Abstract
Human serum paraoxonase-1 (PON1), an enzyme on HDL prevents oxidation of LDL thereby preventing the development of atherosclerosis. Studies done so far have lead to conflicting results. As studies are lacking in North-West Indian Punjabi’s, a distinct ethnic group with high incidence of coronary artery disease, we determined PONase activity in this population. It has been postulated that sudden lowering of serum PONase may lead to precipitation of acute myocardial infarction. We determined serum PONase activity and lipids in 100 patients each of AMI (within 24 h of onset), stable CAD and 100 age and sex matched healthy controls. These were again determined after 6 weeks in AMI patients. The mean serum PONase activity was lowest in AMI patients (23.26 U/ml) followed by stable CAD patients (102.0 U/ml) where as in controls was highest (179.8 U/ml). In patients with AMI, activity was significantly higher at 6 weeks as compared to that after acute event (49.39 %; p < 0.05). Sudden lowering of serum PONase activity in a population which already has lower activity may be one of the risk factors for development of AMI.
Electronic supplementary material
The online version of this article (doi:10.1007/s12291-012-0260-5) contains supplementary material, which is available to authorized users.
Keywords: Paraoxonase-1, Atherosclerosis, Acute myocardial infarction, Coronary artery disease
Introduction
With increasing industrialization in developing countries, the incidence of coronary artery disease (CAD) is rising rapidly in them like in India [1]. Paraoxonase-1 (PON1) an HDL associated Aryl-dialkylphosphatase, protects lipoproteins from peroxidation [2]. In addition it also hydrolyzes the oxidized LDL-C as has been shown both in vivo and in vitro studies [3, 4]. Though it is mainly a lactonase [5], it can hydrolyze a variety of organophosphates and arylesters like phenylacetate [6] as it has inherent paraoxonase and arylesterase activity.
Though PON1 gene has nearly 200 single nucleotide polymorphisms (SNP’s) [7], the widely studied SNP’s are those in coding region i.e. Q192R [rs662] and L55 M [rs854560] [8]. Studies have shown that variations in PON1 polymorphism can be an independent risk factor for CAD and acute myocardial infarction (AMI) [9, 10]. However, results are controversial. Two recently published large case–control studies where PONase activity and genotypes were determined in angiographically proven CAD and controls, concluded that PONase activity was more significantly related with CAD as compared to variations in PON1 genotype [11, 12].
The PONase activity has been shown to be lower after AMI [13]. It is also lower in patients with familial hypercholesterolemia and diabetes mellitus, who are more prone to CAD [14]. This has led to the hypothesis that the lower the PONase activity, higher will be the accumulation of oxidized LDL and risk of CAD. Four large prospective studies undertaken till date to find association between serum PONase activity and CAD risk have been inconclusive. Caperhilly study [15] revealed that low activity predisposed to CAD whereas PRIME and EPIC Norfolk studies [16, 17] could not find any significant difference in activity between CAD patients and controls. The study in Dutch women in contrast concluded that increased PONase activity increased the risk of CAD [18].
Similarly the case control studies of serum PONase activity in AMI patients have shown conflicting results. In some studies, it was found to be significantly lower in patients with AMI as compared to controls [13, 19–21]. However, others failed to reveal any statistically significant difference in it [22–24]. There exists a wide variation in PONase activity in different ethnic groups and within individuals in the same ethnic group [25]. Singh et al. [26] have shown that healthy North-West Indian Punjabi’s have lower PONase activity as compared to Caucasian Whites. In the present study, we determined serum PONase activity and lipid levels in patients with AMI in acute phase and at 6 weeks and compared with that in age and sex matched healthy controls and stable angiographically proven CAD patients in this population which has an high incidence of CAD.
Materials and Methods
Patients
The study included three groups: 100 patients with AMI (with in 24 h of onset), 100 patients with angiographically proven stable CAD and 100 healthy age and sex matched controls. Only those patients of AMI who reached our emergency within 24 h of onset were recruited. Diagnosis of AMI was based on the clinical history (typical anginal pain or its equivalents), ECG changes (ST segment elevation or depression) and elevation of cardiac biomarkers (cardiac troponin I or CK-MB). Patients with diabetes mellitus, chronic renal disease, chronic liver disease, malnutrition, HIV, recent cardiac intervention were excluded from the study. Ninety-five patients had ST elevation AMI, whereas five had non ST elevation AMI. Seventy patients had anterior/antero-lateral wall infarction whereas 30 had inferior wall AMI. According to Killip’s class severity grading, 70 had grade 1 AMI, eight had grade 2, six had grade 3 and 16 had grade 4. Fifty-six patients could be thrombolyzed with streptokinase whereas remaining could not be administered thrombolytic therapy either because they were out of the window period or were having contraindications for thrombolysis. None underwent primary PTCA.
Stable CAD group included patients who were attending the cardiology outpatient of the institute and had significant angiographically proven CAD (≥70 % stenosis in one or more coronary arteries). Patients who had acute coronary event 4 weeks prior to were excluded. Similarly those who had diabetes mellitus, chronic hepatic or renal disease as in the AMI group were excluded. All the patients were allowed to continue with their treatment which included statins, aspirin or clopidogrel or both. The control group included 100 adults who were attending the general medical outpatient for health check up and were found to be healthy.
Of 100 patients with AMI, 13 died within 6 weeks whereas 17 did not turn up for follow up. Of 70, who came for follow up, 28 underwent angioplasty and stent placement and four had coronary bypass surgery. Rest were managed conservatively with drugs alone.
Biochemical Analysis
5 ml of blood was withdrawn by venepuncture in AMI patients within 24 h of onset and at 6 weeks follow up in fasting state, whereas in controls and stable CAD patients, it was collected between 9 and 10 a.m. after overnight fast. After centrifuging the clotted sample at 3,000 rpm for 30 min, serum was separated and stored at −20 °C for further analysis.
The serum PONase activity was measured by a modification of method described by Mackness et al. [27] using paraoxon as substrate. It was measured by adding serum to Tris-HCl buffer (100 mmol/L, pH 8.0) containing 2 mmol/L CaCl2 and 5.5 mmol/L paraoxon (O,O-diethyl-O-p-nitrophenyl phosphate; Sigma Chemical Co.). The rate of generation of p-nitrophenol was determined at 405 nm. The lipids HDL-C, LDL-C, TG, T-CHO and serum creatine kinase were measured by enzymatic method using autoanalyzer (Hitachi modular P) and Roche diagnostic kits.
Statistical Analysis
Statistical analysis was carried out using SPSS (version 17.0) software. All the study variables are shown as mean ± SD or median (range) depending on the shape of the distribution curve. One way Anova test (Kruskal–Wallis test) was used to compare the data between the three study groups. Chi-square test was used for non-continuous variables. Wilcoxon signed rank test and Student’s t test were used to compare the paired groups i.e. AMI group (day 1) and AMI group at follow up (day 42). Spearman’s correlation coefficient was used to test the strength of association between the variables. Stepwise multivariate linear regression analysis was undertaken to determine factors affecting PONase activity. The study was approved by institute’s Ethics Committee. An informed consent was obtained from all the patients and controls.
Results
Subject Characteristics
The demographic, clinical and baseline biochemical characteristics of the study population are shown in Table 1. Of the three groups, AMI group had the highest number of smokers where as CAD group had patients with the highest BMI. Both the CAD and AMI groups had significantly higher number of alcohol consumers and hypertensives as compared to controls. The AMI group at presentation to the hospital had significantly lower SBP and DBP as compared to the other two groups.
Table 1.
Demographic and clinical characteristics of controls, CAD and AMI patients
| Controls (n = 100) | CAD (n = 100) | AMI (n = 100) | p value | |
|---|---|---|---|---|
| Age (years)** | 52.1 ± 9.1 | 55.5 ± 11.7 | 55.4 ± 13.5 | 0.063a |
| Male/Female (n) | 90/10 | 92/8 | 88/12 | 0.641b |
| BMI (kg/m2)** | 24.3 ± 4.2 | 27.3 ± 3.8* | 24.4 ± 4.2† | 0.0001a |
| SBP (mm Hg)** | 122.1 ± 8.1 | 131.8 ± 15.0* | 23.9 ± 2.3*† | 0.0001a |
| DBP (mm Hg)** | 80.9 ± 5.1 | 83.1 ± 6.9 | 70.5 ± 18.7*† | 0.0001a |
| Alcohol consumers | 8 | 22* | 17* | 0.022b |
| Smokers | 26 | 20 | 56*† | 0.0001b |
| Hypertensives | 11 | 59* | 25*† | 0.0001b |
| HDL-C, mg/dL** | 50.18 ± 15.07 | 44.44 ± 18.50* | 39.49 ± 9.94* | 0.0001a |
| LDL-C, mg/dL** | 91.07 ± 24.96 | 91.31 ± 35.85 | 115.65 ± 41.40*† | 0.0001a |
| TG, mg/dL** | 131.74 ± 52.02 | 136.13 ± 54.51 | 151.21 ± 72.71 | 0.060a |
| T-CHO, mg/dL** | 156.57 ± 51.0 | 163.62 ± 56.64 | 172.38 ± 45.77 | 0.094a |
| PONase activity ‡ (U/ml) | 179.8 (53.3–355.0) | 102.0 (21.0–307.5)* | 23.26 (4.7–130.0)*† | 0.0001b |
| PONase/HDL-C ratio ‡ (U/mg) | 36.9 (8.2–82.0) | 25.2 (5.7–69.7)* | 6.3 (0.8–55.8)*† | 0.0001b |
** Values are mean ± SD
‡ Values are median (range)
* p < 0.05 versus Controls
†p < 0.05 versus CAD
aOne way Anova test
bKruskal wallis test
Biochemical Characteristics
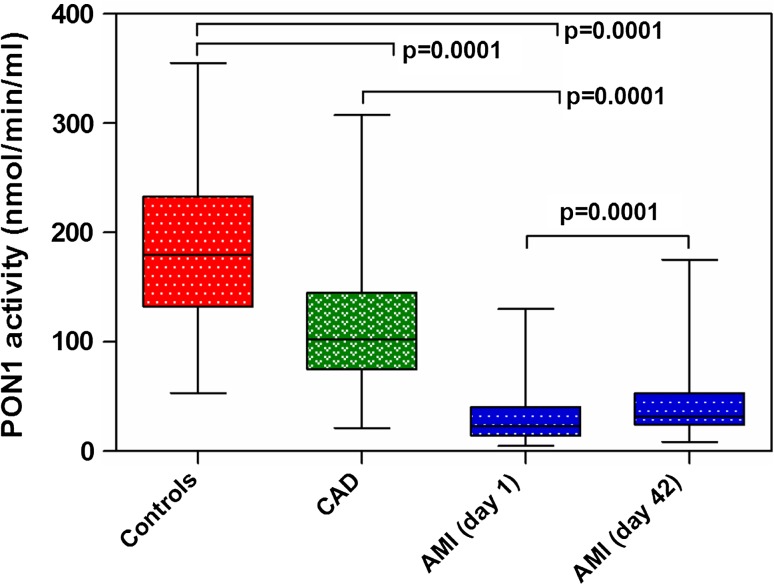
Serum PONase activity was lowest in AMI group (23.26 U/ml), intermediate in CAD group (102.0 U/ml), and highest in healthy controls (179.8 U/ml) with differences between all the three groups being statistically significant (p: AMI vs. CAD <0.05, AMI vs. controls <0.05 and CAD vs. controls <0.05) (Fig. 1). AMI and CAD patients had significantly lower HDL-C levels and lower PONase/HDL ratio as compared to healthy controls. However, no significant difference was observed between HDL-C levels of AMI and CAD patients. However LDL-C levels were significantly higher in AMI patients as compared to CAD patients and controls. Serum TG and T-CHO levels were similar across all the three study groups (Table 1).
Fig. 1.
Box plot showing the PONase activity of the study groups
PONase Activity at Follow up
Figure 1 shows PONase activity of AMI patients at the time of admission and at 6 weeks post AMI. The serum PONase activity was significantly higher at 6 weeks post AMI as compared to that at the time of AMI (49.4 %; p < 0.05). The serum T-CHO and LDL-C levels were significantly lower whereas HDL/PONase ratio was higher. However, no significant difference was observed in serum HDL-C and TG levels (Table 2).
Table 2.
Lipid profile and PONase activity at admission and 6 weeks post AMI
| AMI (day 1) | AMI (day 42) | p values | |
|---|---|---|---|
| HDL-C (mg/dl)** | 39.46 ± 8.6 | 38.32 ± 8.71 | 0.325b |
| LDL-C (mg/dl)** | 116.6 ± 40.2 | 73.8 ± 24.5 | 0.0001b |
| TG (mg/dl)** | 155.9 ± 82.7 | 140.3 ± 54.1 | 0.067b |
| T-CHO (mg/dl)** | 173.7 ± 44.25 | 135.38 ± 29.22 | 0.0001b |
| PONase/HDL-C ratio (U/mg) ‡ | 6.2 (0.8–55.8) | 8.7 (1.7–37.1) | 0.0001a |
** Values are mean ± SD
‡ Values are median (range)
aWilcoxon signed rank test
bPaired student t test
Factors Affecting PONase Activity
On univariate regression analysis, we observed that serum PONase activity was significantly lower in smokers (55.68 U/ml) as compared to those who did not smoke (121.50 U/ml; p = 0.0001). Similarly, it was lower in patients with low HDL-C levels (p = 0.0001) and higher LDL-C levels (p = 0.0001). Age, sex, alcohol consumption, hypertension, BMI, serum T-CHO and TG levels did not affect serum PONase activity (Table 3). However, on multiple linear regression analysis serum PONase activity was found to be independent of all the above variables (data not shown).
Table 3.
Factors affecting PONase activity
| No. of cases | PONase activity | p valuea | ||
|---|---|---|---|---|
| Age | <50 years | 99 | 114.0 (8.35–307.5) | 0.605 |
| ≥50 years | 201 | 95.6 (4.77–355.0) | ||
| Sex | Male | 270 | 98.25 (5.96–355.0) | 0.603 |
| Female | 30 | 96.56 (4.77–315.0) | ||
| Alcohol consumers | Yes | 47 | 98.50 (9.54–287.0) | 0.326 |
| No | 253 | 97.92 (4.77–355.0) | ||
| Smokers | Yes | 102 | 55.68 (8.35–305.0) | 0.0001 |
| No | 198 | 121.50 (4.77–355.0) | ||
| Hypertenion | Yes | 95 | 95.50 (4.77–307.5) | 0.148 |
| No | 205 | 104.0 (5.96–355.0) | ||
| BMI | <25 kg/m2 | 123 | 110.0 (4.77–349.0) | 0.236 |
| ≥25 kg/m2 | 177 | 95.8 (5.96–355.0) | ||
| Total cholesterol | <200 mg/dl | 239 | 98.5 (4.77–342.0) | 0.742 |
| ≥200 mg/dl | 61 | 95.5 (9.54–355.0) | ||
| HDL-C mg/dl | <40 (males) < 50 (females) | 123 | 66.80 (5.96–307.50) | 0.0001 |
| ≥40 (males) ≥ 50 (females) | 177 | 125.0 (4.77–355.0) | ||
| LDL-C | <100 mg/dl | 184 | 98.60 (4.77–315.0) | 0.0001 |
| ≥100 mg/dl | 116 | 95.80 (5.96–355.0) | ||
| TG | <150 mg/dl | 189 | 98.60 (4.77–307.5) | 0.906 |
| ≥150 mg/dl | 111 | 95.80 (5.96–355.0) |
Values are median (range)
aUnivariate regression analysis
AMI patients were subdivided into subgroups based on Killip’s severity staging (1, 2, 3 and 4) and site (Inferior vs. anterior/anterolateral) and whether it was ST elevation (STEMI vs. NSTEMI), and these subgroups were further analyzed. No significant difference in serum PONase activity was observed between the various subgroups (data not shown).
Drugs Affecting PONase Activity
We also assessed the effect of various drugs (aspirin, atorvastatin, beta blockers and ACE inhibitors) on PONase activity. The AMI group patients were divided into subgroups based on the doses of the medications being taken. Changes in PONase activity were calculated and were compared between various subgroups to determine if any of the drugs had a significant effect on PONase activity. No significant difference was observed between various subgroups (data not shown).
Discussion
Serum Paraoxonase 1, an aryl-dialkylphosphatase (EC: 3.1.8.1.) associated with HDL-C, has been shown to protect LDL-C from peroxidation. In addition, it also hydrolyzes the oxidized LDL-C, thereby offering protection from atherosclerosis [2]. In several studies it has been shown that patients with CAD have lower serum PONase activity as compared to healthy controls [2, 11, 28]. Further it has been observed to be even more lower in patients with AMI [29]. However, results are not uniform. Because of the conflicting results and lack of information in North-West Indian Punjabi’s with AMI, current study was undertaken.
In this study, we compared the serum PONase activity between healthy controls, patients with stable CAD and those with AMI together unlike previous studies. As they lie along the increasing severity spectrum of CAD, it can be postulated that serum PONase activity is inversely related to severity of CAD as it was highest in healthy controls, intermediate in stable CAD patients and lowest in AMI patients. The median serum PONase activity of AMI patients was 23.26 U/ml, which is much lower than in patients with stable CAD in a previous study (57.19 U/ml) by Singh et al. [30]. It is also lower as compared to that in other South East Asian [19, 23] as well as West Asian patients with AMI [21, 31–33]. The difference in serum PONase activity in AMI patients of different ethnicities could be due to their differing genetic makeup and possibly due to the differences in the life style and food habits.
We also assessed the various factors affecting the serum PONase activity. On univariate analysis we observed it to correlate with smoking, low HDL-C and high LDL-C. However on multiple linear regression analysis, we found that serum PONase activity was not affected by any of these conventional risk factors for CAD. This non-dependence of PONase activity on lipid parameters in this population has been shown in an earlier study by Gupta et al. [28]. At 6 weeks post AMI, there was no significant increase in HDL-C levels despite nearly 50 % increase in serum PONase activity further supporting that PONase activity can increase irrespective of lipids.
In the study by Sentürk et al. [32] serum PONase activity decreased as the severity of AMI increased (no. of coronary arteries involved and clinical severity based on Gensini scoring system). However, in our study, the PONase activity was similar across all the four Killip’s severity stages. We also could not observe any significant difference in activity between survivors and non-survivors. Though the difference could have resulted from different clinical scoring systems used in two studies, our study shows that serum PONase activity at time of AMI is not a predictor of severity in these patients.
The serum PONase activity was also independent of whether it was STEMI or NSTEMI, which is unlike the study by Serdar et al. [33], where serum PONase activity was higher in STEMI patients as compared to NSTEMI patients. However, three other studies undertaken in the same population later, did not support this and revealed no significant difference in serum PONase activity between STEMI and NSTEMI patients [21, 31, 32]. Study undertaken by Senturk et al. [31] showed no correlation between site of AMI and PONase activity and our results are similar to this study. The results suggest that though low serum PONase activity predisposes to AMI, it does not affect its site or severity. However, further studies are required to have a better understanding of this.
Patients of AMI were followed up till 6 weeks when serum PONase activity and lipids were studied again and it was observed that in AMI patients, PONase activity increased significantly even though it was still markedly lower as compared to the stable CAD patients.
In a prospective study undertaken earlier in AMI patients by Ayub et al. [29] where serum PONase activity was assessed immediately after AMI and at 6 weeks after AMI, showed similar results. It has been postulated that lower PONase activity in AMI patients could be because it decreased acutely at the time of AMI. It is quite possible that this sudden lowering of PONase activity in a population where low activity already existed predisposing them to CAD, led to acute event. The only definite way to prove or disprove this hypothesis would be to do a prospective population study.
We also assessed the effect of various cardiovascular drugs (statins, beta blockers, aspirin and ACE inhibitors) on change in PONase activity (PONase at Follow up—PONase activity at time of AMI). It was observed that none of them had any significant effect on serum PONase activity. In a study done by Nagila et al. [34] in Indian hyperlipidemic patients, showed that treatment with atorvastatin i.e. 10 mg for 3 months significantly increased PONase activity along with improvement in lipid parameters. Two other studies done on hyperlipidemic patients also revealed beneficial effects of hypolipidemic drugs (atorvastatin, gemfibrozil) on serum PONase activity [19, 20]. However, in all these studies, there were no patients with CAD or AMI. Effect of drug treatment of AMI on PONase activity was assessed by Sentürk et al. [32] and like in our study, they found no influence of drugs on PONase activity. In another study where in CAD patients, effect of 6 weeks rosuvastatin 10 mg/day and atorvastatin 20 mg/day on PONase activity was observed, it was observed that rosuvastatin but not atorvastatin significantly increased PONase activity, though both of them caused similar increases in HDL-C [24].
In summary, serum PONase activity was significantly lower in patients of AMI irrespective of age, sex, BMI, hypertension and lipid parameters like HDL-C and it increased with time after AMI in this population. Whether sudden lowering of PONase activity in a population which already has lower activity, is a risk factor for AMI as in this population, needs to be studied prospectively with adequate numbers.
Funding
Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. The funding authority had no role in study design, data collection, analysis, decision to publish or in preparation of the manuscript.
Electronic Supplementary Material
Acknowledgments
The study was carried out at Department of Biochemistry, PGIMER, Chandigarh, India. We are grateful to the volunteers involved in the study, laboratory staff and all those who contributed in terms of time and effort.
Contribution
Author contribution conceived and design the experiments: VNM, NG, KDG and SS. Performed the experiment: NG. Analyzed the data: NG. Contributed reagents/materials/analysis tools: KDG and YSP. Wrote the paper: NG, VNM, SS and KDG. Editing: VNM, NG, GG, SS and KDG.
Abbreviations
- PONase
Paraoxonase-1
- CAD
Coronary artery disease
- AMI
Acute myocardial infarction
- HDL-C
High density lipoprotein cholesterol
- LDL-C
Low density lipoprotein cholesterol
- T-CHO
Total-cholesterol
- TG
Triglycerides
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
Contributor Information
V. Nagarjuna Maturu, Email: arjunjipmer@yahoo.co.in.
Nidhi Gupta, Email: nidhi005@gmail.com.
Gagandip Singh, Email: gagan7130@yahoo.co.in.
Kirandip Gill, Email: kdgill2002@yahoo.co.in.
Yash Paul Sharma, Email: ypspgi@gmail.com.
Surjit Singh, Phone: +91-172-2756672, FAX: +91-0172-2744401, FAX: +91-0172-2275078, Email: surjit51@hotmail.com.
References
- 1.Salonen JT, Ylä-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, et al. Autoantibody against oxidized LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-T. [DOI] [PubMed] [Google Scholar]
- 2.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–480. doi: 10.1161/01.ATV.21.4.473. [DOI] [PubMed] [Google Scholar]
- 3.Cao H, Girard-Globa A, Berthezene F, Moulin P. Paraoxonase protection of LDL against peroxidation is independent of its esterase activity towards paraoxon and is unaffected by the Q–>R genetic polymorphism. J Lipid Res. 1999;40:133–139. [PubMed] [Google Scholar]
- 4.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 5.Khersonsky O, Tawfik DS. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry. 2005;44:6371–6382. doi: 10.1021/bi047440d. [DOI] [PubMed] [Google Scholar]
- 6.Aharoni A, Gaidukov L, Khersonsky O, Mc QGS, Roodveldt C, Tawfik DS. The ‘evolvability’ of promiscuous protein functions. Nat Genet. 2005;37:73–76. doi: 10.1038/ng1482. [DOI] [PubMed] [Google Scholar]
- 7.Richter RJ, Jarvik GP, Furlong CE. Paraoxonase 1 status as a risk factor for disease or exposure. Adv Exp Med Biol. 2010;660:29–35. doi: 10.1007/978-1-60761-350-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–392. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 9.Baum L, Ng HK, Woo KS, et al. Paraoxonase 1 gene Q192R polymorphism affects stroke and myocardial infarction risk. Clin Biochem. 2006;39:191–195. doi: 10.1016/j.clinbiochem.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Morray B, Goldenberg I, Moss AJ, et al. Polymorphisms in the paraoxonase and endothelial nitric oxide synthase genes and the risk of early-onset myocardial infarction. Am J Cardiol. 2007;99:1100–1105. doi: 10.1016/j.amjcard.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Mackness B, Davies GK, Turkie W, Lee E, Roberts DH, Hill E, et al. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001;21:1451–1457. doi: 10.1161/hq0901.094247. [DOI] [PubMed] [Google Scholar]
- 12.Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, et al. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1(192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol. 2000;20:2441–2447. doi: 10.1161/01.ATV.20.11.2441. [DOI] [PubMed] [Google Scholar]
- 13.McElveen J, Mackness MI, Colley CM, Peard T, Warner S, Walker CH. Distribution of paraoxon hydrolytic activity in the serum of patients after myocardial infarction. Clin Chem. 1986;32:671–673. [PubMed] [Google Scholar]
- 14.Mackness MI, Harty D, Bhatnagar D, Winocour PH, Arrol S, Ishola M, et al. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991;86:193–199. doi: 10.1016/0021-9150(91)90215-O. [DOI] [PubMed] [Google Scholar]
- 15.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, et al. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–2779. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 16.Troughton JA, Woodside JV, Yarnell JW, Arveiler D, Amouyel P, Ferrières J, et al. Paraoxonase activity and coronary heart disease risk in healthy middle-aged males: the PRIME study. Atherosclerosis. 2008;197:556–563. doi: 10.1016/j.atherosclerosis.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Birjmohun RS, Vergeer M, Stroes ES, Sandhu MS, Ricketts SL, Tanck MW, et al. Both paraoxonase-1 genotype and activity do not predict the risk of future coronary artery disease; the EPIC-Norfolk Prospective Population Study. PLoS ONE. 2009;4:e6809. doi: 10.1371/journal.pone.0006809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Himbergen TM, van der Schouw YT, Voorbij HA, van Tits LJ, Stalenhoef AF, Peeters PH, et al. Paraoxonase (PON1) and the risk for coronary heart disease and myocardial infarction in a general population of Dutch women. Atherosclerosis. 2008;199:408–414. doi: 10.1016/j.atherosclerosis.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Sivakanesan R, Nagtilak S. Serum paraoxonase activity in normolipidaemic patients with acute myocardial infarction. J Clin Diagn Res. 2008;2:1052–1056. [Google Scholar]
- 20.Kumar A, Nagtilak S, Sivakanesan R, Gunasekera S. Cardiovascular risk factors in elderly normolipidemic acute myocardial infarct patients–a case controlled study from India. Southeast Asian J Trop Med Public Health. 2009;40:581–592. [PubMed] [Google Scholar]
- 21.Kabaroglu C, Mutaf I, Boydak B, Ozmen D, Habif S, Erdener D, Parildar Z, et al. Association between serum paraoxonase activity and oxidative stress in acute coronary syndromes. Acta Cardiol. 2004;59:606–611. doi: 10.2143/AC.59.6.2005242. [DOI] [PubMed] [Google Scholar]
- 22.Lakshmy R, Ahmad D, Abraham RA, Sharma M, Vemparala K, Das S, et al. Paraoxonase gene Q192R & L55 M polymorphisms in Indians with acute myocardial infarction & association with oxidized low density lipoprotein. Indian J Med Res. 2010;131:522–529. [PubMed] [Google Scholar]
- 23.Mohammad PI, Abrar HK, Naseema M, Saleem PI. Human paraoxonase and HDL-Cholesterol in Pakistani patients with acute myocardial infarction and normal healthy adults. Pak J Med Sci. 2007;23:659–664. [Google Scholar]
- 24.Wang X, Fan Z, Huang J, Su S, Yu Q, Zhao J, Hui R, et al. Extensive association analysis between polymorphisms of PON gene cluster with coronary heart disease in Chinese Han population. Arterioscler Thromb Vasc Biol. 2003;23:328–334. doi: 10.1161/01.ATV.0000051702.38086.C1. [DOI] [PubMed] [Google Scholar]
- 25.MacKness B, Mackness MI, Durrington PN, et al. Paraoxonase activity in two healthy populations with differing rates of coronary heart disease. Eur J Clin Invest. 2000;30:4–10. doi: 10.1046/j.1365-2362.2000.00580.x. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Verma M, Nain CK, Leelamma CO, Goel RC. Paraoxonase (PON1) polymorphism & its relation with lipids in north west Indian Punjabis. Indian J Med Res. 1999;110:133–137. [PubMed] [Google Scholar]
- 27.Mackness B, Mackness MI, Arrol S, Turkie W, Julier K, Abuasha B, et al. Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non-insulin dependent diabetes mellitus. Atherosclerosis. 1998;139:341–349. doi: 10.1016/S0021-9150(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 28.Gupta N, Singh S, Maturu VN, Sharma YP, Gill KD. Paraoxonase 1 (PON1) polymorphisms, haplotypes and activity in predicting CAD risk in North-West Indian Punjabis. PLoS ONE. 2011;6:e17805. doi: 10.1371/journal.pone.0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:330–335. doi: 10.1161/01.ATV.19.2.330. [DOI] [PubMed] [Google Scholar]
- 30.Singh S, Venketesh S, Verma JS, Verma M, Lellamma CO, Goel RC. Paraoxonase (PON1) activity in north west Indian Punjabis with coronary artery disease & type 2 diabetes mellitus. Indian J Med Res. 2007;125:783–787. [PubMed] [Google Scholar]
- 31.Senturk T, Sarandol E, Gullulu S, Erdinc S, Ozdabakoglu O, Ozdemir B, Baran I, et al. Serum arylesterase activity is negatively correlated with inflammatory markers in patients with acute coronary syndromes. Saudi Med J. 2009;30:334–339. [PubMed] [Google Scholar]
- 32.Sentürk T, Sarandöl E, Güllülü S, Erdinç S, Ozdemir B, Baran I, et al. Association between paraoxonase 1 activity and severity of coronary artery disease in patients with acute coronary syndromes. Acta Cardiol. 2008;63:361–367. doi: 10.2143/AC.63.3.1020314. [DOI] [PubMed] [Google Scholar]
- 33.Serdar Z, Serdar A, Altin A, Eryilmaz U, Albayrak S. The relation between oxidant and antioxidant parameters and severity of acute coronary syndromes. Acta Cardiol. 2007;62:373–380. doi: 10.2143/AC.62.4.2022281. [DOI] [PubMed] [Google Scholar]
- 34.Nagila A, Permpongpaiboon T, Tantrarongroj S, Porapakkham P, Chinwattana K, Deakin S, et al. Effect of atorvastatin on paraoxonase1 (PON1) and oxidative status. Pharmacol Rep. 2009;61:892–898. doi: 10.1016/s1734-1140(09)70146-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.