Abstract
The evidence for the existence of a heterogeneous population of cancer stem cells (CSCs) responsible for the initiation and maintenance of cancer has been characterized for several tumors recently. Purification and molecular characterization of normal human mammary stem cells from cultured mammospheres has been achieved, providing evidence supporting a model in which breast tumor heterogeneity is a reflection of a number of CSC-like cells in the tumor. A number of experimental methodologies have been developed to characterize epithelial stem cells, including the expression of cell surface or intracellular markers, mammosphere formation, exclusion of fluorescent dye by a side population, retention of the radionucleotide label, etc. Methodologies have also been successfully employed to identify tumorigenic cells within breast cancers. The most important characteristics of stem cells are the capacity for self-renewal and the regulation of the balance between self-renewal and differentiation. In the mammary gland, signaling pathways, such as Hedgehog (Hh), Wnt/β-catenin, and Notch, play a role in embryogenesis and organogenesis and maintenance of tissues in the adult through regulation of the balance between self-renewal and differentiation of stem cells. Breast TAAs include epitopes from proteins, such as carcinoembryonic antigen and NYBR-1, which are involved in tissue differentiation. Targeting BCSCs may be achieved by a number of approaches such as chemotherapy sensitization of BCSCs, differentiating therapy, targeting stem cell elimination, targeting signaling pathways and drug transporters, and inhibition of regulatory pathways involved in self-renewal. Targeting cells which have the potential to metastasize will be an important aspect of the BCSC field as these are the cells that cause the majority of morbidity and mortality from breast cancer.
Keywords: Breast, Cancer stem cells, Metastatic pathways, Hormone receptor expression, Self–renewal, Therapeutic perspectives
Introduction
The classical model for cancer development holds that a series of mutations occurring in a cell can lead to cell transformation [1]. The cancer stem cell (CSC) hypothesis is based on the identification of a unique population of stem cells in the bone marrow [2]. CSCs are tumorigenic multipotential cells with dysregulated self-renewal properties in which upon division, one daughter cell retains stemness and the other becomes committed to a lineage. The CSC fraction typically constitutes 1–5 % of the tumor size [3]. They function in initiation, maintenance, growth, and metastasis of tumors [4, 5] and demonstrate slow cycling and indefinite ability to renew themselves [6]. CSCs can proliferate limitlessly and are more resistant to chemotherapy and apoptosis than somatic cancer cells [7]. The evidence for the existence of a heterogeneous population of adult stem cells responsible for the initiation and maintenance of cancer has been characterized for several tumors recently [8–11]. Pece and colleagues reported the purification and molecular characterization of normal human mammary stem cells from cultured mammospheres, and provided evidence supporting a model in which breast tumor heterogeneity is a reflection of the number of CSC-like cells in the tumor [12]. Researchers have recognized that CSCs comprise not only CD133+ population, but also a heterogeneous CD133− population expressing a wide range of markers [13]. It is generally agreed that CSCs are organized into a hierarchy consisting of cancer “stem cells” at the top of hierarchy capable of sphere formation, and cancer progenitor cells lower in the hierarchy that are incapable of sphere formation but still retain the ability to initiate tumors [9]. A study published by Chen et al., in 2010, presented compelling evidence that CSCs are organized in a three-tiered hierarchy similar to endogenous stem cells, where “stem cells” higher up in the hierarchy could differentiate into a more lineage restricted genitor populations at the bottom of the hierarchy [14]. They further observed that cancer stem cells higher up in the hierarchy are generally slow-dividing sphere forming cells, but gives rise to expansive infiltrative tumor growth in vivo that is well-correlated with high tumor grade. An alternative hypothesis called the “clonal evolution of tumors theory” postulates that cancer originates from mutations occurring in a few cells or a single cell that eventually leads to genomic instability, resulting into uncontrolled and unlimited proliferation of a population of cells [15].
Liang and colleagues [16] demonstrated that drug-induced genomic instability in cancer cells can drive the emergence of CD133+ CSCs. Sharma et al. [17] showed that these CSC populations are not a fixed population, but rather a volatile population arising from the profound epigenetic instability of cancer cells and that the cancer cell stemness, and concomitant resistance to chemotherapy can be turned on and off through DNA methylation, a process through which gene expression is regulated by chemical modification of the chromatin structure suggesting that the tumor stem cell phenotype is highly volatile, and can emerge from seemingly non-stem cancer cells.
Characterization of Epithelial Stem Cells
A number of experimental methodologies have been developed to characterize epithelial stem cells, including the expression of cell surface or intracellular markers, mammosphere formation, exclusion of fluorescent dye by a side population, retention of the radionucleotide label, etc. Methodologies have also been successfully employed to identify tumorigenic cells within breast cancers. Al-Hajj et al. initiated tumors in immunodeficient mice with cells derived from human breast cancer-associated malignant pleural effusions [11]. It was also observed by them that a small proportion of tumorigenic cells could be isolated from dissociated tumor cells by flow cytometry. Following removal of nonepithelial cells, cells with the CD44 + CD24−/loLin- phenotype were 10 to 50-fold enriched in their ability to initiate tumors as well as transfer the tumor to secondary and subsequent hosts as compared to unsorted cells, demonstrating the capacity for self-renewal. HER2 overexpression has been associated with increased expression of the stem cell marker aldehyde dehydrogenase and an increase in fractions of stem cell progenitors in breast tumors [5]. Self-renewal has also been demonstrated by dissociation of mammospheres, reculture, and then reaggregation, and cells from the secondary mammospheres and beyond were almost exclusive bipotent or tripotent [10]. Mammospheres initiated tumors in the cleared mammary fat pad of immunodeficient mice at 1000-fold greater dilutions than established breast cancer derived cell lines, and the breast cancer cell with the capability for long-term self-renewal, enriched within the CD44 + CD24− subset, was also seen [10, 11].
Tumors induced by expression of Wnt pathway express markers of both luminal and basal/myoepithelial lineages [18] and suggest origin from a pluripotent precursor, presumably a mammary epithelial stem cell [19]. This is not seen with other oncogenes such as Neu and H-Ras [20]. Members of the transforming growth factor-β (TGF-β) superfamily, which include bone morphogenetic proteins (BMP), are involved in the control of many different biological processes, including cell proliferation, differentiation, apoptosis, and regulation of invasiveness [21]. TGF-β can also potentiate tumorigenesis and contribute to the progression and invasiveness of various carcinomas [22]. Blockade of TGF-β (signaling) inhibits tumor cell viability, migration, and metastasis [23], including the formation of bone metastases [24]. BMP-7 is expressed in various cell lines, but in a cell line specific manner as the breast cancer cell lines MCF-7 and SK-BR-3 showed
BMP-7 expression on the mRNA level [25]. BMP7 has been proposed as a marker of differentiation in normal and breast cancer cells [26]. BMP-6 and BMP-7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 MAPK activation [27].
Mapping the transcriptional profile of embryonic stem cell-like genes in primary human breast cancer has revealed two classes of tumors: those with an embryonic stem cell-like activated program and those with an embryonic stem cell-like repressed program [28]. Those tumors with an embryonic stem cell-like activated program were associated with poorer differentiated tumors that were more likely to progress to metastasis and death. The CD44+/CD24 low phenotype in human breast tumors has been found to be associated with basal-like tumors, and particularly BRCA1 hereditary breast cancer, and has been linked to expression of CD49f, elevated expression of CK5/14 and EGFR, and low expression of ER, PgR, and HER-2 [29]. Basal-like tumors often have been linked to poorer prognosis. The occurrence of the CD44+/CD24 low phenotype was found to be lower in tumors of luminal type, and particularly HER-2+ tumors, irrespective of ER status [30].
The Epithelial–Mesenchymal Transition Phenomenon
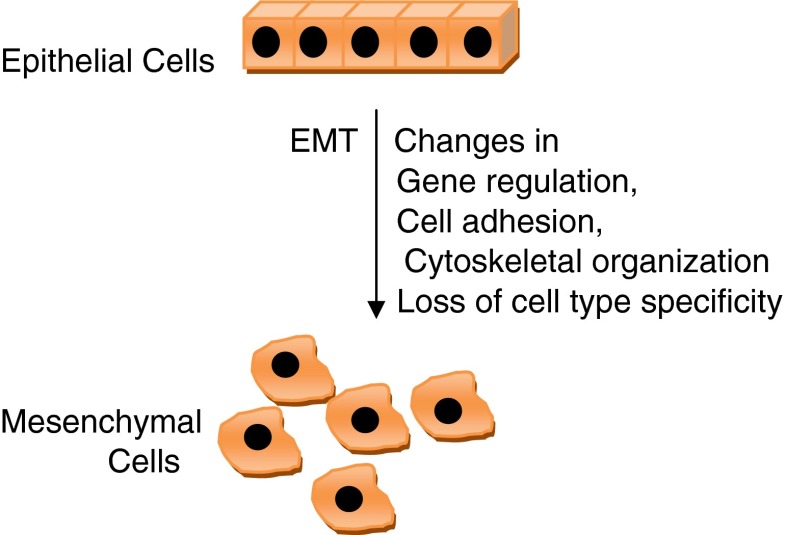
Epithelial–mesenchymal transition (EMT) consists of a rapid and often reversible change of cell phenotype. Epithelial cells loosen cell–cell adhesion structures including adherens junctions and desmosomes, modulate their polarity, and rearrange their cytoskeleton; intermediate filaments typically switch from cytokeratins to vimentin. Cells become isolated, motile, and resistant to apoptosis. In vivo, the EMT process generates poorly differentiated and potentially pluripotent cells rather than true fibroblasts, a differentiated cell type expressing tissue and organ specificity. This intermediate phenotype is linked to the expression of basal cytokeratins (CK5, CK14) and to some level of cell–cell interaction allowing group cell migration [31]. The EMT was initially defined as the cellular remodeling occurring during heart morphogenesis, but the concept was extended to analogous transformation occurring during the formation of mesoderm and neural crest [32, 33], wound healing, mammary tubulogenesis [34], and stemness [35, 36]. Many genes and pathways have been implicated in inducing EMT in tumor cells involving so-called EMT master genes, genes of a family of transcriptional factors including Snail, Twist, Zeb, and E47 [37]. In direct association with cancer progression, several molecules including tyrosine phosphatase Pez [38], ILEI [39], RKIP [40], and CXCR447 appear to control EMT-like phenotypes and tumor metastasis in mouse models [37].
Both human tumors and mouse models of breast tumorigenesis show evidence of EMT or partial EMT. Besides the modification of the phenotype, EMT results in the acquisition of other properties involved in carcinoma progression, such as an increased ability to migrate, a higher resistance to apoptosis, and the already mentioned acquisition of stemness properties [31]. Invasive breast carcinomas are characterized by their strong heterogeneity, reflecting tumor histology and response to therapy. It is likely that the contribution of a process like EMT in cancer progression depends on the tumor type [31]. The best case for a complete EMT taking place during mammary tumor progression is carcinosarcoma or metaplastic carcinomas, where an epithelial and a mesenchymal compartment can be distinguished based on the expression of, respectively, cytokeratins or vimentin intermediate filaments [31]. Cytogenetic studies strongly indicate that these two compartments originate from a common precursor cell population undergoing a full EMT process, giving rise to the mesenchymal component [41]. Recent studies show overexpression of Snail genes in these tumors, correlating with activation of Akt and β-catenin pathways [42]. A more prevalent mammary tumor, the infiltrating lobular carcinoma, is also characterized by the lack of E-cadherin expression, reflecting genomic and epigenetic silencing mechanisms [43, 44]. These tumors express significantly higher levels of a “classic” EMT master gene, Twist [45].
Role of microRNAs in Breast Cancer
microRNAs are noncoding RNA molecules that exert their effect at the translational level and are valuable in regulating gene expression [46]. The Tac1 gene, which is linked to breast and other cancers, could be suppressed by translational inhibition [47]. Three miRNAs have been found that may bind to Tac1: miR-130a, miR-206, and miR-302a [48]. Tac1 regulates breast cancer cell interaction with the mesenchymal stem cells, and microRNAs against Tac1 may affect quiescence of breast cancer cells in the marrow cavity [49].
Breast Cancer Metastatic Pathways
Metastatic spread of tumor cells can be an early event in tumorigenesis [50]. It has been experimentally demonstrated that a small tumorigenic cell population exists in solid tumors of both the breast and the brain which represent around 1–2 % of the total tumor burden and these cells might have a phenotype different from the original tumor-initiating cells [11]. Circulating tumor cells can often be detected in patients with both primary and metastatic diseases, and the presence of these cells is often associated with relatively worse prognosis for survival [51]. CXCR4, a chemokine receptor, expressed by hematopoietic stem cells which bind CXCL12, has been shown to be increased by about four times in mammospheres to be expressed in both metastatic breast cancer cells and neuroblastomas [52]. Additionally, the organs that form the main target of breast cancer metastasis have the highest expression of the ligand CXCL12 [52]. Recently, researchers have identified a subset of CSCs, present in all terminal colon cancer cells and all metastatic cancer cells, having metastatic capacity with a surface marker CD26 [53].
Steroid Hormone Receptor Expression of the Breast Cancer Stem Cells
Recently, it has been shown that the mammary reconstituting cells are ER − and PgR − [54]. Recent experiments have demonstrated a role for BRCA1 being involved in the differentiation of human ER − stem/progenitor cells into ER + luminal epithelial cells [55]. The deletion of BRCA1 results in the prevention of the transition of ER − stem cells into ER + progenitor cells, and heterozygous mutations in the BRCA1 gene predispose women to breast and ovarian cancer, with tumors often being of the basal-like phenotype characterized by a lack of expression of ER, PgR, and HER-2 [56]. A model of breast cancer origin has also been proposed in which ER + tumors are derived from ER + stem cells or ER + early or late progenitor cells and ER − tumors are derived from the more primitive ER − stem cells [57]. Other models have postulated that ER − stem cells, which rarely divide and are also resistant to hormonal therapy, can generate ER + short-term transit-amplifying cells that in turn give rise to ER + differentiated cells [30].
Self-renewal and Differentiation Pathways of Stem Cells
The most important characteristics of stem cells are the capacity for self-renewal and the regulation of the balance between self-renewal and differentiation [30]. In the mammary gland, signaling pathways, such as Hedgehog (Hh), Wnt/β-catenin, and Notch, play a role in embryogenesis and organogenesis and maintenance of tissues in the adult through regulation of the balance between self-renewal and differentiation of stem cells. The Notch family of transmembrane signaling proteins is involved in cell fate development and expressed in stem cells and early progenitor cells [58]. Notch-4 has been shown to suppress differentiation of breast epithelial cells in vitro [59]. A Notch-activating peptide increased secondary and tertiary mammosphere formation tenfold and increased the proportion of cells with multilineage differentiation capacity hundredfold, implying increased mammary stem cell self-renewal and also suggesting that alterations in Notch-4 signaling might play a role in the transition of a healthy stem cell to a CSC [60]. β-Catenin, a downstream target of Wnt signaling pathway, has been identified as a crucial survival signal for mammary epithelial stem cells [61]. Overexpression of Wnt in mouse mammary glands can also lead to increased mammary tumor formation [62]. Mammary tumors arising in mice overexpressing components of this pathway contain cells of both basal/myoepithelial and luminal phenotypes, suggesting an origin from a common precursor [18]. The PITCH membrane protein is a receptor for the Hedgehog (Hh/Patched pathway) family of signaling molecules and has been connected to early embryonic tumorigenesis [63]. Overexpression of components of this pathway, including Shh, Ptch1, and Gli1, is found in a large majority of human breast cancer specimens but not in adjacent normal epithelium consistent with constitutive activation of this pathway [64]. In mice, Ptc-1 haplo-insufficiency leads to abnormal mammary development with ductal dysplasia and hyperplasia by 5 weeks of age [65]. Gli1 heterozygosity results in abnormal mammary development with ductal dysplasia in parous animals [66].
Breast Tumor-Associated Antigens
Breast tumor-associated antigens (TAAs) include epitopes from proteins, such as carcinoembryonic antigen and NYBR-1, which are involved in tissue differentiation [67]. They are overexpressed in breast cancer, or they are shared among a variety of tumors [68]. Breast carcinomas fall into various subtypes associated with different gene expression patterns, phenotypes, and clinical outcomes [69]. However, in a single patient, tumors are heterogenous, with individual tumor cells displaying different phenotypes and TAAs [30].
Mammospheres
Mammospheres have shown expression of markers ESA, CK5, and α6-integrin among many others, which potentially could be used to identify or target breast cancer stem cells (BCSCs) [45, 60]. The activity of aldehyde dehydrogenase-1 (ALDH1), a detoxifying enzyme that may play a role in the differentiation of stem cells, has been detected in both normal and malignant human mammary stem cells and can be used as a predictor for poor clinical outcomes [70]. CD44, another molecule used to identify or target BCSCs, is a membrane receptor involved in cell adhesion, motility, and metastases [71]. CD44 has routinely been used as a marker to purify and enrich BCSCs by selecting for cells that are CD44 + CD24−/lowLin- and ESA + [11]. CD29 (β1-integrin) and CD49f (α6-integrin) expression has also been associated with murine mammary stem cells with a Lin-CD24+ phenotype [20]. Additionally, neither Sca-1 (stem cell antigen) expression nor the side population (SP) phenotype was found to be expressed in the mammary reconstituting Lin-CD29hiCD24+ cell population [20]. However, one report using the human breast cancer line MCF7 has shown that the SP phenotype can be used to identify cells with characteristics of CSCs that express the tumor antigen MUC1, supporting a role for the SP in further analysis of human BCSCs [72]. A recent report has shown that BRCA1-deficient murine breast tumors contain heterogeneous CSC populations [73].
Therapeutic Perspectives Targeting BCSCs
As compared to most cancer cells, CSCs are slow-dividing, have a lower ability to undergo apoptosis and a higher ability of DNA repair, making them more resistant to traditional methods of cancer treatment such as radiation and chemotherapy [30]. In vitro experiments comparing differentiated breast cancer cells grown under monolayer conditions with CD24−/low CD44+ CSCs grown under mammosphere conditions showed that the stem cell-like population was more resistant to radiation [74]. In addition, stem cells express ABC drug transporters, which protect the cell from cytotoxic agents and may lead to multidrug resistance [75]. Targeting BCSCs may be achieved by a number of approaches.
Chemotherapy Sensitization of BCSCs
A wide variety of pathways have been implicated to sensitize BCSCs to the existing chemotherapy, including decreased topoisomerase II expression, reduced sensitivity to apoptosis via alterations in the expression of Bcl2 family members, efflux of chemotherapeutic agents from cancer cells for multidrug resistance, etc. [76]. Several of these mechanisms, in particular resistance to DNA damage and the efflux of toxic compounds, are also advantageous in tissue stem cells, and their activation in malignancy may reflect not only somatic mutation but also their intrinsic activation in the tissue stem cells from which cancer arises. Moreover, as CSCs are at the top of the differentiation hierarchy, they cycle less frequently than other tumor cells and therefore have a greater resistance to antimitotics [19]. Upregulation of cell surface transporters of the ABC family, in particular ABCG2 (breast cancer resistance protein, BCRP), was initially identified as a transcript overexpressed in multiresistant breast cancer [77]. Promising inhibitors for another ABC family transporter implicated in multidrug resistance, P-glycoprotein, are also entering clinical trials [78].
Differentiating Therapy
After debulking of differentiated tumors, targeting of the remaining surviving, often quiescent, CSCs can be achieved by differentiating BCSCs through differentiating therapy or eliminating them via immunotherapy. Retinoic acid and other vitamin A analogues can promote differentiation of epithelial cells and reverse tumor progression through modulation of signal transduction [79]. During metastasis, migrating BCSCs undergo a loss of polarity leading to an epithelial to mesenchymal transition (EMT). Gupta et al. used this attribute of CSCs to develop a high-throughput screen, which successfully identifies small molecules that specifically inhibit CSC proliferation through the induction of differentiation [80]. BMP7 is a potent inhibitor of TGF-β-induced EMT in MDA-231 cancer cells. Decreased BMP7 expression during breast cancer progression may contribute to the acquisition of a bone metastatic phenotype [25].
Targeting Stem Cell Elimination
Present cancer therapy research is mostly focused on targeting specific markers on tumor cells that are overexpressed or mutated and that often represent essential genes/proteins or pathways thought to be important for the development of the tumor.
Traztuzamab targets the HER-2/neu (ErbB2) oncogene which is a member of the epidermal growth factor receptor (EGFR) kinase family and a protein overexpressed on nearly 30 % of breast tumors [81]. Tumors may be not only driven by mutated proteins and inappropriate signaling, but also that epigenetic mechanisms of gene expression of genes involved in “stemness” such as Oct4, Nanog, and Sox2 could be behind tumor formation [82]. Reversal of these epigenetic switches of CSCs could be one novel way to target CSCs [30].
Targeting Signaling Pathways and Drug Transporters
In the mammary gland, Notch, Hedgehog, and Wnt/β-catenin are the three signaling pathways which play a role in stem cell self-renewal and thus represent potential targets for therapy for BCSCs, along with targeting the ABC drug transporters that cancer stem cells use to evade chemotherapy. The use of the cyclopamine to inhibit the Hh signaling pathway has shown some promise in inhibiting the growth of medulloblastoma and could be used in treatments of other tumors [79]. Gunther and colleagues utilized a doxycycline-inducible wnt-1 transgene to show that withdrawal of wnt signaling is sufficient to induce regression of primary mammary tumors and pulmonary metastases [83]. The Wnt pathway can also be inhibited through a variety of other mechanisms. Targeting of β-catenin has received a lot of attention as RA has been shown to inhibit β-catenin activity [84], and tyrosine kinase inhibitors such as imatinib have been shown to downregulate β-catenin signaling [85]. The effects of Notch signaling on stem cells to promote self-renewal and on progenitors promoting their proliferation were completely abrogated by the use of a Notch-4 blocking antibody [86].
Inhibition of Regulatory Pathways Involved in Self-renewal
Recent clinical evidence has established that tumorigenic breast cancer cells with high expression of CD44 and low expression of CD24 are resistant to chemotherapy [87]. Breast cancer patients receiving neoadjuvant chemotherapy had an increase in the CD44+/CD24 low population of cells following treatment. These cells retained the capacity to form mammospheres, thus demonstrating self-renewal, and had an enhanced propensity for forming tumors in SCID/Beige mice compared with pretreatment samples [19]. Treatment of patients with HER-2-positive tumors with lapatinib, an EGFR and HER-2/neu (ErbB-2) dual-tyrosine kinase inhibitor, resulted in nonstatistically significant decreases in the percentage of CD44+/CD24 low population and in the ability for self-renewal as assessed by mammosphere formation [19]. It has been observed that blockading interleukin 8 (IL-8) receptors, such as CXCR1 with the CXCR1 inhibitor repertaxin and alternatively a CXCR1-specific blocking antibody, inhibit some human BCSCs [88].
Immunotherapy Targeting BCSCs
Molecular identification of TAAs can be used as targets for therapy. Dendritic cells (DCs) are professional antigen-presenting cells and initiators of adaptive immunity through processing antigens and presenting epitopes in the context of major histocompatibility complex (MHC) to T cells and play central role to these processes as a result of their role in innate immunity and in generating humoral and cellular immune responses [89]. Evidence for the protective role of the immune system against cancer is seen in the increased incidence of melanoma observed in renal transplant recipients [90]. For breast cancer, it has been observed that DCs and lymphocytic infiltrates in tumors are associated with better prognosis and survival, independent of tumor size [91]. However, in breast cancer, it is also apparent that DC impairment correlates with tumor progression. For instance, co-stimulatory molecule expression and antigen presentation by DCs are decreased in breast cancer patients, with a subsequent impairment in the capacity of these DCs to stimulate T-cell proliferation and secretion of cytokines [92]. DCs of breast cancer patients secrete less interleukin-12 in response to maturation signals [93]. Interestingly, it has also been shown that breast cancer can induce apoptosis in circulating DCs but this process is reversible when the DCs are removed from the inhibitory cancerous environment [94]. These findings suggest a strong role for the immune system in both the progression of disease and as a potential tool to eliminate cancer. Dendritic cell (DC)-based vaccination strategies encompass a variety of different approaches that can be grouped into antigen-defined vaccines and polyvalent vaccines [95]. In one preclinical mouse model, a dendritic cell-based vaccine prevented the outgrowth of a spontaneous breast tumor when used to specifically target a single differentiated HER-2/neu tumor antigen [96]. This vaccine induced the production of anti-neu antibodies and interferon-γ expression by T cells. Although, DC-based vaccines have no serious side effects [97], overall clinical trials with DC-induced antitumor T-cell responses have been disappointing [80, 95]. Most clinical trials using DCs have examined the use of single antigen peptide-loaded DCs [98, 99]. In one trial, using tumor necrosis factor-alpha-matured, monocyte-derived DCs pulsed with MUC1, or HER-2/neu elicited peptide-specific anti-tumor responses, the overall clinical response was modest [100]. The adoptive transfer method has already been used with some success to target cancer [101]. Gene expression comparisons have been conducted and can be used to identify what genes are expressed by the CSC compartment compared with normal stem cells [102].
Chemotherapy Targeting CSCs
Multiple characteristics of CSCs account for resistance to chemotherapy and radiation, including lack of a targetable phenotype and specific oncoprotein expression, high level of MDR1 expression, slow cycling rate, etc. [3]. The use of monoclonal antibodies to target CSCs has been proposed based on the ideas that antibodies can interfere with cancer cell signaling pathways, assist in the delivery of anticancer agents, and facilitate an immune response to tumors [5]. Recently, a compound derived from broccoli, sulforaphane, could help prevent or treat breast cancer by targeting CSCs. Researchers found that sulforaphane targeted and killed CSCs and prevented new tumors from growing [103]. Decitabine, a DNA hypomethylating agent, may target not only active ovarian cancer cells but also CSCs that seem to survive the first treatments [104] (Figs. 1 and 2; Tables 1 and 2).
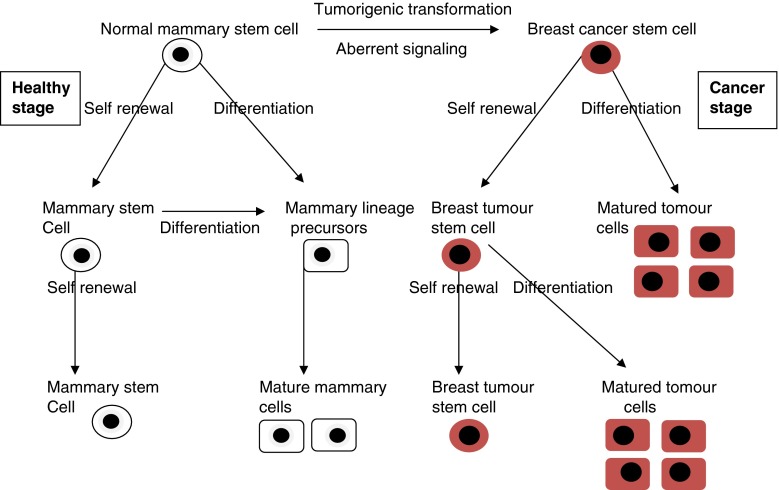
Fig. 1.
Diagrammatic presentation of normal mammary stem cell and breast cancer stem cell
Fig. 2.
Diagrammatic presentation of EMT phenomenon
Table 1.
Breast cancer stem cell (BCSCs) markers
| BCSCs marker | References |
|---|---|
| CD44 + CD24-/loLin- phenotype | [11, 29, 30] |
| HER2 overexpression | [5] |
| Mammosphere formation | [10, 11] |
| Exclusion of fluorescent dye by a side population | [113] |
| Transforming growth factor-β (TGF-β) | [22] |
| BMP-7 expression | [25–27] |
| microRNAs | [33, 34] |
| CXCR4 (Chemokine receptor) | [37] |
| CXCL12 expression | [37] |
| ESA, CK5, and α6- integrin | [45] |
| Aldehyde dehydrogenase-1 (ALDH1) activity | [55] |
| CD44 | [11] |
Table 2.
Therapeutic approaches targeting breast cancer stem cells
| Approach | Targeting pathways/agents |
|---|---|
| Chemotherapy sensitization of BCSCs | Decreased topoisomerase II expression |
| Reduced sensitivity to apoptosis | |
| Efflux of chemical agents | |
| ABCG2 | |
| P-glycoprotein | |
| Differentiating therapy | Vitamin A analogues |
| BMP7 | |
| Targeting stem cell elimination | Traztuzamab |
| Epigenetic mechanisms of gene expression | |
| Targeting signaling pathways and drug transporters | Notch, Hedgehog, and Wnt/β-catenin |
| ABC drug transporters | |
| Inhibition of regulatory pathways involved in self-renewal | Lapatinib |
| Repertaxin | |
| Immunotherapy targeting breast cancer stem cells | Dendritic cell (DC)-based vaccination |
| Chemotherapy targeting cancer stem cells | Monoclonal antibodies |
| Sulforaphane | |
| Decitabine | |
| Targeting metastatic disease | NK1 receptor |
| Tac1 gene | |
| CXCR4 antagonists |
Targeting Metastatic Disease
Targeting cells which have the potential to metastasize among BCSCs will be an important aspect as these are the cells that cause the majority of morbidity and mortality from breast cancer. Chemotherapy shows limited success to target quiescent breast cancer cells in the bone marrow, and several mechanisms of dormancy, as well as tertiary metastasis from the bone marrow, have been proposed [105]. The NK1 receptor is constitutively expressed in neural tissue but inducibly expressed in bone marrow cells and breast epithelial cells [106]. Interactions between peptides of Tac1 gene and NK1 are involved in hematopoietic regulation, depending on the interacting peptide or the signaling receptor [106]. In nontumorigenic MCF12A breast cells, activated nuclear factor-κB (NF-κB) has been shown to suppress Tac1 expression level in the presence of high levels of SDF-1α [49]. Thus, the NK1 receptor offers a valuable pharmacologic target in diseases such as breast cancer, neuroblastoma, and hematological malignancies [107]. Recent investigations on the mechanisms of breast cancer cell metastasis to the marrow cavity have implicated a central role for Tac1. Tac1 appears to regulate the expressions of SDF-1α and CXCR4 on both cancer cells and mesenchymal stem cells [49]. These findings could lead to future studies to define a new method of treatment by targeting the SDF-1α-CXCR4 interactions, and also to target the Tac1 gene. Such treatments could be possible in the near future due to the availability of CXCR4 antagonists [49]. Genes linking to various stages of breast cancer in bone marrow has also been proposed [108]. The promotion of apoptosis by TGF-β has been linked to its interactions with the Survivin gene. TGF-β can downregulate the expression of Survivin at the level of gene transcription, resulting in apoptosis [109]. TGF-β also has the ability to arrest the cell cycle progression in G1 phase via the pRb tumor suppression mechanism, thereby preventing the S phase entry and breast cancer cell proliferation [110]. However, under the regulation ofc-myc, breast cancer cells become less susceptible to the effects of TGF-β [110]. Both NK1 and hematopoietic growth factor inducible neurokinin-1 type (HGFIN) have been linked to tumorigenesis, including breast cancer [107]. HGFIN is a type I transmembrane glycoprotein that maps to the short arm of chromosome 7 and shares structural homology to the NK1 receptor and murine osteoactivin [107]. However, their roles are contrasting. While NK1, in its truncated form, exerts oncogenic properties, HGFIN shows tumor suppressor roles [107]. In breast cancer cells, HGFIN suppresses their growth and migration [48].
Conclusion
There is substantial evidence that proliferation within the normal mammary epithelium is organized in a hierarchical manner. Repopulation experiments and lineage analysis support the existence of pluripotent mammary epithelial stem cells, and cell surface markers have been identified to facilitate their purification. Understanding of the biology of BCSCs should better enable the design of therapies targeted at these CSCs. Gene profiling is a powerful tool in identifying different types of breast cancer with respect to response to therapy, relapse, and metastatic potential. Additionally, targeting universal TAAs such as human telomerase reverse transcriptase and inhibitor of apoptosis proteins might be important for effectively targeting tumors with immunotherapy, as will combining these treatments with ones that target unique stemness-related antigens [111]. Several additional markers and signaling molecules, such as the Hh/patched pathway, as well as the ABCG2 drug transporters could be used as potential targets of therapy for breast cancer [112]. Additionally, markers that are used for migration of BCSCs, such as chemokine receptors, should be explored as potential targets.
In the field of stem cell-targeted therapy, appropriate preclinical models to determine the efficacy of cancer treatment are lacking. It may be important in future studies designed to identify human BCSCs or initiating cells to use improved mouse xenograft models which mimic the human breast microenvironment. Once unique pathways are identified, combination therapy targeting multiple pathways may be more effective than single therapy, based on the observation that CSCs may be heterogeneous with respect to their quiescence state and proliferation capacity as well as the mechanisms underlying their transformation.
References
- 1.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;10(26):2813–2820. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong JF (2007) Probing the biology of cancer stem cells: AACR sheds light on the microenvironment to better target these cells and their pathways. Genet Eng Biotech News 27(10)
- 3.Korkaya H, Paulson A, Iovino F, Wicha MS. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and highgrade oral squamous cell carcinoma. Clin Cancer Res. 2008;14:4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 5.Okamoto OK, Perez JF. Targeting cancer stem cells with monoclonal antibodies: a new perspective in cancer therapy and diagnosis. Expert Rev MolDiagn. 2008;8:387–393. doi: 10.1586/14737159.8.4.387. [DOI] [PubMed] [Google Scholar]
- 6.Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Annabi B, Rojas-Sutterlin S, Laflamme C, Lachambre MP, Rolland Y, Sartelet H, Beliveau R. Tumor environment dictates medulloblastoma cancer stem cell expression and invasive phenotype. Mol Cancer Res. 2008;6:907–916. doi: 10.1158/1541-7786.MCR-07-2184. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Hawkins C, Clarke I, Squire J, Bayani J, Hide T, Henkelman R, Cusimano M, Dirks P. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 10.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG, Di Fiore PP. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140(1):62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Soeda A, Park M, Lee D, et al. Hypoxia promotes expansion of the CD133− positive glioma stem cells through activation of HIF-1α. Oncogene. 2009;28(45):3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Nishimura MC, Bumbaca SM, Kharbanda S, Forrest WF, Kasman IM, Greve JM, Soriano RH, Gilmour LL, Rivers CS, Modrusan Z, Nacu S, Guerrero S, Edgar KA, Wallin JJ, Lamszus K, Westphal M, Heim S, James CD, Vanden Berg SR, Costello JF, Moorefield S, Cowdrey CJ, Prados M, Phillips HS. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17:362–375. doi: 10.1016/j.ccr.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 15.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 16.Liang Y, Zhong Z, Huang Y, Deng W, Cao J, Tsao G, Liu Q, Pei D, Kang T, Zeng YX. Stem-like cancer cells are inducible by increasing genomic instability in cancer cell. J Biol Chem. 2010;285(7):4931–4940. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Welm B, Podsypanina K, Huang S, Chamorro M, Zhang X, Rowlands T, Egeblad M, Cowin P, Werb Z, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci USA. 2003;100:15853–15858. doi: 10.1073/pnas.2136825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch MD, Cariati M, Purushotham AD. Breast cancer, stem cells and prospects for therapy. Breast Cancer Res. 2006;8:211. doi: 10.1186/bcr1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 21.Simic P, Vukicevic S. Bone morphogenetic proteins in development and homeostasis of kidney. Cytokine Growth Factor Rev. 2005;16:299–308. doi: 10.1016/j.cytogfr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 23.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-β signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3:1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 24.Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, ten Dijke P. The tumor suppressor Smad4 is required for transforming growth factor β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 25.Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clézardin P, Papapoulos SE, Cecchini MG, Löwik CW, van der Pluijm G. Bone morphogenetic protein 7 in the development and treatment of bone metastasesfrom breast cancer. Cancer Res. 2007;67(18):8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 26.Schwalbe M, Sanger J, Eggers R, Naumann A, Schmidt A, Hoffken K, Clement JH. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol. 2003;23:89–95. [PubMed] [Google Scholar]
- 27.Takahashi M, Otsuka F, Miyoshi T, Otani H, Goto J, Yamashita M, Ogura T, Makino H, Doihara H. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen-induced proliferation of breast cancer cells by suppressing p38 mitogen-activated protein kinase activation. J Endocrinol. 2008;199(3):445–455. doi: 10.1677/JOE-08-0226. [DOI] [PubMed] [Google Scholar]
- 28.Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. CD44+/CD24-phenotype is enriched in basallike breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrison BJ, Schmidt CW, Sunil R, Lakhani SR, Brent A, Reynolds BA, Lopez JA. Breast cancer stem cells: implications for therapy of breast cancer. Breast Cancer Res. 2008;10:210. doi: 10.1186/bcr2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21(Suppl 7):vii89–vii92. doi: 10.1093/annonc/mdq292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- 33.Thiery J. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis? Recapitulation of morphogenetic processes in cancer. Clin Exp Metastasis. 2007;24:587–597. doi: 10.1007/s10585-007-9114-6. [DOI] [PubMed] [Google Scholar]
- 35.Arnoux V, Come C, Kusewitt DF, Hudson L, Savagner P. Cutaneous wound healing: a partial and reversible EMT. In: Savagner P, editor. Rise and Fall of Epithelial Phenotype: Concepts of Epithelial–Mesenchyme Transition. Berlin: Springer; 2005. pp. 111–134. [Google Scholar]
- 36.Morel A, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyatt L, Wadham C, Crocker LA, Lardelli M, Khew-Goodall Y. The protein tyrosine phosphatase Pez regulates TGFbeta, epithelial-mesenchymal transition, and organ development. J Cell Biol. 2007;178:1223–1235. doi: 10.1083/jcb.200705035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waerner T, Alacakaptan M, Tamir I, Oberauer R, Gal A, Brabletz T, Schreiber M, Jechlinger M, Beug H. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell. 2006;10:227–239. doi: 10.1016/j.ccr.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Beach S, Tang H, Park S, Dhillon AS, Keller ET, Kolch W, Yeung KC. Snail is a repressor of RKIP transcription in metastatic prostate cancer cells. Oncogene. 2008;27:2243–2248. doi: 10.1038/sj.onc.1210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhuang Z, Lininger RA, Man YG, Albuquerque A, Merino MJ, Tavassoli FA. Identical clonality of both components of mammary carcinosarcoma with differential loss of heterozygosity. Mod Pathol. 1997;10:354–362. [PubMed] [Google Scholar]
- 42.Saegusa M, Hashimura M, Kuwata T, Okayasu I. Requirement of the Akt/beta-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of slug. Am J Pathol. 2009;174:2107–2115. doi: 10.2353/ajpath.2009.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, Cornelisse C. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–1925. [PubMed] [Google Scholar]
- 44.Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–1742. [PMC free article] [PubMed] [Google Scholar]
- 45.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Patel SA, Heinrich AC, Reddy BY, Srinivas B, Heidaran N, Rameshwar P. Breast cancer biology: the multifaceted roles of mesenchymal stem cells. J Oncol. 2008;2008:425895. doi: 10.1155/2008/425895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greco SJ, Rameshwar P. MicroRNAs regulate synthesis of the neurotransmitter substance P in human mesenchymal stem cell-derived neuronal cells. Proc Natl Acad Sci USA. 2007;104(39):15484–15489. doi: 10.1073/pnas.0703037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metz RL, Patel PS, Hameed M, Bryan M, Rameshwar P. Role of human HGFIN/nmb in breast cancer. Breast Cancer Res. 2007;9:1–10. doi: 10.1186/bcr1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corcoran KE, Rameshwar P. Nuclear factor-κB accounts for the repressor effects of high stromal cell-derived factor-1α levels on Tac1 expression in nontumorigenic breast cells. Mol Cancer Res. 2007;5:373–381. doi: 10.1158/1541-7786.MCR-06-0396. [DOI] [PubMed] [Google Scholar]
- 50.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 51.Cristofanilli M, Hayes D, Budd G, Ellis M, Stopeck A, Reuben J, Doyle G, Matera J, Allard W, Miller M, Fritsche HA, Hortobagyi GN, Terstappen LW. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 52.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 53.Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, Tan VP, Yau TC, Poon RT, Wong BC. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6(6):603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Vaillant F, Asselin-Labat ML, Shackleton M, Lindeman GJ, Visvader JE. The emerging picture of the mouse mammary stem cell. Stem Cell Rev. 2007;3:114–123. doi: 10.1007/s12015-007-0018-2. [DOI] [PubMed] [Google Scholar]
- 55.Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS. BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA. 2008;105:1680–1685. doi: 10.1073/pnas.0711613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 57.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 58.Gaiano N, Fishell G. The role of Notch in promoting glial and neural stem cell fates. Ann Rev Neurosci. 2002;25:471–490. doi: 10.1146/annurev.neuro.25.030702.130823. [DOI] [PubMed] [Google Scholar]
- 59.Uyttendaele H, Soriano JV, Montesano R, Kitajewski J. Notch4 and Wnt-1 proteins function to regulate branching morphogenesis of mammary epithelial cells in an opposing fashion. Dev Biol. 1998;196:204–217. doi: 10.1006/dbio.1998.8863. [DOI] [PubMed] [Google Scholar]
- 60.Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tepera SB, McCrea PD, Rosen JM. A beta-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci. 2003;116:1137–1149. doi: 10.1242/jcs.00334. [DOI] [PubMed] [Google Scholar]
- 62.Schroeder JA, Adriance MC, McConnell EJ, Thompson MC, Pockaj B, Gendler SJ. ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. J Biol Chem. 2002;277:22692–22698. doi: 10.1074/jbc.M201975200. [DOI] [PubMed] [Google Scholar]
- 63.Hahn H, Wicking C, Zaphiropoulos PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations of the human homolog of drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 64.Kubo M, Nakamura M, Tasaki A, Yamanaka N, Nakashima H, Nomura M, Kuroki S, Katano M. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–6074. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 65.Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Scott MP, Daniel CW. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–5193. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- 66.Hui C, Daniel CW. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev Biol. 2001;238:133–144. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- 67.Theurillat JP, Zurrer-Hardi U, Varga Z, Storz M, Probst-Hensch NM, Seifert B, Fehr MK, Fink D, Ferrone S, Pestalozzi B, Jungbluth AA, Chen YT, Jäger D, Knuth A, Moch H. NY-BR-1 protein expression in breast carcinoma: a mammary gland differentiation antigen as target for cancer immunotherapy. Cancer Immunol Immunother. 2007;56:1723–1731. doi: 10.1007/s00262-007-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pinzon-Charry A, Schmidt C, Lopez JA. Dendritic cell immunotherapy for breast cancer. Expert Opin Biol Ther. 2006;6:591–604. doi: 10.1517/14712598.6.6.591. [DOI] [PubMed] [Google Scholar]
- 69.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lønning P, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 Is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miletti-Gonzalez KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, Apolito K, Shih WJ, Hait WN, Rodriguez-Rodriguez L. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. 2005;65:6660–6667. doi: 10.1158/0008-5472.CAN-04-3478. [DOI] [PubMed] [Google Scholar]
- 72.Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 2008;68:2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- 73.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24- and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10:R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phillips T, McBride W, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 75.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 76.Norgaard JM, Olesen LH, Hokland P. Changing picture of cellular drug resistance in human leukemia. Crit Rev Oncol Hematol. 2004;50:39–49. doi: 10.1016/S1040-8428(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 77.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glyco-protein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 79.Massard C, Deutsch E, Soria JC. Tumour stem cell-targeted treatment: elimination or differentiation. Ann Oncol. 2006;17:1620–1624. doi: 10.1093/annonc/mdl074. [DOI] [PubMed] [Google Scholar]
- 80.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138(4):645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eddy SF, Kane SE, Sonenshein GE. Trastuzumab-resistant HER2-driven breast cancer cells are sensitive to epigallocate-chin-3 gallate. Cancer Res. 2007;67:9018–9023. doi: 10.1158/0008-5472.CAN-07-1691. [DOI] [PubMed] [Google Scholar]
- 82.Ting AH, McGarvey KM, Baylin SB. The cancer epigenome—components andfunctional correlates. Genes Dev. 2006;20:3215–3231. doi: 10.1101/gad.1464906. [DOI] [PubMed] [Google Scholar]
- 83.Gunther EJ, Moody SE, Belka GK, Hahn KT, Innocent N, Dugan KD, Cardiff RD, Chodosh LA. Impact of p53 loss on reversal and recurrence of conditional Wnt-induced tumorigenesis. Genes Dev. 2003;17:488–501. doi: 10.1101/gad.1051603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luu HH, Zhang R, Haydon RC, Rayburn E, Kang Q, Si W, Park JK, Wang H, Peng Y, Jiang W, He TC. Wnt/beta-catenin signaling pathway as a novel cancer drug target. Curr Cancer Drug Targets. 2004;4:653–671. doi: 10.2174/1568009043332709. [DOI] [PubMed] [Google Scholar]
- 85.Zhou L, An N, Haydon RC, Zhou Q, Cheng H, Peng Y, Jiang W, Luu HH, Vanichakarn P, Szatkowski JP, Park JY, Breyer B, He TC. Tyrosine kinase inhibitor STI-571/Gleevec down-regulates the beta-catenin signaling activity. Cancer Lett. 2003;193:161–170. doi: 10.1016/s0304-3835(03)00013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–R615. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 88.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120(2):485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Banchereau J, Steinman R. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 90.Hollenbeak CS, Todd MM, Billingsley EM, Harper G, Dyer AM, Lengerich EJ. Increased incidence of melanoma in renal transplantation recipients. Cancer. 2005;104:1962–1967. doi: 10.1002/cncr.21404. [DOI] [PubMed] [Google Scholar]
- 91.Menard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, Salvadori B, Colnaghi MI, Rilke F. Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 1997;3:817–819. [PubMed] [Google Scholar]
- 92.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 93.Della Bella S, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinzon-Charry A, Maxwell T, McGuckin MA, Schmidt CW, Furnival C, Lopez JA. Spontaneous apoptosis of blood dendritic cells in patients with breast cancer. Breast Cancer Res. 2006;8:R5. doi: 10.1186/bcr1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I: Vaccines for solid tumours. Lancet Oncol. 2004;5:681–689. doi: 10.1016/S1470-2045(04)01610-9. [DOI] [PubMed] [Google Scholar]
- 96.Sakai Y, Morrison BJ, Burke JD, Park JM, Terabe M, Janik JE, Forni G, Berzofsky JA, Morris JC. Vaccination by genetically modified dendritic cells expressing a truncated neu oncogene prevents development of breast cancer in transgenic mice. Cancer Res. 2004;64:8022–8028. doi: 10.1158/0008-5472.CAN-03-3442. [DOI] [PubMed] [Google Scholar]
- 97.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21:873–886. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- 98.Panelli MC, Wunderlich J, Jeffries J, Wang E, Mixon A, Rosenberg SA, Marincola FM. Phase 1 study in patients with metastatic melanoma of immunization with dendritic cells presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. J Immunother. 2000;23:487–498. doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 100.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptidepulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 101.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8+ T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. Stemness: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 103.Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16(9):2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fang F, Balch C, Schilder J, Breen T, Zhang S, Shen C, Li L, Kulesavage C, Snyder AJ, Nephew KP, Matei DE. Cancer, early view (Articles online in advance of print). http://www3.interscience.wiley.com/journal/123500856/abstract [DOI] [PMC free article] [PubMed]
- 105.Taborga M, Corcoran KE, Fernandes N, Ramkissoon SH, Rameshwar P. Gcoupled protein receptors and breast cancer progression: potential drug targets. Mini Rev Med Chem. 2007;7(3):245–251. doi: 10.2174/138955707780059826. [DOI] [PubMed] [Google Scholar]
- 106.Bandari PS, Qian J, Yehia G, Joshi DD, Maloof PB, Potian J, Oh HS, Gascon P, Harrison JS, Rameshwar P. Hematopoietic growth factor inducible neurokinin-1 type: a transmembrane protein that is similar to neurokinin 1 interacts with substance P. Regul Pept. 2003;111(1–3):169–178. doi: 10.1016/s0167-0115(02)00288-4. [DOI] [PubMed] [Google Scholar]
- 107.Rameshwar P. Implication of possible therapies targeted for the tachykinergic system with the biology of neurokinin receptors and emerging related proteins. Recent Pat CNS Drug Discov. 2007;2(1):79–84. doi: 10.2174/157488907779561781. [DOI] [PubMed] [Google Scholar]
- 108.Kretzschmar M. Transforming growth factor-β and breast cancer: transforming growth factor-β/SMAD signaling defects and cancer. Breast Cancer Res. 2000;2:107–115. doi: 10.1186/bcr42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang J, Song K, Krebs TL, Jackson MW, Danielpour D. Rb/E2F4 and Smad2/3 link survivin to TGF-β-induced apoptosis and tumor progression. Oncogene. 2008;27:5326–5338. doi: 10.1038/onc.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oh HS, Moharita A, Potian JG, Whitehead IP, Livingston JC, Castro TA, Patel PS, Rameshwar P. Bone marrow stroma influences transforming growth factor-β production in breast cancer cells to regulate c-myc activation of the preprotachykinin-I gene in breast cancer cells. Cancer Res. 2004;64:6327–6336. doi: 10.1158/0008-5472.CAN-03-3122. [DOI] [PubMed] [Google Scholar]
- 111.Parmiani G, Russo V, Marrari A, Cutolo G, Casati C, Pilla L, Maccalli C, Rivoltini L, Castelli C. Universal and stemness-related tumor antigens: potential use in cancer immunotherapy. Clin Cancer Res. 2007;13:5675–5679. doi: 10.1158/1078-0432.CCR-07-0879. [DOI] [PubMed] [Google Scholar]
- 112.Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357–1360. doi: 10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 113.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel MA. A distinct “side population” of cells with high drug efflux capacity in human tumor cells . Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]