Abstract
Sweet sorghum juice was a cheap and renewable resource, and also a potential carbon source for the fermentation production of lactic acid (LA) by a lactic acid bacterium. One newly isolated strain Lactobacillus salivarius CGMCC 7.75 showed the ability to produce the highest yield and optical purity of LA from sweet sorghum juice. Studies of feeding different concentrations of sweet sorghum juice and nitrogen source suggested the optimal concentrations of fermentation were 325 ml l−1 and 20 g l−1, respectively. This combination produced 142.49 g l−1 LA with a productivity level of 0.90 g of LA per gram of sugars consumed. The results indicated the high LA concentration achieved using L. salivarius CGMCC 7.75 not only gives cheap industrial product, but also broaden the application of sweet sorghum.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-013-0377-0) contains supplementary material, which is available to authorized users.
Keywords: Lactic acid production, Sweet sorghum juice, Mixed sugars, Lactobacillus salivarius
Introduction
Lactic acid (LA) plays an important role in our daily life in that it can be used as an acidulant, flavor enhancer, and preservative in the food [1–4]. In recent decades, lactic acid bacteria (LAB) have been traditionally used for LA production and become the predominant approach for industrial LA production. However, the cost of raw materials, the productivity of LAB, and the purification of fermented LA hindered the development of LA. For example, to reduce production costs, efforts have been made to find an excellent LA-producing strain with the capability to convert agricultural by-products into LA (e.g. [5]). Sweet sorghum juice crashed from stalks is traditionally used for making white sugar [6] or had been examined to produce ethanol through yeast fermentation [7]. Nonetheless, the main component of sweet sorghum juice indicated that it is a desirable and cheap carbon resource for the fermentative production of LA. However, utilization of the juice for LA production has not been widely investigated at least to the best of our knowledge.
In this study, we firstly selected LAB capable of converting sweet sorghum juice to LA; secondly, the production of LA from different concentrations of sweet sorghum juice and nitrogen resource was investigated, respectively; finally, the optional concentrations of these two substrates were studied. Therefore, in this study, we explore the efficient production of LA from sweet sorghum juice through fermentation by a LAB.
Materials and Methods
Materials
Sweet sorghum juice extracted from stalks was obtained from Jinxin Bio-technology Co. Ltd (Inner Mongolia, china), which was composed by glucose, fructose, and sucrose. Corn syrup powder (CSP), yeast extract (YE), beef extract, and peptone were purchased form Tianjin chemical industry Co. Ltd (Tianjin, China). MgSO4·7H2O, MnSO4·H2O, CH3COONa·3H2O, K2HPO4, Tween-80, CaCO3, and diammonium hydrogen citrate (C6H14N2O7) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Reference standard of l-LA with the 99.9 % optical purity was purchased from Sigma-Aldrich Co., Ltd. (Shanghai, China). They were of analytical grade and commercially available.
Microorganism
Fifty LAB strains were isolated from different environmental samples in Qingdao city of Shandong province, China. Strains were obtained and maintained in a modified MRS medium containing (per liter): 20 g glucose, 10 g peptone, 5 g YE, 10 g beef extract, 0.58 g MgSO4·7H2O, 0.25 g MnSO4·H2O, 5 g CH3COONa·3H2O, 2 g K2HPO4, 2 g diammonium hydrogen citrate, 10 g CaCO3, 10 ml Tween-80.
The isolates were first tested for catalase activity. Only catalase-negative isolates were tested for several properties including yield, optical purity and ability to grow and ferment sweet sorghum juice as a primary screening for LA production. The isolate with the highest yield of LA and the higher optical purity was identified by the sequence of 16S/23S rDNA intergenic spacer (ISR) amplified from a primer pair 16S-F/23S-R (16S-F 5′-AGAGTTTGATCMTGGCTCAG, 23S-R 5′-GGTTACCTTGTTACGACTT) [8]. The amplified products were sequenced by Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China) and submitted to the GenBank (GenBank accession number: JX897028). Similarity search was performed in the GenBank database using the BLAST algorithm. This strain was kept in China General Microbiological Culture Collection Center and numbered as CGMCC 7.75.
Media and Culture Conditions
The medium for the seed culture was the MRS medium. The seed culture was prepared as follows: a loop of cells from the fully grown slant was inoculated into 10 ml of the above sterile medium in 15-ml conical flasks and incubated for 12 h at 37 °C without agitation. The inoculum volume was 10 % (v/v).
The medium for the fermentative culture had the following composition (per liter): different concentrations of sweet sorghum juice, different concentrations of nitrogen source composed by the mixture of CSP, YE, and peptone at a ration of 1:1:1 (w:w:w), 75 g CaCO3, 0.58 g MgSO4·7H2O, 0.25 g MnSO4·H2O, 2 g CH3COONa·3H2O, 2 g K2HPO4. Fermentation was carried out in a 500-ml triangle bottle each containing 200 ml medium. The temperature and agitation speed were maintained at 37 °C and 140 rpm, respectively. There was no aeration. Samples were taken at different time points. The concentrations of LA and residuals were analyzed as described below. The same experiment was conducted three times to obtain repeatable results which were described here.
Effects of Sweet Sorghum Juice and Nitrogen Source
To study the effect of sweet sorghum juice, four concentrations, 250, 300, 350, and 400 ml l−1, were experimented with the nitrogen source concentration being 15 g l−1, respectively. Information about these combinations was presented in the electronic supplementary Table S1 (online resource). To study the effect of nitrogen source, four concentrations, 10, 15, 20, and 25 g l−1, were experimented with the concentration of sweet sorghum juice being 300 ml l−1, respectively. Information about these combinations was presented in the electronic supplementary Table S2 (online resource).
To find the optimal concentration for the efficient production of LA, three concentration levels of sweet sorghum juice and nitrogen source were designed, respectively. The concentrations of sweet sorghum juice were at three levels: 275, 300, and 325 ml l−1. The concentrations of nitrogen source were evaluated with three levels: 18, 20, and 22 g l−1 (Table S3, online resource).
Fermentations were carried out as described by above methods. Analysis was done by below methods.
Analytical Methods
Sucrose was hydrolyzed into reducing sugar with 3 M sulfuric acid. The initial (no hydrolyzed sweet sorghum juice) and total concentration (hydrolyzed) of reducing sugars was measured by the Di-nitrosalicylic method. The value derived from the total concentration minus the initial concentration of reducing sugars was the concentration of sucrose from sweet sorghum juice. The concentration of residual reducing sugars and sucrose was measured by this method, respectively. The concentration of LA was measured by EDTA titration after calibration. The optical purity of LA was measured using high-performance liquid chromatography (Prominence LC-20A, Japan,) equipped with a chiral column (Daicel MA+, Daicel Inc., Shanghai, China) and a tunable UV detector at 210 nm. The mobile phase was 2 mM CuSO4, which was pumped at a flow rate of 0.50 ml/min (30 °C). All the experiments were carried out three times and the mean values are reported. The data obtained was analyzed by Analysis of Variance using SAS package (version 9.0, SAS Institute, Cary, USA) statistical software.
Results
Characterization and Identification of Strain CGMCC 7.75
In the MRS medium using sweet sorghum juice as the carbon source, strain CGMCC 7.75 showed the highest yield and better optical purity of LA (about 91.7 %). This isolate was then selected for detailed study. Strain CGMCC 7.75 was gram positive, short rod-shaped or globular, no spore, closely arranged, 2.5–5.5 μm in length, and 0.7–0.9 μm in width. This strain was a homofermentative LA producer, which could utilize glucose, fructose and sucrose as the carbon sources to produce LA. The 16S/23S IGS sequence showed the biggest similarity to the sequence of reference strain Lactobacillus salivarius CECT 5713 (GenBank accession number: CP002034) available in the NCBI. Accordingly, we concluded that the strain CGMCC 7.75 was a strain of L. salivarius.
Influence of Feeding Different Concentrations of Sweet Sorghum Juice and Nitrogen Source
Four concentrations of sweet sorghum juice with the fixed 15 g l−1 nitrogen source level were used to find the proper concentration for the effective production of LA. Kinetic parameters of LA and residual sugars during fermentations at different time points were measured and analyzed (online resources of Fig. S1 and Table S1). Obviously, the maximum specific LA productivity of stain CGMCC 7.75 was almost at the 300 ml l−1 sweet sorghum juice level. In this combination, fermentation was almost completed within 42 h and produced 118.49 g l−1 LA (0.81 g of LA per gram of sugars consumed) with small amounts of residual sugars (5.18 g l−1 reducing sugars and 25.00 g l−1 sucrose).
Four concentrations of nitrogen source with the fixed 300 ml l−1 sweet sorghum juice level were used to find the proper concentration for the effective production of LA. Kinetic parameters of LA and residual sugars during fermentations at different time points were measured and analyzed (online resources of Fig. S2 and Table S2). Obviously, the maximum specific LA productivity was almost at the 20 g l−1 nitrogen source level. In this combination, fermentation was almost completed within 48 h and produced 130.49 g l−1 LA (0.89 g of LA per gram of sugars consumed) with small amounts of residual sugars (4.87 g l−1 reducing sugars and 13.90 g l−1 sucrose).
Optimal Concentrations for LA Fermentation
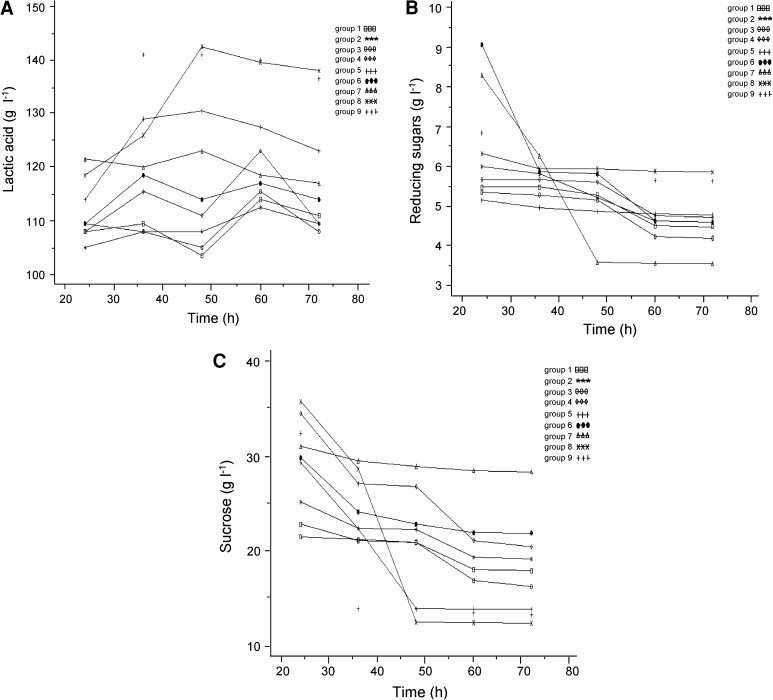
In an attempt to obtain higher production of LA, the optimal concentrations of sweet sorghum juice and nitrogen source were given a further investigation. Base on the above results, three levels of sweet sorghum juice were designed at 300 ± 25 ml l−1; three levels of nitrogen source were designed at 20 ± 2 g l−1. Therefore, nine groups were combined. Kinetic parameters of LA and residual sugars during fermentations at different time points were analyzed (Fig. 1). Firstly, with the fixed 275 ml l−1 sweet sorghum juice level, group 1, 2, and 3 were used to find the proper concentration of nitrogen source (Fig. 1, online resource Table S3). Obviously, the maximum specific LA productivity was obtained at the 22 g l−1 nitrogen source concentration level. In this combination, fermentation was almost completed within 60 h and produced 112.49 g l−1 LA (0.84 g of LA per gram of sugars consumed) with small amounts of residual sugars (4.63 g l−1 reducing sugars and 19.35 g l−1 sucrose). Secondly, with the fixed 300 ml l−1 sweet sorghum juice level, group 4, 5, and 6 were used to find the proper concentration of nitrogen source (Fig. 1, online resource Table S3). In these three groups, the maximum specific LA productivity was almost at the 20 g l−1 nitrogen source level. In this combination, fermentation was almost completed within 48 h and produced 130.49 g l−1 LA (0.89 g of LA per gram of sugars consumed) with small amounts of residual sugars (4.87 g l−1 reducing sugars and 13.90 g l−1 sucrose). Finally, with the fixed 325 ml l−1 sweet sorghum juice level, group 7, 8, and 9 were used to find the proper concentration of nitrogen source (Fig. 1, online resource Table S3). In these three groups, the maximum specific LA productivity was almost at the 20 g l−1 nitrogen source. In this combination, fermentation was almost completed within 48 h and produced 142.49 g l−1 LA (0.90 g of LA per gram of sugars consumed) with small amounts of residual sugars (5.94 g l−1 reducing sugars and 12.48 g l−1 sucrose). Therefore, the optimal concentration of sweet sorghum juice and nitrogen source was 325 ml l−1 and 20 g l−1, respectively. This combination produced 142.49 g l−1 LA with a productivity level of 0.90 g of LA per gram of sugars consumed. Indeed, this combination resulted in small amounts of residual sugars, which will simplify the purification of LA.
Fig. 1.
Kinetics of lactic acid and residual sugars produced by L. salivarius CGMCC 7.75 at three concentration levels of sweet sorghum juice and nitrogen source. A Yields of lactic acid; B amounts of residual reducing sugars; C amounts of residual sucrose. Numbers following groups correspond to the groups in the first column in the Electronic supplementary Table S3 and were represented by different symbols
Discussion
Only small LAB had bee reported to have higher yields of LA from the simultaneous consumption of mixed sugars than from glucose alone. For example, Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 can effectively produce LA from cane sugar molasses [9]. Moreover, efficient production of LA by LAB is often repressed by carbon catabolite, which is controlled by a complex regulatory mechanism [10]. Thus, for industrialization of LA production from sweet sorghum juice, L. salivarius CGMCC 7.75 should consume mixed sugars simultaneously and break through carbon catabolite repression as much as possible. Fermentation by L. salivarius CGMCC 7.75 with different concentrations of sweet sorghum juice at fixed concentrations of nitrogen source levels, i.e., 15, 18, 20, or 22 g l−1 nitrogen source levels, exhibited different utilisation of mixed sugars. For example, the proper concentration of nitrogen source at the level of 20 g l−1 significantly improved the utilization of sucrose from sweet sorghum juice and reduced inhibitory effects of the substrate. In contrast, amounts of sucrose consumption decreased when other concentrations of nitrogen source were fed (Table S3). This may be due to product inhibition or exhaustion of one restricting nutrient or the combined effect of both as suggested by Cock and de Stouvenel [11]. In this study, L. salivarius CGMCC 7.75 proved to be a promising strain enabling production of LA from sweet sorghum juice. This strain with a proper concentration of substrates will lead to almost complete conversion of sweet sorghum juice to LA. From an economical point of view, using strain CGMCC 7.75, the cost of LA production from sweet sorghum juice will be reduced due to the supplement of an inexpensive agricultural resource.
Electronic Supplementary Material
Kinetics of lactic acid and residual sugars produced by L. salivarius CGMCC 7.75 using different concentrations of sweet sorghum juice at the fixed 15 g l-1 nitrogen source. (A) yields of lactic acid; (B) amounts of residual reducing sugars; (C) amounts of residual sucrose. Numbers following groups correspond to the groups in the first column in the Electronic supplementary Table S1 and were represented by different symbols. Supplementary material 1 (DOC 73 kb)
Kinetics of lactic acid and residual sugars produced by L. salivarius CGMCC 7.75 using different concentrations of nitrogen source at the fixed 300 ml l-1 sweet sorghum juice. (A) Yields of lactic acid; (B) amounts of residual reducing sugars; (C) amounts of residual sucrose. Numbers following groups correspond to the groups in the first column in the Electronic supplementary Table S2 and were represented by different symbols. Supplementary material 2 (DOC 72 kb)
Acknowledgments
This research was supported by the National Science & Technology Pillar Program in 12th Five-year Plan of China (2011BAD17B04).
References
- 1.Patel S, Majumder A, Goyal A. Potentials of exopolysaccharides from lactic acid bacteria. Indian J Microbiol. 2012;52:3–12. doi: 10.1007/s12088-011-0148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thumu SCR, Halami PM. Acquired resistance to macrolide–lincosamide–streptogramin antibiotics in lactic acid bacteria of food origin. Indian J Microbiol. 2012;52:530–537. doi: 10.1007/s12088-012-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manisseri C, Gudipati M. Prebiotic activity of purified xylobiose obtained from Ragi (Eleusine coracana, Indaf-15) Bran. Indian J Microbiol. 2012;52:251–257. doi: 10.1007/s12088-011-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Özdemir GB, Biyik HH. Isolation and characterization of a bacteriocin-like substance produced by Geobacillus toebii strain HBB-247. Indian J Microbiol. 2012;52:104–108. doi: 10.1007/s12088-011-0227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LM, Zhao B, Liu B, Yu B, Ma CQ, Su F, Hua DL, Li QG, Ma YH, Xu P. Efficient production of l-lactic acid from corncob molasses, a waste by-product in xylitol production, by a newly isolated xylose utilizing Bacillus sp. strain. Bioresour Technol. 2010;20:7908–7915. doi: 10.1016/j.biortech.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Gnansounou E, Dauriat A, Wyman CE. Refining sweet sorghum to ethanol and sugar: economic trade-offs in the context of North China. Bioresour Technol. 2005;96:985–1002. doi: 10.1016/j.biortech.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Rooney WL, Blumenthal J, Bean B, Mullet JE. Designing sorghum as a dedicated bioenergy feedstock. Biofuels Bioprod Bioref. 2007;1:147–157. doi: 10.1002/bbb.15. [DOI] [Google Scholar]
- 8.Berthier F, Ehrlich SD. Rapid species identification within two groups of closely related lactobacilli using PCR primers that target the 16S/23S rRNA spacer region. FEMS Microbiol Lett. 1998;161:97–106. doi: 10.1111/j.1574-6968.1998.tb12934.x. [DOI] [PubMed] [Google Scholar]
- 9.Dumbrepatil A, Adsul M, Chaudhari S, Khire J, Gokhale D. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl Environ Microbiol. 2008;74:333–335. doi: 10.1128/AEM.01595-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JH, Shoemaker SP, Mills DA. Relaxed control of sugar utilization in Lactobacillus brevis. Microbiology. 2009;155:1351–1359. doi: 10.1099/mic.0.024653-0. [DOI] [PubMed] [Google Scholar]
- 11.Cock LS, de Stouvenel AR. Lactic acid production by a strain of Lactococcus lactis subs lactis isolated from sugar cane plants. Electron J Biotechnol. 2006;9:40–45. doi: 10.2225/vol9-issue1-fulltext-10. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kinetics of lactic acid and residual sugars produced by L. salivarius CGMCC 7.75 using different concentrations of sweet sorghum juice at the fixed 15 g l-1 nitrogen source. (A) yields of lactic acid; (B) amounts of residual reducing sugars; (C) amounts of residual sucrose. Numbers following groups correspond to the groups in the first column in the Electronic supplementary Table S1 and were represented by different symbols. Supplementary material 1 (DOC 73 kb)
Kinetics of lactic acid and residual sugars produced by L. salivarius CGMCC 7.75 using different concentrations of nitrogen source at the fixed 300 ml l-1 sweet sorghum juice. (A) Yields of lactic acid; (B) amounts of residual reducing sugars; (C) amounts of residual sucrose. Numbers following groups correspond to the groups in the first column in the Electronic supplementary Table S2 and were represented by different symbols. Supplementary material 2 (DOC 72 kb)