Abstract
During an epidemic outbreak in a human population, susceptibility to infection can be reduced by raising awareness of the disease. In this paper, we investigate the effects of three forms of awareness (i.e., contact, local, and global) on the spread of a disease in a random network. Connectivity-correlated transmission rates are assumed. By using the mean-field theory and numerical simulation, we show that both local and contact awareness can raise the epidemic thresholds while the global awareness cannot, which mirrors the recent results of Wu et al. The obtained results point out that individual behaviors in the presence of an infectious disease has a great influence on the epidemic dynamics. Our method enriches mean-field analysis in epidemic models.
Keywords: Epidemic spread, Complex network, Behavioral response, Heterogeneous transmission
Introduction
The effect of individual awareness (or risk perception) in the context of an infectious disease outbreak in a human population has been under investigation for a few years [1, 2]. Human responses to disease outbreaks are sometimes decisive factors. For example, when aware of a disease in their vicinity, people can take precautionary measures such as wearing masks, frequent hand washing, and evading contact with infected individuals to reduce the risk of infection and lower the possibility of disease transmission [3–5]. The behavioral change triggered in a population corresponds to the information obtained from the circumstances [6]. The information taken from a social or spatial neighborhood is called local information, while information that comes from the news media and public health authorities is called global information. Both sources of information have strong impacts on epidemic dynamics. We refer the readers to [6, 7] for comprehensive surveys of related results.
To investigate the effect of behavioral response, two kinds of awareness, global awareness, which increases with the overall disease prevalence, and local awareness, which increases with the fraction of infected contacts, were studied in [1, 8]. Global and local awareness were described by exponential functions of respective global and local information. By using a mean-field approximation, it was shown that the network topology, homogeneous random network or scale-free network, has an intrinsic impact on the existence of a critical value (in terms of global and local awareness) that stops the epidemics. In [9], a third kind of awareness, called contact awareness, which increases with the individual contact number, was proposed. By using a linear formulation of awareness, the authors showed that both the local and contact awareness can raise the epidemic threshold (hence, inhibit the epidemic from spreading), while the global awareness cannot. The precise functioning of awareness, nevertheless, is still not well understood. One of the goals in this paper is to understand the role of the aforementioned three forms of awareness by providing a more flexible yet analytically tractable framework.
In most of the existing relevant literature (including the work mentioned above), it is assumed that the transmission rate is constant for all individuals. To describe the vast spectrum of disease propagation strategies, the degree-correlated transmission rates were examined in [10]. It was shown that the connectivity-dependent infection scheme can yield threshold effects even in scale-free networks where they would otherwise be unexpected (see e.g., [11, 12]). Therefore, for a more realistic epidemic model, the degree-correlated transmission rates should be taken into account.
In view of the above considerations, in this paper we investigate the impact of global, local, and contact awareness on epidemic spreading with degree-correlated transmission rates. Our model is based on an SIS epidemiological process where, at a given time, each individual can be susceptible (S) or infected (I). The contact network of the population is modeled by a configuration model (described below) where nodes represent individuals and edges indicate potential contacts between individuals. Building on a continuous mean-field approach and the Lyapunov stability theory, we establish the epidemic dynamics and derive the epidemic threshold. The function of awareness is expressed by a non-linear function (the linear function used in [9, 13] can be viewed as a special case) that provides additional flexibility in applications. Through numerical simulation on scale-free networks, we confirm that both local and contact awareness can raise the epidemic threshold while global awareness can only decrease the final epidemic size. However, the influence degree of the awareness is shown to be closely related to the heterogeneous transmission rates.
The rest of the paper is organized as follows. We describe the model and establish the epidemic dynamics by mean-field analysis in Section 2. We determine the epidemic threshold in Section 3 and present numerical simulations in Section 4. Finally, we conclude the paper in Section 5.
Model and mean-field analysis
We use a modified SIS (susceptible-infected-susceptible) model to study the epidemic dynamics on a network consisting in n individuals. The contact network is defined as a configuration model [12, 14], where only the network’s degree distribution (that is, the distribution, pk, which governs the probability that a node will have degree k) is specified and the edges are made by random pairing. Configuration model networks are increasingly used for infectious diseases in complex networks, which yield to analytical treatment and allow for heterogeneous contact levels [15]. Data-driven studies reveal that the accuracy of such models is mostly high; see e.g., [16, 17]. In the simplest SIS model, each individual is either in a susceptible state or an infected state. A susceptible individual, say node i, becomes infected upon contact with a single infected individual, say node j, at some infection rate. The infection rate along the edge from 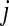 to i can be expressed as AiTj, where Ai is the admission rate of node i describing the rate that susceptible node i would actually admit an infection through an edge connected to an infected node and Tj is the transmission rate of node j meaning the rate that infected node j would actually transmit an infection through an edge connected to a susceptible node [10, 15]. Once infected, a node recovers (i.e., returns to the susceptible state) at rate γ.
to i can be expressed as AiTj, where Ai is the admission rate of node i describing the rate that susceptible node i would actually admit an infection through an edge connected to an infected node and Tj is the transmission rate of node j meaning the rate that infected node j would actually transmit an infection through an edge connected to a susceptible node [10, 15]. Once infected, a node recovers (i.e., returns to the susceptible state) at rate γ.
In case of no awareness, the admission rate Ai is usually assumed to be 1 and the transmission rate Ti = β for all nodes i. As mentioned above, we will consider the degree-correlated transmission rate βk [10], which is defined as the transmission rate of a node with degree k. In addition, we modulate the admission rate Ai by some multiplicative factors. First, let 0 ≤ ψk ≤ 1 be a decreasing function, which represents the contact awareness of a node with degree k. Naturally, an individual having a larger contact number has a higher risk of being infected [9]. This factor of contact awareness reflects an individual’s risk perception based on the contact information. Second, let 0 ≤ ϕk ≤ 1 with ϕ0 = 1 being a decreasing function accommodating the local and global epidemic information. Specifically, for a node, say i, with degree k, let 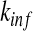 be the number of its infected neighbors. The local awareness of node i is given by
be the number of its infected neighbors. The local awareness of node i is given by 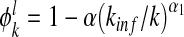 for some precaution level, 0 ≤ a ≤ 1 and α1 is a positive integer reflecting the use of special prophylaxis [1]. The quantity ρ is taken to be representative of the global infection density, that is, the fraction of infected individuals over the whole population. The global awareness of a node with degree k is supposed to be
for some precaution level, 0 ≤ a ≤ 1 and α1 is a positive integer reflecting the use of special prophylaxis [1]. The quantity ρ is taken to be representative of the global infection density, that is, the fraction of infected individuals over the whole population. The global awareness of a node with degree k is supposed to be 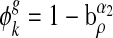 with 0 ≤ b ≤ 1 and α2 similarly being a k positive integer. The parameters α1 and α2 embody the impact strength of the local and global epidemic information on the admission rate. The role of them will be clear in the following. For a susceptible node i with degree k and one of its infected neighbor j, the modified infection rate along the edge from j to i can be written as
with 0 ≤ b ≤ 1 and α2 similarly being a k positive integer. The parameters α1 and α2 embody the impact strength of the local and global epidemic information on the admission rate. The role of them will be clear in the following. For a susceptible node i with degree k and one of its infected neighbor j, the modified infection rate along the edge from j to i can be written as
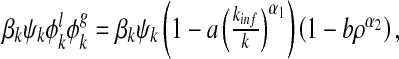 |
1 |
where βk is the transmission rate of node i, and kinf is the number of node i’s infected neighbors.
Note that both the infection density ρ and the number of infected neighbors kinf evolve with respect to time t. We mention that the functions 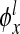 and
and 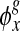 can be viewed x as an approximation of the exponential function
can be viewed x as an approximation of the exponential function 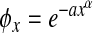 analyzed in [1, 8]. Setting α1 = α2 = 1, we readily reproduce the linear functions used in the work [9].
analyzed in [1, 8]. Setting α1 = α2 = 1, we readily reproduce the linear functions used in the work [9].
At time t, let θ(t) be the probability that a randomly chosen edge points to an infected individual. Let ρk(t) be the infection density among nodes having degree k. As in [18], we obtain
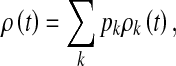 |
2 |
and
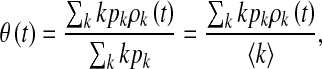 |
3 |
where 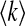 is the average degree of the network.
is the average degree of the network.
Denote Xk to be a random variable counting the number of infected neighbors of a node with degree k. Thus, Xk follows a binomial distribution Bin(k, θ(t)) with [19]
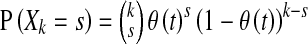 |
4 |
for 0 ≤ s ≤ k. Given a susceptible individual with degree k, who has s infected neighbors, the probability of infection is
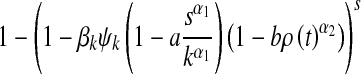 |
5 |
by using (1). Thus, the probability that a susceptible node with degree k becomes infected is shown to be given by
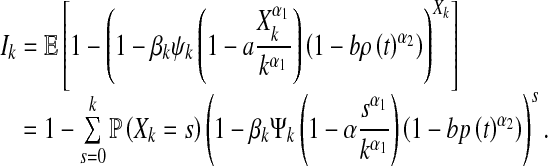 |
6 |
Hence, the discrete-time epidemic dynamics can be described as
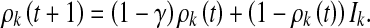 |
7 |
Considering an infinitesimal interval (t, t + h], similarly as in [9, 20], we can transform (6) and (7) into
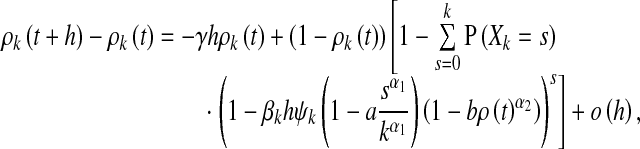 |
8 |
where the probability P(Xk = s) is given by (4).
By employing L’ Hospital’s rule, we obtain
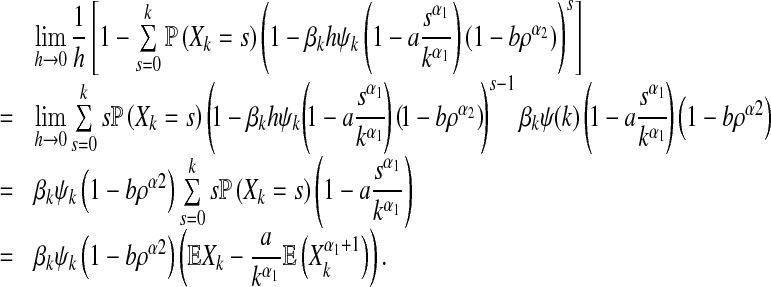 |
9 |
The moment-generating function of Xk is defined for all ℓ ∈ (− ∞ , ∞ ) by 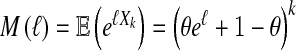 . It is well known that by differentiation at ℓ = 0,
. It is well known that by differentiation at ℓ = 0,
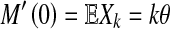 |
10 |
and
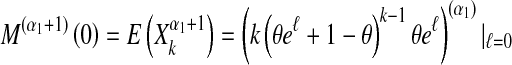 |
11 |
for any positive integer α1.
Dividing both sides of (8) by h and letting h → 0, we obtain
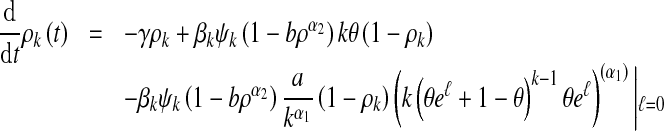 |
12 |
employing (9), (10) and (11), where ρ and θ are given by (2) and (3), respectively.
For α1 = α2 = 1 and βk ≡ β, (12) reduces to (9) obtained in [9]. The consistency confirms that (12) is valid. Notice that the above system (12) (k = 1, ⋯ , n) is highly involved. However, we will see in the next section that a neat formulation of the epidemic threshold can be derived. Without loss of generality, we will set the recovery rate γ = 1 in the following.
Epidemic threshold
In this section, we determine the epidemic threshold in terms of the connectivity-correlated (i.e., k-dependent) transmission rates βk. In the simplest networked SIS model, the epidemic threshold corresponds to a critical value of infection rate βc (or the reproductive ratio R0), above which the disease in question spreads, while below it the disease dies out [21]. The critical value has been shown to rely on the infection and recovery rates of a disease, as well as the topology of the host population through which it spreads [22–26]. By studying the local stability of the infection-free equilibrium, we will present the dependency of awareness on the epidemic threshold. Our results also have implications for the dissemination of a computer virus/worm across the Internet as well as opinions/rumors/news in social networks.
To start with, we establish a linearization system of (12). On omitting higher powers of ρk and noting that γ = 1, we obtain
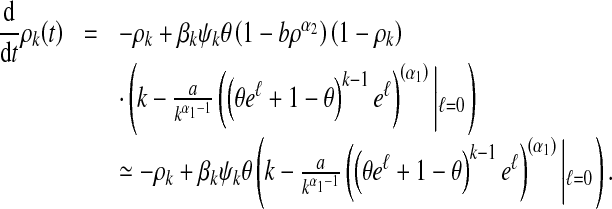 |
13 |
Notice that
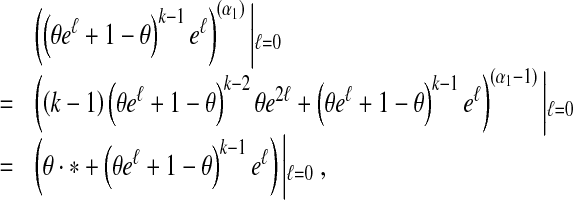 |
14 |
where * represents an unspecified or unknown quantity. Therefore, we have linear differential equations for k = 1, ⋯, n
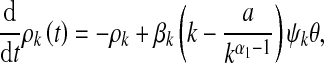 |
15 |
which implies that the Jacobian matrix of (12) can be calculated as
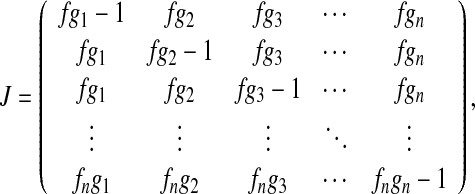 |
16 |
where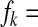 βk(k − a/kα1 − 1) ψk and gk = kpk/
βk(k − a/kα1 − 1) ψk and gk = kpk/ 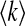 for k = 1, ⋯, n.
for k = 1, ⋯, n.
By basic determinant transformations (see e.g., [9, Lemma 1]), we obtain the n eigenvalues of J from the characteristic equation det (J − λI) = 0 as λ1 = ⋯ = λn − 1 = −1 and 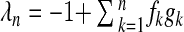 . The trivial solution ρk ≡ 0 of system (12) (which is the infection-free equilibrium) is locally stable if and only if λn < 0, which yields
. The trivial solution ρk ≡ 0 of system (12) (which is the infection-free equilibrium) is locally stable if and only if λn < 0, which yields
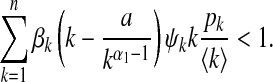 |
17 |
Hence, if (17) holds, the disease dies out; otherwise, the disease spreads. This expression shows that local and contact awareness play a pivotal role in determining whether an epidemic spreads in a population, while the global awareness is independent of the epidemic threshold. The same result was observed in [9].
In what follows, we study the epidemic threshold by instantiating the above general correlated transmission rates in two special examples.
In the first example, we set βk ≡ β. This infection scheme implies that an infected individual can transmit the infection from all of its edges with the same rate. This example has been addressed in [9] and it has relevance for many of the respiratory infectious diseases such as the 2003 severe acute respiratory syndrome (SARS) [27] and the 2009 influenza A (H1N1) [28]. Introducing βk = β into (17), we obtain the threshold for containing the disease as
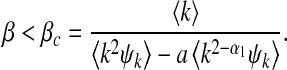 |
18 |
If we set α1 = 1, the above threshold reduces to that deduced in [9, Eq. (11)].
Next, we consider a reciprocal infection scheme where 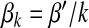 . Here, the transmission rate is connectivity correlated. This scheme reflects the infection dynamics of some macroparasite diseases where infected agents have a limited pathogen reservoir and the more the agent contacts the less would be the chance of transmission per contact (or per capita) [10]. Substituting
. Here, the transmission rate is connectivity correlated. This scheme reflects the infection dynamics of some macroparasite diseases where infected agents have a limited pathogen reservoir and the more the agent contacts the less would be the chance of transmission per contact (or per capita) [10]. Substituting 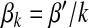 into (17) yields
into (17) yields
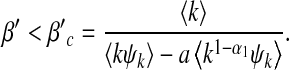 |
19 |
We mention that this infection scheme also has implications in cyber security. In peer-to-peer (P2P) file-sharing networks (e.g., Napster and Kazaa), every node has a limited upload capacity. The larger the connectivity, the slower each one of its neighbors can download. The probability of successful downloading would thus be inversely proportional to the connectivity. Another plausible scenario is the denial of service (DoS) attacks, which flood a target computer system with bogus requests, making it unable to provide normal services to legitimate users.
The variance 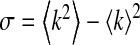 of the degree in a network is an indicator of the degree of asymmetry [12]. Compared with regular graphs or classical random graphs, scale-free networks have much larger σ and their degree distributions are asymmetric. To take a look at the effect of σ on the epidemic threshold, for simplicity, we set ψk = 1 and α1 = 1.
of the degree in a network is an indicator of the degree of asymmetry [12]. Compared with regular graphs or classical random graphs, scale-free networks have much larger σ and their degree distributions are asymmetric. To take a look at the effect of σ on the epidemic threshold, for simplicity, we set ψk = 1 and α1 = 1.
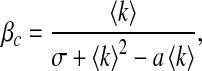 |
20 |
and
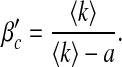 |
21 |
It is clear that the threshold βc decreases with respect to σ while β′c remains unchanged. This suggests that, in our first infection scheme where βk = β, an epidemic is more inclined to occur for asymmetric networks and that in a reciprocal infection scheme the asymmetry has no influence on the epidemic threshold. For scale-free networks, a similar result was observed in [10] without considering awareness.
Simulation study
To complement the theoretical analysis carried out in the previous section, we now investigate the impact of awareness on the epidemic thresholds (18) and (19) by numerical simulations.
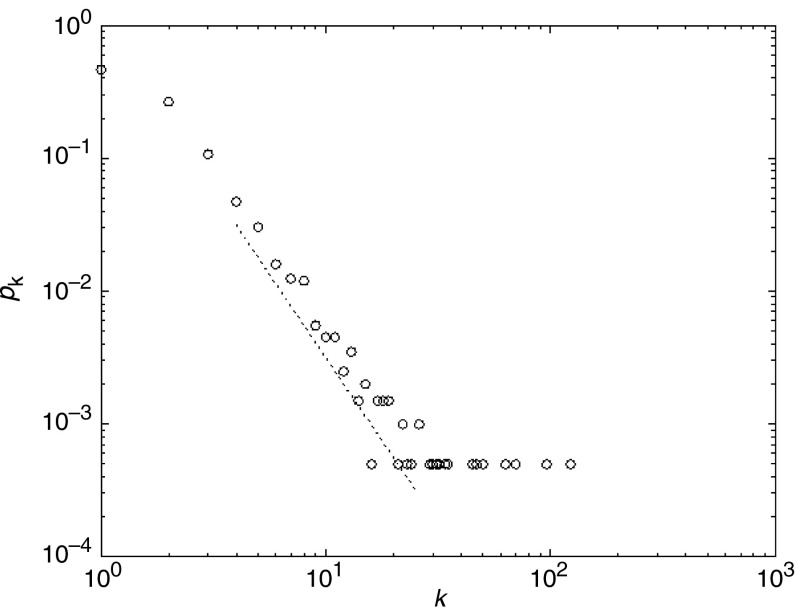
Simulations are performed on a scale-free network of n = 2,000 nodes with degree distribution 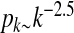 (see Fig. 1). The graph is generated by using the
(see Fig. 1). The graph is generated by using the
configuration_model
in NetworkX [29]. This degree exponent is a typical value for networks seen in the real world [30]. Initially 1% of the nodes are infected. We iterate the SIS process until convergence to a steady/equilibrium state. Following [9], we choose the contact awareness as a power-law function 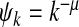 , where μ ≥ 0 . Hence, it follows from (18) and (19) that we obtain the thresholds
, where μ ≥ 0 . Hence, it follows from (18) and (19) that we obtain the thresholds
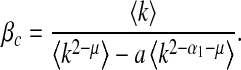 |
22 |
and
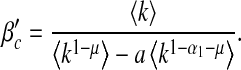 |
23 |
Fig. 1.
The degree distribution of a scale-free network with degree distribution 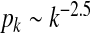 used in the simulations. The slope of the dotted line is − 2.5
used in the simulations. The slope of the dotted line is − 2.5
First, we examine the dependence of βc and 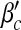 on local and global awareness, namely the parameters a, b, α1 and α2. We fix μ = 0.6. The epidemic threshold βc is measured by calculating the final infected portion ρ for each β from 0 to 1 in steps of 0.01 and the epidemic threshold
on local and global awareness, namely the parameters a, b, α1 and α2. We fix μ = 0.6. The epidemic threshold βc is measured by calculating the final infected portion ρ for each β from 0 to 1 in steps of 0.01 and the epidemic threshold 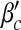 is measured by calculating ρ for each β′ starting from 0 in steps of 0.1. If ρ > 0.0025, we accept the corresponding value of β (or β′) as the threshold value.
is measured by calculating ρ for each β′ starting from 0 in steps of 0.1. If ρ > 0.0025, we accept the corresponding value of β (or β′) as the threshold value.
To reduce the fluctuation, for each β (or β′), we calculate the average of ρ over 10 simulation runs with different initial infected nodes.
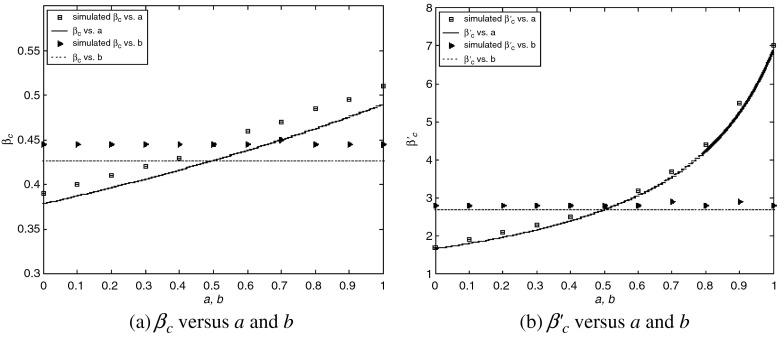
In Fig. 2 we show the results of calculations of the epidemic thresholds βc and 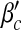 from the exact formulas (22) and (23), compared with explicit simulations of the model with α1 = α2 = 1. We find that both βc and
from the exact formulas (22) and (23), compared with explicit simulations of the model with α1 = α2 = 1. We find that both βc and 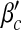 are almost unchanged for different b, while they increase with a. An intuitive interpretation is that a higher level of precaution measures adopted by individuals (i.e., larger a) can decrease the likelihood of an epidemic outbreak (i.e., larger βc and
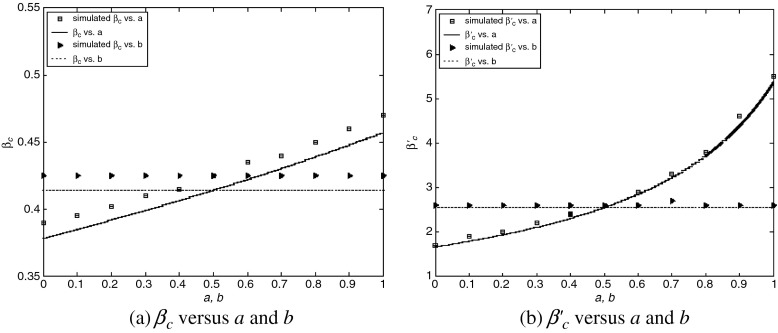
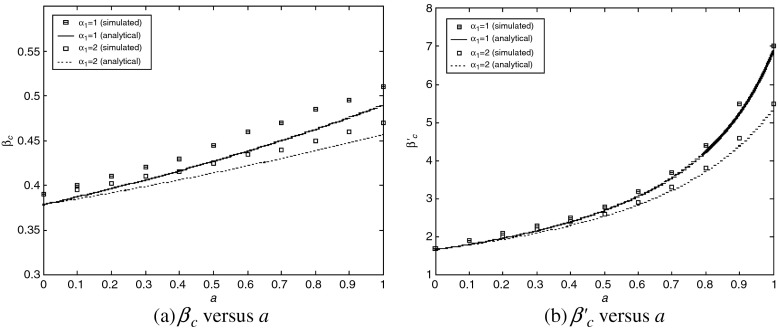
are almost unchanged for different b, while they increase with a. An intuitive interpretation is that a higher level of precaution measures adopted by individuals (i.e., larger a) can decrease the likelihood of an epidemic outbreak (i.e., larger βc and 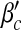 ). The simulated values are slightly larger than the theoretical predictions, which is likely due to a finite-size effect [9, 10]. We illustrate the epidemic thresholds for α1 = 2, α2 = 1 in Fig. 3 and those for α1 = 1, α2 = 2 in Fig. 4. We find similar behaviors as observed in Fig. 2. By comparing Fig. 2 with Fig. 3 and comparing Fig. 2 with Fig. 4, we see that the epidemic thresholds βc and
). The simulated values are slightly larger than the theoretical predictions, which is likely due to a finite-size effect [9, 10]. We illustrate the epidemic thresholds for α1 = 2, α2 = 1 in Fig. 3 and those for α1 = 1, α2 = 2 in Fig. 4. We find similar behaviors as observed in Fig. 2. By comparing Fig. 2 with Fig. 3 and comparing Fig. 2 with Fig. 4, we see that the epidemic thresholds βc and 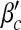 decrease with α1; while, they are almost unchanged for different α2. (The dependency between
decrease with α1; while, they are almost unchanged for different α2. (The dependency between 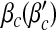 and α1 is re-plotted in Fig. 5 for the sake of comparison). These observations agree well with our analytical solutions. The decrease of epidemic thresholds with respect to α1 has an important epidemiological implication. Nodes with exposure to a lot of infectious contacts (corresponding to a high value of kinf /k) in a network may fail to be infected due to their increased perception of the risk or safety measures (here, smaller α1) and thus stopping the epidemic spreading. In the real world, medical doctors and care/sex workers should adopt strong safety measures, which may efficiently contain the disease transmission. A similar phenomenon was observed in Bagnoli et al. [1] for a network with a power-law in-degree distribution and an exponential out-degree distribution using degree-independent transmission rates (corresponding to our Fig. 5a). The ineffectiveness of factor α2 as well as b, nevertheless, indicates the incapability of altering an epidemic threshold for a global influence over the population.
and α1 is re-plotted in Fig. 5 for the sake of comparison). These observations agree well with our analytical solutions. The decrease of epidemic thresholds with respect to α1 has an important epidemiological implication. Nodes with exposure to a lot of infectious contacts (corresponding to a high value of kinf /k) in a network may fail to be infected due to their increased perception of the risk or safety measures (here, smaller α1) and thus stopping the epidemic spreading. In the real world, medical doctors and care/sex workers should adopt strong safety measures, which may efficiently contain the disease transmission. A similar phenomenon was observed in Bagnoli et al. [1] for a network with a power-law in-degree distribution and an exponential out-degree distribution using degree-independent transmission rates (corresponding to our Fig. 5a). The ineffectiveness of factor α2 as well as b, nevertheless, indicates the incapability of altering an epidemic threshold for a global influence over the population.
Fig. 2.
Epidemic thresholds as a function of a and b with 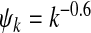 , α1 = 1 and α2 = 1. (a) is for βc and (b) is for
, α1 = 1 and α2 = 1. (a) is for βc and (b) is for 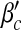 . When considering the thresholds versus a, we set b = 0.5; when considering the thresholds versus b, we set a = 0.5. Solid and dotted lines are the exact solutions from (22) and (23)
. When considering the thresholds versus a, we set b = 0.5; when considering the thresholds versus b, we set a = 0.5. Solid and dotted lines are the exact solutions from (22) and (23)
Fig. 3.
Epidemic thresholds as a function of a and b with 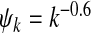 , α1 = 2 and α2 = 1. (a) is for βc and (b) is for
, α1 = 2 and α2 = 1. (a) is for βc and (b) is for 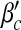 . When considering the thresholds versus a, we set b = 0.5; when considering the thresholds versus b, we set a = 0.5. Solid and dotted lines are the exact solutions from (22) and (23)
. When considering the thresholds versus a, we set b = 0.5; when considering the thresholds versus b, we set a = 0.5. Solid and dotted lines are the exact solutions from (22) and (23)
Fig. 4.
Epidemic thresholds as a function of a and b with 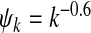 , α1 = 1 and α2 = 2. (a) is for βc and (b) is for
, α1 = 1 and α2 = 2. (a) is for βc and (b) is for 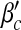 . When considering the thresholds versus a, we set b = 0.5; when considering the thresholds versus b, we set a = 0.5. Solid and dotted lines are the exact solutions from (22) and (23)
. When considering the thresholds versus a, we set b = 0.5; when considering the thresholds versus b, we set a = 0.5. Solid and dotted lines are the exact solutions from (22) and (23)
Fig. 5.
Epidemic thresholds as a function of a with 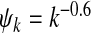 , α2 = 1 and b = 0.5. The data are copied from Figs. 2 and 3
, α2 = 1 and b = 0.5. The data are copied from Figs. 2 and 3
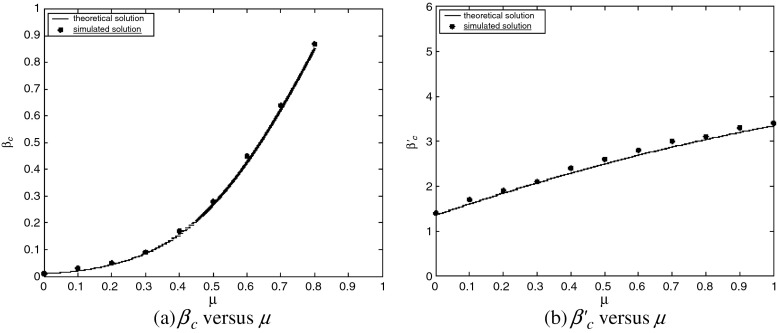
Next, we examine the dependence of βc and 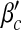 on contact awareness, namely the parameter μ. We show the changes of βc and
on contact awareness, namely the parameter μ. We show the changes of βc and 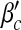 with respect to μ in Fig. 6. Simulated solutions are slightly raised from the expected values again due to a finite-size effect. In Fig. 6a, the value of βc is absent when μ is close to 1. This is because βc =
with respect to μ in Fig. 6. Simulated solutions are slightly raised from the expected values again due to a finite-size effect. In Fig. 6a, the value of βc is absent when μ is close to 1. This is because βc = 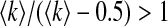 exceeding the range of β.
exceeding the range of β.
Fig. 6.
Epidemic thresholds as a function of μ with 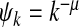 , a = b = 0.5 and α1 = α2 = 1. (a) is for βc and (b) is for
, a = b = 0.5 and α1 = α2 = 1. (a) is for βc and (b) is for 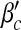
Conclusions
This paper has addressed the impact of awareness on epidemic outbreaks by proposing a mean-field approach accommodating heterogeneous transmission rates. Our analysis is based on an SIS epidemiological process in random networks modeled by a configuration model. Theoretical and numerical results show that both the contact and local awareness can raise the epidemic threshold, while the global awareness cannot. Our results confirm and further extend the previous observations in [1, 8, 10] to more general forms of awareness as well as degree correlated transmission rates. We found that the non-linear effect encoded in parameter α1 of local awareness implies that individuals who are exposed to many infectious contacts can effectively contribute to disease control by increasing their awareness of risk.
In the present work, we implicitly assumed that the disease is visible at the same moment it becomes infective. However, from a practical point of view, the information reaction may experience time delay or retardation for an individual. Oscillatory behavior may be displayed if we take delayed/periodic updating mechanisms into account [31]. Applications of the techniques described here are also possible for other network structures, for example, the dynamic contact networks [32], etc. Finally, we mention that a relevant issue we have not addressed is the risk estimation. The form of risk functions in (1) might have implications for disease control [33].
References
- 1.Bagnoli F, Lió P, Sguanci L. Risk perception in epidemic modeling. Phys. Rev. E. 2007;76:61904. doi: 10.1103/PhysRevE.76.061904. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson N. Capturing human behaviour. Nature. 2007;446:733. doi: 10.1038/446733a. [DOI] [PubMed] [Google Scholar]
- 3.Blendon RJ, Benson JM, DesRoches CM, Raleigh E, Taylor-Clark K. The public’s response to severe acute respiratory syndrome in Toronto and the United States. Clin. Infect. Dis. 2004;38:925–931. doi: 10.1086/382355. [DOI] [PubMed] [Google Scholar]
- 4.Fenichel EP, Castillo-Chavez C, Ceddia MG, Chowell G, Parra PAG, Hickling J, Holloway G, Horan R, Morin B, Perrings C, Springborn M, Velazquez L, Villalobos C. Adaptive human behavior in epidemiological models. Proc. Natl. Acad. Sci. U. S. A. 2011;108:6306–6311. doi: 10.1073/pnas.1011250108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiss IZ, Cassell J, Recker M, Simon PL. The impact of information transmission on epidemic outbreaks. Math. Biosci. 2010;225:1–10. doi: 10.1016/j.mbs.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Funk S, Salathé M, Jansen VAA. Modelling the influence of human behaviour on the spread of infectious diseases: a review. J. R. Soc. Interface. 2010;7:1247–1256. doi: 10.1098/rsif.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perra N, Balcan D, Gonçalves B, Vespignani A. Towards a characterization of behavior-disease models. PLoS ONE. 2011;6:23084. doi: 10.1371/journal.pone.0023084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitchovitch S, Liò P. Risk perception and disease spread on social networks. Procedia Comput. Sci. 2010;1:2339–2348. doi: 10.1016/j.procs.2010.04.264. [DOI] [Google Scholar]
- 9.Wu Q, Fu X, Small M, Xu X-J. The impact of awareness on epidemic spreading in networks. Chaos. 2012;22:13101. doi: 10.1063/1.3673573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olinky R, Stone L. Unexpected epidemic thresholds in heterogeneous networks: the role of disease transmission. Phys. Rev. E. 2004;70:30902. doi: 10.1103/PhysRevE.70.030902. [DOI] [PubMed] [Google Scholar]
- 11.May RM, Lloyd AL. Infection dynamics on scale-free networks. Phys. Rev. E. 2001;64:66112. doi: 10.1103/PhysRevE.64.066112. [DOI] [PubMed] [Google Scholar]
- 12.Newman MEJ. Networks: An Introduction. New York: Oxford University Press; 2010. [Google Scholar]
- 13.Shang Y. Discrete-time epidemic dynamics with awareness in random networks. Int. J. Biomath. 2013;6:1350007. doi: 10.1142/S1793524513500071. [DOI] [Google Scholar]
- 14.Molloy M, Reed B. A critical point for random graphs with a given degree sequence. Random Struct. Algoritm. 1995;6:161–179. doi: 10.1002/rsa.3240060204. [DOI] [Google Scholar]
- 15.Newman MEJ. Spread of epidemic disease on networks. Phys. Rev. E. 2002;66:16128. doi: 10.1103/PhysRevE.66.016128. [DOI] [PubMed] [Google Scholar]
- 16.Meloni, S., Perra, N., Arenas, A., Gómez, S., Moreno, Y., Vespignani, A.: Modeling human mobility responses to the large-scale spreading of infectious diseases. Sci. Rep. 1, Art. 62 (2011) [DOI] [PMC free article] [PubMed]
- 17.Meyers LA, Pourbohloul B, Newman MEJ, Skowronski DM, Brunham RC. Network theory and SARS: predicting outbreak diversity. J. Theor. Biol. 2005;232:71–81. doi: 10.1016/j.jtbi.2004.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastor-Satorras R, Vespignani A. Epidemic spreading in scale-free networks. Phys. Rev. Lett. 2001;86:3200–3203. doi: 10.1103/PhysRevLett.86.3200. [DOI] [PubMed] [Google Scholar]
- 19.Nagy V. Mean-field theory of a recurrent epidemiological model. Phys. Rev. E. 2009;79:66105. doi: 10.1103/PhysRevE.79.066105. [DOI] [PubMed] [Google Scholar]
- 20.Reed WJ. A stochastic model for the spread of a sexually transmitted disease which results in a scale-free network. Math. Biosci. 2006;201:3–14. doi: 10.1016/j.mbs.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Diekmann O, Heesterbeek JAP. Mathematical Epidemiology of Infectious Diseases. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- 22.Moslonka-Lefebvre M, Pautasso M, Jeger MJ. Disease spread in small-size directed networks: epidemic threshold, correlation between links to and from nodes, and clustering. J. Theor. Biol. 2009;260:402–411. doi: 10.1016/j.jtbi.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Pastor-Satorras R, Vespignani A. Epidemic dynamics and endemic states in complex networks. Phys. Rev. E. 2001;63:66117. doi: 10.1103/PhysRevE.63.066117. [DOI] [PubMed] [Google Scholar]
- 24.Serrano A, Bogu~~ M. Percolation and epidemic thresholds in clustered networks. Phys. Rev. Lett. 2006;97:88701. doi: 10.1103/PhysRevLett.97.088701. [DOI] [PubMed] [Google Scholar]
- 25.Taylor M, Taylor TJ, Kiss IZ. Epidemic threshold and control in a dynamic network. Phys. Rev. E. 2012;85:16103. doi: 10.1103/PhysRevE.85.016103. [DOI] [PubMed] [Google Scholar]
- 26.Shang Y. Mixed SI(R) epidemic dynamics in random graphs with general degree distributions. Appl. Math. Comput. 2013;219:5042–5048. doi: 10.1016/j.amc.2012.11.026. [DOI] [Google Scholar]
- 27.Smith RD. Responding to global infectious disease outbreaks: lessons from SARS on the role of risk perception, communication and management. Soc. Sci. Med. 2006;63:3113–3123. doi: 10.1016/j.socscimed.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaRussa P. H1N1 influenza: what have we learned? Semin. Respir. Crit. Care Med. 2011;32:393–399. doi: 10.1055/s-0031-1283279. [DOI] [PubMed] [Google Scholar]
- 29.Hagberg, A.A., Schult, D.A., Swart, P.J.: Exploring network structure, dynamics, and function using NetworkX. In: Proc. of the 7th Python in Science Conference, pp. 11–16 (2008)
- 30.Barabási A-L. Scale-free networks: a decade and beyond. Science. 2009;325:412–413. doi: 10.1126/science.1173299. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhang W, Sun G, Zhou T, Wang B. Time-delayed information can induce the periodic outbreaks of infectious diseases. Sci. Sin. Phys. Mech. Astron. 2012;42:631–638. doi: 10.1360/132011-240. [DOI] [Google Scholar]
- 32.Bansal S, Read J, Pourbohloul B, Meyers LA. The dynamic nature of contact networks in infectious disease epidemiology. J. Biol. Dyn. 2010;4:478–489. doi: 10.1080/17513758.2010.503376. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Zhang J, Li P, Small M, Wang B. Risk estimation of infectious diseases determines the effectiveness of the control strategy. Physica D. 2011;240:943–948. doi: 10.1016/j.physd.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]