Abstract
Aeromonads are inhabitants of aquatic ecosystems and are described as being involved in intestinal disturbances and other infections. The purpose of this study was to investigate the production of N-acyl-homoserine lactone (AHL) signal molecules and some virulence factors, including hemolysins, proteases, extracellular nucleases production and cytotoxicity by waterborne Aeromonas hydrophila. A total of 24 strains isolated from fresh-water or diseased fish were used in the study. The majority A.hydrophila strains produce two AHL molecules (21/24), one is N-butanoyl homoserine lactone (BHL), and the other is N-hexanoyl homoserine lactone (HHL) according to thin-layer chromatography analysis. Among the virulence factors tested, more than 83 % of the isolates produced β haemolysin when inoculated on sheep blood agar, only 50 % of the isolates displayed DNase activity, 75 % of the isolates shown proteolytic activity on skimmed milk plate, and cytotoxic activity was detected in 20 of 24 of the isolates. The strains producing AHLs possessed one or more virulence factors. In conclusion, the production of quorum sensing signal molecules is common among the strains that we examined, and there seems to some relationships between quorum sensing signal production and virulence factors in A. hydrophila.
Keywords: Aeromonas hydrophila, N-acy homoserine lactone, Quorum sensing signal molecule, Virulence factors
Introduction
In most bacteria, a global level of regulation exists involving intercellular communication via the production and response to cell density dependent signal molecules. This cell-density-dependent regulation has been termed quorum sensing (QS) [1, 2]. This mechanism has been implicated in regulating the virulence factors in a number of bacterial pathogens, such as Vibrio cholerae, the bacterium responsible for the severe diarrheal disease cholera; Pseudomonas aeruginosa, the opportunistic pathogen responsible for death in cystic fibrosis patients and high mortality rates in immunocompromised individuals; and Staphylococcus aureus, a major culprit of infections in surgical wounds [3, 4]. Consequently, improved understanding of quorum-sensing regulation should provide more insight into combating these pathogens by using either traditional chemotherapy or emerging technologies such as quorum-sensing inhibitors or regulated degradation of quorum-sensing signals [5–8].
Aeromonas hydrophila is a ubiquitous gram-negative bacterium, which has been implicated as a causative agent of motile aeromonad septicemia in a variety of aquatic animals especially freshwater fish species [9]. It causes gastrointestinal and extraintestinal infections in humans, including septicemia, wound infections, gastroenteritis and peritonitis [10]. A number of virulence factors have been identified in A. hydrophila, such as, adhesins, S-layers, exotoxins such as hemolysins, enterotoxin, proteases, amylases, and lipases [11, 12]. A. hydrophila has been found to have homologues of the V. fischeri quorum sensing genes luxI and luxR, designated ahyI and ahyR. The A. hydrophila quorum sensing system is of particular interest as the quorum sensing regulated phenotypes such a extracellular proteases are decreased in the presence of long chain N-acyl-l-homoserine lactones (AHLs) such as 3-oxo-C12-HSL [13].
In the present study, all the waterborne isolates of A. hydrophila collected were investigated for their AHL signal molecule profile and their virulence factors including hemolysins, proteases, extracellular nucleases production and cytotoxicity. The purpose of the study was to elucidate any possible correlation between AHL production and phenotype of the micro-organisms.
Materials and Methods
Bacterial Strains and Culture Conditions
The A. hydrophila strains used in this study (Table 1) were isolated from fresh water or diseased fish, and was identified by standard biochemical diagnostic kits (Microbact Identification kit24E) and 16S rDNA PCR confirmed. A. hydrophila strains were grown in LB medium at 28 °C with shaking. Chromobacterium violaceum CV026 (a mini-Tn5 mutant) which was used as an indicator strain for the detection of C4 and C6-HSLs, was kindly supplied by Dr. McLean (Texas State University) and was grown in LB medium at 30 °C. Media were solidified with 1.5 % (wt/vol) agar as needed. Antibiotics were added as required at the following final concentrations: kanamycin, 50 μg mL−1. Bacterial growth was monitored turbidimetrically at 600 nm.
Table 1.
Production of N-acyl-homoserine lactones (AHLs) and virulence factors of Aeromonas hydrophila
| Strains | AHLs | Protease | Hemolysin | Nucleases | Cytotoxicity |
|---|---|---|---|---|---|
| YJ-1 | + | + | + | + | + |
| HAE-1 | + | + | + | + | + |
| HAE-2 | + | + | + | – | + |
| B-1 | + | – | + | + | + |
| B-2 | + | + | + | – | + |
| S-1 | + | + | + | – | + |
| S-2 | + | + | + | + | + |
| M-13 | + | + | + | – | + |
| P1 | – | + | – | – | – |
| NL-1 | + | + | + | + | + |
| NL-2 | + | – | – | – | – |
| TPS-30 | + | + | + | + | + |
| TPS-49 | + | – | + | – | + |
| X-1 | + | + | + | + | + |
| BJ | + | – | + | + | + |
| BC | + | + | + | + | + |
| GML | + | + | + | + | + |
| P-2 | – | – | – | – | – |
| BX-50 | + | + | + | – | + |
| BH-50 | + | + | + | – | + |
| W-1 | – | – | – | – | – |
| AN-1 | + | + | + | + | + |
| LS-4 | + | + | + | – | + |
| J-1 | + | + | + | + | + |
Bioassay for Detection of AHLs
The AHL detection was applied by cross-streaking test strains against C. violaceum CV026 on nutrient agar plate, in which the purple pigment violacein can be restored in response to the presence of AHL molecules. Briefly, strain CV026 was streaked at the center of the nutrient agar plate, the target bacteria were streaked on the same plate against CV026 line, if the target bacteria have AHL-producing ability, diffusible AHL produced by the target bacteria induces strain CV026 to produce a purple pigment [14].
To evaluate the profiles of AHLs produced by the test isolates, bacterial culture supernatant were extracted and subjected to analytical thin-layer chromatography (TLC). A 10 ml sample of culture supernatant was extracted twice with three times volume of ethyl acetate and then dried in a fume hood. The residues of extraction were then dissolved in 100 l of HPLC-grade ethyl acetate. Analytical TLC was performed on C18 reversed-phase TLC plates (Whatman, Clifton, NJ USA). Chromatograms were developed with methanol water (60:40, v/v), then air-dried in a fume hood. The TLC plate was then overlaid with a thin film of agar seeded with the AHL reporter strain C. violaceum CV026 that produces the purple colour violacein in response to AHLs with N-acyl side chain between four and eight carbons in length (e.g., BHL). After incubation of the plate at 30 °C for 24 h, AHLs were located as purple spots on a white background. All the experiments were performed at least twice.
Molecular Detection of the luxRI Homologs
Oligonucleotides ahyR-F(5-TCAGATGTCTCCATTTCAGTGTT-3) and ahyR-R(5-CCATGACTGTCAATTGCAGGATC-3) according to the gene AhyR (X89469) were used to amplify the internal fragment of ahyR gene from A. hydrophila. The product was about 900 bp.
Detection of Virulent Factors of A. hydrophila
Some extracellular enzyme activities were detected by patching bacteria on LB agar plates supplemented with different substrates [15]. All strains were tested induplicate, and when results were different, a third experiment was carried out to resolve the discrepancies.
Hemolytic activity was tested on agar base (Oxoid) supplemented with 5 % sheep erythrocytes. The culture was streaked onto the plates and incubated at 27 °C for 24–36 h. The presence of a clear colourless zone surrounding the colonies indicated β-hemolytic activity.
Protease production and proteolytic activity was detected on 1.2 % agar plates supplemented with 10 % (v/v) sterile skimmed milk (105 °C for 30 min). The cultures were streaked on the skim milk agar plates and incubated at 27 °C for 24–36 h. Proteolytic strains caused a clearing zone around the colonies.
Extracellular nucleases (DNases) were determined on DNase agar plates (Difco) with 0.005 % methyl green. The culture was streaked onto the plates and incubated at 27 °C for 24–36 h, A pink halo around the colonies indicated nuclease activity.
Cytotoxicity of A. hydrophila strains was assayed with epithelioma papillosum cyprini (EPC) cells as described previously [13]. The EPC cells were grown as a monolayer at 25 C in Eagle’s minimum essential medium (MEM; Sigma) supplemented with 10 % fetal calf serum, and harvested with trypsin ethylenediaminetetraacetic acid. A 900 μl aliquot of the cell suspension was inoculated to each well in a 24-well culture plate (Costar, U.S.A.). After incubation for 24 h, 100 μl of filtered supernatant of A. hydrophila culture was added to EPC cell culture, and the EPC cells were inspected under microscopy for the morphologic damage.
Results
Production of AHL Molecules
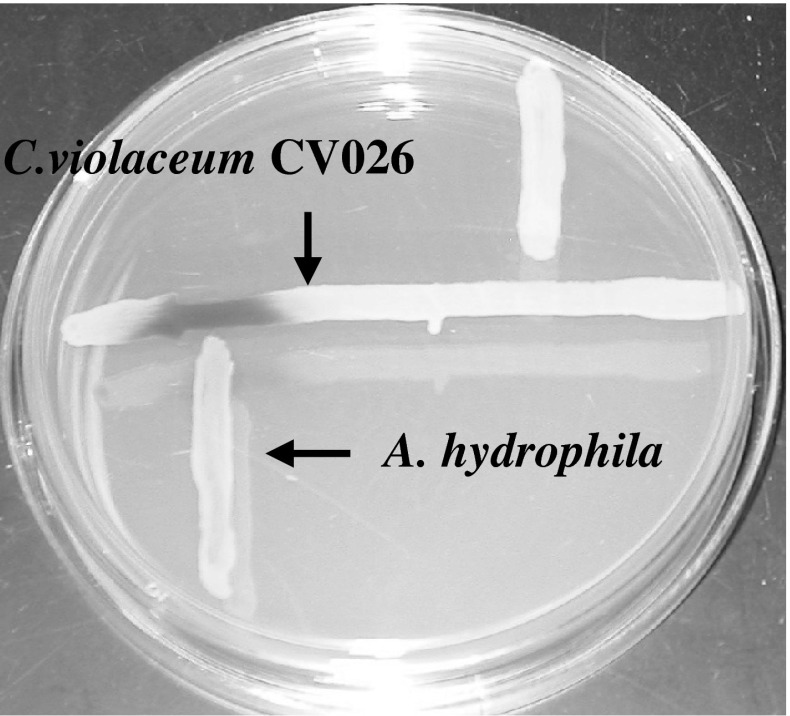
The use of AHL biosensor, C. violaceum CV026, in combination with cross-streaking test allowed for the screening AHLs productions from the test strains (Fig. 1). Among the A. hydrophila isolates obtained in the present study, 87.5 % of the isolates can produce AHL molecules (21/24). Only three of the test strains can not produce AHLs (Table 1).
Fig. 1.
The detection of N-acyl-homoserine lactones (AHLs) production by test strains with biosensors. Cross-streaks of test strains (vertical) against biosensor Chromobacterium violaceum CV026 (horizontal) showing the production of AHL in A. hydrophila
To characterise the AHL molecules produced by waterborne A. hydrophila, TLC was performed with the monitor bacteria and different AHL standards were used. The TLC profiles demonstrated that in most of test strains two AHL molecules was produced, one of which had a shape and retention factor (Rf) value similar to N-butanoyl homoserine lactone (BHL), and the other similar to N-hexanoyl homoserine lactone (HHL) (Fig 2).
Fig. 2.
Detection of AHLs by thin layer chromatography (TLC) with the Chromobacterium violaceum CV026 strain used as a biosensor. Lane 1, N-butanoyl-l-homoserine lactone, lane 2, N-hexanoyl-l-homoserine lactone, lane 3, Aeromonas hydrophila extract
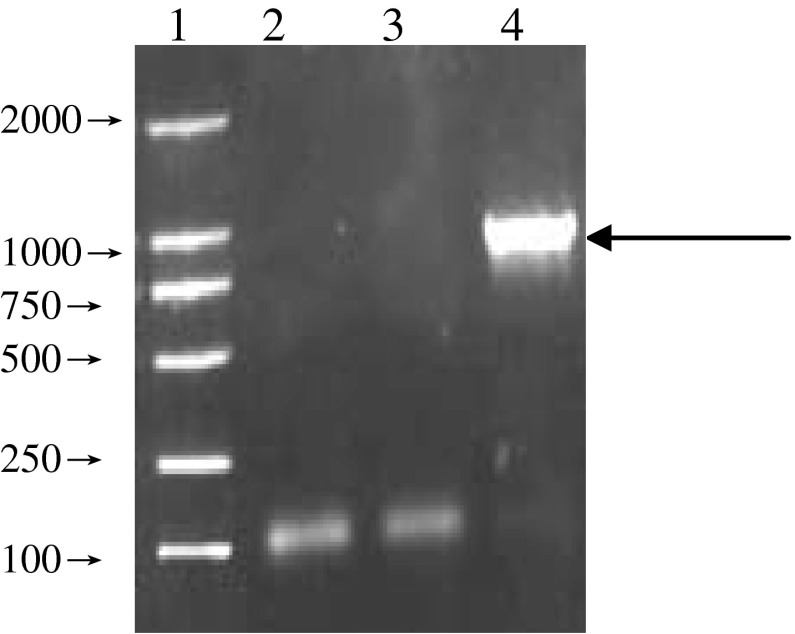
All the twenty strains were detected the AhyR gene by PCR amplification. The amplification of the AhyR produced a reproducible single DNA fragment (~900 bp) from seventeen strains (Fig 3). The three exceptions were w1, P1 and P2 which were isolated from pond.
Fig. 3.
PCR results of AhyR gene of Aeromonas hydrophila 1, Marker, 2, W-1, 3, P-1, 4, YJ-1
Virulent Factors of A. hydrophila
The virulence profiles of the 24 Aeromonas isolates with respect to their hemolytic activity, DNase and protease production, and cytotoxic effect are summarized in Table 1. The results show that majority (more than 83 % (20/24)) of the fresh water environmental isolates and clinical strains in this study displayed hemolytic activity. The isolates showed β-hemolytic activity on sheep blood agar. The results of present study indicate the difference in the expression of protease and DNase by A. hydrophila isolates. Only 12 of the 24 Aeromonas strains used in this study displayed DNase activity. Proteolytic activity was detected in 18 of 24 (75 %) of the isolates. Cytotoxic activity was detected in 20 of 24 of the isolates (Table 1).
Discussion
The present study has demonstrated that most of the water borne isolates of A. hydrophila produce quorum sensing signal molecules, other studies have also showed that Aeromonas spp. can produce AHLs [16, 17]. Based on TLC profiling we found that BHL and HHL were produced by waterborne A. hydrophila. It has been reported that environmental conditions determine the AHL production in A. hydrophila, Medina-Martinez et al. [18, 19] reported that environmental factors (temperature and composition of medium (glucose concentration)) affect AHLs production. Styp von Rekowski et al. [20] results shown that AHL-mediated QS was not required for A. hydrophila during colonization and degradation of organic particles in lake water microcosms. Thus, it can be presumed the production of quorum-sensing signal molecules of A. hydrophila in different environments may be quite different.
The virulence in A. hydrophila is complex and involves multiple virulence factors, which may work in concert to enable the bacteria to colonize, gain entry, establish, replicate, and cause damage in host tissues and to evade the host defense system and spread, eventually killing the host [21]. It has been shown before that blocking the AHL-mediated quorum sensing system of A. hydrophila with signal molecule analogues decreased exoprotease production and disrupt biofilm formation [22–24]. Among the Aeromonas isolates obtained in the present study, most of the isolates can produce quorum sensing molecules and some of the virulence factors, and the strains which can not produce quorum sensing molecules also can not produce virulence factors, from the results it has to be borne in mind that the production of quorum-sensing signal molecules is consistence with the production of virulence factors. Previous studies have shown that disrupt the QS signaling decreased the virulent of pathogenic bacteria [25–27].The results from ours may also predict that inhibit the quorum sensing will be a new pathway to combat Aeromonas spp. infection.
In conclusion, strains of A. hydrophila isolated from water environment produce more than one AHL molecule, and the AHLs producing strain possessed more than virulence factors. The findings of AHL signal molecules present in the A. hydrophila reveal that analogues of AHL may be useful or blocking quorum-sensing controlled virulence of these bacteria and hence prevent the infectious diseases for aquaculture [28, 29].
Acknowledgments
This work was supported by State Administration for Entry and Exit Inspection and Quarantine of P. R. China (2012IK011). The authors are much indebted to Dr. RJC McLean, Department of Biology, Texas State University, for generous provision of the AHL bioassay strain and for his helpful comments on the manuscript.
References
- 1.Põllumaa L, Alamäe T, Mäe A. Quorum sensing and expression of virulence in pectobacteria. Sensors. 2012;12(3):3327–3349. doi: 10.3390/s120303327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens AM, Schuster M, Rumbaugh KP. Working together for the common good: cell–cell communication in bacteria. J Bacteriol. 2012;194(9):2131–2141. doi: 10.1128/JB.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology. 2010;156(Pt 8):2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 4.Gospodarek E, Bogiel T, Zalas-Wiecek P. Communication between microorganisms as a basis for production of virulence factors. Pol J Microbiol. 2009;58(3):191–198. [PubMed] [Google Scholar]
- 5.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37(2):121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 6.Ronald PC. Small protein-mediated quorum sensing in a gram-negative bacterium: novel targets for control of infectious disease. Discov Med. 2011;12(67):461–470. [PubMed] [Google Scholar]
- 7.Kalia VC (2013) Quorum sensing inhibitors: an overview. Biotechnol Adv 31(2):224–245 [DOI] [PubMed]
- 8.Ni NT, Li MY, Wang JF, Wang BH. Inhibitors and antagonists of bacterial quorum sensing. Med Res Rev. 2009;29:65–124. doi: 10.1002/med.20145. [DOI] [PubMed] [Google Scholar]
- 9.Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23(1):35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daskalov H. The importance of Aeromonas hydrophila in food safety. Food Control. 2006;17:474–483. doi: 10.1016/j.foodcont.2005.02.009. [DOI] [Google Scholar]
- 11.Kozlova EV, Khajanchi BK, Sha J, Chopra AK. Quorum sensing and c-di-GMP-dependent alterations in gene transcripts and virulence-associated phenotypes in a clinical isolate of Aeromonas hydrophila. Microb Pathog. 2011;50(5):213–223. doi: 10.1016/j.micpath.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khajanchi BK, Fadl AA, Borchardt MA, Berg RL, Horneman AJ, Stemper ME, Joseph SW, Moyer NP, Sha J, Chopra AK. Distribution of virulence factors and molecular fingerprinting of Aeromonas species isolates from water and clinical samples: suggestive evidence of water-to-human transmission. Appl Environ Microbiol. 2010;76(7):2313–2325. doi: 10.1128/AEM.02535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift S, Karlyshev AV, Fish L, Durant EL, Winson MK, Chhabra SR, Williams P, Macintyre S, Stewart GS. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J Bacteriol. 1997;179:5271–5281. doi: 10.1128/jb.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu WH, Vattem DA, Maitin V, Barnes MB, McLean RJ. Bioassays of quorum sensing compounds using Agrobacterium tumefaciens, and Chromobacterium violaceum. Methods Mol Biol. 2011;692:3–19. doi: 10.1007/978-1-60761-971-0_1. [DOI] [PubMed] [Google Scholar]
- 15.Chu WH, Jiang Y, Liu YW, Zhu W. Role of the quorum-sensing system in biofilm formation and virulence of Aeromonas hydrophila Africa. J Microbiol Res. 2011;5(32):5819–5825. [Google Scholar]
- 16.Jangid K, Kong R, Patole M, Shouche Y. luxRI homologs are universally present in the genus Aeromonas. BMC Microbiol. 2007;7:93. doi: 10.1186/1471-2180-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan KG, Puthucheary SD, Chan XY, Yin WF, Wong CS, Too WS, Chua KH. Quorum sensing in Aeromonas species isolated from patients in Malaysia. Curr Microbiol. 2011;62(1):167–172. doi: 10.1007/s00284-010-9689-z. [DOI] [PubMed] [Google Scholar]
- 18.Medina-Martinez MS, Uyttendaele M, Demolder V, Debevere J. Effect of temperature and glucose concentration on the N-butanoyl-l-homoserine lactone production by Aeromonas hydrophila. Food Microbiol. 2006;23:534–540. doi: 10.1016/j.fm.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Medina-Martinez MS, Uyttendaele M, Demolder V, Debevere J. Influence of food system conditions on N-acyl-l-homoserine lactones production by Aeromonas spp. Int J Food Microbiol. 2006;112:244–252. doi: 10.1016/j.ijfoodmicro.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Styp von Rekowski K, Hempel M, Philipp B. Quorum sensing by N-acylhomoserine lactones is not required for Aeromonas hydrophila during growth with organic particles in lake water microcosms. Arch Microbiol. 2007;189:475–482. doi: 10.1007/s00203-007-0338-2. [DOI] [PubMed] [Google Scholar]
- 21.Yu HB, Zhang YL, Lau YL, Yao F, Vilches S, Merino S, Tomas JM, Howard SP, Leung KY. Identification and characterization of putative virulence genes and gene clusters in Aeromonas hydrophila PPD134/91. Appl Environ Microbiol. 2005;71:4469–4477. doi: 10.1128/AEM.71.8.4469-4477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlova EV, Popov VL, Sha J, Foltz SM, Erova TE, Agar SL, Horneman AJ, Chopra AK. Mutation in the S-ribosylhomocysteinase (luxS) gene involved in quorum sensing affects biofilm formation and virulence in a clinical isolate of Aeromonas hydrophila. Microb Pathog. 2008;45:343–354. doi: 10.1016/j.micpath.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Garde C, Bjarnsholt T, Givskov M, Jakobsen TH, Hentzer M, Claussen A, Sneppen K, Ferkinghoff-Borg J, Sams T. Quorum sensing regulation in Aeromonas hydrophila. J Mol Biol. 2010;396(4):849–857. doi: 10.1016/j.jmb.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Natrah FM, Alam MI, Pawar S, Harzevili AS, Nevejan N, Boon N, Sorgeloos P, Bossier P, Defoirdt T. The impact of quorum sensing on the virulence of Aeromonas hydrophila and Aeromonas salmonicida towards burbot (Lota lota L.) larvae. Vet Microbiol. 2012;159(1–2):77–82. doi: 10.1016/j.vetmic.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Annapoorani A, Jabbar AKKA, Musthafa SKS, Pandian SK, Ravi A. Inhibition of quorum sensing mediated virulence factors production in urinary pathogen serratia marcescens PS1 by marine sponges. Indian J Microbiol. 2012;52(2):160–166. doi: 10.1007/s12088-012-0272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HF, Tu FP, Gui ZH, Lu XH, Chu WH. Antibiotic resistance profiles and quorum sensing-dependent virulence factors in clinical isolates of Pseudomonas Aeruginosa. Indian J Microbiol. 2013 doi: 10.1007/s12088-013-0370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakkiyaraj D, Sivasankar C, Pandian SK. Anti-pathogenic potential of coral associated bacteria isolated from Gulf of Mannar against Pseudomonas aeruginosa. Indian J Microbiol. 2012 doi: 10.1007/s12088-012-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Defoirdt T, Boon N, Bossier P, Verstraete W. Disruption of bacterial quorum sensing: an unexplored strategy to fight infections in aquaculture. Aquaculture. 2004;240:69–88. doi: 10.1016/j.aquaculture.2004.06.031. [DOI] [Google Scholar]
- 29.Natrah FMI, Defoirdt T, Sorgeloos P, Bossier P. Disruption of bacterial cell-to-cell communication by marine organisms and its relevance to aquaculture. Mar Biotechnol. 2011;13(2):109–126. doi: 10.1007/s10126-010-9346-3. [DOI] [PubMed] [Google Scholar]