In this study the mean interval of analysis of enzymes following death was 9.9 h (range 1.5–17.5 h, Table 1). Though some of the enzymes are likely to be labile, a number of enzymes including acid hydrolases will survive for several hours and their activity can be measured [1]. It can be concluded that the methods used for the assay of acid hydrolases [8] are highly reliable and that influences of post mortal sampling time can be neglected.
One more important observation to this work is that there was no significant difference in age group samples for protein and enzymatic activity. However the difference was shown to the region wise of the normal autopsy brain samples.
In the present study it was noted that carboxyl esterase activity is significantly higher in gray matter (two-tailed P value is less than 0.0001) as compared to white matter regions of frontal, parietal, temporal and occipital regions of the human brain (Table 2; Fig. 1a). However, slightly higher values of this enzyme were noted in the combined gray and white matter regions of thalamus, hippocampus and cerebellum (Table 3). Such a finding for non specific esterase has been reported earlier by Schiffer et al. [26]. Indeed they observed that, α-naphthol esterase activity is present in neurons and in the cytoplasm of astrocytes, but little activity is found in white matter. Thus the higher activity of carboxyl esterase in gray matter rich tissues could be attributed to the fact that gray matter represents primarily the soma of the neurons, where as the white matter predominantly represent axon and glial tissue compartments. Further, Zhang et al. [9] observed that in the central nervous system, carboxylesterase-2 expression was confined to capillary endothelial cells, consistent with the enzyme having a role to protect the central nervous system from toxic esters and perhaps being a component of a blood–brain barrier system.
In contrast to carboxyl esterases, the physiological significance, if any, of the observed differential distribution of ‘acid’ and ‘alkaline’ phosphatases in ‘white’ and ‘gray’ matter rich tissues is rather difficult to explain (Table 2; Fig. 1b, c). The frontal, parietal, temporal and occipital regions showed higher acid phosphatase activity in the white matter (8.18 ± 0.519) as compared to gray matter (5.56 ± 0.458) in the four regions of the brain. On the other hand, the specific activity of alkaline phosphatase was lower (1.40 ± 0.23) in white matter as compared to gray matter (3.59 ± 0.27) (Fig. 1c; Table 2). In the combined gray and white matter regions of thalamus, hippocampus and cerebellum showed 2.38 ± 0.502 nm/min/mg of protein (Table 3). Apparently, these two enzymes with different pH optimum may have distinct functions in different sub cellular domains/compartments of brain tissue. Possibly, further studies involving immuno-cytochemistry and/or histochemical staining of neural tissues may throw more light on the differential tissue distribution of ‘acid’ and ‘alkaline’ phosphatase(s). While the observed (relative) high specific activities of many of the hydrolytic enzymes in gray matter rich tissues could be in the membranous fraction of cell bodies of the gray matter, the significance of acid phosphatase being high in white matter rich fraction of brain tissue remains to be explained. The relative differential distribution of acid and alkaline phosphatase(s) between white and gray matter was conspicuous in this study. Earlier studies on these enzymes in ox and human brain [27] reported a similar pattern of acid and alkaline phosphatases activity.
An intensive study of glycosidases in nervous tissue was carried out in the present study by utilizing 4-methylumbelliferyl-β-d-glycosides. This assay is simple and convenient to perform, and permits the measurement of the enzyme activity in as little as 1 pg of neural tissue. This provides insight into some properties of the three glycosidases with regard to sub cellular organelles. It was interesting to note in the present study that higher activity of β-galactosidase, β-glucosidase and β-hexosaminidase were noted in the cerebellum as compared to other regions (except for pineal gland) of the human brain (Table 3; Fig. 1). One earlier study has reported the relatively higher distribution of β-galactosidase and β-glucuronidase in the granular layer of cerebellum [28]. In contrast to the cerebellum, activity of β-galactosidase was higher in gray matter of frontal, parietal, temporal and occipital regions (198.43 ± 35.23) (Table 2; Fig. 1d), while β-glucosidase showed higher activity of 178.83 ± 17.15 in white matter as compared to gray matter (Table 2; Fig. 1e). However β-glucosidase did not showed statistically much difference in white (163.86 ± 32.9) and gray (151.4 ± 41.67) matter of the occipital region.
Amongst all the six enzymes, β-hexosaminidase showed the highest activity gray matter of frontal, parietal, temporal and occipital regions (2495.65 ± 211.411; Table 2; Fig. 1f). The two-tailed P value for this enzyme was less than 0.0001 and by conventional criteria; this difference is considered to be extremely statistically significant. Similarly the cerebellum (3818.09 ± 509.22), thalamus (3082 ± 492.76) and hippocampus (2962.40 ± 477.37) showed high activity for this enzyme.
The most significant finding in the present study was the high activity of all hydrolytic enzymes (except for carboxyl esterase) noted in the pineal gland as compared all other regions of the human brain (Table 3; Fig. 1). Such a finding has not been hitherto reported in human brain tissue samples. If the specific activities of these enzymes are to be considered as any functional index, then pineal gland may be more metabolically active tissue with respect to the hydrolytic function as compared to the other regions of the brain. This observation possibly reflects the differential metabolic disposition of hydrolytic enzymes in the pineal gland as compared to the rest of the brain. Therefore, it would be interesting to look into the role of hydrolytic enzymes in pineal gland.
To summarize, among all the hydrolytic enzymes studied, brain tissue was found to have high activity of hexosaminidase followed by β-galactosidase and β-glucosidase, (160–250 units) Carboxyl esterase (50–70 units), Acid phosphatase and alkaline phosphatase 2–10 units (Fig. 1). The observed high specific activity of β-hexosaminidase (2,700–3,300 units), as compared to other enzymes in brain tissue, may explain the metabolic significance of this enzyme with respect to the catabolism of gangliosides [29]. Except for carboxyl esterase activity localized mainly in microsomes, which was found to be comparable between the pineal gland and rest of the brain regions, other hydrolytic enzymes studied were found to be several folds higher in pineal gland. The present report is the first comparative investigation of the levels of several hydrolases in different regions of white matter, gray matter and mixture of both in autopsy human brain.
Native PAGE for protein in brain tissue supernatant of pineal gland showed intense bands which thereby indicating high protein content in this tissue. Further, it was observed the supernatant exhibited higher specific activity of enzymes, which confirms the higher total activity of the five hydrolytic enzymes in the pineal gland.
Electronic supplementary material
References
- 1.Robins E, Smith DE, Daesch GE, Payne KE. The validation of the quantitative histochemical method for use on post mortem material—II: the effects of fever and uremia. J Neurochem. 1958;3:19–27. doi: 10.1111/j.1471-4159.1958.tb12605.x. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey RB, Smith KR, Jr., Crafts DC, Chung HD, Fredericks M. Hydrolytic enzyme activities of the nervous system. Arch Neurol. 1980;37(6):356–359. doi: 10.1001/archneur.1980.00500550058007. [DOI] [PubMed] [Google Scholar]
- 3.Wielgat P, Walczuk U, Szajda S, Bien M, Zimnoch L, Mariak Z, Zwierz K. Activity of lysosomal exoglycosidases in human gliomas. J Neurooncol. 2006;80(3):243–249. doi: 10.1007/s11060-006-9188-z. [DOI] [PubMed] [Google Scholar]
- 4.Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, and Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances maik most neurons in populations at risk to degenerate in Alzheimer’s disease. J Neurosci 1996; 16(1):186–l99. [DOI] [PMC free article] [PubMed]
- 5.Futerman AH, Gerrit VM. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–565. doi: 10.1038/nrm1423. [DOI] [PubMed] [Google Scholar]
- 6.Guner G, Kokoglu E, Guner A. Acid and alkaline phosphatase activities in homogenates and subcellular fractions of human brain tumors. Cancer Lett. 1985;29(3):339–343. doi: 10.1016/0304-3835(85)90145-4. [DOI] [PubMed] [Google Scholar]
- 7.Wolleman M. Hydrolytic enzymes. In: Wollemann M (ed) Biochemistry of brain tumors. The Macmillan press Ltd., London; 1974, pp. 123–158.
- 8.Friede RL, Knoller M. A quantitative mapping of acid phosphatase in the brain of the rhesus monkey. J Neurochem. 1965;12:150–441. doi: 10.1111/j.1471-4159.1965.tb04244.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Xu G, McLeod HL. Comprehensive evaluation of carboxylesterase-2 expression in normal human tissues using tissue array analysis. Appl Immunohistochem Mol Morphol. 2002; 10(4): 374–80. [DOI] [PubMed]
- 10.Sinha L and Sinha AK. Lysosomal Hydrolases in developing human brain regions. J Neurochem 1980;1080–1086. [DOI] [PubMed]
- 11.Hirsch HE. Differential determination of Hexosaminidases A and B and two forms of β-galactosidase, in the layers of the human cerebellum. J Neurochem. 1972;19(6):1513–1517. doi: 10.1111/j.1471-4159.1972.tb05095.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AR, Randoll RJ. Protein measurement with the Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 13.Hartree EF. Determination of proteins: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- 14.Gomori G. The distribution of phosphatase in normal organs and tissues. J Cell Comp Physiol. 1941;17:71–83. doi: 10.1002/jcp.1030170108. [DOI] [Google Scholar]
- 15.Asperen VK. A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol 1962;8:401–16.
- 16.Lam WK, Lai LC, Yam LT. Tartrate-resistant (Band 5) acid phosphatase activity measured by electrophoresis on acrylamide gel. Clin Chem. 1978;24(2):309–312. [PubMed] [Google Scholar]
- 17.Fernley H. Mammalian alkaline phosphatases. In: P. Boyer, editor. The Enzymes, 3rd edn. New York: Academic Press. 1971. p. 417.
- 18.McComb RB, Bowers GN Jr., Posen S. Alkaline phosphatase. New York: Plenum Press, c1979:1926.
- 19.Falck B. Observations on the possibilities of the cellular localization of monoamines by a fluorescence method. Acta Physiol Scand. 1962;56(Suppl):197. [Google Scholar]
- 20.Peters SP, Coyle P, Glew RH. Differentiation of β-glucocerebrosidase from β-glucosidase in human tissues using sodium taurocholate. Arch Biochem Biophys. 1976;175:569–582. doi: 10.1016/0003-9861(76)90547-6. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien JS, Okada S, Chen A, Fillerup DL. Tay Sach’s disease: detection of heterozygotes and homozygotes by serum hexosaminidase assay. N Engl J Med. 1970;283(1):15–20. doi: 10.1056/NEJM197007022830104. [DOI] [PubMed] [Google Scholar]
- 22.Leaback DH, Walker PG. Studies on glucosaminidase 4. The fluorimetric assay of N-acetyl-β-glucosaminidase. Biochem J. 1961;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Hojring N, Svensmark O. Carboxylesterases with different substrate Specificity in human brain extracts. J Neurochem. 1976;27(2):523–528. doi: 10.1111/j.1471-4159.1976.tb12277.x. [DOI] [PubMed] [Google Scholar]
- 25.Hardy JA, Dodd PR. Metabolic and functional studies on post-mortem human brain. Neurochem Int 1983; 5(3): 253–66. (Commentary). [DOI] [PubMed]
- 26.Schiffer D, Vesco C, Piazza L. Contribution to the histochemical demonstration and distribution of phosphatases and non-specific esterase in human nervous tissue. Psychiat Neurol (Basel) 1962;144:34–37. doi: 10.1159/000129570. [DOI] [PubMed] [Google Scholar]
- 27.Fleischhacker HH. Studies on the brain phosphatases. J Ment Sci. 1938;84:947–959. [Google Scholar]
- 28.Hirsch HE, Robins E. Fed Proc. 1961;20:342. [Google Scholar]
- 29.Tallman JF, Johnson WG, Brady RO. The metabolism of Tay-Sachs ganglioside: catabolic studies with lysosomal enzymes from normal and Tay-Sachs brain tissue. J Clin Invest. 1972;51(9):2339–2345. doi: 10.1172/JCI107045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prabha.M, Ravi V, Shetty KT, Swamy NR. Pineal gland of elevated hydrolytic enzyme activities and similar carboxyl esterase activity to the rest of the brain regions. Proceedings of the National conference in Chemistry. Sept 27–29, Bangalore, 2006.
- 31.Prabha M. A Comparative biochemical study on hydrolytic enzymes in normal postmortem human brain, brain tumors and in their derived cell lines. Dissertation. Bangalore University; 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.