Abstract
Monosodium glutamate (MSG) is a popular flavour enhancer used in food industries; however, excess MSG is neurotoxic. Oxidative stress is well documented in MSG induced neurotoxicity. The compounds having antioxidant and anti-inflammatory properties reportedly possess beneficial effects against various neurotoxic insults. Calendula officinalis Linn. flower extract (COE) is known for its potent antioxidant and anti-inflammatory activities. Hence, this present study has been designed to evaluate the neuroprotective effect of COE on MSG-induced neurotoxicity in rats. Adult Wistar rats were administered systemically for 7 days with MSG and after one h of MSG injection, rats were treated with COE (100 and 200 mg/kg) orally. At the end the treatment period, animals were assessed for locomotor activity and were sacrificed; brains were isolated for estimation of LPO, GSH, CAT, TT, GST, Nitrite and histopathological studies. MSG caused a significant alteration in animal behavior, oxidative defense (raised levels of LPO, nitrite concentration, depletion of antioxidant levels) and hippocampal neuronal histology. Treatment with COE significantly attenuated behavioral alterations, oxidative stress, and hippocampal damage in MSG-treated animals. Hence, this study demonstrates that COE protects against MSG-induced neurotoxicity in rats. The antioxidant and anti-inflammatory properties of COE may be responsible for its observed neuroprotective action.
Keywords: Excitotoxicity, Marigold, MSG, Neuroprotective, Nitrative stress, Oxidative stress
Introduction
Sodium salt of glutamate (MSG) is one of the popular flavour enhancer used by various food industries [1]. MSG has been reported to produce symptoms such as numbness, weakness, flushing, sweating, dizziness, headache and showed increased oxidative stress in brain [2, 3]. Use of MSG as a flavour has been questioned due to its toxic effects, as manifested by the Chinese restaurant syndrome [2], but in many countries there are no limitations on the amount of MSG that can be added to food. Hence, people ingest large doses of MSG [4] that increases the glutamate concentration in blood.
Glutamate is the most abundant excitatory neurotransmitter in the brain. Excessive amounts of glutamate may act as a potent neurotoxin through increasing the excitability and by activating the proteolytic enzymes [5]. Earlier animal studies have reported that the administration of MSG causes damage to hypothalamic neurons of brain [6]. In addition, MSG causes alteration in mitochondrial lipid peroxidation (LPO) and antioxidant status in different regions of the brain, namely the cerebral hemispheres, cerebellum, brain stem and diencephalon [2]. Hence, intraperitoneal injections of MSG in rodents provide a successful model to screen the drugs on brain oxidative stress and excitotoxicity induced neuronal damage.
Historically, herbs have always played a significant role in treating illness or preventing disease. Over the past several decades, scientific literature and popular media articles on adverse drug effects have increased the interest in natural products by the general public [7]. Many plant extracts, formulations and phytoconstituents with antioxidant and anti inflammatory properties have been reported to protect the neurons in different animal models [3, 8]. Our lab has been screening a number of plant species and phytoconstituents in this line [9, 10].
Calendula officinalis Linn. (Asteraceae) commonly known as marigold is a herb of ancient medicinal repute in traditional and homeopathic medicine [11]. It is used as a component in various foods like chowders, soups, stews, roast meats, chickens and sea foods. Fresh flowers of C. officinalis are chopped into salad, and dried petals are used in tea and as flavor in cakes, cookies and puddings, and to colour the butters and cheeses [11, 12]. C. officinalis reportedly possesses antibacterial, antifungal, antiviral, antimutagenic, hepatoprotective, renoprotective, free radical scavenger, anti-inflammatory and central nervous system properties [13–15]. However, there is no literature found on marigold as neuroprotective, hence, the present study has been undertaken with the aim to determine the neuroprotective activity of marigold flower extract against oxidative stress and excitotoxicity induced neuronal damage in rats.
Materials and Methods
Chemicals
1-Chloro-2,4-dinitrobenzene (CDNB) was obtained from Sigma Aldrich, St. Louis, USA. MSG, reduced glutathione (GSH), 5,5′-dithiobis-2-nitrobenzoic acid, thiobarbituric acid and trichloroacetic acid were purchased from Hi-Media Laboratories Pvt. Ltd, Mumbai, India. All the other chemicals used were of analytical grade.
Plant Material and Extraction
The fresh flowers of C. officinalis L., were collected from herbal garden of University of Agricultural Sciences, Dharwad, Karnataka, India. The plant was identified and authenticated by Prof. K. Siddappa, Department of Botany, Sree Siddaganga College of Arts, Science and Commerce, Tumkur, Karnataka, India and a voucher specimen of the same was kept in the college herbarium.
The shade-dried flowers were extracted exhaustively with 70 % methanol by cold maceration process for 14 days. The obtained extract was concentrated to get a thick brown semisolid paste (yield 18.66 %).
Animals and Experimental Design
Adult female Wistar rats (190–220 g) bred in animal house of Sree Siddaganga College of Pharmacy, Tumkur, Karnataka, India were used. The animals were housed under standard laboratory conditions, maintained on a 12 h light:12 h dark cycle and had a free access to food and water. The experimental protocols were approved by the Institutional Animal Ethics Committee (SSCPT/IAEC/73/2009–2010) and conducted according to CPCSEA guidelines, Government of India.
Accurately weighed quantity of hydro-alcoholic (70 % methanol) extract of C. officinalis (COE) was suspended in distilled water using 1 % Tween 80 v/v and was administered orally to experimental animals. MSG was freshly prepared in saline (adjusted to pH 7.4) and administered by intra-peritoneal route to rats for a period of seven days to induce neurotoxicity.
Every day, COE was administered one h post MSG injection. The dose of MSG and COE were selected based on previous literatures [3, 11]. During the treatment period, rats were observed for behavioral changes for 50 min daily. On the 8th day after locomotor activity assessment, rats were sacrificed; brains were removed for estimation of LPO, GSH, CAT, TT, GST, nitrite and histopathological studies.
Locomotor Activity
The locomotor activity was recorded by using actophotometer (INCO Pvt. Ltd., Ambala, India). Before locomotor task, animals were placed individually in the activity meter for 2 min for habituation. Thereafter, locomotor activity was recorded using actophotometer for a period of 5 min [16].
Biochemical Estimations
Preparation of Tissue Homogenates
The animals were sacrificed under deep ether anesthesia and perfused transcardially with ice-cold saline. The whole brain dissected out, blotted dry and immediately weighed. A 10 % brain homogenate was prepared with ice-cold phosphate buffered saline using Teflon-glass homogenizer. The homogenate was centrifuged at 10,000 rpm at 4 °C for 15 min and the pellet discarded. The supernatant obtained was used for biochemical estimations.
Total Protein
The protein content of the brain homogenate was determined by the method of Lowry et al. [17] using bovine serum albumin as standard.
Lipid Peroxidation (LPO)
The extent of LPO was determined by measuring the amount of malanodialdehyde (MDA) formed. 0.1 mL homogenate was taken and to this 1 mL of TBA reagent containing equal proportions of 0.375 % TBA, 15 % TCA and 0.25 N HCl was added and placed in a boiling water bath for 30 min. Then the mixture was placed in crushed ice for 10 min followed by centrifugation at 6,000 rpm for 5 min. The absorbance of the clear pink colour supernatant was measured at 532 nm against appropriate blank. The results were expressed as nmol MDA/mg protein [18].
Reduced Glutathione (GSH)
GSH was measured according to the method of Ellman [19]. Briefly, 0.5 mL of homogenate was mixed with 0.1 mL of 25 % TCA to precipitate proteins and centrifuged at 4,000 rpm for 5 min. Then 0.3 mL of the supernatant was mixed with 0.5 mL of 0.1 M phosphate buffer (pH 7.4) and 0.2 mL of 10 mM DTNB. This mixture was incubated for 10 min and the absorbance was measured at 412 nm against appropriate blank. The values were expressed as nmol/mg protein.
Total Thiols (TT)
Briefly, 0.2 mL of brain tissue supernatant was mixed with 0.36 mL of 0.1 M phosphate buffer (pH 7.4), 40 μL of 10 mM DTNB and 1.4 mL of methanol. This mixture was incubated for 10 min and the absorbance was measured at 412 nm against appropriate blank and the values are expressed as nmol/mg protein [20].
Glutathione-S-Transferase (GST)
The GST activity was measured as described by Habig et al. [21]. Briefly, 0.85 mL of phosphate buffer (pH 7.4), 50 μL homogenate and 50 μL of 1 mM CDNB were mixed in a cuvette, to which 50 μL of 10 mM GSH was added to initiate the reaction. The rate of formation of GSH-CDNB complex was monitored for 5 min at 340 nm. The results were expressed as nmol of CDNB conjugate formed/min/mg protein.
Catalase (CAT)
Briefly, to 0.95 mL of 10 mM H2O2 in 60 mM phosphate buffer (pH 7.0), 50 μL of the brain tissue supernatant was added and the rate of degradation of H2O2 was monitored at 240 nm for 60 sec and expressed as units/mg protein [22].
Nitrite
The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide (NO), was estimated by using the principle of Griess reaction [23]. Briefly, equal volumes of tissue homogenate and Griess reagent (5 % phosphoric acid containing 0.1 % NEDD (N,N-(1-naphythyl) ethylene-diamine dihydrochloride), 1 % sulfanilamide) were mixed and incubated in dark for 10 min at room temperature. Then the absorbance of the reaction mixture was determined at 540 nm. The concentration of nitrite in the supernatant was determined from a sodium nitrite (NaNO2) standard curve. The amount of nitrite was expressed as nmol/g tissue.
Statistical Evaluation
The data were expressed as mean ± SEM and statistical comparisons were performed by one-way ANOVA followed by Turkey’s post-test using Graph Pad Prism version 5.0, USA.
Histopathological Studies
A section of the brain was fixed with 10 % formalin and embedded in paraffin wax and cut into sections of 5-μm thickness. The sections were stained with hematoxylin and eosin dye and hippocampal neurons were observed for morphological changes.
Results
Locomotor Activity
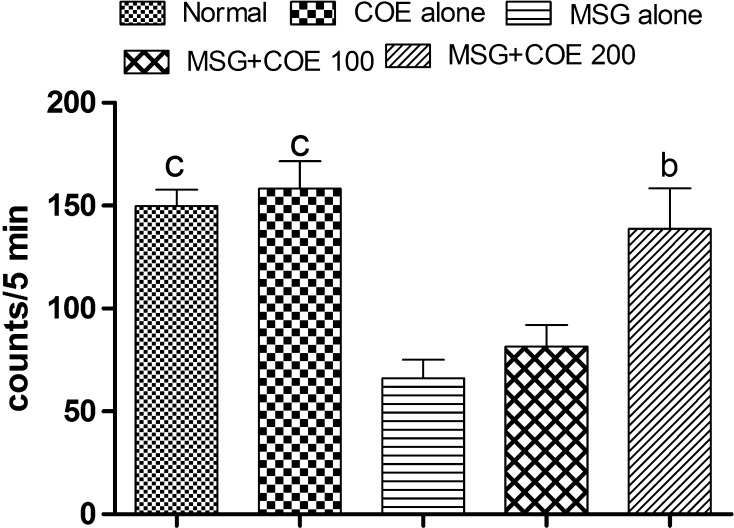
Administration of MSG for 7 days resulted in significant (p < 0.001) decrease in movement of animals (measured as counts/5 min) when compared to normal control animals. The locomotor count in MSG alone treated animals showed 66.00 ± 9.13, which was 55.94 % less when compared to normal control animals (149.80 ± 8.00). Treatment with COE (100 and 200 mg/kg) to MSG treated rats increased the locomotor counts (81.67 ± 10.48 and 138.80 ± 19.62 respectively) when compared to MSG alone treated rats. Treatment with COE 200 mg/kg to MSG treated animals showed significantly (p < 0.01) improved locomotor activity when compared with MSG alone treated animals (Fig. 1).
Fig. 1.
Effect of COE on ambulatory behavior in MSG treated rats. Each bar represents the mean ± SEM (n = 6), bp < 0.01, cp < 0.001 when compared to MSG alone treated rats. One-way ANOVA followed by Tukeys post test
Biochemical Estimations
Lipid Peroxidation
The LPO levels (measured as nmol of MDA/mg protein) were found to be significantly (p < 0.001) higher in MSG treated rats (3.96 ± 0.31) when compared with normal control animals (1.98 ± 0.98). Treatment with COE (100 and 200 mg/kg) significantly (p < 0.01 and p < 0.05 respectively) attenuated the increased LPO level in MSG treated rats when compared with MSG alone treated animals. LPO level in COE 100 and 200 mg/kg treated rats were found to be 2.51 ± 0.32 and 2.87 ± 0.22 respectively (Table 1).
Table 1.
Effect of COE on oxidative stress parameters in MSG treated rats
| Treatment | GSH (nmol/mg protein) | TT (nmol/mg protein) | GST (nmol CDNB-GSH/min/mg protein) | CAT (U/mg protein) | LPO (nmol MDA/mg protein) | Nitrite (nmol/g tissue) |
|---|---|---|---|---|---|---|
| Normal control | 3.85 ± 0.85c | 28.41 ± 1.77b | 26.03 ± 2.30c | 15.71 ± 0.76a | 1.98 ± 0.10c | 25.71 ± 1.08c |
| COE alone | 4.40 ± 0.68c | 28.62 ± 2.06c | 27.36 ± 3.18c | 15.49 ± 2.13a | 2.04 ± 0.14c | 25.04 ± 1.30c |
| MSG alone | 0.48 ± 0.80 | 16.77 ± 1.84 | 9.13 ± 0.51 | 8.01 ± 0.43 | 3.96 ± 0.31 | 35.25 ± 1.86 |
| MSG + COE 100 mg/kg | 2.72 ± 0.14a | 17.16 ± 2.11 | 19.20 ± 1.98a | 16.81 ± 2.78b | 2.52 ± 0.32b | 28.17 ± 1.15b |
| MSG + COE 200 mg/kg | 1.788 ± 0.07 | 25.34 ± 1.84a | 20.58 ± 1.59b | 15.14 ± 1.87a | 2.88 ± 0.22a | 28.69 ± 0.9a |
Each values are expressed as mean ± SEM (n = 6), ap < 0.05, bp < 0.01, cp < 0.001 when compared with MSG alone treated animals. One-way ANOVA followed by Tukeys post test
Glutathione
There was a significant (p < 0.001) depletion in GSH levels (measured as nmol/mg protein) found in MSG alone treated rats (0.48 ± 0.08) when compared with normal control animals (3.85 ± 0.85). Treatment with COE 100 mg/kg significantly (p < 0.05) protected the animals against MSG induced GSH depletion. GSH levels in COE 100 and 200 mg/kg treated rats were found to be 2.71 ± 0.14 and 1.78 ± 0.07 respectively (Table 1).
Total Thiols
Administration of MSG alone significantly (p < 0.01) reduced the TT level when compared with normal rats (measured as nmol/mg protein). MSG alone treated rats showed 40.97 % reduced level of TT (16.77 ± 1.85) than normal animals (28.41 ± 1.77). Treatment with COE (100 and 200 mg/kg) increased the level of TT content to 17.16 ± 2.11 and 25.34 ± 1.84 respectively. The content of TT in COE higher dose treated rats was statistically significant (p < 0.05) when compared with MSG alone treated animals (Table 1).
Glutathione-S-Transferase
GST activity (measured as nmol CDNB conjugate formed/min/mg protein) was significantly (p < 0.001) reduced in the MSG treated rats (9.12 ± 0.51) when compared with normal control animals (26.03 ± 2.27). A significant rise (p < 0.05 and p < 0.01) in the enzyme activity was found in the COE 100 and 200 mg/kg treated rats (19.20 ± 1.97 and 20.58 ± 1.58 respectively) when compared with MSG control animals (Table 1).
Catalase
A significant (p < 0.05) depletion in catalase activity (around 49 %) was observed in the MSG treated animals (8.01 ± 0.43) when compared with normal rats (15.71 ± 0.76). Both the tested doses of COE significantly (p < 0.01 and 0.05 respectively) restored the catalase activity. The catalase activity in COE 100 and 200 mg/kg treated rats were found to be 16.81 ± 2.78 and 15.14 ± 1.87 respectively (Table 1).
Tissue Nitrite Content
Nitrite content (measured as nmol/g tissue) in the tissue was significantly (p < 0.001) elevated in MSG alone treated rats (35.25 ± 1.86) when compared with normal control rats (25.71 ± 1.08). Treatment with COE (100 and 200 mg/kg) showed a significant (p < 0.01 and p < 0.05) reduction in nitrite content and the values were found to be 28.17 ± 1.15 and 28.69 ± 0.91 respectively (Table 1).
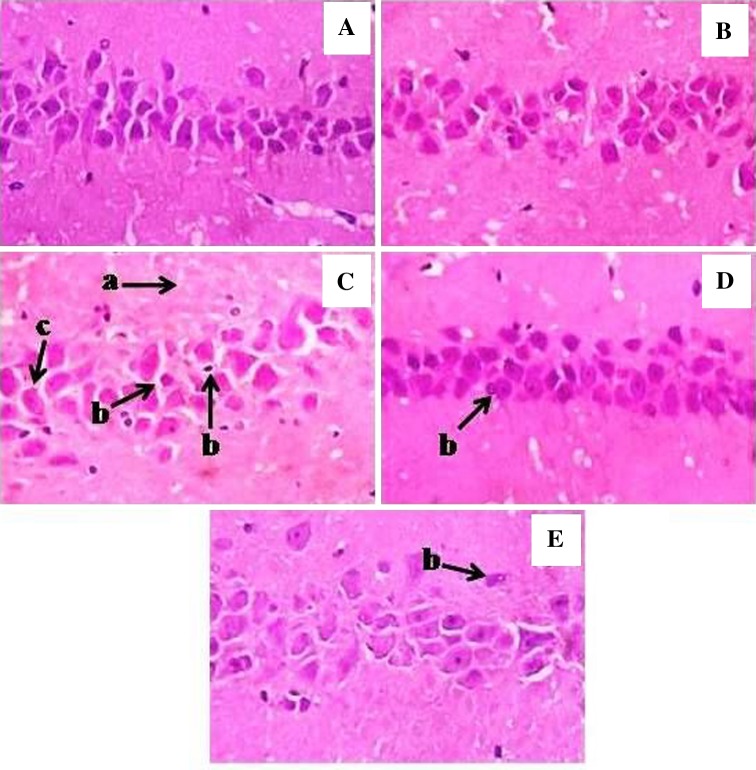
Histopathological Observations
Sections studied from the hippocampus of normal control rats showed intact layers of CA1, CA2, CA3 and CA4 regions with intermingled neuropil fibers and irregular shaped processes of astroglial cells. On the other hand, MSG alone treated rat brain sections showed decreased CA1 layer, darkly stained shrunken cells and mildly separated interconnected neuropil fibers. The brain sections from COE 100 mg/kg treated animals showed normal CA1 layer, astroglial cells and the interconnected neuropil fibers. In the COE 200 mg/kg treated animals, normal CA1 layer, astroglial cells, markedly reduced population of shrunken cells and unremarkable interconnected neuropil fibers were observed. However, the hippocampal histology of COE alone treated rats was found to be normal (Fig. 2).
Fig. 2.
Histopathological changes in hippocampus region of experimental rat brain sections. A Normal control; B COE alone; C MSG alone; D MSG + COE 100 mg/kg; E MSG + COE 200 mg/kg. Arrows indicates mildly separated interconnected neuropil fibers (a), shrunken and darkly stained cells (b), decreased CA1 layer & dispersed astroglial cells (c)
Discussion
Glutamate induced cell death is associated with both apoptotic and necrotic changes, where excitotoxic and oxidative pathways are the two distinct pathways through which glutamate induces neuronal death. Excitotoxicity involves the over activation of glutamate receptors that leads to rapid and slow triggered cytotoxic events [2]. Over activation of NMDA and AMPA receptors by glutamate leads to release of Ca++ from the intracellular stores, which causes over activation of mitochondria and enzymes like phospholipase and protein kinases leading to the degradation of proteins and membranes, thus impairing cellular survival [5]. Glutamate has also been reported to disrupt the glutathione-cystine antiporter system and thereby causing a drop in the glutathione levels allowing the generation of neurotoxic reactive oxygen species (ROS) [2].
Several studies have reported that MSG could cause severe behavioral abnormalities such as increased irritability, hypoactivity, and deficits in spontaneous alternation behavior [24]. In agreement with the above reports, the animals administered with MSG showed aggressive behavior on the initial days of the treatment followed by calmness and hypoactivity. COE at both the tested doses normalized the general behavior and increased the locomotor activity as compared to MSG control rats indicating the protective action of COE against MSG induced neurotoxicity. Furthermore, MSG treatment for 1 week depleted the GSH, GST, TT, and catalase activity and increased the MDA and nitrite levels in the brain tissues indicating the increased oxidative and nitrative stress in these animals.
Oxidative stress is a characteristic feature in a number of neurodegenerative disorders [25]. The brain is particularly vulnerable to oxidative stress injury because of its high rate of oxidative metabolic activity, high content of polyunsaturated fatty acids, relatively low antioxidant capacity, and the abundance of redox-active transition metal ions and nonreplicating nature of its neuronal cells [26]. In order to scavenge ROS, different defense systems exist in the brain, such as enzymatic (superoxide dismutase, glutathione peroxidase and catalase), non enzymatic (glutathione and uric acid) and dietary antioxidants. If ROS are not effectively eliminated, they can cause peroxidation of cell membrane phospholipids, proteins and DNA [27]. Hence, antioxidants are the first line of defense against free radical damage and are critical for maintaining health. Due to this, antioxidant supplements and antioxidants containing diet are now being recognized as an important means of improving free radical protection [28].
In the present study, treatment with both the tested doses of COE significantly attenuated the glutamate induced excitation and oxidative stress in rat. Administration of COE to the MSG treated animals showed significantly increased levels of GSH, CAT, TT, GST and decreased levels of LPO and nitrite when compared with MSG alone treated rats, these findings are in accord to the earlier reports demonstrating antioxidant property of COE [14]. Hence, these results indicate use of C. officinalis flower containing foods may protect the body from free radical injury.
Certain regions of brain are highly enriched in non-heme iron, which is catalytically involved in the production of ROS [29] and thus increasing the risk of neurodegenerative diseases. Reports suggest that the regions like cortex and hippocampus are more susceptible to oxidative damage when compared with cerebellum [30]. The present study also evidenced loss of neuronal structure, necrosis in hippocampal region of MSG alone treated rats, and this observation well supports the above findings. Both the tested doses of COE reversed the histopathological changes produced by MSG administration confirming the protective effect of COE against MSG induced excitotoxic neuronal damage in hippocampus.
The anti-inflammatory effect of COE was reported by Korengath et al. [15]. Several anti-inflammatory compounds are known to protect against hippocampal neuronal loss in different animal models [31]. Owing to the fact that COE has anti-inflammatory action, this mode of action may be drawn for the observed neuroprotective effect of the extract.
The flowers of C. officinalis L. contain flavonoids like quercetin, isorhamnetin, isoquercetin, calendoflaside, calendoflavoside, calendoflavobioside, rutin. The flowers are also rich in carotenoids like α-carotene, β-carotene and quinones like plastoquinone, phylloquinone, and α-tocopherol [32]. Flavonoids, carotenoids and quinones are well documented for its beneficial effect in protecting the organs against various toxic insults. Presence of such active compounds might be responsible for the neuroprotective activity by the C. officinalis.
Conclusion
Conclusively, the present study demonstrates that hydro alcoholic extract of C. officinalis flowers possess neuroprotective effect against MSG induced brain damage in rats. The protective effect of COE might be attributed to its antioxidant and anti-inflammatory properties. However, further research is required to unravel the specific mode of action.
Acknowledgments
We are gratefully acknowledging the financial support by the Vision Group on Science and Technology, Government of Karnataka under the programme for Establishment of Centre of Excellence in Herbal Drug Development (VGST/PRMG/CESEM-1/2009-10/199).
References
- 1.Veni NK, Karthika D, Devi MS, Rubini MF, Vishalini M, Pradeepa YJ. Analysis of monosodium l-glutamate in food products by high-performance thin layer chromatography. J Young Pharm. 2010;2:297–300. doi: 10.4103/0975-1483.66795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farombi EO, Onyema OO. Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol. 2006;25:251–259. doi: 10.1191/0960327106ht621oa. [DOI] [PubMed] [Google Scholar]
- 3.Ramanathan M, Sivakumar S, Anandvijayakumar PR, Saravanababu C, Rathinavel PP. Neuroprotective evaluation of standardized extract of Centella asiatica in monosodium glutamate treated rats. Indian J Exp Biol. 2007;45:425–431. [PubMed] [Google Scholar]
- 4.Diniz YS, Faine LA, Galhardi CM, Rodrigues HG, Ebaid GX, Burneiko RC, Cicogna Monosodium glutamate in standard and high-fiber diets: metabolic syndrome and oxidative stress in rats. Nutrition. 2005;21:749–755. doi: 10.1016/j.nut.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Weil ZM, Norman GJ, DeVries AC, Nelson RJ. The injured nervous system: a Darwinian perspective. Prog Neurobiol. 2008;86:48–59. doi: 10.1016/j.pneurobio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meldrum B. Amino acids as dietary excitotoxins: a contribution to understanding neurodegenerative disorders. Brain Res Rev. 1993;18:293–314. doi: 10.1016/0165-0173(93)90014-Q. [DOI] [PubMed] [Google Scholar]
- 7.Cooper R, Kronenberg F. Botanical medicine: from bench to bedside. 2. Maryland: Mary Ann Liebert; 2008. [Google Scholar]
- 8.Shukla PK, Khanna VK, Ali MM, Maurya R, Khan MY, Srimal RC. Neuroprotective effect of Acorus calamus against middle cerebral artery occlusion induced ischaemia in rat. Hum Exp Toxicol. 2006;25:187–194. doi: 10.1191/0960327106ht613oa. [DOI] [PubMed] [Google Scholar]
- 9.Thippeswamy BS, Nagakannan P, Shivasharan BD, Mahendran S, Veerapur VP, Badami S. Protective effect of embelin from Embelia ribes Burm. against transient global ischemia induced brain damage in rats. Neurotox Res. 2011;20:379–386. doi: 10.1007/s12640-011-9258-7. [DOI] [PubMed] [Google Scholar]
- 10.Nagakannan P, Shivasharan BD, Thippeswamy BS, Veerapur VP, Bansal P. Protective effect of hydroalcoholic extract of Mimusops elengi Linn. flowers against middle cerebral artery occlusion induced brain injury in rats. J Ethnopharmacol. 2012;140:247–254. doi: 10.1016/j.jep.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Cetkovic GS, Djilas SM, Brunet C, Tumbas VT. Antioxident properties of marigold extracts. Food Res Int. 2004;37:643–650. doi: 10.1016/j.foodres.2004.01.010. [DOI] [Google Scholar]
- 12.Duke JA. Pot marigold an herbal florin for health. New Rochelle: Mary Ann Liebert Inc.; 2008. [Google Scholar]
- 13.Duke JA. Handbook of medicinal herb. 2. New York: CRC Press; 1992. [Google Scholar]
- 14.Preethi K, Chandran, Kuttan R. Effect of Calendula officinalis flower extract on acute phase proteins, antioxidant defence mechanism and granuloma formation during thermal burns. J Clin Biochem Nutr. 2008;43:58–64. doi: 10.3164/jcbn.2008043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korengath CP, Kuttan G, Kuttan R. Anti-inflammatory activity of flower extract of Calendula officinalis L. and its possible mechanism of action. Indian J Exp Biol. 2009;47:113–120. [PubMed] [Google Scholar]
- 16.Kulkarni SK. Hand book of experimental pharmacology. New Delhi: Vallabh Prakashan; 1999. [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Gelvan D, Saltman P. Different cellular targets of Cu- and Fe-catalyzed oxidation observed using a Cu-compatible thiobarbiturate acid assay. Biochim Biophys Acta. 1990;1035:353–360. doi: 10.1016/0304-4165(90)90100-B. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak J, Lindsay R. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 21.Habig WH, Pabst MJ, Jarkoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 22.Claiborne A. Handbook of methods for oxygen radical research. London: CRC Press; 1985. [Google Scholar]
- 23.Green LC, Wagner DA, Glogowski J, Skipper L, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 24.Sanabria ER, Pereira MFS, Dolnikoff MS, Andrade IS, Ferreira AT, Cavalheiro EA, Fernandes MJ. Deficit in hippocampal long-term potentiation in monosodium glutamate treated rats. Brain Res Bull. 2002;59:47–51. doi: 10.1016/S0361-9230(02)00837-7. [DOI] [PubMed] [Google Scholar]
- 25.Andersen JK. Oxidative stress in neurodegeneration: cause or consequences. Nat Med. 2004;10:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 26.Evans PH. Free radicals in brain metabolism and pathology. Br Med Bull. 1993;49:577–587. doi: 10.1093/oxfordjournals.bmb.a072632. [DOI] [PubMed] [Google Scholar]
- 27.Ilhan A, Gurel A, Armutcu F, Kamisli S, Iraz M. Antiepileptogenic and antioxidant effects of Nigella sativa oil against pentylenetetrazol-induced kindling in mice. Neuropharmacology. 2005;49:456–464. doi: 10.1016/j.neuropharm.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Veerapur VP, Thippeswamy BS, Prabhakar KR, Nagakannan P, Shivasharan BD, Bansal P, Sneha SD, Mishra B, Priyadarsini KI, Unnikrishnan MK. Antioxidant and renoprotective activities of Ficus racemosa Linn. stem bark: bioactivity guided fractionation study. Biomed Prev Nutr. 2011;1:273–281. doi: 10.1016/j.bionut.2011.06.002. [DOI] [Google Scholar]
- 29.Hill JM, Switzer RC. The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 1984;11:595–603. doi: 10.1016/0306-4522(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 30.Venkataraman P, Muthuvel R, Krishnamoorthy G, Arunkumar A, Sridhar M, Srinivasan N, Balasubramanian K. PCB (Aroclor 1254) enhances oxidative damage in rat brain regions: protective role of ascorbic acid. Neurotoxicology. 2007;28:490–498. doi: 10.1016/j.neuro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Candelario-Jalil E, Álvarez D, González-Falcón A, García-Cabrera M, Martínez-Sánchez G, Merino N, Giuliani A, León OS. Neuroprotective efficacy of nimesulide against hippocampal neuronal damage following transient forebrain ischemia. Eur J Pharmacol. 2002;453:189–195. doi: 10.1016/S0014-2999(02)02422-6. [DOI] [PubMed] [Google Scholar]
- 32.Muley BP, Khadabadi SS, Banarase NB. Phytochemical constituents and pharmacological activities of Calendula officinalis Linn. (Asteraceae): a review. Trop J Pharm Res. 2009;8:455–465. doi: 10.4314/tjpr.v8i5.48090. [DOI] [Google Scholar]