Abstract
A new device called Buzzy® has been recently presented that combines a cooling ice pack and a vibrating motor in order to relieve the venipuncture pain. The aim of this study was to evaluate the impact of Buzzy® use during diagnostic blood specimen collection by venipuncture for routine immunochemistry tests. Blood was collected from 100 volunteers by a single, expert phlebotomist. A vein was located on the left forearm without applying tourniquet, in order to prevent any interference from venous stasis, and blood samples were collected using a 20-G straight needle directly into 5 mL vacuum tubes with clot activator and gel separator. In sequence, external cold and vibration by Buzzy® was applied on the right forearm—5 cm above the chosen puncture site—for 1 min before venipuncture and continued until the end of the same procedure already done in the left forearm. The panel of tests included the following: glucose, total cholesterol, HDL-cholesterol, triglycerides, total protein, albumin, c-reactive protein, urea, creatinine, uric acid, alkaline phosphatase, amylase, AST, ALT, g-glutamyltransferase, lactate dehydrogenase, creatine kinase, total bilirubin, phosphorus, calcium, magnesium, iron, sodium, potassium, chloride, lipase, cortisol, insulin, thyroid-stimulating hormone, total triiodothyronine, free triiodothyronine, total thyroxine, free thyroxine and haemolysis index. Clinically significant differences between samples were found only for: total protein, albumin and transferrin. The Buzzy® can be used during diagnostic blood specimens collection by venipuncture for the majority of the routine immunochemistry tests. We only suggest avoiding this device during blood collection when protein, albumin and transferrin determinations should be performed.
Keywords: Clinical chemistry tests, Laboratory error, Patient safety, Phlebotomy, Preanalytical variability, Reproducibility of results
Introduction
Laboratory diagnostics is an essential part of the clinical decision making, provided that a high degree of quality is established throughout the total testing process [1]. Continuous monitoring and management of preanalytical errors (i.e., by quality indicators) are crucial for improving the quality of laboratory performance, and are also necessary for all clinical laboratories accredited on the basis of the international organization for standardization 15189 document [2–6]. Moreover, quality indicators in laboratory medicine are fundamental tools for quantifying the quality of a selected aspect of care by comparing it against a defined criterion [7]. For private laboratories the level of patient satisfaction with the diagnostic blood collection service is a very important quality indicator. A new device called Buzzy® has been recently presented that combines a cooling ice pack and a vibrating motor in order to relieve the venipuncture pain in adults and children, thus aimed at increasing patient satisfaction during blood collection [8–10]. The aim of this study was to evaluate the impact of Buzzy® use during diagnostic blood specimens collection by venipuncture for routine immunochemistry tests. We hypothesized that a combination of cold and vibration near venipuncture site might be a new source of preanalytical variability.
Methods
Study Design
A group of 100 healthy adults of both sexes, volunteers for this study, from a private laboratory called Bioanalise in Teresina city, Piaui, Brazil, were evaluated in one workday. Each volunteer provide an informed consent for being enrolled in this study, which was also carried out in agreement with the Declaration of Helsinki and under the terms of all relevant local legislation.
Collection of Diagnostic Blood Specimens
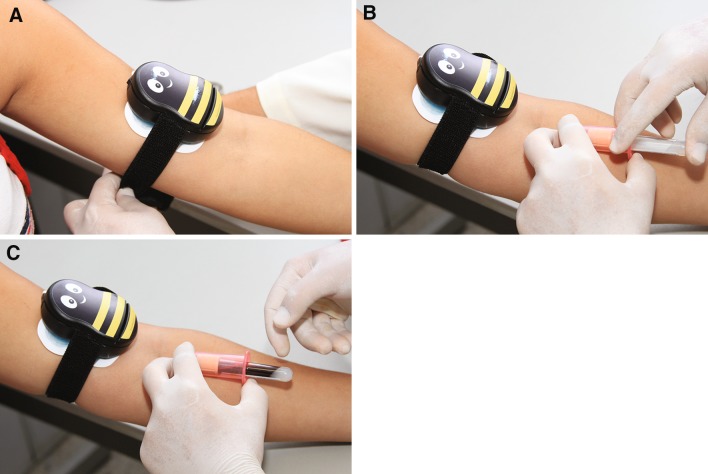
The collection of all diagnostic blood specimens was performed from 8.00 to 11.00 AM by a single, expert phlebotomist, according to the phlebotomy guidelines [11, 12]. All volunteers, after 12-hour fasting, were maintained seated for 15 min prior to phlebotomy in order to eliminate possible interferences of blood distribution due to the posture [13]. After that, a vein was located on the left forearm without applying tourniquet using a subcutaneous tissue transilluminator device (Venoscópio IV plus, Duan do Brasil, Sao Paulo, Brazil), in order to prevent any interference from venous stasis [14–16], and blood samples were collected using a 20-G straight needle (BD Vacuntainer) directly into 5 mL SST II Advance® vacuum tubes with clot activator and acrylic gel separator. To eliminate any potential interference due to either the contact phase or the tissue factor, ~2 mL of blood were preliminarily collected in a discard tube without additive (Vacuette®, Greiner Bio-One GmbH, Kremsmünster, Austria). In sequence, external cold and vibration by Buzzy® (MMJ Labs, Atlanta, GA, USA) was applied on the right forearm—5 cm above the chosen puncture site—for 1 min before venipuncture and continued until the end of the same procedure already done in the left forearm (Fig. 1). Buzzy® is a reusable 8 × 5 × 2.5 cm plastic bee-like device containing a battery-powered vibrating motor with an 18 g solid frozen ice pack underneath. Blood collection was accurately standardized, including the use of needles and vacuum tubes of the same lot.
Fig. 1.
Using the Buzzy® device during diagnostic blood specimen collection by venipuncture a Buzzy® application 1 min before venipuncture b venipuncture c diagnostic blood specimen collection by venipuncture with vacuum tube system
Processing of Diagnostic Blood Specimens
Pre-centrifugation and Centrifugation
All the serum tubes were left upright for 45 min at room temperature (20 °C) to allow complete blood clotting before centrifugation [17]. Then all samples were centrifuged at 1,500 g for 10 min at room temperature (according to the instructions of the manufacturers).
Diagnostic Blood Specimens Storage
All serum samples were separated, stored in aliquots and kept frozen at −70 °C until measurement. Samples did not show any sign of hemolysis by visual inspection. No specimen was discarded due to unsatisfactory attempts, difficult venous access, missing veins, manifest hemolysis or lipaemia.
Laboratory Testing
All frozen serum aliquots were thawed at the same time. The clinical chemistry and immunochemistry tests were performed in duplicate immediately after thawing on the same instrument Cobas® 6000 <c501> and <e601> module (Roche Diagnostics GmbH, Penzberg, Germany), according to the manufacturer’s specifications and using proprietary reagents. The panel of tests included the following: glucose (GLU), total cholesterol (COL), HDL cholesterol (HDL), triglycerides (TG), total protein (TP), albumin (ALB), c-reactive protein (CRP), urea, creatinine (CRE), uric acid (UA), alkaline phosphatase (ALP), amylase (AMYL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), g-glutamyltransferase (GGT), lactate dehydrogenase (LDH), creatine kinase (CK), total bilirubin (TBil), phosphorus (P), calcium (Ca), magnesium (Mg), iron (Fe), sodium (Na), potassium (K), chloride (Cl), lipase, cortisol, insulin, thyroid-stimulating hormone (TSH), total triiodothyronine (T3), free triiodothyronine (FT3), total thyroxine (T4), free thyroxine (FT4) and haemolysis index (HI). The instrument was calibrated against appropriate proprietary reference standard materials and verified with the use of proprietary quality controls. Our evaluation of the within-run precision by internal quality control on the Cobas® 6000 <c501> and <e601> module (Roche Diagnostics GmbH) showed low coefficients of variation—CVA (Table 1).
Table 1.
Impact of Buzzy® on routine immunochemistry laboratory testing
| Diagnostic blood specimen collection procedures | |||||||
|---|---|---|---|---|---|---|---|
| Tests | Units | Desirable Bias (%) | CVa | Buzzy® | Gold standard | Mean % difference | p value |
| COL** | (mmol/L) | 4.0 | 1.8 | 4.73 ± 0.7 | 4.58 ± 0.7 | 3.2 | 0.0002 |
| HDL* | (mmol/L) | 5.2 | 4.3 | 1.35 (1.11–1.64) | 1.30 (1.07–1.63) | 3.7 | 0.0079 |
| TG** | (mmol/L) | 10.7 | 2.0 | 1.55 ± 0.6 | 1.51 ± 0.6 | 2.6 | 0.0101 |
| TP** | (g/L) | 1.2 | 1.2 | 80.1 ± 4.4 | 78.9 ± 3.6 | 1.5 | 0.0244 |
| ALB** | (g/L) | 1.3 | 1.2 | 48.9 ± 3.7 | 47.9 ± 3.4 | 2.0 | 0.0135 |
| CRP* | (mg/L) | 21.8 | 1.3 | 50.0 (50.0–50.1) | 49.8 (49.8–50.0) | 0.4 | 0.7500 |
| UREA** | (mmol/L) | 5.5 | 2.6 | 9.35 ± 1.8 | 9.64 ± 1.7 | −3.1 | 0.0004 |
| CRE* | (μmol/L) | 3.8 | 2.5 | 73.4 (61.9–108.7) | 72.5 (61.9–105.2) | 1.2 | 0.7764 |
| UA** | (μmol/L) | 4.9 | 1.0 | 299.7 ± 92.5 | 300.2 ± 89.2 | −0.2 | 0.8494 |
| ALP** | (μkat/L) | 6.4 | 2.7 | 1.19 ± 0.3 | 1.14 ± 0.3 | 4.2 | 0.0232 |
| Lipase* | (μkat/L) | 10.1 | 2.8 | 2.12 (1.34–2.91) | 2.08 (1.30–2.93) | 1.9 | 0.2483 |
| AMYL** | (μkat/L) | 7.4 | 0.8 | 1.29 ± 0.3 | 1.24 ± 0.3 | 3.9 | 0.0069 |
| AST* | (μkat/L) | 5.4 | 1.2 | 0.49 (0.37–0.60) | 0.44 (0.37–0.61) | 10.2 | 0.8217 |
| ALT** | (μkat/L) | 12.0 | 1.3 | 0.61 ± 0.2 | 0.58 ± 0.2 | 4.9 | 0.1253 |
| GGT* | (μkat/L) | 10.8 | 1.6 | 0.54 (0.37–0.81) | 0.53 (0.37–0.80) | 1.8 | 0.0232 |
| LDH** | (μkat/L) | 4.3 | 1.5 | 7.32 ± 1.0 | 7.19 ± 1.2 | 1.8 | 0.1132 |
| CK** | (μkat/L) | 11.5 | 3.3 | 2.25 ± 1.5 | 2.14 ± 1.4 | 4.9 | 0.0035 |
| TBil* | (μmol/L) | 11.4 | 2.2 | 10.3 (5.1–13.7) | 8.6 (5.1–13.7) | 16.5 | 0.1736 |
| P** | (mmol/L) | 3.2 | 3.0 | 1.30 ± 0.2 | 1.32 ± 0.2 | −1.5 | 0.0717 |
| Ca** | (mmol/L) | 0.8 | 0.7 | 2.37 ± 0.1 | 2.36 ± 0.1 | 0.4 | 0.5320 |
| Mg** | (mmol/L) | 1.8 | 1.2 | 0.81 ± 0.07 | 0.81 ± 0.06 | 0.0 | 0.7192 |
| Fe* | (μmol/L) | 8.8 | 2.6 | 14.1 (12.6–18.0) | 14.9 (12.4–17.2) | −5.7 | 0.6817 |
| Transferrin** | (g/L) | 1.3 | 1.2 | 2.31 ± 0.3 | 2.27 ± 0.3 | 1.7 | 0.0266 |
| Na** | (mmol/L) | 0.3 | 1.0 | 144.3 ± 1.9 | 144.3 ± 1.8 | 0.0 | 1.000 |
| K** | (mmol/L) | 1.8 | 1.5 | 4.0 ± 0.4 | 4.0 ± 0.3 | 0.0 | 0.6590 |
| Cl* | (mmol/L) | 0.5 | 1.0 | 102 (101–103) | 102 (101–103) | 0.0 | 0.2755 |
| GLU* | (mmol/L) | 2.2 | 1.5 | 4.66 (4.27–5.11) | 4.66 (4.33–5.11) | 0.0 | 0.9987 |
| Cortisol** | (nmol/L) | 12.5 | 3.7 | 223.9 ± 99.0 | 198.4 ± 104.2 | 11.4 | 0.0317 |
| Insulin* | (pmol/L) | 15.5 | 5.9 | 66.0 (30.0–125.8) | 66.4 (24.2–110.5) | −0.6 | 0.3757 |
| TSH** | (mU/L) | 7.8 | 3.5 | 1.33 ± 0.6 | 1.28 ± 0.6 | 3.8 | 0.0074 |
| T3** | (nmol/L) | 4.8 | 2.1 | 2.01 ± 0.2 | 2.00 ± 0.2 | 0.5 | 0.2723 |
| FT3* | (pmol/L) | 4.8 | 2.2 | 0.054 (0.050–0.059) | 0.055 (0.052–0.059) | −1.8 | 0.6909 |
| T4** | (nmol/L) | 3.0 | 1.3 | 90.7 ± 14.4 | 89.6 ± 14.5 | 1.2 | 0.1661 |
| FT4* | (pmol/L) | 3.3 | 1.4 | 13.4 (12.0–15.2) | 13.2 (11.7–15.2) | 1.5 | 0.3265 |
| HI* | NA | NA | 0.8 | 4.07 ± 0.22 | 4.02 ± 0.20 | 1.2 | 0.7573 |
The bold p values are statistically significant (p < 0.05) and bold mean % differences represent clinically significant variations, when compared with desirable bias [19]
* Non-normal distribution; the values are presented as median (interquartile range); p value represents the significance by Wilcoxon ranked-pairs test
** Normal distribution; the values are presented as mean ± standard deviation; p value represents the significance by paired student’s t test
NA not available [19], ALB albumin, ALP alkaline phosphatase, ALT alanine aminotransferase, AMYL amylase, AST aspartate aminotransferase, Ca calcium, CK creatine kinase, Cl chloride, COL total cholesterol, CRE creatinine, CRP c-reactive protein, Fe iron, FT3 free triiodothyronine, FT4 free thyroxine, GGTg-glutamyltransferase, GLU glucose, HDL HDL-cholesterol, HI haemolysis index, K potassium, LDH lactate dehydrogenase, Mg magnesium, Na sodium P phosphorus, TBil total bilirubin, TG triglycerides, TP total protein, TSH thyroid-stimulating hormone, T3 total triiodothyronine, T4 total thyroxine, UA uric acid
Statistical Analysis
The significance of the differences between samples was assessed by paired Student’s t test after checking for normality (with D’Agostino-Pearson’s omnibus test). As non-normal distribution was found for HDL, CRP, CRE, lipase, AST, GGT, TBil, Fe, Cl, GLU, insulin, FT3 and FT4 results were assessed by Wilcoxon ranked-pairs test using licensed statistical software (GraphPad Prism® version 5.01, La Jolla, CA, USA). A p value <0.05 was considered statistically significant. Mean % differences were determined according to the formula: mean % difference = [(Buzzy procedure + Gold standard procedure)/Buzzy procedure] × 100 Finally, the biases from gold standard- and Buzzy-venipuncture procedure (mean % differences) were compared with the current desirable quality specifications, derived from biological variation [18, 19] according to the formulae:
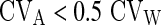 |
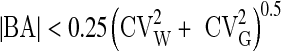 |
where CVA is the percent analytical coefficient of variation, CVW is the within subject biological variation, CVG is the between subject biological variation, BA is the bias.
Results
Since our aim was to ascertain whether any differences could be observed when Buzzy was employed vs the standard procedure and whether they might be clinically relevant, we based the criteria for evaluating the analytical performance upon desirable quality specifications as suggested [18], by employing the above reported formulae.
Statistically significant differences between standard-(without tourniquet) and Buzzy-venipuncture procedure (adequately assessed as reported in Methods by paired Student’s t test or Wilcoxon ranked-pairs test, p value <0.05) were found for: COL, HDL, TG, TP, ALB, UREA, ALP, AMYL, GGT, CK, transferrin, cortisol and TSH (Table 1). The main analytes shown increased concentration when cold and vibration by Buzzy® were applied before diagnostic blood specimen collection (Table 1). Moreover clinically significant differences were found only for: TP, ALB, transferrin, AST and TBil (the last two without statistical significance).
Discussion
For a long time the preanalytical phase has been considered as the “dark side of the moon” [20–27]. Presently in order to start a “light-side” period in the preanalytical phase and in patient safety, the attention of the laboratory professionals has been focused on some procedures previously regarded as consolidated, such as: (i) furniture changes among different manufacturers of syringes for blood gas analyses, which can represent new sources of laboratory variability; similar considerations apply to not validated vacuum tubes[28–31]; (ii) transport boxes unable to guarantee the maintenance of the temperature during blood specimens transportation [32]; (iii) traditionally accepted although incorrect paradigms ranging from the filling of vacuum tubes to the mixing procedures which appear unsupported by accurate experimental verification; e.g., recently it has been observed that there is no apparent need to mix all blood specimens after collection into vacuum tube systems by venipuncture [33, 34]. On the other hand, apparently incorrect vigorous mixing of the primary blood vacuum tubes immediately after collection does not promote laboratory variability [35]; more so, no clinical impact has been observed in routine and specialized coagulation laboratory testing when the vacuum tubes are incompletely filled (when filled more than 90 % but <100 %) [36]. Obviously the ability of the phlebotomist to put the patient at ease is considered very important by outpatient [37]. Oatey and Stiller after evaluating inpatients showed that 28 % agree that generally, the venipuncture procedure is painful [38]. The new tool called Buzzy® reportedly relieves the sensation of pain during venipuncture [8–10]. However our results showed statistically significant increase of several analytes when the blood specimens were collected using Buzzy® in comparison with the standard procedure (Table 1). Nevertheless, clinically significant differences, due to results consistently and systematically higher after Buzzy procedure than after gold standard were observed only for TP, ALB, transferrin, AST and TBil—the last two without statistical significance—when comparison is made with the current quality specifications for bias, derived from biological variation [19, 39]. Obviously the quality specifications derived from biological variation [19] are considered both very important and useful in the daily practice by the quality managers of the medical laboratories [40–43]. Even in this case caring physicians unaware of the real patient situation might initiate (or delay) appropriate treatments as a consequence of the use of this new device. Direct skin cooling causes a vasoconstriction [44, 45]. From a practical point of view, the cold-induced hemoconcentration [46] promotes the exit of water, diffusible ions and low molecular weight substances from the vessel thereby increasing the concentration of various blood analytes at the punctured site thus potentially influencing the laboratory results interpretation (e.g. TP, ALB and transferrin). As known, calcium is the fifth most common element in the body and the most prevalent cation. Moreover this cation is present in three physicochemical states in plasma: (i) 50 % is free (ionized); (ii) 10 % is complexed with small diffusible inorganic and organic anions (e.g. bicarbonate, lactate, phosphate and citrate); (iii) 40 % is bound to plasma proteins (where ~80 % is associated with albumin and the remaining 20 % associated with globulines) [47]. This explains why the hemoconcentration due to Buzzy application during diagnostic blood specimen collection by venipuncture can affect ALB results without significant effects on calcium concentration, since calcium is only partially bound to proteins. Fuller et al. [48] in a systematic review showed that vibration increases muscle perfusion with the magnitude of increase positively related to the vibratory load applied. Our results showed that the vibration promoted by Buzzy® could have an effect on the muscle (see CK), but possibly without clinical impact when comparing Buzzy® versus gold standard procedure, as no clinically significant differences are observed.
In conclusion the Buzzy® can be used during diagnostic blood specimens collection by venipuncture for the majority of the routine immunochemistry tests. We only suggest to avoid this device during blood collection when TP, ALB and transferrin determinations should be performed. Future investigations should be planned to cover other areas of clinical laboratory (e.g. haematology and coagulation).
Acknowledgments
The authors acknowledge Drs. David Valentim and Sylvio J. C. Romano, respectively technical director and president of Bioanalise (Teresina, Piaui, Brazil) for authorizing to perform this validation in their clinical laboratory. Special thanks to Mss. Marise D. R. Campelo and Katharyne S. A. Tajra for their skilful technical support, and to Mr. Flavio S. Gomes for his dedication in collecting all the diagnostic blood specimens for routine immunochemistry tests presented in this work.
Disclosure
All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Plebani M, Lippi G. To err is human. To misdiagnose might be deadly. Clin Biochem. 2010;43(1–2):1–3. doi: 10.1016/j.clinbiochem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Medical laboratories: particular requirements for quality and competence ISO document 15189. 2. Geneva: International Organization for Standardization; 2007. [Google Scholar]
- 3.Lippi G, Guidi GC. Preanalytic indicators of laboratory performances and quality improvement of laboratory testing. Clin Lab. 2006;52(9–10):457–462. [PubMed] [Google Scholar]
- 4.Simundic AM, Nikolac N, Vukasovic I, Vrkic N. The prevalence of preanalytical errors in a Croatian ISO 15189 accredited laboratory. Clin Chem Lab Med. 2010;48(7):1009–1014. doi: 10.1515/cclm.2010.221. [DOI] [PubMed] [Google Scholar]
- 5.Simundic AM, Bilic-Zulle L, Nikolac N, Supak-Smolcic V, Honovic L, Avram S, et al. The quality of the extra-analytical phase of laboratory practice in some developing European countries and Mexico: a multicentric study. Clin Chem Lab Med. 2011;49(2):215–228. doi: 10.1515/cclm.2011.034. [DOI] [PubMed] [Google Scholar]
- 6.Guzel O, Guner EI. ISO 15189 accreditation: requirements for quality and competence of medical laboratories, experience of a laboratory I. Clin Biochem. 2009;42(4–5):274–278. doi: 10.1016/j.clinbiochem.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Plebani M, Sciacovelli L, Marinova MM, J Chiozza M. Quality indicators in laboratory medicine: a fundamental tool for quality and patient safety. Clin Biochem. 2012 doi: 10.1016/j.clinbiochem.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 8.Inal S, Kelleci M. Relief of pain during blood specimen collection in pediatric patients. MNC Am J Matern Child Nurs. 2012;37(5):339–345. doi: 10.1097/NMC.0b013e31825a8aa5. [DOI] [PubMed] [Google Scholar]
- 9.Baxter A, Cohen L, McElvery H, Lawson M, von Baeyer C. An integration of vibration and cold relieves venipuncture pain in a pediatric emergency department. Pediatr Emerg Care. 2011;27(12):1151–1156. doi: 10.1097/PEC.0b013e318237ace4. [DOI] [PubMed] [Google Scholar]
- 10.Baxter A, Leong T, Mathew B. External thermomechanical stimulation versus vapocoolant for adult venipuncture pain: pilot data on a novel device. Clin J Pain. 2009;25(8):705–710. doi: 10.1097/AJP.0b013e3181af1236. [DOI] [PubMed] [Google Scholar]
- 11.Procedures for the collection of diagnostic blood specimens by venipuncture. CLSI H3–A6 document. 6. Clinical Laboratory Standards Institute: Wayne; 2007. [Google Scholar]
- 12.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Impact of the phlebotomy training based on CLSI/NCCLS H03–A6: procedures for the collection of diagnostic blood specimens by venipuncture. Biochem Med (Zagreb) 2012;22(3):342–351. doi: 10.11613/BM.2012.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guder WG, Narayanan S, Wisser H, Zawta B. Diagnostic samples: from the patient to the laboratory: the impact of preanalytical variables on the quality of laboratory results. 4. New York: Wiley-Blackwell; 2009. p. 124. [Google Scholar]
- 14.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Mangueira C, Sumita N, et al. New ways to deal with known preanalytical issues: use of transilluminator instead of tourniquet for easing vein access and eliminating stasis on clinical biochemistry. Biochem Med (Zagreb). 2011;21(2):152–159. doi: 10.11613/BM.2011.024. [DOI] [PubMed] [Google Scholar]
- 15.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Scartezini M, Guidi GC, et al. Transillumination: a new tool to eliminate the impact of venous stasis during the procedure for the collection of diagnostic blood specimens for routine haematological testing. Int J Lab Hematol. 2011;33(5):457–462. doi: 10.1111/j.1751-553X.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 16.Lima-Oliveira G, Salvagno GL, Lippi G, Montagnana M, Scartezini M, Picheth G, et al. Elimination of the venous stasis error for routine coagulation testing by transillumination. Clin Chim Acta. 2011;412(15–16):1482–1484. doi: 10.1016/j.cca.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Procedures for the handling and processing of blood specimens for common laboratory tests. CLSI H18–A4 document. 4. Clinical Laboratory Standards Institute: Wayne; 2010. [Google Scholar]
- 18.Hyltoft Petersen P, Fraser CG. Strategies to set global analytical quality specifications in laboratory medicine: 10 years on from Stockholm consensus conference. Accred Qual Assur. 2010;15:323–330.
- 19.Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59(7):491–500. doi: 10.1080/00365519950185229. [DOI] [PubMed] [Google Scholar]
- 20.Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem. 2007;53(7):1338–1342. doi: 10.1373/clinchem.2007.088344. [DOI] [PubMed] [Google Scholar]
- 21.Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem. 1997;43(8 Pt 1):1348–1351. [PubMed] [Google Scholar]
- 22.Lippi G, Guidi GC, Mattiuzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006;44(4):358–365. doi: 10.1515/CCLM.2006.073. [DOI] [PubMed] [Google Scholar]
- 23.Bowen RA, Hortin GL, Csako G, Otanez OH, Remaley AT. Impact of blood collection devices on clinical chemistry assays. Clin Biochem. 2010;43(1–2):4–25. doi: 10.1016/j.clinbiochem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Gren B. Incorrect guidelines for venipuncture affect the analytical results. Scand J Clin Lab Invest. 2009;69(8):815–816. doi: 10.3109/00365510903307970. [DOI] [PubMed] [Google Scholar]
- 25.Lippi G, Guidi GC. Risk management in the preanalytical phase of laboratory testing. Clin Chem Lab Med. 2007;45(6):720–727. doi: 10.1515/CCLM.2007.167. [DOI] [PubMed] [Google Scholar]
- 26.Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48(5):691–698. [PubMed] [Google Scholar]
- 27.Plebani M. Pre-analytical errors and patient safety. J Med Biochem. 2012;31:265–270. doi: 10.2478/v10011-012-0014-1. [DOI] [Google Scholar]
- 28.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Different manufacturers of syringes: a new source of variability in blood gas, acid-base balance and related laboratory test? Clin Biochem. 2012;45(9):683–687. doi: 10.1016/j.clinbiochem.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Pre analytical management: serum vacuum tubes validation for routine clinical chemistry. Biochem Med (Zagreb) 2012;22(2):180–186. doi: 10.11613/BM.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Poli G, Solero GP, et al. K3EDTA vacuum tubes validation for routine hematological testing. ISRN Hematol. 2012;2012:875357. doi: 10.5402/2012/875357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Picheth G, Guidi GC. Sodium citrate vacuum tubes validation: preventing preanalytical variability in routine coagulation testing. Blood Coagul Fibrinolysis. 2013;24(3):252–5. [DOI] [PubMed]
- 32.Lippi G, Lima-Oliveira G, Nazer SC, Moreira ML, Souza RF, Salvagno GL, et al. Suitability of a transport box for blood sample shipment over a long period. Clin Biochem. 2011;44(12):1028–1029. doi: 10.1016/j.clinbiochem.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 33.Parenmark A, Landberg E. To mix or not to mix venous blood samples collected in vacuum tubes? Clin Chem Lab Med. 2011;49(12):2061–2063. doi: 10.1515/CCLM.2011.705. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Plebani M. Primary blood tubes mixing: time for updated recommendations. Clin Chem Lab Med. 2012;50(4):599–600. doi: 10.1515/cclm-2012-0120. [DOI] [PubMed] [Google Scholar]
- 35.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Gelati M, Volanski W, et al. Effects of vigoros mixing of blood vacuum tubes on laboratory test results. Clin Biochem. 2013;46(3):250–254. doi: 10.1016/j.clinbiochem.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 36.Lippi G, Salvagno GL, Montagnana M, Lima-Oliveira G, Guidi GC, Favaloro EJ. Quality standards for sample collection in coagulation testing. Semin Thromb Hemost. 2012;38(6):565–575. doi: 10.1055/s-0032-1315961. [DOI] [PubMed] [Google Scholar]
- 37.Cembrowski GS, Strauss S, Waldeland LJ, Kropp E, Adlis SA. Are phlebotomy services completely satisfying our patient customers? 1995 institute: frontiers in laboratory practice research. 1996:198-208.
- 38.Oatey A, Stiller K. An evaluation of the level of satisfaction with a dedicated inpatient service at an rehabilitation centre. Int J Nurs Pract. 2009;15(6):553–559. doi: 10.1111/j.1440-172X.2009.01779.x. [DOI] [PubMed] [Google Scholar]
- 39.St Laurent RT. Evaluating agreement with a gold standard in method comparison studies. Biometrics. 1998;54(2):537–545. doi: 10.2307/3109761. [DOI] [PubMed] [Google Scholar]
- 40.Ricos C, Cava F, Garcia-Lario JV, Hernandez A, Iglesias N, Jimenez CV, et al. The reference change value: a proposal to interpret laboratory reports in serial testing based on biological variation. Scand J Clin Lab Invest. 2004;64(3):175–184. doi: 10.1080/00365510410004885. [DOI] [PubMed] [Google Scholar]
- 41.Westgard J. Biological variation database specifications. 2010 January 2012 [cited 2010 11/13]; Available from: http://www.westgard.com/biodatabase1.htm.
- 42.Cembrowski GS, Tran DV, Higgins TN. The use of serial patient blood gas, electrolyte and glucose results to derive biologic variation: a new tool to assess the acceptability of intensive care unit testing. Clin Chem Lab Med. 2010;48(10):1447–1454. doi: 10.1515/cclm.2010.286. [DOI] [PubMed] [Google Scholar]
- 43.Plebani M, Lippi G. Biological variation and reference change values: an essential piece of the puzzle of laboratory testing. Clin Chem Lab Med. 2012;50:189–190. doi: 10.1515/cclm.2011.751. [DOI] [PubMed] [Google Scholar]
- 44.Johnson J. Mechanisms of vasoconstriction with direct skin cooling in humans. Am J Physiol Heart Circ Physiol. 2007;292:H1690–H1691. doi: 10.1152/ajpheart.00048.2007. [DOI] [PubMed] [Google Scholar]
- 45.Pergola P, Kellogg D, Johnson J, Kosiba W, Solomon D. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol Heart Circ Physiol. 1993;265:H785–H792. doi: 10.1152/ajpheart.1993.265.3.H785. [DOI] [PubMed] [Google Scholar]
- 46.Austin A, Patterson S, Kanel RV. Hemoconcentration and hemostasis during acute stress: interacting and independent effects. Ann Behav Med. 2011;42:153–173. doi: 10.1007/s12160-011-9274-0. [DOI] [PubMed] [Google Scholar]
- 47.Risteli J, Winter WE, Kleerekoper M, Risteli L. Bone and mineral metabolism. In: Burtis CA, Edwards RA, Bruns DE, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5. Amsterdam: Elsevier; 2012. pp. 1733–1801. [Google Scholar]
- 48.Fuller J, Thomson R, Howe P, Buckley J. Effect of vibration on muscle perfusion: a systematic review. Clin Physiol Funct Imaging. 2013;33(1):1–10. doi: 10.1111/j.1475-097X.2012.01161.x. [DOI] [PubMed] [Google Scholar]