Abstract
Porcine reproductive and respiratory syndrome (PRRS) is considered one of the most important infectious diseases to affect the swine industry and characterized by reproductive failure in late term gestation in sows and respiratory disease in pigs of all ages. The GP5a gene, encoding RNA-dependent RNA polymerase, is generally regarded as fairly conserved when compared to other viral proteins. It plays an important role in the process of duplication and transcription carried out by Porcine reproductive and respiratory syndrome virus (PRRSV). We firstly expressed and purified the GP5a protein of PRRSV. This provides a good method for the purification of expressed proteins and the preparation of the corresponding antibodies.
Keywords: Porcine reproductive and respiratory syndrome virus, GP5a, Purification, Expression
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is characterized by severe reproductive failure and respiratory distress, and has been recognized as one of the most serious infectious diseases in swine since it was first identified in North America in 1987 [1]. Porcine reproductive and respiratory syndrome virus (PRRSV), the causative agent of this disease, belongs to the Arteriviridae family [2, 3].
The PRRSV genome is a 15 kb polyadenylated RNA molecule that contains short 5′ and 3′ terminal untranslated regions (UTRs), and encodes at least two large replicase genes (ORF1a and ORF1b), and eight structural protein genes. These proteins consist of envelope-associated proteins and a nucleocapsid (N) protein that are proposed to segregate into three groups: major structural components (GP5, membrane protein (M) and N), minor glycoproteins (GP2, GP3, and GP4), and small hydrophobic proteins (envelope [E] and ORF5a proteins) [4]. Among these, the ORF5a protein is a recently discovered novel structural protein. This protein, composed of 43–64 amino acids, is encoded by an alternative ORF of the subgenomic (sg) mRNA coding for the major envelope glycoprotein GP5, and these two structural proteins are likely translated from the same sg mRNA via a leaky ribosomal scanning mechanism [5, 6]. Further experiments using a reverse genetics system for equine arteritis virus (EAV) revealed that the ORF5a protein is dispensable for EAV infection, but its inactivation results in reduced virus titer and plaque morphology [7]. Although there are already several reports revealing that the presence of ORF5a in all arteriviruses implicates that it has potential significance in arterivirology, there are still a lot of details that need to be clarified.
In order to gain further understanding of ORF5a, prokaryotic expression and antibody preparation of the ORF5a gene was performed. We report here the first successful expression and purification of PRRSV ORF5a; this detailed molecular knowledge of viral ORF5a may also contribute to the identification of selective inhibitors of this important class of viral protein.
Materials and Methods
Strains, Vectors, and Main Reagents
In this study, we used the Escherichia coli strains DH5a and BL21 (DE3), the expression vector pET-32a (+), and the plasmids pMD18-T-ORF5a, which were preserved in the author’s laboratory. Platinum pfx DNA polymerase was purchased from Invitrogen. Restriction enzymes, DNA markers, and isopropyl β-D-1-Thiogalactopyranoside (IPTG) were purchased from TaKaRa. T4 DNA Ligase and protein molecular weight markers were purchased from Fermentas. Plasmid Mini Kits and Gel Extraction Kits were purchased from OMEGA. Ni Sepharos 6 Fast Flow was purchased from GE Healthcare. PCR amplification of the ORF5a gene based on the ORF5a sequence and the primers for the amplification of the ORF5a gene were designed using the biological software Oligo 6.71 (Wojciech and piotr Rychlik, USA) and synthesized by Invitrogen. The forward primer was 5′-CGCAAGCTTATTTAAACTGCTAGCCGCCAG-3′, and the reverse primer was 5′-GCCTCGAGATCTCAGGCAATCATGAG-3′. These primers contained the EcoR I and Xho I restriction sites (underlined), respectively. The plasmid pMD-18T-ORF5a was used as the template, PCR reactions (100 μL/tube) were performed using 10 μL of 10× pfx buffer, 8 μL of dNTP mix (10 mM), 2 μL of MgSO4 (50 mM), 2 μL of platinum pfx DNA polymerase, 2 μL of each primer (10 μM), 1 μL of DNA template, and 73 μL of ultrapure water. The conditions of the PCR amplification were initial denaturation at 94 °C for 3 min, followed by 30 consecutive cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 68 °C for 120 s, and then a final extension at 68 °C for 10 min. The amplified products were analyzed by electrophoresis on a 1 % agarose gel.
Construction of the Expression Plasmids pET-32a (+)-ORF5a
The PCR products of the ORF5a genes were digested by EcoR I and Xho I. They were directionally ligated into the previously EcoR I- and Xho I-digested expression vector, pET-32a(+). The ligation mixture was transformed into competent E. coli DH5a for storage. The positive colony was identified by restriction analysis and sequencing analysis. The extracted positive plasmids were transformed into the competent E. coli strain BL21 (DE3).
Protein Expression, Purification, and Polyclonal Antibody Production
The pET-32a- ORF5a-positive cloning strains were inoculated into 5 mL of LB/Amp liquid medium and cultivated overnight. The 50 μL cultures were inoculated with 5 mL of LB/Amp for activation. When the bacterium reached the logarithmic phase (at OD600 of 0.4–0.6), IPTG (final concentration 1.0 mmol/L) was added in order to induce the expression of the ORF5a proteins. The level of protein expression was analyzed by SDS-PAGE. The non-induced and vector control cultures were analyzed in parallel. In order to increase the production of the recombinant proteins, the expression conditions, including the duration of induction, the concentrations of IPTG, and the composition of the binding buffer (formula of binding buffer with compounds: 20 mM Na3PO4, 0.5 M NaCl, 20 mM imidazole, 0.5 % β-mercaptoethanol, 1.3 M urea, 0.5 % Tween 20, 3 % glycerol, 1 % SDS, pH 7.4; formula of binding buffer without compounds: 20 mM Na3PO4, 0.5 M NaCl, 20 mM imidazole, pH 7.4) were optimized. The ORF5a proteins were purified by Ni Sepharos 6 Fast Flow. The samples from the Ni-column were assessed by SDS-PAGE. The purified proteins were used to immunize a New Zealand rabbit to raise antibodies. The antiserum was collected by artery sampling and stored at −70 °C.
Western-blot Analysis of the Purification of the ORF5a Antigens
Western-blot analysis was used to evaluate the protein expression of ORF5a, as previously described. The purification samples were subjected to SDS-PAGE with a 10 % gel and electro-transferred to a nitrocellulose membrane. Nonspecific antibody-binding sites were blocked with 5 % skimmed milk in TBST overnight at 4 °C. The membranes were incubated with a 1:1000 dilution of rabbit antiserum to the ORF5a protein at 37 °C for 1 h and then washed 4 times with TBST (5 min each). The blot was probed with a 1:8000 dilution of IRDye 800CW Goat anti-rabbit IgG (H + L) secondary antibody for 1 h in the dark at 37 °C. Then, the membrane was washed 5 times with TBST and then twice with TBS. The blot was analyzed using the Odyssey Infrared Imaging System (LI-COR).
Results
Amplification of Gene Fragments of ORF5a
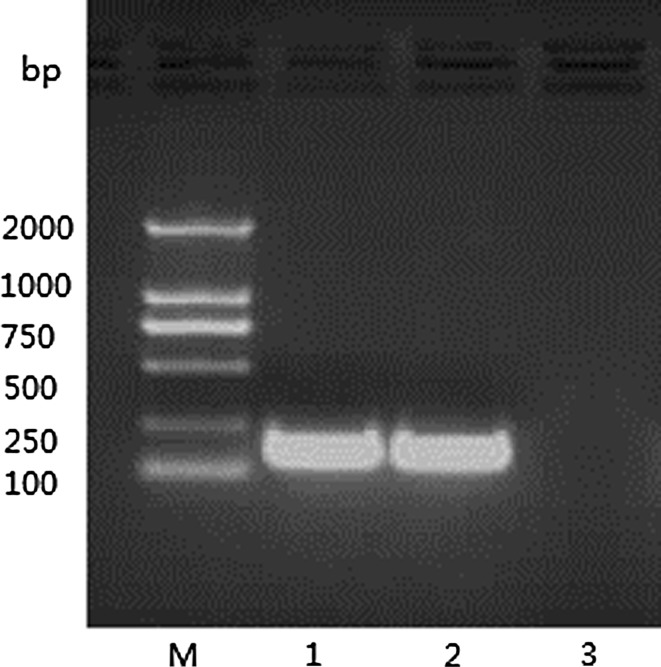
Specific bands of about 150 bp in size were amplified with the specific primers XH-ORF5a-F and XH-ORF5a-R with the cDNA of the PRRSV-GDXH as a template. The results conform with the expected fragment size (Fig. 1).
Fig. 1.
The amplification results of the ORF5a M Marker DL2000, 1–2 ORF5a PCR reactions, 3 negative control
Enzyme Digestion of Recombinant Plasmid pET-32a-ORF5a
The recombinant plasmid pET-32a-ORF5a identified by PCR by using specific primers XH-ORF5a-F and XH-ORF5a-R, and 150 bp fragments were amplified. The same 150 bp fragments were identified through double digestion with restriction endonucleases EcoR I and Xho I. (Fig. 2).
Fig. 2.
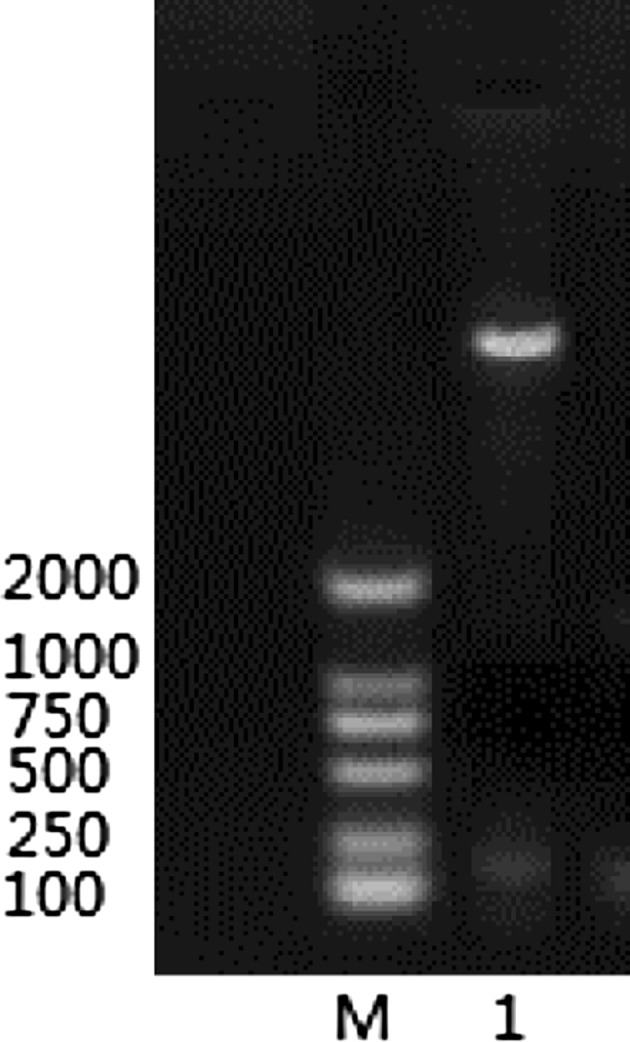
The results of double digestion M Marker DL2000, 1 PET32a-ORF5a EcoR I and X ho I double digestion
Results of ORF5a Sequencing
The bacilli identified as positive were sequenced by Shanghai Invitrogen Biotechnology Co., Ltd. The sequencing results showed that the target gene ORF5a did not exhibit any nucleotide mutations compared with XH-GD (Accession NO. EU624117) (Fig. 3).
Fig. 3.
The sequencing results of positive bacterium
Optimized Time of Inducing Expression by SDS-PAGE Analysis
The cloned ORF5a fragment encoding a protein with 47 amino acids, with a molecular size of 5 kDa, along with the expression vector pET-32a with 17 kDa plus the tag, and a termination codon were inserted in the downstream primer of the amplified ORF5a fragment. Based on the calculation results, the theoretical value of the fusion protein was about 20 kDa.
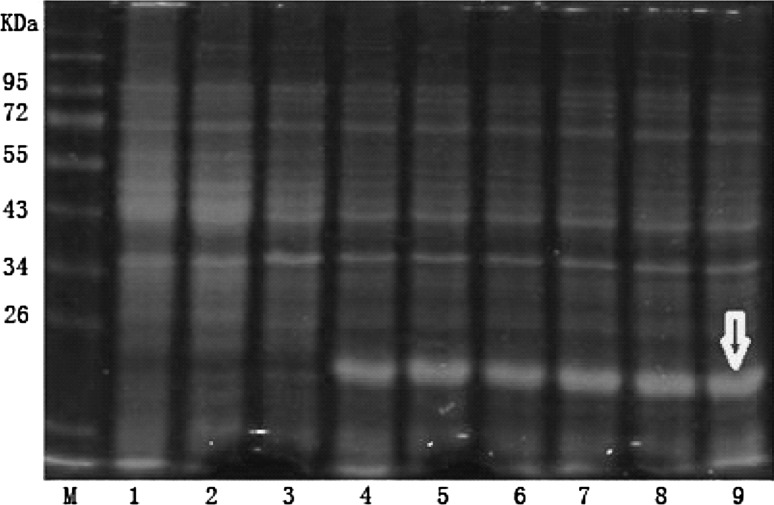
After being transformed into BL21 competent cells, the recombinant pET-32a-ORF5a plasmids identified as positive were induced with IPTG, and SDS-PAGE electrophoresis was performed, as compared with the empty expression vector of pET-32a. The bacteria containing the recombinant plasmid pET-32a-ORF5a presented with about 20 kDa apparent specificity protein bands of recombinant protein with the expected size (Fig. 4). Judging from the effect of different induction times, the amount of protein reaches its peak point after 4 h induction. As induction time increases, the expression amount remains almost constant; thus a 4 h induction time for the expression of the recombinant protein was selected.
Fig. 4.
SDS-PAGE results of the different time IPTG induction of fusion protein expressionM protein molecular weight standards, 1 vector pET-32a before induction, 2 vector pET-32a induction, 3 recombinant plasmid pET-32a-ORF5a before induction, 4 the recombinant plasmid pET-32a-ORF5a induced after 1 h, 5 2 h after induction of the recombinant plasmid pET-32a-ORF5a, 6 recombinant plasmid ppET-32a-ORF5a after induction 3 h, 7 the recombinant plasmid pET-32a-ORF5a induced after 4 h, 8 after the induction of the recombinant plasmid pET-32a-ORF5a 5 h, 9 6 h after the induction of the recombinant plasmid pET-32a-ORF5a
SDS-PAGE Analysis of the Different Concentrations of IPTG Induction
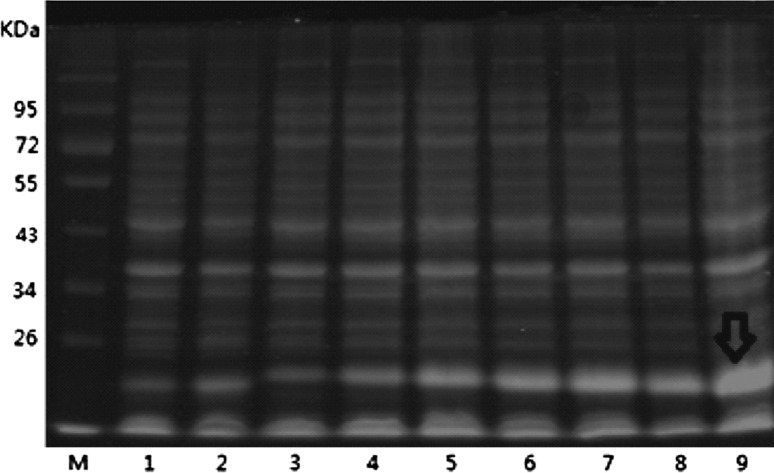
Positive recombinant bacterium were cultured at 37° C until the OD600 reached 0.4–0.6, and then final concentrations of 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 mmol/L IPTG were added to the bacteria for induction at 37° C for 4 h, the results were analyzed by SDS-PAGE, and the final IPTG induction concentration was selected with 1.0 mmol/L IPTG (Fig. 5).
Fig. 5.
SDS-PAGE results of different concentrations of IPTG induction of fusion protein expressionM protein molecular weight standards, 1 vector pET-32a IPTG induction concentration of 0.2 mmol/L, 2 vector pET-32a IPTG induction concentration of 1.0 mmol/L, 3 recombinant plasmid pET-32a-ORF5a IPTG induction concentration mmol/L, 4 The recombinant plasmid pET-32a-ORF5a IPTG induction concentration of 0.2 mmol/L, 5 The recombinant plasmid pET-32a-ORF5a IPTG induction concentration of 0.4 mmol/L, 6 The recombinant plasmid pET-32a-ORF5a IPTG induction concentration of 0.6 mmol/L, 7 The recombinant plasmid pET-32a-ORF5a IPTG induction concentration of 0.8 mmol/L, 8 recombinant plasmid pET-32a-ORF5a IPTG induction concentration of 1.0 mmol/L, 9 The recombinant plasmid pET-32a-ORF5a IPTG induction concentration 1.2 mmol/L
Soluble Analysis of the Protein
Equal volumes of sonicated bacteria in the supernatant and pellet were tested by SDS-PAGE electrophoresis (Fig. 6). The results showed that the expression of the fusion protein presented in the supernatant—i.e., the target protein is soluble.
Fig. 6.
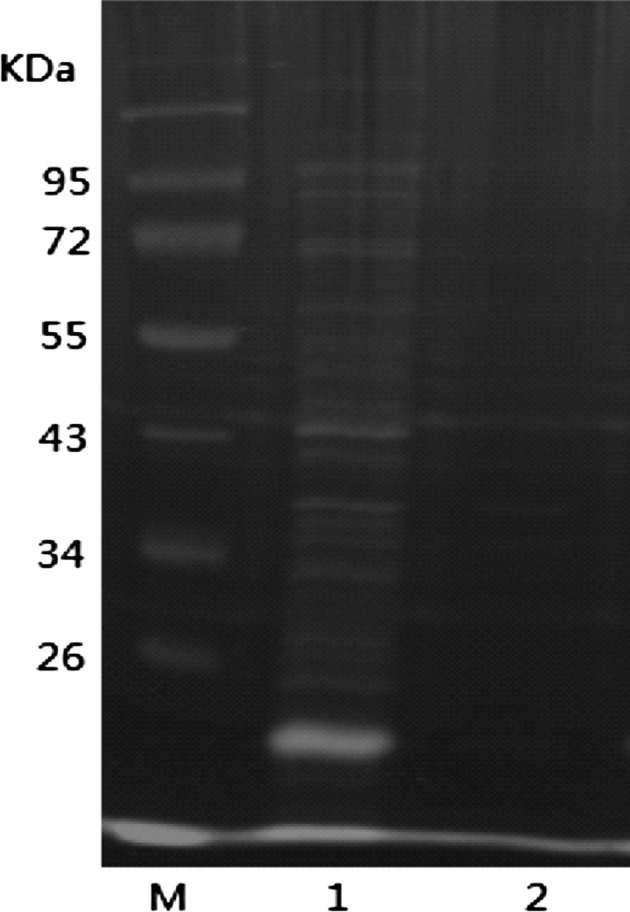
The analysis results of the soluble fusion protein M protein molecular weight standard substance, 1 bacterial supernatant after sonication, precipitate after 2 bacterial sonication
Western-blot Identification of Expressed Proteins
After SDS-PAGE electrophoresis of the protein in the gel, it was transfected by electroporation instrument, transferred to the NC membrane, and identified by Western-Blot analysis. The results showed that the recombinant protein with the HIS tag exhibits a monoclonal antibody specificity binding reaction (Fig. 7).
Fig. 7.
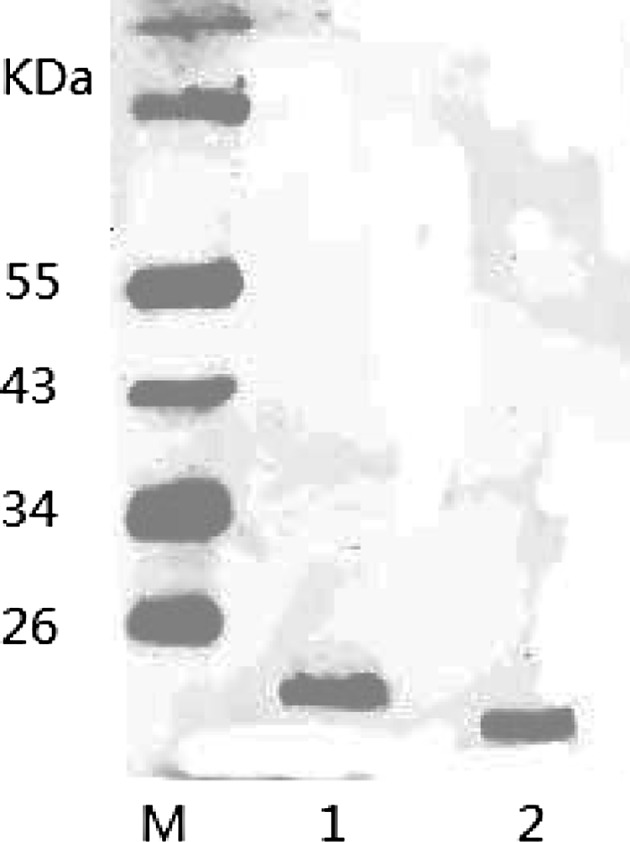
Fusion protein western-blot identified M protein marker, 1 pET-32a-ORF5a induction group, 2 negative control
Discussion
Recently, it was shown that the ORF5a protein is a novel structural protein in PRRSV, which is encoded by an alternative ORF5a that is present in all arteriviruses [6]. Inactivation of ORF5a expression by reverse genetics yielded a severely crippled EAV mutant, suggesting that the ORF5a protein is not essential, but that it is important for EAV viability [5]. We report here the first successful expression and purification of PRRSV ORF5a; such detailed molecular knowledge of viral ORF5a also may contribute to the identification of selective inhibitors of this important class of viral protein.
PRRS is characterized by severe reproductive failure in sows, including early farrowing of stillborn piglets and late term absorption, respiratory distress in piglets and growing pigs, as well as an influenza-like disease in grown/finished swine [7, 8]. PRRS continues to be a serious threat, causing an economically significant impact on the swine industry worldwide [9]. Although commercial vaccines against PRRSV are available, traditional control strategies and conventional vaccines have failed to provide sustainable disease control [10, 11]. Since the first report in 1995, PRRS has become an increasing threat in China [12]. In particular, the devastating outbreak of HP-PRRSV in 2006 affected more than 2,000,000 pigs, with about 400,000 fatal cases [13, 14].
In this study, we report the expression in E. coli and purification of the PRRSV ORF5a. A gene encoding the full-length PRRSV ORF5a protein was cloned in the pET-32a(+) vector for expression in E. coli. Optimal conditions for soluble protein expression were determined according to the procedure described by Cao [19], which resulted in production of a predominant protein of the expected size (20 kDa) (Fig. 2a). The ORF5a-His protein was purified to near homogeneity using a Ni–NTA chromatography column. EAV ORF5a-His eluted at its expected molecular size, demonstrating its monomeric state.
We also constructed the plasmid pGEX-6p-1-ORF5a. The level of protein in the supernatant did not increase greatly, and when it comes to further immunifaction, it may be more troublesome, so we chose the prokaryotic expression vector pET-32a(+). We also tested other E. coli strains such as the lasotta strain, but the results did not demonstrate any major differences. In addition, these compounds may promote the solubility of proteins. It has not yet been verified whether or not these proteins have enzymatic activity. We found that the compound “0.5 % b-mercaptoethanol, 1.3 M urea, 0.5 % Tween 20, 3 % glycerol, 1 % SDS” can greatly increase the levels of soluble proteins. This can provide a useful method for the purification of proteins and the preparation of the corresponding antibodies. Further research is needed to determine whether these proteins exhibit enzyme activity.
The anti-ORF5a polyclonal antibody may be one useful tool for further analysis of PRRSV ORF5a. The ORF5a protein we obtained from the prokaryotic expression system should be researched further. Many questions about the function of ORF5a still need to be confirmed in the future. The ability to purify a minor structure protein will facilitate its further functional and structural characterization and will provide insight into the molecular basis of this minor structural protein. Such detailed molecular knowledge of the viral ORF5a protein also may contribute to the identification of selective inhibitors of this important class of viral protein.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China (Nos. 31272564, 31201876), the Joint Fund of NSFC-Guangdong of China (U0931003), and the Modern Agricultural Industry Technology System (CARS-36).
Footnotes
Chunya Wei and Zhen Huang contributed equally to this work.
References
- 1.Keffaber KK. Reproductive failure of unknown etiology. Am Assoc Swine Pract Newsl. 1989;1:1–10. [Google Scholar]
- 2.Allende R, Laegreid WW, Kutish GF, Galeota JA, Wills RW, Osorio FA (2000) Porcine reproductive and respiratory syndrome virus: description of persistence in individual pigs upon experimental infection. J Virol 74:10834–10837 [DOI] [PMC free article] [PubMed]
- 3.Meulenberg JJ, Hulst MM, de Meijer EJ, Moonen PL, den Besten A, de Kluyver EP, Wensvoort G, Moormann RJ (1993) Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology 192:62–72 [DOI] [PMC free article] [PubMed]
- 4.Tian D et al (2012) Arterivirus minor envelope proteins are a major determinant of viral tropism in cell culture. J Virol 86:3701–3712 [DOI] [PMC free article] [PubMed]
- 5.Firth AE, et al. Discovery of a small arterivirus gene that overlaps the GP5 coding sequence and is important for virus production. J Gen Virol. 2011;92:1097–1106. doi: 10.1099/vir.0.029264-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson CR, Griggs TF, Gnanandarajah J, Murtaugh MP (2011) Novel structural protein in porcine reproductive and respiratory syndrome virus encoded in an alternative open reading frame 5 present in all arteriviruses. J Gen Virol 92:1107–1116 [DOI] [PMC free article] [PubMed]
- 7.Tian KG, Yu XL, Zhao TZ et al (2007) Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One 6:e526 [DOI] [PMC free article] [PubMed]
- 8.Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ (2005) Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 227:385–392 [DOI] [PubMed]
- 9.Garner MG, Whan IF, Gard GP, Phillips D et al (2001) The expected economic impact of selected exotic diseases on the pig industry of Australia. Rev Sci Technol 20(3):671–685 [DOI] [PubMed]
- 10.Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM Stockhofe-Zurwieden N (2009) Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 27:3704–3718 [DOI] [PubMed]
- 11.Labarque G, Reeth KV, Nauwynck H, Drexler C, Van Gucht S, Pensaert M (2004) Impact of genetic diversity of European-type porcine reproductive and respiratory syndrome virus strains on vaccine efficacy. Vaccine 22:4183–4190 [DOI] [PubMed]
- 12.Guo B-Q, Chen Z-S, Liu W-X, Cui Y-Z (1996) Isolation of PRRS virus from fetuses suspicious of PRRS. Chinese J Animal Poult Infect Dis 1–5
- 13.Tong GZ, Zhou YJ, Hao XF, Tian ZJ, An TQ, Qiu HJ (2007) Highly pathogenic porcine reproductive and respiratory syndrome, China. Emerg Infect Dis 13:1434–1436 [DOI] [PMC free article] [PubMed]
- 14.Wang XR, Ma HY, Zhu WG et al (2008) Investigation and isolations of isolates of PRRSV in “Swine high fever disease” pig herds. Chinese J Vet 44(3):39240
- 15.Ke Y, Wang Y, Yuan X et al (2012) Altered transcriptome of the B-melitensis vaccine candidate 16 M delta vjbR, implications for development of genetically marked live vaccine. Indian J Microbiol 52(4):575–580 [DOI] [PMC free article] [PubMed]
- 16.Das J, Jha DK, Policegoudra RS, et al. Isolation and characterization of antidermatophytic bioactive molecules from Piperlongum L. leaves. Indian J Microbiol. 2012;52(4):624–629. doi: 10.1007/s12088-012-0303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]