Abstract
Probiotic is a preparation containing microorganisms that confers beneficial effect to the host. This work assessed whether oral administration of Bacillus amyloliquefaciens SC06 (Ba) could decrease bacterial translocation in weaned mice. Weaned C57BL/6 were randomly allocated into three groups: group I as the control group, group II were treated with 0.85 % NaCl. Group III was administered with probiotic Ba 1 × 109 CFU/day dissolved in 100 μl of 0.85 % NaCl for 30 days. Mice were then sacrificed, and tissue were cultured to determine bacterial translocation. Meanwhile, splenic CD4+T cells, CD8+T cells, B cells, and macrophages were analysised by FACS. Our results showed that probiotic Ba significantly reduced bacteria translocation compared with the control group and 0.85 % NaCl group (P < 0.05), lower levels of bacteria were detected in the MLN, liver, spleen, and kidney of mice. Moreover, significant increase in percentage and number of macrophages were observed in the spleen of Ba-treated mice compared with the control and 0.85 % NaCl groups. Together, these data indicated that Ba could decrease bacterial translocation in weaned mice. This effect seems to be correlated with the changes of macrophage numbers.
Keywords: Bacillus amyloliquefaciens SC06, Bacterial translocation, Macrophage
Introduction
Weaning is a critical stage of gut development and postnatal growth in mammals, including rodents, pigs, and humans. Animals at the weaning stage become more susceptible to infection and intestinal disorders. Therefore, it might lead to bacterial translocation (BT) during this critical stage [8]. BT is defined as the passage of viable bacteria from the gastro-intestinal tract to extra-intestinal sites, such as the mesenteric lymph node (MLN) complex, liver, spleen, kidney, and bloodstream. Three primary mechanisms promote BT from the gastrointestinal tract: (a) intestinal bacterial overgrowth, (b) immunodeficiencies, and (c) increased intestinal permeability [5, 24]. BT may cause the ingress of viable bacteria and their antigens with the development of sepsis, initiation of the cytokines mediated multiorgan dysfunction syndrome (MODS), systemic inflammatory response syndrome (SIRS), and death [10]. Probiotics are defined as non-pathogenic live microorganisms that seem to promote gut health and regulate intestinal homeostasis [9, 23]. There are substantial researches focusing on modulation of the intestinal microbiota and host inflammatory responses by probiotic bacteria [23]. However, fewer studies have studied whether the availability of genus Bacillus reduce the BT.
Bacteria of the genus Bacillus can produce a large number of antimicrobial peptides with different chemical structures, such as bacteriocins, bacteriocin-like substances, and lipopeptides [2, 17, 25]. Bacillus amyloliquefaciens (Ba) has been a major workhorse for the production of a variety of commercially important enzymes and metabolites for the past decades [21]. Recent studies focused on the antifungal proteins or antimicrobial factors from B. amyloliquefaciens [2, 3, 28]. In this study, this work assessed whether oral administration of B. amyloliquefaciens SC06 (Ba) could decrease BT in weaned mice.
Materials and Methods
Mice and Reagents
C57BL/6 were purchased from Shanghai Slac Animal Inc. and maintained in Experimental Animal Center of Zhejiang University. Experimental protocols for animal studies were approved by the Institutional Animal Care and Use Committee of Zhejiang University.
Bacterial Strains and Medium
The probiotic B. amyloliquefaciens SC06 (Ba) was isolated from soil and kept at China Center for Type Culture Collection (CCTCC No: M 2012280). Luria–Bertani (LB) medium (per liter: peptone 10 g, yeast extract 5 g, NaCl 10 g, pH 7.2) was used in inoculum culture. 5 ml LB medium was inoculated with strain Ba, and shaken at 180 rpm for 10 h at 37 °C. After 10 h cultivation, Ba were collected by centrifugation, washed three times with PBS.
Bacterial Translocation
Mice weaned on day 23, and then raised under conventional environment. The mice were divided into three groups. Group I as the control group, group II were treated with 0.85 % NaCl. Group III was administered with probiotic Ba 1 × 109 CFU/day dissolved in 100 μl of 0.85 % NaCl. After 30 days, mice were anesthetized and MLN, liver, spleen, and lungs were removed under sterile conditions. The collected tissues were weighed and homogenized in 500 μl of sterile saline. Aliquots of the homogenate from each tissue were plated onto Tryptic Soy Broth (TSB) agar plates. The plates were examined after aerobic incubation at 37 °C for 24–48 h. Representative colonies were expressed as colony forming unit per gram of organ tissue (CFU/g tissue).
Flow Cytometric Analysis
To detect splenic CD4+T cells, CD8+T cells, B cells, and macrophages, cells were incubated with phycoerythrin-labeled antibodies against mouse CD4+T, CD8+T, F4/80 and B220 (eBioscience) for 30 min on ice, washed, and analyzed in a FACScalibur flow cytometer (Becton–Dickinson) [7].
Statistical Analysis
All data are expressed as mean ± SD of at least three independent experiments. Statistical analyses were performed using Student’s two-tailed t test. Values of P < 0.05 were considered significant.
Results
Oral Administration of B. amyloliquefaciens SC06 Decreases the BT in Weaned Mice
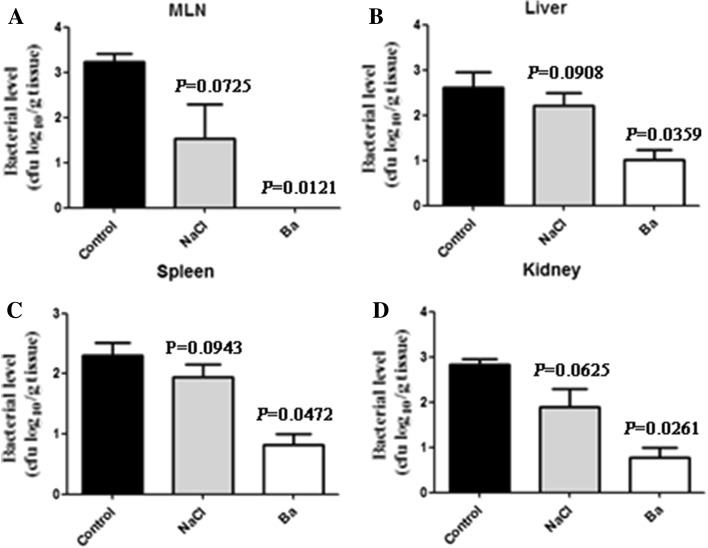
In weaned mice, there were no significant differences between the control group and 0.85 % NaCl treatment group in bacterial levels of the MLN, liver, spleen, and kidney, respectively (P > 0.05) (Fig. 1a–d). Interestingly, oral administration of probiotic Ba significantly reduced bacteria translocation compared with the control group. Lower levels of bacteria were detected in MLN, liver, spleen, and kidney of mice (P < 0.05) (Fig. 1a–d). These results indicated that probiotic Ba ameliorated the maternal separation-induced BT.
Fig. 1.
The level of bacterial translocation was assessed in weaned mice. a Weaned mice of bacterial translocation to MLN in mice without Ba (controls and mice with 0.85 % NaCl, n = 6) and mice with Ba (n = 6). b Weaned mice of bacterial translocation to liver in mice without Ba (controls and mice with 0.85 % NaCl, n = 6) and mice with Ba (n = 6). c Weaned mice of bacterial translocation to spleen in mice without Ba (controls and mice with 0.85 % NaCl, n = 6) and mice with Ba (n = 6). d Weaned mice of bacterial translocation to kidney in mice without Ba (controls and mice with 0.85 % NaCl, n = 6) and mice with Ba (n = 6). Data are mean ± SD for at least three independent experiments. *P < 0.05 (t test)
CD4+T, CD8+T and B Cells Proportion and Macrophage Number in the Spleen of Mice
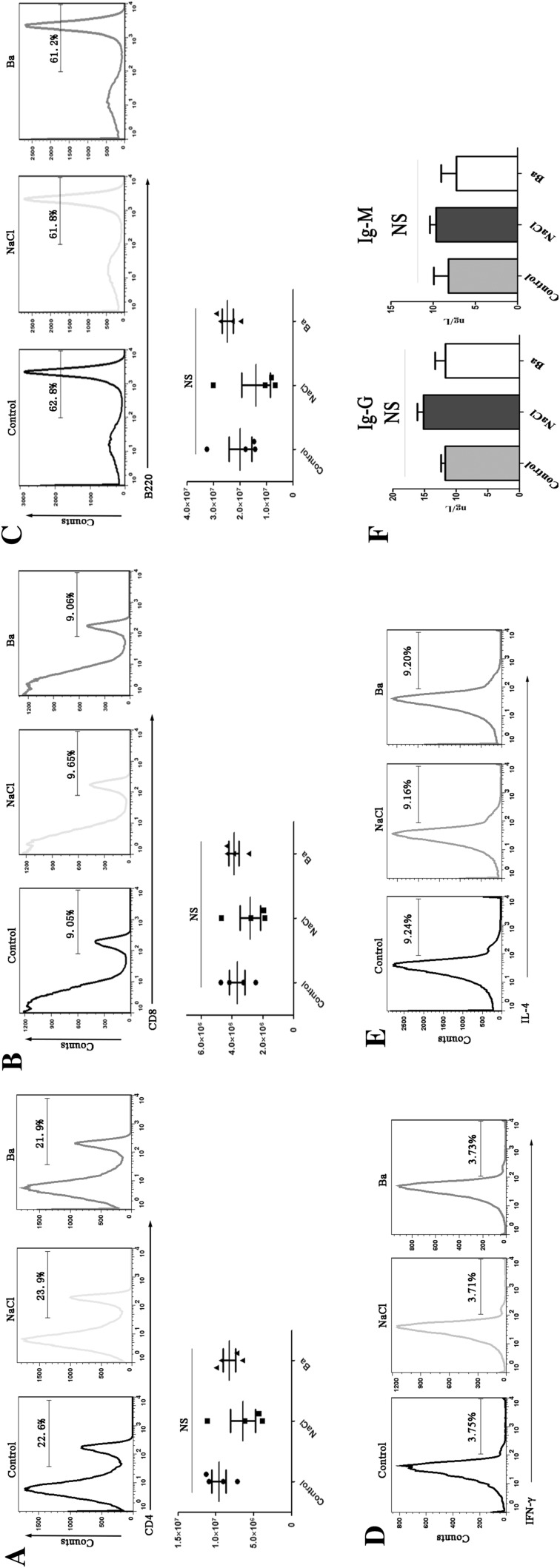
The percentage and absolute number of splenic CD4+T, CD8+T and B cells were determined by FACS after weaned mice were treated with the Ba supplement. As shown in Fig 2, after 30 days, no statistical difference in proportion and absolute number of splenic T (CD4+ and CD8+) and B cells were noted between the groups. Moreover, the percentage of Th1 and Th2 did not show differences in the spleen when the mice were treated with Ba. Meanwhile, there have no statistically change in the IgG and IgM levels (relative to B cells function) in intestinal mucosa among the groups.
Fig. 2.
Effect of Ba on CD4+T, CD8+T, and B cells of spleen in weaned mice. a CD4+T cells proportion and number of spleen in weaned mice. b CD8+T cells proportion and number of spleen in weaned mice. c B cells proportion and number of spleen in weaned mice. d Th1 cells proportion of spleen in weand mice. e Th2 cells proportion of spleen in weand mice. f Mucosal IgG and IgM levels in weaned mice. Data are mean ± SD for at least three independent experiments; *P < 0.05 (t test)
Thus, probiotic Ba may have no immunomodulation of the T (CD4+ and CD8+) and B cells in weaned mice.
Macrophages Proportion and Macrophage Number in the Spleen of Mice
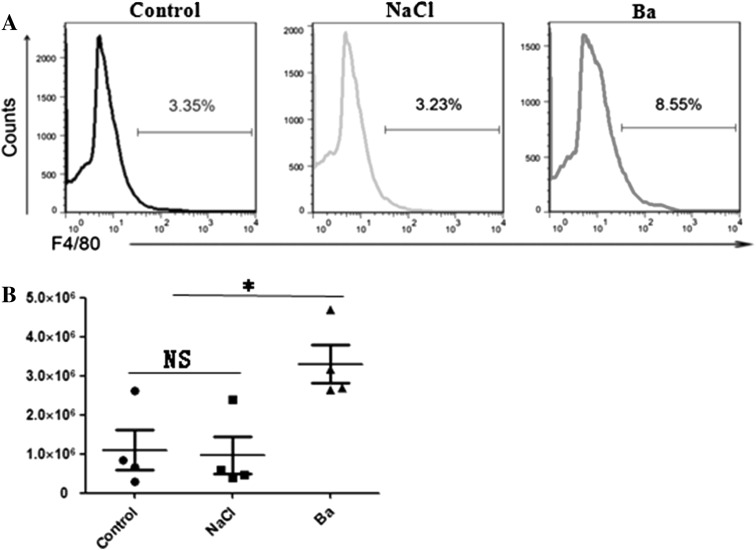
At the same time, we tested the effect of supplemented Ba on the percentage and number of macrophages in spleen of weaned mice. As seen in Fig. 3, dramatically increase in the percentage and number of macrophages were observed in the spleen of Ba-treated mice compared with the control and 0.85 % NaCl group (P < 0.05). These data suggested that administration of Ba could decrease the BT in weaned mice, which might be relevant to the changes in macrophages numbers and function.
Fig. 3.
Effect of Ba on macrophage of spleen in weaned mice. a Macrophage proportion in spleen. b Macrophage number in spleen. Data are mean ± SD for at least three independent experiments. *P < 0.05 (t test)
Discussion
The microflora plays a very important role in maintaining the normal intestinal ecological environment and supplying preferred fuels to the intestinal wall, consequently supporting the intestinal barrier [20]. Changes in diet, stress, the use of antibiotics and excessive hygiene all bring changes in the microbiological ecosystem. Bacterial translocation is caused by the passage of viable pathogens from the gastrointestinal tract through the mucosal epithelium to other sites such as the MLN, spleen, liver, and blood [5]. In the present study, we tested the effect of oral administration of Ba on BT in weaned mice. The results demonstrated a significant reduction of BT after oral administration of Ba. Studies have shown that disruption of intestinal microflora balance may increase the incidence of BT by modifying intestinal barrier function [20]. The effect of dietary fibre and lactobacilli on BT has been investigated in several studies. In models of cytotoxic drug-induced gut injury and liver injury, pectin and oat fibre were shown to reduce BT [1, 16]. Administration of live lactobacilli either orally or by enema will further reduce the translocation [1]. The effect is different for different lactobacilli and the strongest effect was found with Lactobacillus plantarum and Lactobacillus rhamnosus [15]. The mechanisms underlying the decreased translocation are not illustrated. One effect may be mediated via an action on the intestinal wall and its permeability. A study using the Using chamber technique found that Lactobacilli will significantly reduce an Escherichia coli-induced increase in permeability [14].
The immune system of mammals includes a complex array of cells and molecules, which interact to provide protection from challenge by pathogenic microbes (bacteria, viruses, parasites). Cells of innate immune system act as the first line of defence against pathogens but are not overly specific in their ability to recognize their target [6]. Inflammation is a complex biological response to infection or injury that involves many different cell types, mediators, and stimuli [18, 26]. Key players in the innate immune response include the phagocytic cells like neutrophils, monocytes and macrophages. Macrophages are able to produce cytokines recruiting other inflammatory cells such as neutrophils. Macrophages recognize an array of stimuli from endogenous and exogenous sources and respond with remarkable phenotypic plasticity [18, 19]. It is well established that macrophages undergo along 2 distinct pathways of activation under different cytokine microenvironments: the classical (M1) pathway and the alternative (M2) pathway. Activation of macrophages can express distinct functional programs under the different micro-environmental signals. M2 macrophages function in resolving inflammation while promoting cell proliferation and wound healing. M1 macrophages possess an enhanced phagocytic and clear intracellular pathogens in pathological conditions such as infections [4, 11, 14]. M1 appeared in irradiated mice, and were shown to be resistant against Enterococcus faecalis translocation and subsequent sepsis. M2 were responsible for the impaired resistance of mice irradiated with gamma-rays to BT and subsequent sepsis. In contrast, M1 appeared in irradiated mice, and were shown to be resistant against BT [13]. Our previous study indicated that probiotic Ba facilitated polarization of M1 macrophages and enhanced its phagocytic capacity in mouse bone marrow-derived macrophages [12]. Thus, it would be interesting to further explore the role that Ba mediated the M1 macrophage polarization in weaned mice. Many studies have shown that each probiotic appears to influence the immune system in a particular fashion [9, 21, 22]. Comparative genomic analyses of the different strains could provide useful information for determining the strain-specific factors that would explain these differences. More detailed studies are needed to determine the precise action modes of probiotic Ba on both mucosal and systemic immunity. The use of transgenic or knockout mice and other animal models would allow a better understanding of these mechanisms [27].
In summary, our results suggest that administration of B. amyloliquefaciens SC06 could reduce BT in weaned mice. This effect seems to be correlated with the change of macrophage numbers. Although these studies do not cover the entire range of potential immune responses affected by probiotic Ba, they do, importantly, demonstrate that the probiotic play a critical role in the pathogenesis and progression of intestinal disorders involving probiotic-mediated macrophages proliferation.
Acknowledgments
We are grateful to Dr. Linrong Lu (Zhejiang University School of Medicine) for generous support and insightful guidance. This study was supported by the grants from Specialized Research Fund for the Doctoral Program of Higher Education (No. 20110101110101).
Footnotes
Jian Ji and Shenglan Hu contributed equally to this work.
References
- 1.Adawi D, Kasravi FB, Molin G, Jeppsson B. Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology. 1997;25:642–647. doi: 10.1002/hep.510250325. [DOI] [PubMed] [Google Scholar]
- 2.Benitez L, Correa A, Daroit D, Brandelli A. Antimicrobial activity of Bacillus amyloliquefaciens LBM 5006 is enhanced in the presence of Escherichia coli. Curr Microbiol. 2011;62:1017–1022. doi: 10.1007/s00284-010-9814-z. [DOI] [PubMed] [Google Scholar]
- 3.Benitez LB, Velho RV, de Souza DMA, Segalin J, Brandelli A. Antimicrobial factor from Bacillus amyloliquefaciens inhibits Paenibacillus larvae, the causative agent of American foulbrood. Arch Microbiol. 2012;194:177–185. doi: 10.1007/s00203-011-0743-4. [DOI] [PubMed] [Google Scholar]
- 4.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 5.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol Rev. 2009;227:248–263. doi: 10.1111/j.1600-065X.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown KL, Christenson K, Karlsson A, Dahlgren C, Bylund J. Divergent effects on phagocytosis by macrophage-derived oxygen radicals. J Innate Immun. 2009;1:592–598. doi: 10.1159/000235583. [DOI] [PubMed] [Google Scholar]
- 8.Cheung QC, Yuan Z, Dyce PW, Wu D, DeLange K, Li J. Generation of epidermal growth factor-expressing Lactococcus lactis and its enhancement on intestinal development and growth of early-weaned mice. Am J Clin Nutr. 2009;89:871–879. doi: 10.3945/ajcn.2008.27073. [DOI] [PubMed] [Google Scholar]
- 9.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. doi: 10.1111/j.1365-2672.1989.tb05105.x. [DOI] [PubMed] [Google Scholar]
- 10.Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill–evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25:741–757. doi: 10.1111/j.1365-2036.2006.03174.x. [DOI] [PubMed] [Google Scholar]
- 11.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Husheng L, Zhiwen C, Liwei F. Probiotic Bacillus amyloliquefaciens mediate M1 macrophage polarization in mouse bone marrow-derived macrophages. Arch Microbiol. 2013 doi: 10.1007/s00203-013-0877-7. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Nakamura K, Cornforth M, Suzuki F. Role of M2b macrophages in the acceleration of bacterial translocation and subsequent sepsis in mice exposed to whole body [137Cs] gamma-irradiation. J Immunol. 2012;189:296–303. doi: 10.4049/jimmunol.1200350. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 15.Mao Y, NSAD Comparison of the effects of different strains of Lactobacillus in reducing bacterial translocation on methotrexate-induced enterocolitis in rats. Dig Surg. 1997;14:284–291. doi: 10.1159/000172560. [DOI] [Google Scholar]
- 16.Mao Y, Nobaek S, Kasravi B, Adawi D, Stenram U, Molin G, Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996;111:334–344. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- 17.Marahiel MA, Nakano MM, Zuber P. Regulation of peptide antibiotic production in Bacillus. Mol Microbiol. 1993;7:631–636. doi: 10.1111/j.1365-2958.1993.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 19.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nettelbladt CG, Katouli M, Bark T, Svenberg T, Mollby R, Ljungqvist O. Bulking fibre prevents translocation to mesenteric lymph nodes of an efficiently translocating Escherichia coli strain in rats. Clin Nutr. 1998;17:185–190. doi: 10.1016/S0261-5614(98)80055-1. [DOI] [PubMed] [Google Scholar]
- 21.Oggioni MR, Ciabattini A, Cuppone AM, Pozzi G. Bacillus spores for vaccine delivery. Vaccine. 2003;21(Suppl 2):S96–S101. doi: 10.1016/S0264-410X(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 22.Pagnini C, Saeed R, Bamias G, Arseneau KO, Pizarro TT, Cominelli F. Probiotics promote gut health through stimulation of epithelial innate immunity. Proc Natl Acad Sci USA. 2010;107:454–459. doi: 10.1073/pnas.0910307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–672. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steffen EK, Berg RD. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983;39:1252–1259. doi: 10.1128/iai.39.3.1252-1259.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Lou J, Ouyang C, Chen W, Liu Y, Liu X, Cao X, Wang J, Lu L. Ras-related protein Rab10 facilitates TLR4 signaling by promoting replenishment of TLR4 onto the plasma membrane. Proc Natl Acad Sci USA. 2010;107:13806–13811. doi: 10.1073/pnas.1009428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong JH, Hao J, Cao Z, Qiao M, Xu H, Bai Y, Ng TB. An antifungal protein from Bacillus amyloliquefaciens. J Appl Microbiol. 2008;105:1888–1898. doi: 10.1111/j.1365-2672.2008.03917.x. [DOI] [PubMed] [Google Scholar]