Women with human immunodeficiency virus (HIV)–1 subtype C had significantly higher genital tract viral loads compared to women with HIV-1 subtype B and men with HIV-1 subtype C or B. Women in general were significantly less likely to have genital tract viral load below the lower limit of quantification compared to men.
Keywords: HIV-1 genital tract RNA, HIV-1 subtypes B and C, antiretroviral drugs
Abstract
Background. Combination antiretroviral therapy (cART) reduces genital tract human immunodeficiency virus type 1 (HIV-1) load and reduces the risk of sexual transmission, but little is known about the efficacy of cART for decreasing genital tract viral load (GTVL) and differences in sex or HIV-1 subtype.
Methods. HIV-1 RNA from blood plasma, seminal plasma, or cervical wicks was quantified at baseline and at weeks 48 and 96 after entry in a randomized clinical trial of 3 cART regimens.
Results. One hundred fifty-eight men and 170 women from 7 countries were studied (men: 55% subtype B and 45% subtype C; women: 24% subtype B and 76% subtype C). Despite similar baseline CD4+ cell counts and blood plasma viral loads, women with subtype C had the highest GTVL (median, 5.1 log10 copies/mL) compared to women with subtype B and men with subtype C or B (4.0, 4.0, and 3.8 log10 copies/mL, respectively; P < .001). The proportion of participants with a GTVL below the lower limit of quantification (LLQ) at week 48 (90%) and week 96 (90%) was increased compared to baseline (16%; P < .001 at both times). Women were significantly less likely to have GTVL below the LLQ compared to men (84% vs 94% at week 48, P = .006; 84% vs 97% at week 96, P = .002), despite a more sensitive assay for seminal plasma than for cervical wicks. No difference in GTVL response across the 3 cART regimens was detected.
Conclusions. The female genital tract may serve as a reservoir of persistent HIV-1 replication during cART and affect the use of cART to prevent sexual and perinatal transmission of HIV-1.
Blood plasma and genital tract human immunodeficiency virus type 1 (HIV-1) RNA concentrations are predictors of risk for heterosexual [1, 2] and perinatal HIV-1 transmission [3–5], and differences in blood plasma viral load (BPVL) between men and women with similar CD4+ cell counts have been observed [6–11]. It is unknown whether there are sex-related differences in genital tract viral load (GTVL), because few clinical trials in which GTVL has been measured have enrolled sufficient numbers of both men and women. Some studies have suggested, however, that women are less likely to durably suppress GTVL compared to men [12, 13]. This may be related to known differences in antiretroviral (ART) penetration found between men and women and among different specific ART drugs [14, 15] or to differences in coinfections or the immunologic milieu between men and women (reviewed in [16]). Blood and seminal plasma (SP) viral loads (VLs) may be higher with HIV-1 subtype C infection compared with other subtypes [17–20] and might, in part, explain the rapid spread of HIV-1 in areas of the world where subtype C predominates. The effect of antiretroviral therapy on GTVL of different HIV-1 subtypes is unknown.
MATERIALS AND PARTICIPANTS
The AIDS Clinical Trials Group (ACTG) study A5185s was a substudy of the A5175, PEARLS Trial (Prospective Evaluation of Anti-retroviral Combinations for Treatment Naive, HIV Infected Persons in Resource-Limited Settings; ClinicalTrials.gov NCT00084136) [21]. Antiretroviral-naive participants with CD4+ lymphocytes <300/μL were randomly assigned (1:1:1) to 1 of 3 open-label combination antiretroviral (cART) regimens: efavirenz plus co-formulated lamivudine-zidovudine (EFV + 3TC/ZDV); or atazanavir plus didanosine EC plus emtricitabine (ATV + DDI + FTC); or efavirenz plus co-formulated emtricitabine-tenofovir DF (EFV + FTC/TDF). All participants meeting the entry criteria for PEARLS at participating sites were offered enrollment to A5185s and provided separate informed consent. A5185s was designed to evaluate differences in GTVL between men and women and between HIV-1 subtypes B and C, as well as the effect of cART on GTVL.
Specimen Collection and Processing
Genital secretions and blood plasma specimens were obtained at study entry and at weeks 48 and 96. For men, semen was collected at the clinic or within 2 hours prior to the clinic visit by masturbation following a prescribed abstinence of 48 hours. Following liquefaction, the total semen volume was determined and the sample centrifuged at 600g for 15 minutes. For women, a pelvic examination was performed with collection of 2 endocervical wicks (Tear-Flo Diagnostic Test Strip, Odyssey, Bartlett, TN). Each wick was cut at the “15” mark and the 2 distal round ends, which delivered 12 μL each, were placed into a 2-mL cryovial containing 500 μL of NASBA Buffer solution (bioMérieux, Durham, NC) and stored at −70°C. Women refrained from any kind of sexual activity, douching, or insertion of intravaginal products for at least 48 hours prior to the collection of cervical specimens. Genital specimens were processed within 4 hours of collection and stored at −70°C. Specimens were shipped to the University of Washington ACTG Virology Specialty Laboratory, Seattle, for HIV-1 RNA quantification.
Laboratory Testing
Blood plasma HIV-1 RNA and CD4+ cell counts were measured in PEARLS [21]. HIV-1 RNA was extracted from SP or cervical wicks (CWs) and quantified using an independently validated in-house real-time polymerase chain reaction assay, with a lower limit of quantification (LLQ) of 120 copies/mL (SP) and 1575 copies/mL (CW) and an upper limit of quantification of 5 000 000 (SP) and 52 500 000 copies/mL (CW). The difference in the LLQ between SP and CW was due to the requirement for sample dilution during specimen processing. We did not control for HIV-1 DNA; the assay precision was <0.24 log10 nucleic acid copies/mL for genital specimens from men and women [22, 23].
HIV-1 subtype was determined by DNA sequence analysis of reverse transcriptase and protease in 103 participants who were also included in a case-cohort study of virologic failures in the main study (S. Eshleman, unpublished observations). Of these, 29 had HIV-1 subtype B, 71 had C, 2 had F1, and 1 had R12. All 71 participants infected with subtype C were from a subtype C–dominant country, whereas 91% (29/32) of participants from a subtype B–dominant country were infected with subtype B. For the remaining 228 participants in the A5185s substudy, HIV-1 subtype was imputed using the dominant subtype in the country where the participant was enrolled. Overall, this resulted in 128 participants with subtype B, 200 with subtype C, and 3 with neither subtype C or B; the latter 3 were excluded from all analyses.
Statistical Analyses
Baseline characteristics were compared among the 4 sex/subtype groups using the Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical variables. The Spearman correlation was used to measure the association between GTVL and BPVL. A median regression model was used to compare baseline GTVL among sex/subtype groups by including main effects for sex and subtype as well as an interaction term for sex and subtype. The model was extended by including BPVL as a covariate to assess the association between GTVL and BPVL, and by also adding age and presence of a sexually transmitted infection (STI) to evaluate whether the interaction of sex and subtype persisted when adjusted for BPVL, age, and proportion with an STI. Change in the proportion of women with VL below the LLQ from baseline to weeks 48 and 96 was evaluated using the McNemar test. Logistic regression was used to evaluate differences according to sex/subtype and among randomized treatments at weeks 48 and 96 in the proportion of participants with GTVL below the LLQ.
RESULTS
Baseline Characteristics
Between May 2005 and November 2006, 328 individuals (158 men and 170 women; 128 infected with HIV subtype B and 200 with subtype C) of the 1571 (21%) total PEARLS participants enrolled in A5185s and provided baseline genital secretions. Participants were from 16 sites in 7 countries. The distribution of men and women varied across countries. Enrollment in Brazil, India, Peru, and the United States included more men than women, whereas enrollment in the African countries included more women than men (Table 1). Thus, men were predominantly enrolled in countries in which the dominant subtype is B and women in countries in which the dominant subtype is C. Among men, 87 (55%) had subtype B and 71 (45%) had subtype C, whereas among women, 41 (24%) had subtype B and 129 (76%) had subtype C (Table 1). Selected baseline characteristics of participants for each subtype by sex are shown in Table 1.
Table 1.
Selected Baseline Characteristics by Sex and HIV-1 Subtype
| Sex and Subtype |
||||||
|---|---|---|---|---|---|---|
| Characteristic | Male Subtype B (n = 87) | Male Subtype C (n = 71) | Female Subtype B (n = 41) | Female Subtype C (n = 129) | Total (N = 328) | P Value |
| Age, y | ||||||
| Median | 34 | 34 | 33 | 32 | 33 | .0682a |
| Q1, Q3 | 29, 42 | 30, 40 | 27, 40 | 27, 37 | 28, 39 | |
| Country | ||||||
| Brazil | 40 (46%) | 0 (0%) | 12 (29%) | 0 (0%) | 52 (16%) | <.0001b |
| India | 0 (0%) | 19 (27%) | 0 (0%) | 9 (7%) | 28 (9%) | |
| Malawi | 0 (0%) | 36 (51%) | 0 (0%) | 82 (64%) | 118 (36%) | |
| Peru | 26 (30%) | 0 (0%) | 19 (46%) | 0 (0%) | 45 (14%) | |
| South Africa | 0 (0%) | 11 (15%) | 0 (0%) | 27 (21%) | 38 (12%) | |
| United States | 21 (24%) | 0 (0%) | 10 (24%) | 0 (0%) | 31 (9%) | |
| Zimbabwe | 0 (0%) | 5 (7%) | 0 (0%) | 11 (9%) | 16 (5%) | |
| Screening CD4+ count, cells/mm3 | ||||||
| Median | 196 | 189 | 171 | 193 | 193 | .9002a |
| Q1, Q3 | 90, 259 | 121, 248 | 121, 234 | 133, 237 | 123, 243 | |
| Plasma HIV-1 viral load, log10 copies/mL | ||||||
| Median | 5.0 | 5.0 | 4.9 | 4.9 | 4.9 | .1722a |
| Q1, Q3 | 4.5, 5.4 | 4.6, 5.5 | 4.5, 5.3 | 4.5, 5.2 | 4.5, 5.3 | |
| Genital secretion HIV-1 viral load, log10 copies/mL | ||||||
| Median | 3.8 | 4.0 | 4.0 | 5.1 | 4.4 | <.0001a |
| Q1, Q3 | 2.7, 4.7 | 3.0, 4.8 | 3.2, 4.9 | 4.4, 5.5 | 3.4, 5.2 | |
| Sexually transmitted infection | ||||||
| Yes | 30 (34%) | 0 (0%) | 7 (17%) | 4 (3%) | 41 (13%) | <.0001b |
| No | 57 (66%) | 71 (100%) | 34 (83%) | 125 (97%) | 287 (88%) | |
| Randomized treatment group | ||||||
| ZDV/3TC + EFV | 21 (24%) | 26 (37%) | 12 (29%) | 42 (33%) | 101 (31%) | .3166b |
| DDI + FTC + ATV | 30 (34%) | 26 (37%) | 18 (44%) | 40 (31%) | 114 (35%) | |
| TDF/FTC + EFV | 36 (41%) | 19 (27%) | 11 (27%) | 47 (36%) | 113 (34%) | |
Abbreviations: 3TC, lamivudine; ATV, atazanavir; DDI, didanosine; EFV, efavirenz; FTC, emtricitabine; HIV-1, human immunodeficiency virus type 1; TDF, tenofovir; ZDV, zidovudine.
a Kruskal-Wallis test.
b χ2 test.
The median age for participants in the substudy was 33 years (25th–75th percentiles, 28–39 years; Table 1), varying between 32 and 33 years for women with subtypes C and B, to 34 for men with either subtype (P = .068). The median screening CD4+ cell count was 193 cells/μL (25th–75th percentiles, 123–243 cells/μL; Table 1) with no significant difference among the 4 sex/subtype groups (P = .90). The median baseline blood plasma HIV-1 RNA was 4.9 (25th–75th percentiles, 4.5–5.3) log10 copies/mL, also with no significant difference among the 4 sex/subtype groups (P = .17).
The proportion of participants with STIs reported at baseline also varied significantly among the 4 sex/subtype groups (P < .0001), reflecting higher prevalence among participants with subtype B (34% for men and 17% for women) than subtype C (0% for men and 3% for women). Among the 328 participants with complete baseline GTVL, there were 50 STIs reported among 41 participants (30 males with subtype B, 7 females with subtype B, and 4 females with subtype C). Of these 50 events, 19 were syphilis, 15 were genital warts, 11 were gonorrhea, 2 were trichomoniasis, 2 were unspecified venereal disease, and 1 was chlamydial infection. There was no significant difference in baseline GTVL between those with and without STIs (P = .107). These results, however, should be interpreted with caution given the small number of female participants with STIs.
Baseline GTVL by Subtype and Sex
HIV-1 RNA was detected in 99% of baseline blood samples, but in only 82% of SP samples and 86% of CW samples. The correlation of baseline BPVL and GTVL was 0.38 for men and 0.27 for women (P < .001 for both). There was no evidence that the association between GTVL and BPVL varied by sex or subtype; in regression analysis, median GTVL increased by 0.58 log10 copies/mL for each 1 log10 copies/mL increase in BPVL (95% confidence interval [CI], .38–.77 log10 copies/mL).
There was a significant difference in GTVL among the 4 sex/subtype groups (P < .001; Table 1). Women with subtype C had the highest median GTVL (5.1 log10 copies/mL) whereas women with subtype B and men with subtype C or B had similar median GTVL (4.0, 4.0, and 3.8 log10 copies/mL, respectively). The difference in median GTVL between men and women varied significantly according to subtype (sex by subtype interaction P = .030). The estimated difference between men and women with subtype B was not significant (difference in medians: 0.27 log10 copies/mL higher for women; 95% CI, −.34 to .88; P = .38), whereas there was a significant difference between men and women with subtype C (1.03 log10 copies/mL higher for women; 95% CI, .77–1.29; P < .001). The higher GTVL for subtype C was not driven by one particular country: the median GTVL for men and for women with subtype C was 4.0 and 5.1 log10 copies/mL in Malawi, 4.0 and 5.0 log10 copies/mL in South Africa, 4.1 and 5.4 log10 copies/mL in India, and 4.8 and 5.0 log10 copies/mL in Zimbabwe. The difference in median GTVL according to sex/subtype persisted in analysis adjusted by BPVL, age, and STI at baseline (interaction P = .037), and so was not explained by the observed differences in BPVL, age, and proportion with an STI according to sex/subtype.
Effect of cART on GTVL
Among the 328 participants with baseline GTVL measurements, 40 (12%; 17 men and 23 women) were lost to follow-up or died before 96 weeks of follow-up. A total of 269 participants (82%; 142 men and 127 women) had evaluable results at week 48, and 250 participants (76%; 126 men and 124 women) had results at week 96. Two hundred twenty-nine participants (70%; 122 men and 107 women) had results at all 3 time points.
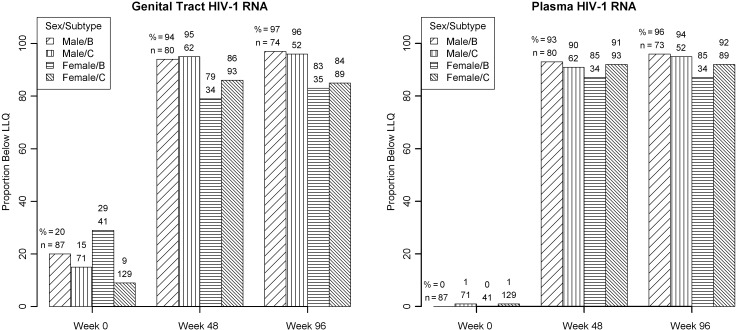
Overall, 52 (16%) of the substudy participants had GTVL below the assay LLQ (120 copies/mL for men and 1575 copies/mL for women) at baseline. Of the 87 and 71 men with subtypes B and C, 17 (20%) and 11 (15%) had a baseline GTVL below the LLQ (P = .51), and the corresponding figures for the 41 and 129 women with subtypes B and C were 12 (29%) and 12 (9%), respectively (P = .001; Figure 1).
Figure 1.
Proportion of participants with genital tract and blood plasma human immunodeficiency virus type 1 (HIV-1) RNA levels below the assay lower limit of quantification by weeks after starting antiretroviral therapy by sex/HIV-1 subtype. Abbreviations: HIV-1, human immunodeficiency virus type 1; LLQ, lower limit of quantification.
The proportion of participants with a GTVL below the LLQ at week 48 (90%) and week 96 (90%) was significantly increased compared to baseline (P < .001 at both times). However, women were significantly less likely than men to have GTVL below the LLQ at both times despite the fact that the LLQ for CW samples was >10 times higher than that for SP samples. There was no evidence that the difference between men and women varied by subtype (interaction P = .83 and P = .68 at weeks 48 and 96, respectively). Specifically, at week 48, 84% of women and 94% of men had GTVL below the LLQ (P = .006 comparing women to men, adjusted for subtype; Figure 1). The corresponding proportions at week 96 were 84% and 97% (P = .002). In contrast, only 2 (1%) had a BPVL below the assay LLQ (400 copies/mL) at baseline. For weeks 48 and 96, 91% and 93%, respectively, of participants had BPVL below the LLQ with similar results in each of the 4 sex/subtype groups (Figure 1). There was no significant difference among the 3 treatment groups in the proportion of participants with GTVL below the LLQ at week 48 (P = .57) or week 96 (P = .73) (data not shown).
Effect of Hormonal Contraception
Information on hormonal contraception was available for 168 women. At baseline, 30 women (18%) had been on hormonal contraception for at least 14 days, primarily on medroxyprogesterone (73%). The median GTVL among these women was 5.0 log10 copies/mL compared with 4.9 log10 copies/mL among women not on hormonal contraception or on hormonal contraception for <14 days (P = .49). There was no evidence that the difference between these 2 groups depended on HIV-1 subtype (interaction P = .30). There were also no significant differences in median age, baseline CD4 count, or baseline BPVL between these 2 groups. At weeks 48 and 96, the proportion of women on hormonal contraception had increased to 63% and 69%, respectively, with about three-quarters of these on medroxyprogesterone. At weeks 48 and 96, 84% (67 of 80 and 71 of 85, respectively) of women on hormonal contraception had GTVL less than the LLQ compared with 85% (39 of 46 and 33 of 39, respectively) not on hormonal contraception (P = .88 at both times).
DISCUSSION
This study demonstrates that despite similar BPVL between men and women and between HIV subtypes B and C, GTVLs were significantly higher at baseline in women infected with subtype C compared to women infected with subtype B and men infected with either subtype. The relationship between GTVL and subtype among women was not associated with hormonal contraception use or STIs, and women with either subtype were significantly less likely to have GTVL below the LLQ at weeks 48 and 96.
In a study of early HIV infection in 188 women, Morrison et al [19] found that Zimbabwean (subtype C) infection was associated with increased cervical VLs compared to Ugandan women infected with subtypes A or C. Women infected with subtype D also had increased cervical VLs, but this was not significant [19]. However, VLs in these women were much lower (mean cervical HIV RNA = 1.64 log10 copies/mL) compared to those observed in this study (mean female GTVL = 4.79 log10 copies/mL).
In a large study of heterosexual HIV-1–serodiscordant couples from 7 African countries (n = 2521) with median CD4+ cell count of 496, median endocervical VLs of 3.20 log10 copies/mL (n = 1805) and 2.57 log10 copies/mL in SP (n = 716) were reported by Baeten et al [24]. These values are much lower than those observed in our study (median female GTVL, 4.92 log10 copies/mL; median SP VL, 3.91 log10 copies/mL). Additionally, although the same laboratory tested genital secretions from both Baeten et al and this study, sampling techniques for the women differed.
Antiretrovirals were very effective in decreasing VLs in both compartments in all participants. At weeks 48 and 96, 89% and 90%, respectively, of all participants had BPVLs that were below the limit of quantification. However, in this study, men were significantly more apt to have a GTVL that was below the limit of quantitation, regardless of subtype and in spite of the difference in the LLQ of the assay for SP (120 copies/mL) compared to the female genital tract (1575 copies/mL). Similar results have been reported previously [12, 13]. Differences in genital tract drug penetration between men and women [14, 15] might explain this finding. Of the drugs used in the study, TDF, 3TC, FTC, DDI, and ZDV have higher seminal plasma-to-blood plasma ratios whereas levels of EFV and ATV are negligible. In women, FTC, 3TC, and ZDV accumulate in the genital tract, whereas ATV and EFV have lower levels in the genital tract than in blood. Blood plasma to cervical secretion ratios for DDI and TDF have varied [15]. The 3 randomized treatment groups had comparable proportions of participants with VLs that were below the limit of quantification both at weeks 48 and 96.
Recently, Cohen et al [25] reported that early initiation of ART reduces the rate of sexual transmission in people with a CD4+ cell count between 350 and 550 cells/µL. Baeten et al [24] observed that genital HIV RNA independently predicted transmission after adjusting for BPVL. In the absence of ART, 59%–67% of the genetically linked transmissions in these 2 studies have been from women to men. Because participants in our study were more immunocompromised (median CD4+ cell count, 194) and GTVL was 1.3–1.7 log10 copies/mL greater than those in the Baeten et al study, the risks for sexual transmission are presumably greater in the absence of cART.
The strengths of the present study are the relatively large number of participants from ethnically and geographically diverse areas of the world infected with different HIV-1 subtypes. These locations coincide with all but 2 of the sites where PEARLS was conducted and with 6 of 13 HPTN 052 sites [25]. However, A5185s study participants had <300 CD4 cells/μL at entry and are thus more representative of individuals starting antiretrovirals under current World Health Organization treatment guidelines. The same laboratory performed the genital tract HIV RNA assays for the Partners in Prevention HSV/HIV Transmission Study [25], minimizing one potential confounder when trying to make comparisons between studies.
Our study has several important limitations. Participation in A5185s was voluntary and, as a consequence, there may be some selection bias. Assignment of subtype for 69% of the participants was based on country of residence, and there may have been some misclassification. However, we think the likelihood of misclassification is small, as all participating countries except Brazil have a dominant subtype comprising 96%–100% of the sequences for that country [26]. Additionally, there was a nonrandom distribution of HIV subtypes between men and women, and the differences observed were largely driven by the higher GTVL observed in women infected with subtype C from all countries. There were differences in the lower limit of detection of the GTVL assays, although that does not explain the significantly higher GTVL observed in women. We did not measure or control for HIV proviral DNA, which might have contributed to the higher GTVL found in women. Although GTVL can be affected by STIs and bacterial vaginosis, few STIs were recorded and information on bacterial vaginosis was not collected. Unmeasured coinfections and inflammation in women may have led to higher GTVL measurements.
In summary, we have demonstrated that GTVL differs according to sex and HIV subtype, with higher levels in women with subtype C virus. Moreover, women are less likely to suppress GTVL after initiating cART. The female genital tract may serve as a reservoir of persistent HIV-1 replication during cART and affect the use of cART to prevent sexual and perinatal transmission of HIV-1.
Notes
Acknowledgments. The authors thank the A5185s study participants who volunteered their time and efforts, and acknowledge the contributions of the following A5185s investigators: Karin L. Klingman, MD, National Institutes of Health, Bethesda; Apsara Nair, MS, Frontier Science and Technology Research Foundation, Amherst; Ann Walawander, MA, Frontier Science & Technology Research Foundation, Amherst; Laura M. Smeaton, MS, Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston; Victor De Gruttola, PhD, Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston; Ana I. Martinez, RPh, National Institutes of Health, Bethesda; Edith Swann, PhD, National Institutes of Health, Bethesda; Ronald L. Barnett, PhD, ACTG Operations Center, Social & Scientific Systems, Inc, Silver Spring; Barbara Brizz, BSN, MHSEd, ACTG Operations Center, Social & Scientific Systems, Inc. Silver Spring; Yvette Delph, MD, ACTG Operations Center, Social & Scientific Systems, Inc, Silver Spring; Nikki Gettinger, MPH, ACTG Operations Center, Social & Scientific Systems Inc, Silver Spring; Ronald T. Mitsuyasu, MD, UCLA CARE Center, Los Angeles; Susan Eshleman, MD, Johns Hopkins University, Baltimore; Steven Safren, PhD, Harvard Medical School, Boston; Adriana Andrade, MD, MPH, Division of Infectious Diseases, John Hopkins University, Baltimore; David W. Haas, MD, Infectious Diseases, Vanderbilt University, Nashville; Farida Amod, MBChB, FCPath, FCP, Department of Medicine, Nelson R. Mandela School of Medicine, Durban; Vladimir Berthaud, MD, Infectious Disease, Vanderbilt University Medical Center, Nashville; Robert C. Bollinger, MD, Division of Infectious Diseases, John Hopkins University, Baltimore; Yvonne Bryson, MD, Pediatric Infectious Disease Department, UCLA School of Medicine, Los Angeles; David Celentano, ScD, MHS, Department of Epidemiology, Johns Hopkins School of Hygiene and Public Health, Baltimore; David Chilongozi, CO, MPH, UNC HIVNET, UNC Project, Lilongwe, Malawi; Myron Cohen, MD, University of North Carolina, Chapel Hill; Ann C. Collier, MD, University of Washington, ACTU, Harborview Medical Center, Seattle; Judith Silverstein Currier, MD, MSc, University of California, Los Angeles; Joseph Eron, MD, Division of Infectious Diseases, Department of Medicine, University of North Carolina; Cynthia Firnhaber, MD, University of the Witwatersrand, South Africa; Charles Flexner, MD, Johns Hopkins University Hospital, Baltimore; Joel E. Gallant, MD, MPH, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore; Roy M. Gulick, MD, MPH, The Cornell Clinical Trials Unit, New York; Scott M. Hammer, MD, Division of Infectious Diseases, Columbia Presbyterian Medical Center, NY; Irving Hoffman, PA, MPH, University of North Carolina, Chapel Hill; Peter Kazembe, MBCHB, FRCP(C), Baylor College of Medicine-Abbott Fund Children's Clinical Centre of Excellence, Lilongwe, Malawi; Johnstone Kumwenda, MBBS, College of Medicine, Blantyre, Malawi; Newton Kumwenda, MPH, PhD, Johns Hopkins Project, Malawi; Javier R. Lama, MD, MPH, Investigaciones Medicas en Salud (INMENSA), Lima, Peru; Jody Lawrence, MD, University of California, San Francisco, Adult AIDS Clinical Trials Unit; Chiedza Maponga, PharmD, DaTIS, Medical University of Zimbabwe; Francis Martinson, MD, UNC Project, Lilongwe; Kenneth Mayer, MD, Division of Infectious Diseases, Brown University School of Medicine, Memorial Hospital of Rhode Island, Pawtucket; Karin Nielsen, MD, UCLA School of Medicine, Los Angeles; Richard B. Pendame MD, MPH, Malawi; Bharat Ramratnam, MD, Laboratory of Retrovirology, Division of Infectious Diseases, Brown University Medical School, Providence; James F. Rooney, MD, Gilead Sciences, Inc, Foster City; Jorge Sanchez, MD, Asociación Civil Impacta Salud y Educación, Lima, Peru; Ian Sanne, University of Witwatersrand, Johannesburg, South Africa; Robert T. Schooley, MD, University of California, San Diego; Wendy Snowden, GlaxoSmithKline, Research Triangle Park; Suniti Solomon, MD, YRG Centre for AIDS Research and Education, India; Steve Tabet, MD, University of Washington, Harborview Medical Center, Seattle; Taha Taha, MD, Johns Hopkins University, School of Hygiene and Public Health, Baltimore; Jonathan Uy, MD, Bristol-Myers Squibb Company, Plainsboro; Charles van der Horst, MD, Department of Medicine, University of North Carolina, Chapel Hill; Christine Wanke, MD, Tufts University School of Medicine, Boston; Joan Gormley, BSN, The Miriam Hospital, Immunology Center, Providence; Cheryl J. Marcus, RN, BSN, University of North Carolina, Chapel Hill; Beverly Putnam, RN, MSN, University of Colorado Health Sciences, Denver; Smanga Ntshele, Community Advisory Board Member, Durban; Edde Loeliger, MD, Clinical Development and Medical Affairs, Greenford, Middlesex; Keith A. Pappa, PharmD, GlaxoSmithKline, Infectious Diseases Medicine, Triangle Park; Nancy Webb, MS, Frontier Science and Technology Research Foundation, Inc, Amherst; David L. Shugarts, MA, University of Colorado Health Sciences, Denver; Mark A. Winters, MS, Stanford University Medical Center, Division of Infectious Disease, Stanford; Renard S. Descallar, Joseph Steele, Michael Wulfsohn, Farideh Said, Yue Chen, John C Martin, Norbert Bischofberger, Andrew Cheng, and Howard Jaffe, MD, Gilead Sciences, Foster City; Jabin Sharma, MHS, YRG Centre for AIDS Research and Education, India; S. Poongulali, MBBS, DGO, MSc, YRG Centre for AIDS Research and Education, Chennai, India; Sandra Wagner Cardoso, Instituto de Pesquisa Clinica Evandro Chagas-Fiocruz, Brazil; Deise Lucia Faria, Instituto de Pesquisa Clinica Evandro Chagas-Fiocruz, Brazil; Sima Berendes, MD, College of Medicine, Blantyre, Malawi; Kelly Burke, MPH, Blantyre, Malawi; Cecelia Kanyama, MBBS, Kamuzu Central Hospital, Lilongwe, Malawi; Virginia Kayoyo, Kamuzu Central Hospital, Lilongwe, Malawi; Wadzanai P. Samaneka, MD, University of Zimbabwe College of Health Sciences, Harare; Anthony Chisada, MD, University of Zimbabwe College of Health Sciences, Harare; Breno Santos, MD, Hospital Conceicao, Porto Alegre, Brazil; Alberto La Rosa, MD, Asociacion Civil Impacta Salud y Educacion, Miraflores, Lima, Peru; Rosa Infante, MD, Investigaciones Medicas en Salud, INMENSA, Lima, Peru; Henry H. Balfour, MD, University of Minnesota, Minneapolis; Beth Mullan, University of Minnesota, Minneapolis; Ge-Youl Kim, BSN; Washington University, St Louis, Missouri; Michael K. Klebert, PhD, RN, ANP-BC, Washington University, St Louis, Missouri; Donna Mildvan, MD, Beth Israel Medical Center, New York; Manuel Revuelta, MD, Beth Israel Medical Center, New York; P. Jan Geiseler, MD, University of Southern California; Los Angeles; Bartolo Santos, RN, University of Southern California, Los Angeles; Eric S. Daar, MD, Harbor–UCLA, Los Angeles; Ruben Lopez, MD, Harbor–UCLA, Los Angeles; Laurie Frarey, ANP-BC, University of North Carolina, Chapel Hill; David Currin, RN, CCRC, University of North Carolina, Chapel Hill; David H. Haas, MD, Vanderbilt University, Nashville; Vicki L. Bailey, RN, Vanderbilt University, Nashville; Pablo Tebas, MD, Hospital of the University of Pennsylvania, Philadelphia; Larisa Zifchak, RN, Hospital of the University of Pennsylvania, Philadelphia; Beverly E. Sha, MD, Rush University Medical Center, Chicago; Janice M. Fritsche, MS, APRN, BC, Rush University Medical Center, Chicago. We thank the members of the National Institute of Allergy and Infectious Diseases (NIAID) multinational drug monitoring safety board for their careful oversight and thoughtful recommendations during the design and conduct of this study.
Author contributions. All authors were fully responsible for all aspects of manuscript development.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health.
Financial support. The project described was supported by the NIAID (award number U01AI068636, AI68634, and AI38858), and by the National Institute of Mental Health and the National Institute of Dental and Craniofacial Research. The pharmaceutical sponsors (Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline) provided study drug, and Gilead Sciences provided funding to purchase study drug that was not otherwise available. Sites were supported by the following grants from the NIAID: AI069424, AI069428, AI027661, AI069495, AI069471, AI046370, AI069423, AI069439, AI69467, AI069463, AI069438, AI069432, AI069436, AI069518, and AI069476-01. Additional funding was provided by the University of Washington Center for AIDS Research, a National Institutes of Health–funded program (P30 AI027757), which is supported by the NIAID, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, and National Center for Complementary and Alternative Medicine.
Potential conflicts of interest. M. D. H. has served as a paid data monitoring committee member for Boehringer Ingelheim, Medicines Development, Pfizer, and Tibotec. S. A. F. has been a paid consultant for Gen-Probe, ViiV Healthcare, and bioMONTR. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 2.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17:901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 4.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385–93. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 5.John GC, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–12. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 6.Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 7.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–72. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 8.Napravnik S, Poole C, Thomas JC, Eron JJ., Jr Gender difference in HIV RNA levels: a meta-analysis of published studies. J Acquir Immune Defic Syndr. 2002;31:11–9. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly CA, Bartley LM, Ghani AC, et al. Gender difference in HIV-1 RNA viral loads. HIV Med. 2005;6:170–8. doi: 10.1111/j.1468-1293.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 10.Lingappa JR, Kahle E, Mugo N, et al. Partners HSV-2/HIV-1 Transmission Study Team. Characteristics of HIV-1 discordant couples enrolled in a trial of HSV-2 suppression to reduce HIV-1 transmission: the Partners study. PLoS One. 2009;4:e5272. doi: 10.1371/journal.pone.0005272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grinsztejn B, Smeaton L, Barnett R, et al. PEARLS study team of the ACTG. Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4(+) T-cell count. Antivir Ther. 2011;16:1057–62. doi: 10.3851/IMP1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Günthard HF, Havlir DV, Fiscus S, et al. Residual human immunodeficiency virus (HIV) type 1 RNA and DNA in lymph nodes and HIV RNA in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J Infect Dis. 2001;183:1318–27. doi: 10.1086/319864. [DOI] [PubMed] [Google Scholar]
- 13.Cu-Uvin S, DeLong AK, Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–97. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 14.Nicol MR, Kashuba AD. Pharmacologic opportunities for HIV prevention. Clin Pharmacol Ther. 2010;88:598–609. doi: 10.1038/clpt.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther. 2011;16:1149–67. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 16.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type 1 infection in the genital tract of men and women. AIDS. 2003;17:455–80. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 17.Dyer JR, Kazembe P, Vernazza PL, et al. High levels of human immuno-deficiency virus type 1 in blood and semen of seropositive men in sub-Saharan Africa. J Infect Dis. 1998;177:1742–6. doi: 10.1086/517436. [DOI] [PubMed] [Google Scholar]
- 18.Neilson JR, John GC, Carr JK, et al. Subtypes of human immunodeficiency virus type 1 and disease stage among women in Nairobi, Kenya. J Virol. 1999;73:4393–403. doi: 10.1128/jvi.73.5.4393-4403.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison CS, Demers K, Kwok C, et al. Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS. 2010;24:573–82. doi: 10.1097/QAD.0b013e32833433df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novitsky V, Ndung'u T, Wang R, et al. Extended high viremics: a substantial fraction of individuals maintain high plasma viral RNA levels after acute HIV-1 subtype C infection. AIDS. 2011;25:1515–22. doi: 10.1097/QAD.0b013e3283471eb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell TB, Smeaton LM, Kumarasamy N, et al. PEARLS study team of the ACTG. Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: a randomized clinical trial in diverse multinational settings. PLoS Med. 2012;9:e1001290. doi: 10.1371/journal.pmed.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baeten JM, Strick LB, Lucchetti A, et al. Herpes simplex virus (HSV)–suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuckerman RA, Lucchetti A, Whittington WL, et al. HSV suppression reduces seminal HIV-1 levels in HIV-1/HSV-2 co-infected men who have sex with men. AIDS. 2009;23:479–83. doi: 10.1097/QAD.0b013e328326ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeten JM, Kahle E, Lingappa JR, et al. Partners in Prevention HSV/HIV Transmission Study Team. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen MS, Chen YQ, McCauley M, et al. HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Los Alamos National Laboratory. HIV Sequence Database. http://www.hiv.lanl.gov/components/sequence/HIV/geo/geo.comp . Accessed 4 April 2013.