Abstract
It is clear that cancer is one of the most mortal diseases in the world and the most prevalent among women is breast cancer. As hydroxyurea (HU)—a drug which is used in chemotherapy—has many adverse effects in long-term despite of its therapeutic properties, we made use of nano drug delivery technology in order to reduce adverse effects and increase therapeutic index. Thus, liposomation is a novel way in drug delivery systems. In this study a mixture of phosphatidylcholine and cholesterol was mixed and HU was added to the resultant mixture. The mean diameter of the nanoliposomal HU measured with the Zeta Sizer device (equal to 402.5 nm) and its encapsulation efficiency was 70.8 %. Besides, using dialysis, the pattern of drug release from nanoliposomes has been studied and the results showed that the drug release of nanoliposomal drug within 28 h was equal to 25.85 %. This study showed that the cytotoxicity effect of nanoliposomal drug is more than that of the standard drug.
Keywords: Breast cancer, Nano drug delivery, Hydroxyurea, Liposomal
Introduction
Of the major health problems in the world, cancer can be noted [1]. Breast cancer is the most common type of cancer among women worldwide [2]. It takes thousands of lives every year. Factors affecting fertility, environment, lifestyle and physical activity explain this trend [3]. Iran ranks first in breast cancers diagnosed among women [4]. Unfortunately, there is a shortage of studies on pathologic clinical features, stages and age distribution of this disease [5, 6].
Current therapies for the treatment of breast cancer can be surgery, radiation and chemotherapy [1]. Hydroxyurea (HU) medicine is used in the breast cancer treatment by chemotherapy. HU is an anti-cancer drug which is used in the treatment of hematologic malignancies, sickle cell anemia, myeloproliferative, breast cancer and other diseases [7–9]. Long-term use of HU is associated with adverse reactions in the blood and skin such as hyperpigmentation sclera of the eye, and atrophy of the skin with alopecia [10, 11]. Various methods have been used in order to reduce side effects and increase treatment efficiency.
Nanotechnology has created a revolution in cancer diagnosis and treatment [12]. Drug carriers at the nano scale are utilized for clinical applications of which the liposome could be mentioned. Liposomes are concentric phospholipid two layers vesicles of both hydrophilic and hydrophobic regions. Hydrophilic drugs are in the water box and hydrophobic and amphiphilic drugs are placed in bilayer phospholipids [13].
The aim of this study is to nanoliposome HU to improve therapeutic index and reduce its side effects.
Materials and Methods
Materials
Ethanol and isopropanol and RPMI 1640 medium were purchased from Merck Co. and Invitrogen Co., respectively. Cholesterol, HU, phosphatidylcholine and MTT solution (0.5 mg/ml) were purchased from Sigma Chemical Co., USA. MCF-7 cells were obtained from the Cell Bank Pasteur Institute of Iran.
Preparation of the Pegylated Nanoliposomal Drug
In order to prepare nanoliposome, phosphatidylcholine and cholesterol (10 to 1 ratio) were dissolved in 100 ml of 98 % ethanol (room temperature, 300 rpm), until a clear yellow suspension was achieved.
Ethanol was removed to prepare the nanoliposomal drug by using the rotary evaporator at 50 °C, 90 rpm (Company Heidolph, Germany), and then a defined volume of physiological serum and 8 mg HU was added to obtained gelos and mixed (room temperature, 24 h, 300 rpm). Also, the drug-free control was prepared for this formulation. Then the solution was sonicated for 5 min (model Bandelin Sonorex Digitec, 60 Hz) to reduce the size of nanoliposomes and increase drug loading efficiency.
Size Measurement of Nanoliposomes
The mean diameter of nanoliposomes was measured by Zeta Sizer device (Malvern Instruments Ltd).
Encapsulation Efficiency
To check the amount of encapsulated HU, 1 ml (0.32 mg) of the nanoliposomal formulation of HU was centrifuged (13,000 rpm at 4 °C) for 2 h. Then using a spectrophotometer (Model UV—1601PC company SHIMADZU), nanoliposomal drug supernatant absorbance was measured at 214 nm. Then by Formula 1, the encapsulation efficiency was calculated.
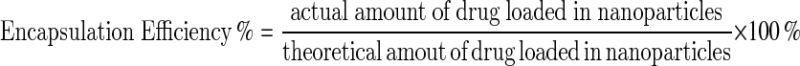 |
1 |
In order to plot the standard curve, different concentrations of HU were prepared and the absorbance was measured by spectrophotometry at 214 nm.
In Vitro Release Study
To review the nanoliposomal drug release, 0.32 mg of the nanoliposomal HU formulation was poured in dialysis bag then put in 100 ml phosphate buffered saline (PBS) pH 7.4 while it was placed on the magnetic stirrer (24 h at 37 °C). The amount of released drug in the PBS buffer was measured by spectrophotometry at 214 nm and release percentage of HU was obtained using the standard curve.
Toxicological Effects Evaluation of Drug Concentration
Hundred microliter of suspension containing 10,000 MCF-7 cells was poured in 96 well plates and incubated at 37 °C under CO2 5 % for 24 h. Then, the supernatant was removed and different concentrations of the nanoliposomal drug formulation and its control were decanted on the cells and incubated for 24 h in the above condition. Then the supernatant was removed and the MTT solution (0.5 mg/ml) was added. After 3 h of incubation, the amethyst crystal (formazan) was dissolved in 100 μl Isopropanol. The light absorbance at 570 nm wavelength was measured (spectrophotometer Model Power Eave XS) and the IC50 was calculated using the pharm software.
Statistical Analysis
The results are expressed as mean ± standard deviation (SD, n = 3). The data were statistically analyzed by one-way analysis of variance using IBM Statistics SPSS software version 19, and significant difference was set at p < 0.05.
Results
Size Measurement of Nanoliposomes
The mean diameter of liposomes for nanoliposomal HU was 402.5 nm.
Encapsulation Efficiency
Drug encapsulation percentage was calculated according to the standard curve. According to the drug loading efficiency formula (Formula 1), the amount of unencapsulated (free) drugs was calculated. Regarding the initial drug concentration of the prepared formulation, the percentage of encapsulated drug for the liposomal drug was obtained 70.8.
In Vitro Release Study
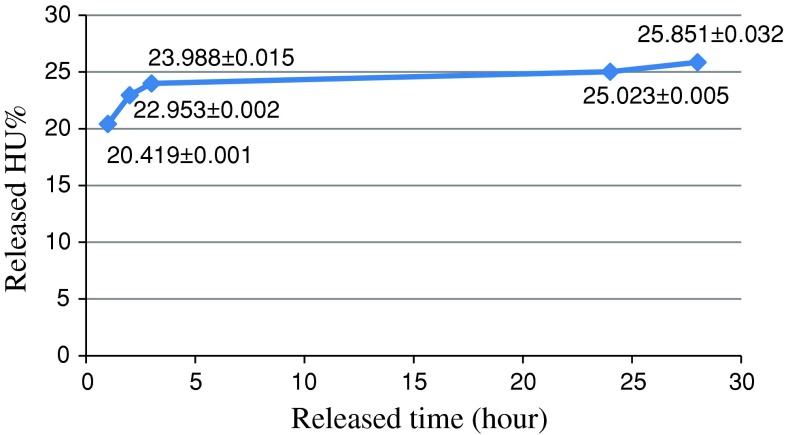
The amount of HU released from the prepared drug formulation in PBS buffer during time periods of 1, 2, 3, 24 and 28 h was obtained using the standard curve (Fig. 1). The amount of drug released in PBS buffer for the formulation of the nanoliposomal HU was 25.85 % after 28 h.
Fig. 1.
The release of HU from formulations liposomal HU in PBS buffer within 28 h
Evaluation Toxicological Effects of Drug Concentration
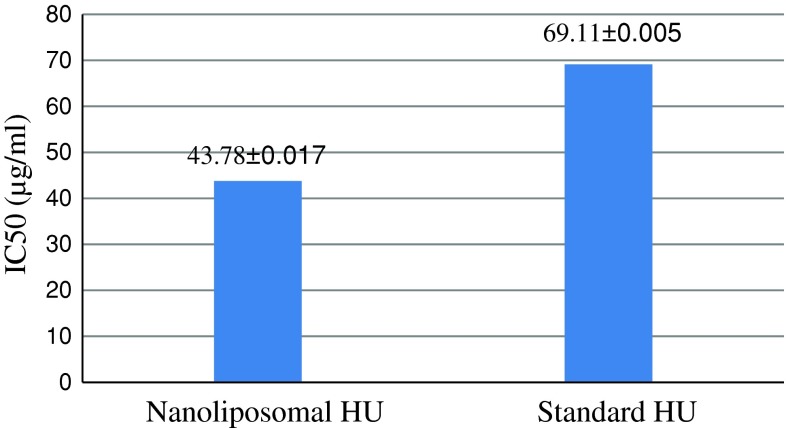
The cytotoxicity of the nanoliposomal HU formulation was investigated in different concentrations using MTT assay which results are shown in Fig. 2.
Fig. 2.
The values of IC50 (μg/ml) for nanoliposomal formulations of HU and standard HU
Discussion
Targeted delivery is a novel treatment to treat a variety of diseases; some of the targeted drug delivery is based on drug delivery using lipid nano-carriers. Liposome is a kind of these nano-carriers.
In 1996, Kirpotin et al. [14] research led to the construction of immunoliposomes containing anti-cancer drugs. In 2001, Cerald [15] studied cytotoxicity of encapsulated doxorubicin in the liposome on heart which results showed that the cardiotoxicity of liposomal doxorubicin was significantly decreased. In 2012, Chang et al. [16] in their study on the effect of the liposomal Curcuminoid drug on MCF-7, MDA-MB-435 and MDA-MB-23 cell lines concluded that the IC50 value of the liposomated Curcuminoid drug was lower than that of the non liposomated drug, so the survival percentage of cancer cells was decreased by liposomating this drug.
In the present study, some experiments have been designed to measure cell cytotoxicity of free form of HU drug and compare it with the liposomal form by MTT assay.
The results of the liposome diameter measurements using Zeta sizer device confirmed the particle size in the nano scale [17]. Also, investigating the drug loading efficiency showed that the encapsulation efficiency of the nanoliposomated HU drug was 70.836 %, which is fairly reasonable.
To determine the pattern of drug release at different times, the dialysis method was used. Nanoparticles of the prepared formulation for releasing encapsulated HU under in vitro conditions was detected (Fig. 1). HU release from nanoparticles was consisted of an initial phase of rapid release and then continued by the slow release phase. As can be seen in the Fig. 1 it could be said that most of the drug release was occurred in the first 2 h. The results showed that using nanoliposomes has a significant role in the drug release because the drug spends more time to pass through phospholipids layers.
The cytotoxic effect of nanoliposomal HU was perused by MTT assay. The results indicated that the IC50 belonged to nanoliposomal HU was less than that of standard drug. This phenomenon seems to stem from the effect of liposome on more stability and slower drug releasing of nanoliposomal formulation.
References
- 1.Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29:402–417. doi: 10.1016/j.biotechadv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Vo AT, Millis RM. Epigenetics and breast cancers. Obstet Gynecol Int. 2012;2012:602720. doi: 10.1155/2012/602720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathy NB, Yip CH, Taib NA, Hartman M, Saxena N, Iau P, et al. Singapore–Malaysia breast cancer working group. Breast cancer in a multi-ethnic asian setting: results from the Singapore–Malaysia hospital-based breast cancer registry. Breast. 2011;20:75–80. doi: 10.1016/j.breast.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6:359–363. [PubMed] [Google Scholar]
- 5.Harirchi I, Ebrahimi M, Zamani N, Jarvandi S, Montazeri A. Breast cancer in Iran: a review of 903 case records. Public Health. 2000;114:143–145. doi: 10.1038/sj.ph.1900623. [DOI] [PubMed] [Google Scholar]
- 6.Rezaianzadeh A, Peacock J, Reidpath D, Talei A, Hosseini SV, Mehrabani D. Survival analysis of 1148 women diagnosed with breast cancer in Southern Iran. BMC Cancer. 2009;9:168. doi: 10.1186/1471-2407-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, et al. Systematic review: hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meo A, Cassinerio E, Castelli R, Bignamini D, Perego L, Cappellini MD. Effect of hydroxyurea on extramedullary haematopoiesis in thalassaemia intermedia: case reports and literature review. Int J Lab Hematol. 2008;30:425–431. doi: 10.1111/j.1751-553X.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaccaria E, Cozzani E, Parodi A. Secondary cutaneous effects of hydroxyurea: possible pathogenetic mechanisms. J Dermatolog Treat. 2006;17:176–178. doi: 10.1080/09546630600780494. [DOI] [PubMed] [Google Scholar]
- 10.Sirieix ME, Debure C, Baudot N, Dubertret L, Roux ME, Morel P, et al. Leg ulcers and hydroxyurea: forty-one cases. Arch Dermatol. 1999;135:818–820. doi: 10.1001/archderm.135.7.818. [DOI] [PubMed] [Google Scholar]
- 11.Daoud MS, Gibson LE, Pittelkow MR. Hydroxyurea dermopathy: a unique lichenoid eruption complicating long-term therapy with hydroxyurea. J Am Acad Dermatol. 1997;36:178–182. doi: 10.1016/S0190-9622(97)70276-7. [DOI] [PubMed] [Google Scholar]
- 12.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. [DOI] [PubMed]
- 13.Riaz M. Liposomes preparation methods. Pak J Pharm Sci. 1996;9:65–77. [PubMed] [Google Scholar]
- 14.Kirpotin D, Park JW, Hong K, Zalipsky S, Li WL, Carter P, et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro. Biochemistry. 1997;36:66–75. doi: 10.1021/bi962148u. [DOI] [PubMed] [Google Scholar]
- 15.Cerald B. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin compared with conventional doxorubicin in randomized, multicente trial of mefustatic breast cancer. J Oncol. 2001;1444–54. [DOI] [PubMed]
- 16.Chang CC, Yang WT, Ko SY, Hsu YC. Liposomal curcuminoids for transdermal delivery: iontophoresis potential for breast cancer chemotherapeutics. Dig J Nanomater Biostruct. 2012;7:59–71. [Google Scholar]
- 17.Mansour HM, Rhee YS, Wu X. Nanomedicine in pulmonary delivery. Int J Nanomed. 2009;4:299–319. doi: 10.2147/IJN.S4937. [DOI] [PMC free article] [PubMed] [Google Scholar]