Abstract
Liver transplantation is a therapeutic option of choice for acute and chronic end-stage liver disease. Indications, contraindications, and surgical procedures for the liver transplantation have become well established. In most part of the world, the main source of liver for transplantation remains the donation after brain death (DBD), but in view of increasing death on the waiting list due to shortage of brain dead organs other options such as split liver transplantation, living donor liver transplantation (LDLT), and donation after cardiac death (DCD) have been used. In the pretransplantation era, liver failure was nearly universally fatal, with mortality from fulminant hepatic failure of 80–90 %, and 1-year mortality in decompensated cirrhosis of more than 50 %. In contrast, liver transplantation patient survival is presently more than 85 % at 1 year and more than 70 % at 5 years, emphasizing the clinical benefit of liver transplantation for either acute or chronic liver failure.
Keywords: Liver transplantation, End-stage liver disease, Living donor
Introduction
Liver transplantation is now the treatment of choice for selected groups of patients with end-stage liver disease or liver failure. Liver failure can be either acute (fulminant or subfulminant failure) or chronic (decompensated cirrhosis). In the pretransplantation era, liver failure was nearly universally fatal, with mortality from fulminant hepatic failure of 80–90 %, and 1-year mortality in decompensated cirrhosis of more than 50 %. In contrast, following liver transplantation, patient survival is more than 85 % at 1 year and more than 70 % at 5 years [1]. Thus, the liver transplantation has become the standard of care for either acute or chronic liver failure.
Liver Transplantation for Acute Liver Failure
Acute liver failure (often used synonymously with fulminant liver failure) is defined as an acute hepatic decompensation without history of chronic liver disease, which has progressed from the onset of jaundice to the development of hepatic encephalopathy within weeks [2]. Acute liver failure includes fulminant with the onset of encephalopathy within 8 weeks and subfulminant hepatic failure with the onset of encephalopathy up to 26 weeks from the onset of jaundice—a difference that reflects the greater predominance of brain edema and intracranial hypertension in patients with the shorter interval. Both drug-induced hepatic failure and an indeterminate etiology seem to be more commonly associated with a longer interval (Table 1).
Table 1.
Common etiology of acute liver failure
| Hepatitis non A–E |
| Hepatitis B |
| Hepatitis E |
| Drug induced |
| Hepatitis A |
| Others—Wilson’s disease, acute fatty liver of pregnancy, Budd–Chiari syndrome, autoimmune hepatitis, ischemic injury |
Liver transplantation is currently the best therapeutic option for acute irreversible liver failure. Commonly used criteria for selecting patients with acute liver failure for liver transplantation are given in Box 1.
Box 1. Criteria for transplantation in acute liver failure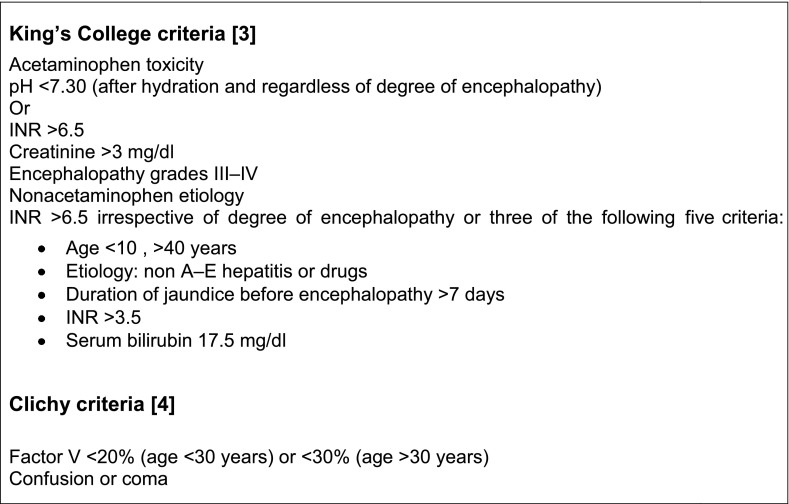
Early referral to a liver transplantation center is essential due to the following reasons:
It is difficult to predict which patients will recover spontaneously.
Deterioration can occur suddenly.
The chance of receiving a liver transplant increases with early placement on the waiting list.
Once brainstem herniation has occurred, patients are not salvageable by any means, including liver transplantation. The survival following liver transplantation for acute liver failure is about 90 % [5].
Liver Transplantation for Chronic Liver Disease
Cirrhosis
Cirrhosis due to any etiology should be considered for liver transplantation (Table 2). Transplantation is usually considered once complications of cirrhosis develop. Certain metabolic diseases can present with liver failure without cirrhosis. This occurs more commonly in the pediatric population, but can occasionally extend into young adulthood. Some congenital abnormalities (e.g., urea cycle enzyme deficiencies, familial hypercholesterolemia, and familial amyloidosis) can present with such severe extrahepatic manifestations that liver transplantation is recommended in the absence of hepatic disease (Table 3). Finally, miscellaneous chronic disorders may require transplantation in the absence of both cirrhosis and hepatic failure.
Table 2.
Causes of cirrhosis
| Hepatocellular diseases |
| Chronic hepatitis |
| Hepatitis B |
| Hepatitis D |
| Hepatitis C |
| Autoimmune |
| Drug-induced (e.g., nitrofurantoin and alpha-methyldopa) |
| Steatohepatitis |
| Alcohol |
| Obesity |
| Drug-induced (e.g., amiodarone) |
| Vascular disease |
| Chronic Budd–Chiari syndrome |
| Inborn errors of metabolism |
| Hemochromatosis |
| a1-Antitrypsin deficiency |
| Wilson’s disease |
| Glycogen storage disease type I/III |
| Cholestatic diseases |
| Disease of intrahepatic bile ducts |
| Biliary atresia |
| Primary biliary cirrhosis |
| Drug-induced disease (e.g., chlorpromazine and tolbutamide) |
| Familial cholestasis, Byler’s syndrome, arteriohepatic dysplasia |
| Cystic fibrosis |
| Disease of extrahepatic bile ducts |
| Primary sclerosing cholangitis |
| Secondary biliary cirrhosis |
Table 3.
Liver abnormality requiring liver transplantation without cirrhosis
| Congenital abnormalities |
| Urea cycle enzyme deficiency |
| Homozygous hypercholesterolemia |
| Primary hyperoxaluria |
| Familial amyloidotic polyneuropathy |
| Developmental abnormalities |
| Polycystic liver disease |
| Caroli’s disease |
Primary Liver Cancer
About 80 % of people with hepatocellular carcinoma (HCC) have cirrhosis. HCC is curable by surgery only if the cancer is small and the patient has adequate hepatic reserve. In patients with hepatic insufficiency or severe portal hypertension, liver transplantation may also be curative for the cancer and the underlying hepatic disorder. Surgery or liver transplantation is contraindicated if there is evidence of metastases. The currently accepted criterion for selecting patients with HCC for liver transplantation is the Milan criteria where 80 % 2-year recurrence-free survival after transplantation has been reported [6]. As per this criterion, patients with HCC having single tumor of less than 5 cm and maximum three lesions each of less than 3 cm without vascular invasion or metastatic disease are selected for transplantation. Cholangiocarcinoma is now another potential indication for liver transplantation. The preferred situation is in patients with primary sclerosing cholangitis who have cytologic evidence of intrahepatic carcinoma. Certain secondary liver cancers, such as neuroendocrine tumors and salivary gland tumors, may be treated by liver transplantation. Such lesions need to be low-grade malignancies without extrahepatic spread to diminish the risk of recurrence after transplantation.
Exclusion Criteria (Contraindications)
Although liver transplantation is not absolutely contraindicated at any specific chronologic age, evaluation of physiologic age requires a thorough clinical assessment, including evaluation of cardiac function. Coexisting medical conditions need to be excluded. Uncontrolled sepsis outside the biliary tract constitutes an absolute contraindication to liver transplantation. Metastatic hepatobiliary or extrahepatic malignancies also constitute absolute contraindications. For extrahepatic cancers, a waiting period of 5 years after treatment of a solid organ tumor and 2 years after treatment of a hematologic malignancy is recommended. Although HIV infection is no longer a contraindication, the presence of AIDS is a contraindication to transplantation because post-transplant immunosuppression accelerates the course of AIDS. Irreversible brain damage and multiorgan failure also preclude liver transplantation.
Psychosocial Assessment
Assessment of the patient’s lifestyle, psychologic stability (including his or her perception of disability), and extent of family support requires interaction with psychiatric and social work support services. This evaluation is critical for patients with alcoholic liver disease. The ability to abstain from alcohol after transplantation is predicted by the ability to abstain before transplantation for at least 6 months [7], and a family and friend support structure.
Selection Criteria and Listing Process
The decision to proceed with transplantation requires careful assessment of the etiology and stage of liver disease, complications of cirrhosis, potential contraindications, and a comprehensive psychosocial evaluation. The severity of liver disease is assessed by the MELD (model of end-stage liver disease) score [8]. This score, based on objective laboratory values, predicts the 3-month mortality of patients awaiting liver transplantation. The MELD score incorporates serum creatinine, serum bilirubin, and INR. There are MELD calculators available online at several websites.
Recipient Operation
When a suitable donor is identified, a rapid evaluation of the recipient is performed to exclude potential contraindications (e.g., infectious and cardiovascular) that may have arisen during the waiting period.
Standard Surgical Technique
The recipient operation consists of total hepatectomy of the native liver followed by implantation of the donor liver. The native hepatectomy can be technically difficult, especially in patients with previous upper abdominal operations and severe portal hypertension. The ligamentous attachments of the liver are divided, followed by skeletonization of the hilar structures (bile duct, hepatic artery, and portal vein) to prepare for implantation of the new liver. The inferior vena cava (IVC) is encircled above and below the liver to achieve full vascular control. Committing the patient to transplant, the bile duct and hepatic artery are divided. Vascular clamps are placed on the portal vein and the IVC below and above the liver, and the liver is removed by transecting the portal vein and the IVC. The retrohepatic IVC is removed with the liver. The donor liver is surgically prepared for implantation on the back table. Anastomoses are constructed between the donor liver and the recipient patient in the following sequence: suprahepatic IVC, infrahepatic IVC, and portal vein anastomosis. Once the portal vein is anastomosed, the clamps are removed in sequence and the liver is perfused with portal venous inflow. Some centers use venous–venous bypass, and other centers never use it, but most centers use venous–venous bypass in selected patients, especially for a difficult hepatectomy (hemorrhage, cardiovascular instability). Venous–venous bypass requires cannulation of a lower extremity vein (typically the femoral vein), an upper extremity or neck vein, and the portal vein. This circuit allows decompression of the portal venous system and maintenance of cardiac preload during the anhepatic phase [9]. The hepatic artery is typically connected to the recipient hepatic artery. Donor iliac arteries are routinely harvested during the donor procedure. These arteries can be used to construct a conduit between the recipient aorta and the donor hepatic artery or celiac axis. Once the liver is arterialized and the hepatic artery demonstrates satisfactory flow, hemostasis is achieved, and the bile duct is reconstructed using an end-to-end choledochocholedochostomy. If the recipient bile duct is not appropriate for end-to-end reconstruction, a Roux-en-Y choledochojejunostomy is performed.
Alternative Technique
Piggyback Procedure
The recipient hepatectomy can be altered to leave the recipient retrohepatic IVC in situ. The donor IVC is then anastomosed side-to-side to the recipient IVC, and the remaining structures are anastomosed in the standard fashion [10].
Split-Liver Procedure
The use of split livers has become an option for selected donor livers for most liver recipients. The liver is typically split along the falciform ligament separating the left lateral segment (Couinaud segments II and III) from the remaining liver. The main hilar vascular and biliary structures are retained with the right side of the liver. The left lateral segment is typically transplanted into a child and the remaining liver is transplanted into an adult. In this procedure, it is important to secure hemostasis at the cut hepatic surface and to check carefully for biliary leaks at this surface. Split-liver transplants, when performed on properly selected recipients using suitable donor livers, have survival results comparable with whole livers, but have a slightly higher rate of surgical complications [11, 12].
Postoperative Care
Major considerations in the immediate postoperative period include liver function, postoperative bleeding, and general considerations.
Liver Function
Indicators of good early graft function include normalization of prothrombin time and transaminase levels. In addition, clearance of lactic acidosis, awakening from the anesthetized state, and good renal function provide further affirmation of liver function. Primary nonfunction of the graft will lead to graft failure and need for retransplantation [13].
Postoperative Bleeding
Significant coagulopathy can be present following hepatic revascularization, attributed to fibrinolysis, heparin-like effect, thrombocytopenia, and coagulation factor deficiencies. Usually, coagulopathy is reversed by the time of abdominal closure with a functioning graft. If ongoing bleeding, despite correction of coagulopathy and rewarming of the patient, is suspected, especially if hemodynamic instability and oliguria are present, the patient should undergo reoperation to evacuate a hematoma and to identify and stem the ongoing bleeding. Postoperative bleeding is high in the differential diagnosis of early postoperative hypotension and oliguria.
General Consideration
Pulmonary management consists of standard ventilatory support. Pulmonary complications are common in the postoperative period because of the large surgical incision, the debilitated state of the patient, and frequent pleural effusions. Pulmonary care is highly important following extubation. Laboratory testing includes careful attention to the serum levels of glucose and electrolytes. In addition to attention to sodium and potassium levels, magnesium levels are typically low and magnesium supplementation is required. The ionized calcium level should be frequently determined and normalized. The transaminase levels and prothrombin time should normalize during the first 24 h. In some centers, a baseline Doppler ultrasound to assess patency of the hepatic artery is performed within the first 24 h after transplantation.
Liver Function Abnormality
Vascular Complications
Hepatic artery thrombosis is the most common vascular complication after liver transplantation [14]. Hepatic artery thrombosis presents with various liver test abnormalities, including very subtle elevations in the serum transaminases, and may not be diagnosed in the early postoperative period and manifest later with biliary complications, such as bile leaks, bilomas, liver abscesses, and biliary strictures . An abnormal trend in liver function tests should be investigated immediately with Doppler ultrasound and, if the hepatic arterial signal is not clearly seen, a hepatic arteriogram and radiologic or operative intervention should be performed. Retransplantation may be necessary, especially if liver function is severely compromised in the early postoperative period. Hepatic artery thrombosis is usually related to technical complications. Optimal flow should be ensured during implantation, even using quantitative measurements, to avoid this complication [15]. Portal venous thrombosis is uncommon, but can occur in the setting of significant portal vein stenosis or previous portal vein thrombosis in the recipient, especially in the pediatric recipient. Typically, severe elevations in the serum transaminase levels occur early postoperatively. Ascites is a manifestation of delayed portal vein thrombosis. Also, acute portal hypertension manifested by variceal bleeding should alert the clinician to possible acute portal vein thrombosis. In the acute setting, thrombectomy should be attempted to try to save the graft, although retransplantation may be necessary. Venous outflow obstruction causing a Budd–Chiari-like congestion of the liver can occur. In the early postoperative period, a significant elevation in the transaminase levels results from the acute congestion, requiring surgical correction, whereas delayed manifestations consist primarily of ascites and manifestations of portal hypertension, which may be addressed by endovascular techniques.
Biliary Tract Complications
Anastomotic biliary leaks can occur early postoperatively, resulting in localized or generalized peritonitis. Biliary output from the drains and elevation in serum bilirubin disproportionate with the elevation of the other liver function tests raise this diagnostic possibility. Early leaks are best treated by reoperation and revision to a Roux-en-Y choledochojejunostomy. Localized leaks may be treated with endoscopic retrograde cholangiopancreatography (ERCP) and stenting [16]. Delayed complications include bile duct anastomotic strictures and intrahepatic biliary strictures, sometimes related to hepatic artery thrombosis. These strictures are usually dilated and stented at ERCP. When these interventions fail, biliary reconstruction with a Roux-en-Y choledochojejunostomy may be necessary. Dysfunctional motility of the bile duct and sphincter of Oddi may cause functional obstruction without mechanical obstruction [17]. These problems manifest later in the postoperative period. Also, biliary casts and stones can form, especially with prolonged ischemia, and cause biliary obstruction requiring ERCP intervention.
Rejection
Rejection can occur in the first few days after transplantation, especially if induction immunosuppressive therapy is not used. The liver function test abnormalities can be hepatocellular or cholestatic. Diagnosis is made by liver biopsy because the clinical signs and symptoms of rejection (including fever, elevation of the bilirubin or transaminase levels, malaise, and increased ascites) are highly variable, nonspecific, and unreliable. Acute cellular rejection usually occurs between the fourth and fourteenth days after transplantation and rarely occurs more than 3 months after transplantation. Some patients are asymptomatic, whereas others experience profound symptoms caused by liver allograft failure. The diagnosis of allograft rejection is confirmed by histologic examination of a liver biopsy. Classic histologic findings include a portal infiltrate consisting of mixed inflammatory cells. The presence of eosinophils, lymphocyte-mediated bile duct injury, and endothelialitis can be diagnostic.
Infection
Abnormal liver function tests secondary to infection most commonly arise from viral infections, including cytomegalovirus hepatitis, and recurrence of previous viral hepatitides. Cytomegalovirus hepatitis is diagnosed by the presence of inclusion bodies with clusters of polymorphonuclear cells. Evidence of tissue invasion is often associated with symptoms of fever, general malaise, and myalgias. The diagnosis is established by positive antigenemia tests or polymerase chain reaction. Treatment consists of reduction in immunosuppression and antiviral agents, such as ganciclovir. Bacterial or fungal systemic infections may also result in abnormal liver function tests, usually with a cholestatic pattern. Hepatic abscesses may occur and result in abnormal liver function tests. They typically result from hepatic artery thrombosis and are diagnosed radiologically. ERCP may be helpful in delineating the extent of biliary duct disruption.
Postoperative Care
Immunosuppression
Baseline immunosuppression is instituted in the immediate postoperative period. It typically consists of a calcineurin inhibitor, either cyclosporine or tacrolimus, mycophenolate mofetil, and corticosteroids. Corticosteroids are administered initially as intravenous, and switched to oral prednisone once the patient tolerates oral feedings [18]. In addition to immunosuppressive agents, prophylaxis is initiated against Pneumocystis carinii pneumonia and against cytomegalovirus. Antifungal prophylaxis is achieved with swish and swallow of a nystatin suspension or other similar topical antifungals. Fluconazole or itraconazole can be used in the early postoperative period for prophylaxis against systemic fungal infections. Standard antibacterial prophylaxis necessitates coverage of gram-negative and anaerobic agents typically present in bile.
Early Outpatient Care
When the patient tolerates an oral diet and can ambulate, he or she can be discharged and closely followed in an outpatient clinic. Typically, blood work is obtained three times weekly, and the patients are examined weekly, according to a standard, established protocol. At clinic visits the patient’s medications are carefully reviewed to avoid errors. Abnormalities of liver function tests or of other laboratory tests are investigated. The standard evaluation of abnormal liver function tests includes an abdominal ultrasound with Doppler examination to look for hepatic vascular patency, dilatation of the biliary tree, and abnormalities within the hepatic parenchyma, such as liver abscesses. If the abdominal ultrasound is unremarkable, the next step usually consists of percutaneous liver biopsy to exclude rejection or infection. The immunosuppressive agents have side effects. Side effects of the calcineurin inhibitors include nephrotoxicity, neurotoxicity, hyperkalemia, hypomagnesemia, hypertension, and tremor. Tacrolimus can also induce new-onset diabetes, and is more prone to cause abdominal pain and diarrhea than cyclosporine. Both calcineurin inhibitors are metabolized through the cytochrome P-450 system. Their serum levels are increased by erythromycin; antifungal agents, such as ketoconazole, fluconazole, and itraconazole; and calcium channel blockers, such as diltiazem, verapamil, and nicardipine. Drugs that decrease their serum levels include antiseizure medications, such as phenytoin, phenobarbital, and carbamazepine, and many antituberculosis medications, such as isoniazid, rifampin, and rifabutin. Twelve-hour serum trough levels are measured and monitored closely to guide the dosage of administration. Azathioprine and mycophenolate mofetil primarily cause leukopenia. The dosage of these agents must be adjusted according to the leukocyte count. The availability of granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor has made leukopenia much easier to manage in these patients.
Live-Donor Liver Transplantation
During the past decade, the gap between the number of adult patients who need liver transplantation and the number of donated organs has greatly increased all over the world. In India cadaver donation is still a rarity. Attempts to address the inadequate supply of donor organs for transplant have included the use of marginal donors (old age, poor hemodynamics, or chronic viral infection). Living donors have also been used to address this need. In India majority of the transplant being done is from living donors. Use of a living donor graft was first used for pediatric recipient more than a decade ago [10]. This option decreases the waiting list mortality, and produces excellent recipient results with a low risk of morbidity and mortality in the donor. This concept was extended to adult LDLT [11]. The adult LDLT procedure usually involves transplantation of the right hepatic lobe from an adult donor to the recipient.
Potential donors are blood group compatible and are completely healthy. Their hepatic size and anatomy should also be compatible with right lobe liver transplantation. Donor surgery involves removal of the right lobe with or without the middle hepatic vein. Once harvested liver lobe is flushed with preservative fluid and venous reconstruction is done and prepared for implantation. The recipient operation involves IVC preserving hepatectomy and anastomosis of donor right side vascular and biliary structures to corresponding recipient structures.
Summary
Quality-of-life studies have shown that most patients have an excellent quality of life following transplantation, with 1-year patient survival at 90 % and 5-year patient survival at 75 %. De novo malignancies and recurrence of native disease, however, remain as significant challenges. The most significant current hurdle is a dramatic donor organ shortage, although living donor transplants are being increasingly used. The discrepancy between needy recipients and available organ donors necessitates more aggressive and innovative management algorithms for the complications of cirrhosis.
References
- 1.Riordan SM, Williams R. Perspectives on liver failure: past and future. Semin Liver Dis. 2008;28:137–141. doi: 10.1055/s-2008-1073113. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou JP. Fulminant and sub-fulminant hepatic failure: definition and causes. In: Williams R, Hughes RD, editors. Acute liver failure: improved understanding and better therapy. London: Mitre; 1991. pp. 6–10. [Google Scholar]
- 3.O’Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–445. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 4.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97–106. doi: 10.1055/s-2008-1040593. [DOI] [PubMed] [Google Scholar]
- 5.Ascher NL, Lake JR, Emond JC, et al. Liver transplantation for fulminant hepatic failure. Arch Surg. 1993;128:677–682. doi: 10.1001/archsurg.1993.01420180079015. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nüssler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2007;13:197–205. doi: 10.1002/lt.20934. [DOI] [PubMed] [Google Scholar]
- 8.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 9.Chari RS, Gan TJ, Robertson KM, Bass K, Camargo CA, Jr, Greig PD, et al. Venovenous bypass in adult orthotopic liver transplantation: routine or selective use? J Am Coll Surg. 1998;186:683–690. doi: 10.1016/S1072-7515(98)00101-X. [DOI] [PubMed] [Google Scholar]
- 10.Jovine E, Mazziotti A, Grazi GL, Ercolani G, Masetti M, Morganti M, et al. Piggy-back versus conventional technique in liver transplantation: report of a randomized trial. Transpl Int. 1997;10:109–112. doi: 10.1111/j.1432-2277.1997.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 11.Broelsch CE, Emond JC, Whitington PF, et al. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368–375. doi: 10.1097/00000658-199009000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes J, Gerber D, Mazariegos GV, et al. Split-liver transplantation: a comparison of ex vivo and in situ techniques. J Pediatr Surg. 2000;35:283–289. doi: 10.1016/S0022-3468(00)90026-5. [DOI] [PubMed] [Google Scholar]
- 13.Greig PD, Woolf GM, Sinclair SB, et al. Treatment of primary liver graft nonfunction with prostaglandin E1. Transplantation. 1989;48:447–453. doi: 10.1097/00007890-198909000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Langnas AN, Marujo W, Stratta RJ, Wood RP, Shaw BW., Jr Vascular complications after orthotopic liver transplantation. Am J Surg. 1991;161:76–82. doi: 10.1016/0002-9610(91)90364-J. [DOI] [PubMed] [Google Scholar]
- 15.Abbasoglu O, Levy MF, Testa G, et al. Does intraoperative hepatic artery flow predict arterial complications after liver transplantation? Transplantation. 1998;66:598–601. doi: 10.1097/00007890-199809150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Todo S, Furukawa H, Kamiyama T. How to prevent and manage biliary complications in living donor liver transplantation? J Hepatol. 2005;43:22–27. doi: 10.1016/j.jhep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Douzdjian V, Abecassis MM, Johlin FC. Sphincter of Oddi dysfunction following liver transplantation: screening by bed-side manometry and definitive manometric evaluation. Dig Dis Sci. 1994;39:253–256. doi: 10.1007/BF02090194. [DOI] [PubMed] [Google Scholar]
- 18.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]


