Abstract
Previous studies have identified differences in gait kinetics between healthy older and young adults. However, the underlying factors that cause these changes are not well understood. The objective of this study was to assess the effects of age and speed on the activation of lower-extremity muscles during human walking. We recorded electromyography (EMG) signals of the soleus, gastrocnemius, biceps femoris, medial hamstrings, tibialis anterior, vastus lateralis, and rectus femoris as healthy young and older adults walked over ground at slow, preferred and fast walking speeds. Nineteen healthy older adults (age, 73 ± 5 years) and 18 healthy young adults (age, 26 ± 3 years) participated. Rectified EMG signals were normalized to mean activities over a gait cycle at the preferred speed, allowing for an assessment of how the activity was distributed over the gait cycle and modulated with speed. Compared to the young adults, the older adults exhibited greater activation of the tibialis anterior and soleus during mid-stance at all walking speeds and greater activation of the vastus lateralis and medial hamstrings during loading and mid-stance at the fast walking speed, suggesting increased coactivation across the ankle and knee. In addition, older adults depend less on soleus muscle activation to push off at faster walking speeds. We conclude that age-related changes in neuromuscular activity reflect a strategy of stiffening the limb during single support and likely contribute to reduced push off power at fast walking speeds.
Keywords: Aging, Gait, Speed, Lower extremity, Muscles, EMG
1. Introduction
Joint kinetic measures have proven effective in distinguishing the changes in gait mechanics associated with aging. Notably, older adults exhibit decreased peak ankle plantar flexor power during push off (McGibbon and Krebs, 2004; Winter, 1991; Winter et al., 1990), accompanied by either increased peak hip extensor power during early-stance (McGibbon and Krebs, 2004) and/or increased peak hip flexor power generation during late-stance (McGibbon and Krebs, 2004; Judge et al., 1996). This distal to proximal shift in power production (DeVita and Hortobagyi, 2000) exists even among active, healthy older adults, and seems to be more pronounced at faster walking speeds (Silder et al., 2008).
While age-related changes in joint kinetics are well documented, the underlying factors that drive these changes are not well understood. In particular, it is unclear whether biomechanical changes in muscle or adaptations in neuromuscular activity play a more prominent role. For example, it is feasible that muscle activation patterns are unchanged with age and that the changes in joint kinetics are simply a result of age-related muscle remodeling. Sarcopenia is a well documented effect of aging (Frontera et al., 2000, 1991), and a decrease in muscle strength and power is generally associated with this loss of muscle mass (Frontera et al., 2000, 1991; Visser et al., 2002). Such structural changes alone could diminish power output when the plantar flexors contract during the push off phase of walking. Sarcopenia is also characterized by an overall decrease in the number of muscle fibers and cross-sectional area due to fatty and connective tissue replacement of the muscle fibers (Huang et al., 1999; Lexell, 1995; Luff, 1998; Proctor et al., 1998). This non-contractile tissue replacement may cause increased joint stiffness. Since hip flexor tightness is known to contribute substantially to the hip power generated during pre-swing (Whittington et al., 2008), it is feasible that an increase in passive hip joint stiffness could allow for enhanced energy storage in the passive hip flexors during mid to late stance. The release of this energy during pre-swing could then contribute to the increased hip flexor power output observed experimentally in older adult gait.
Alternatively, the changes in gait mechanics may arise via neurally mediated changes in muscle excitation patterns. It has previously been shown that older adults exhibit increased coactivation of knee muscles during walking and that this may contribute to increased metabolic costs for locomotion (Mian et al., 2006). Increased levels of cocontraction have also been observed about the ankle in older adults during isometric exertions (Patten and Kamen, 2000) and challenging postural tasks (Benjuya et al., 2004; Manchester et al., 1989). However, it is not known whether similar ankle muscle cocontraction strategies are employed about the ankle during the single support phase of walking. If present, this could increase active ankle joint stiffness while contributing to a decrease in net power output about the ankle. In this scenario, the greater hip power output in older adults may reflect a compensatory mechanism, whereby an increased neural drive of the hip muscles is used to compensate for decreased power output at the ankle (McGibbon, 2003).
An assessment of muscle activities in the lower limb muscles is important to gain additional insights into the relative influence of biomechanical and neural factors on older adult gait. In this study, we investigated the modulation of lower-extremity electromyography (EMG) signals with walking speed in healthy young and healthy older adults. We hypothesized that older adults would exhibit increased activation of ankle dorsi- and plantar flexors during the single support phase of gait, consistent with ankle muscle coactivation that is observed during challenging postural tasks. We also hypothesized that the distal to proximal shift in power output would be reflected by changes in neuromuscular activity at faster walking speeds, such that older adults would exhibit decreased plantar flexor activity during push off accompanied by increased hip muscle activity during early and late stance.
2. Materials and methods
Nineteen healthy older adults (7 males, 12 females; age, 73 ± 5 years) and 18 healthy young adults (8 males, 10 females; age, 26 ± 3 years) participated in this study (Table 1). Subjects were excluded based on current or history of orthopedic diagnosis, musculoskeletal trauma, persistent joint pain, and any known cardiac, neural, gait impairment, or balance issues. Older adults were screened by a geriatrician to determine additional exclusion criteria based on cognitive impairment (score <24 on the Mini-Mental State Exam, MMSE) (Folstein et al., 1975), plantar sensory impairment (unable to perceive 5.07 Semmes–Weinstein (10-g) monofilament), or loss of vibratory sensation at the great toe (unable to perceive vibration at the great metatarsal–phalangeal joint with a 128 Hz tuning fork). Physical activity levels of the older adults were assessed using the CHAMPS questionnaire (Stewart et al., 2001). The Dynamic Gait Index was used as a clinical assessment of gait and dynamic balance with a score below 19 (maximum possible score of 24) correlated to an increased risk of falling (Whitney et al., 2000). Each subject gave informed consent in agreement with a protocol approved by the University of Wisconsin’s Health Sciences Institutional Review Board.
Table 1.
Average (SD) characteristics of the adults who participated in the study.
| Young adults (n = 18) |
Older adults (n = 19) |
|||
|---|---|---|---|---|
| 8 Men | 10 Women | 7 Men | 12 Women | |
| Age (years) | 27 (4) | 25 (2) | 73 (4) | 72 (6) |
| Body mass (kg) | 81 (7) | 61 (7) | 74 (8) | 67 (12) |
| Height (m) | 1.84 (0.09) | 1.66 (0.06) | 1.75 (0.07) | 1.66 (0.09) |
Subjects were first asked to perform repeated walking trials at their preferred speed over a 12 m walkway. Sacral marker kinematics were recorded in these trials and used to determine the average preferred speed over a gait cycle. Subjects then performed a total of fifteen walking trials, five trials each at 80% (slow), 100% (preferred), and 120% (fast) of their preferred speed, with the ordering of speed randomized. Trials were accepted if the measured speed, as determined from the sacral marker kinematics, was within 5% of the target speed. If not, subjects were asked to repeat the trial and instructed whether to slow down or speed up relative to the most recent trial.
Whole body motion was tracked using 42 motion capture markers, 23 of which were placed on palpable anatomical landmarks. The additional 19 markers were used to enhance segment tracking and reduce skin motion artifact (Cappozzo et al., 1995). Kinematic data were collected at 100 Hz using an eight-camera passive motion capture system (Motion Analysis Corporation, Santa Rosa, CA) and processed with motion capture software (EVaRT v5.0). Ground reaction forces were synchronously recorded at 2000 Hz for two successive foot strikes using three force plates (Model BP400600, AMTI, Watertown, MA) imbedded in the middle of the walkway. EMG signals for the soleus, gastrocnemius, biceps femoris, medial hamstrings, tibialis anterior, vastus lateralis, and rectus femoris were also synchronously recorded at 2000 Hz using preamplified single differential electrodes with 10 mm inter-electrode distance (DE-2.1, DelSys, Inc, Boston, MA). The electrodes were coated with conducting gel prior to application and interfaced with an amplifier/processor unit (CMRR > 85 dB at 60 Hz; input impedance > 100 MΩ). The electrode locations were determined by the same investigator for each subject using standard EMG electrode locations that placed the electrodes at the center of the muscle belly (Basmajian, 1989).
The EMG signals were processed using MATLAB (MATLAB R2006a, MathWorks, Inc., Natick, MA). The data was first passed through a 10–500 Hz sixth order Butterworth bandpass filter and full wave rectified (Konrad, 2005). To create a linear envelope, the data was subsequently passed through a sixth order Butterworth low-pass filter with a 6 Hz cutoff frequency. Heel contact times were determined from the vertical ground reaction force traces using a 10 N threshold. Rectified EMG data were interpolated to obtain a data series of 1001 points over the gait cycle, from heel contact to the subsequent heel contact of the same limb. The EMG signals for each subject were normalized to the mean signal for each muscle over the entire gait cycle of that subject’s preferred speed walking trials.
For each of the seven muscles recorded, an average EMG trace for each subject at each speed was obtained by ensemble averaging across all five trials. We then computed the average normalized EMG activity within selected phases of the gait cycle (Perry, 1992): loading (0–10% of the gait cycle), mid-stance (10–30%), terminal stance and pre-swing (30–60%), initial swing (60–73%), and terminal swing (87–100%). For each muscle, average EMG activities were computed for the phases needed to test our hypotheses. To assess the potential presence of cocontraction during stance, we quantified and compared the average muscle activities from all muscles during loading, mid-stance and terminal stance/pre-swing. To assess the potential role that neural factors play in the distal to proximal shift in power production, we also quantified rectus femoris activity during initial swing and the biceps femoris and medial hamstring EMG activities during terminal swing. A repeated measures ANOVA was used to assess the effects of age (young subjects, older subjects) and speed (slow, preferred, and fast) on the normalized muscle activities in these phases (STATISTICA 6, StatSoft, Inc., Tulsa, OK). Post hoc analyses were subsequently performed using Tukey’s HSD test to evaluate significant speed–age interactions. Statistical significance was defined at p < 0.05.
3. Results
There were no significant differences between the young and older adults with respect to preferred walking speed, cadence, or the percent of the gait cycle spent in swing (Table 2). The older adults tested in this study (CHAMPS score = 11,772) were substantially more active than typical older adults (CHAMPS score = 3386,Stewart et al., 2001). In addition, all older adults displayed normal gait and dynamic balance (Dynamic Gait Index score = 23.8 (0.7)Whitney et al., 2000).
Table 2.
Mean (SD) walking speed, cadence and percent of gait cycle spent in swing phase for both groups at each of the walking speeds tested.
| Young adults |
Older adults |
|||||
|---|---|---|---|---|---|---|
| Slow | Preferred | Fast | Slow | Preferred | Fast | |
| Walking speed (m/s) |
1.07 (0.10) |
1.33 (0.13) |
1.60 (0.13) |
1.06 (0.10) |
1.32 (0.13) |
1.58 (0.16) |
| Cadence (steps/min) |
98 (11) | 112 (10) | 122 (10) | 100 (6) | 115 (7) | 125 (9) |
| Swing phase (% gait cycle) |
32 (1) | 34 (1) | 35 (1) | 32 (2) | 34 (2) | 35 (2) |
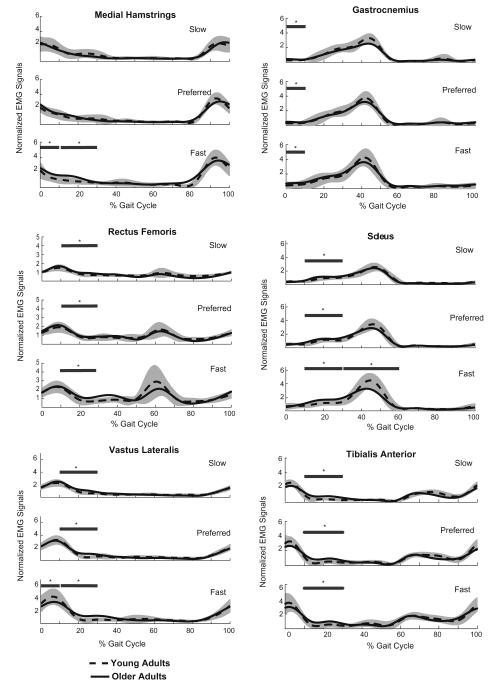
Table 3 summarizes the mean EMG values and significant factors for each gait phase tested, and Fig. 1 displays the EMG curves for muscles that showed significant differences between older and young adults.
Table 3.
Mean (SD) normalized electromyography activities for each group during select phases of the gait cycle. Each muscle’s activity level was normalized to the mean rectified activity it exhibited at the preferred walking speed. A two-way, repeated measures ANOVA was used to determine the influence of age and walking speed on the muscle activities observed.
| Phase of gait cycle | Muscle | Slow |
Preferred |
Fast |
p Values |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young adults | Older adults | Young adults | Older adults | Young adults | Older adults | Speed | Age | Speed*Age | ||
| Loading response (0–10%) | TA | 2.14 (0.40) | 1.97 (0.31) | 2.61 (0.48) | 2.30 (0.36) | 2.82 (0.80) | 2.54 (0.74) | 0.000 | 0.068 | 0.801 |
| SOL | 0.48 (0.15) | 0.44 (0.15) | 0.52 (0.19) | 0.57 (0.18) | 0.73 (0.38) | 0.80 (0.42) | 0.000 | 0.697 | 0.553 | |
| GAS | 0.29 (0.11) | 0.41 (0.18) | 0.32 (0.15) | 0.47 (0.17) | 0.51 (0.39) | 0.77 (0.55) | 0.000 | 0.013 | 0.514 | |
| BF | 1.70 (0.77) | 1.81 (0.65) | 1.73 (0.84) | 2.12 (0.80) | 1.89 (0.97) | 2.35 (0.93) | 0.038 | 0.166 | 0.416 | |
| VL | 2.06 (0.47) | 2.15 (0.47) | 2.90 (0.63) | 2.86 (0.47) | 3.96 (1.17) | 3.24 (0.98) | 0.000 | 0.234 | 0.011 | |
| MH | 2.11 (0.81) | 1.97 (0.62) | 1.66 (0.51) | 1.90 (0.61) | 1.46 (0.51) | 1.98 (0.80) | 0.006 | 0.263 | 0.009 | |
| RF | 1.31 (0.25) | 1.43 (0.34) | 1.69 (0.45) | 1.93 (0.50) | 2.11 (0.56) | 2.16 (0.60) | 0.000 | 0.328 | 0.351 | |
| Mid-stance (10–30%) | TA | 0.44 (0.22) | 0.83 (0.41) | 0.49 (0.21) | 0.92 (0.35) | 0.55 (0.29) | 0.96 (0.41) | 0.092 | 0.000 | 0.941 |
| SOL | 0.86 (0.26) | 1.07 (0.23) | 0.99 (0.28) | 1.21 (0.34) | 1.08 (0.35) | 1.57 (0.47) | 0.000 | 0.001 | 0.054 | |
| GAS | 1.14 (0.49) | 1.08 (0.36) | 1.20 (0.41) | 1.17 (0.45) | 1.27 (0.53) | 1.50 (0.62) | 0.001 | 0.755 | 0.101 | |
| BF | 0.71 (0.34) | 0.88 (0.56) | 0.76 (0.36) | 0.99 (0.59) | 0.89 (0.49) | 1.30 (0.69) | 0.000 | 0.100 | 0.086 | |
| VL | 1.13 (0.26) | 1.44 (0.40) | 1.31 (0.25) | 1.55 (0.30) | 1.39 (0.31) | 1.76 (0.49) | 0.000 | 0.001 | 0.577 | |
| MH | 1.06 (0.56) | 0.90 (0.51) | 0.87 (0.35) | 0.98 (0.59) | 0.82 (0.34) | 1.25 (0.61) | 0.219 | 0.395 | 0.000 | |
| RF | 0.92 (0.19) | 1.09 (0.26) | 1.06 (0.20) | 1.19 (0.26) | 1.11 (0.36) | 1.44 (0.39) | 0.000 | 0.006 | 0.170 | |
| Terminal stance and pre-swing (30–60%) |
TA | 0.29 (0.14) | 0.32 (0.17) | 0.39 (0.15) | 0.38 (0.16) | 0.58 (0.36) | 0.57 (0.25) | 0.000 | 0.992 | 0.846 |
| SOL | 1.71 (0.35) | 1.69 (0.24) | 2.13 (0.29) | 1.98 (0.22) | 2.67 (0.53) | 2.23 (0.52) | 0.000 | 0.048 | 0.007 | |
| GAS | 1.96 (0.33) | 1.69 (0.31) | 2.06 (0.32) | 1.96 (0.29) | 2.31 (0.44) | 2.22 (0.68) | 0.000 | 0.172 | 0.381 | |
| BF | 0.50 (0.23) | 0.48 (0.23) | 0.61 (0.26) | 0.45 (0.24) | 0.84 (0.70) | 0.58 (0.30) | 0.002 | 0.137 | 0.180 | |
| VL | 0.47 (0.21) | 0.54 (0.23) | 0.55 (0.18) | 0.55 (0.19) | 0.66 (0.35) | 0.76 (0.21) | 0.000 | 0.352 | 0.438 | |
| MH | 0.49 (0.18) | 0.42 (0.21) | 0.49 (0.22) | 0.39 (0.17) | 0.55 (0.30) | 0.52 (0.25) | 0.007 | 0.338 | 0.507 | |
| RF | 0.65 (0.16) | 0.64 (0.16) | 0.83 (0.18) | 0.83 (0.26) | 1.20 (0.42) | 1.23 (0.49) | 0.000 | 0.983 | 0.949 | |
| Initial swing (60–73%) | RF | 0.77 (0.27) | 0.62 (0.22) | 1.18 (0.33) | 1.05 (0.39) | 1.93 (0.88) | 1.50 (0.66) | 0.000 | 0.084 | 0.168 |
| Terminal swing (87–100%) | BF | 1.90 (0.66) | 1.79 (0.71) | 2.63 (0.62) | 2.46 (0.83) | 3.06 (0.89) | 3.28 (1.32) | 0.000 | 0.928 | 0.194 |
| MH | 2.14 (0.73) | 2.06 (0.54) | 2.95 (0.61) | 2.73 (0.79) | 3.40 (0.81) | 3.27 (1.19) | 0.000 | 0.541 | 0.822 | |
Note: TA = tibialis anterior; SOL = soleus; GAS = gastrocnemius; BF = biceps femoris; VL = vastus lateralis; MH = medial hamstrings; RF = rectus femoris.
Fig. 1.
Ensemble averaged electromyographic activities for the young and older adults at the slow, preferred and fast walking speeds. The shaded portion represents plus and minus one standard deviation of the young adults’ data. The horizontal lines above the curves indicate phases of the gait cycle where the average normalized activities over a phase of the gait cycle differed significantly (*p < 0.05) between the age groups, as determined by Tukey’s post hoc tests. The phases of the gait cycle that were statistically compared for each muscle are given in Table 3.
3.1. Loading phase
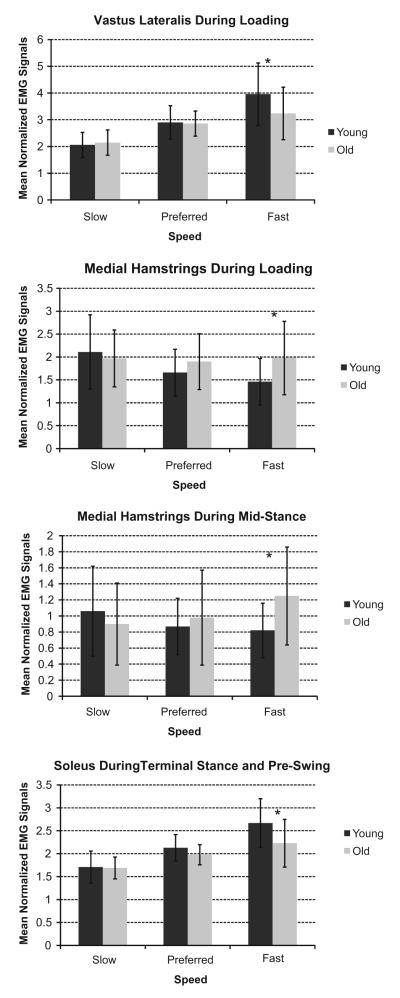
Tibialis anterior, soleus, biceps femoris, and rectus femoris activities showed a speed effect, with activity increasing as speed increased. The older adults used significantly more gastrocnemius activity than the young adults across all three speeds (p = 0.013), with activity significantly increasing for both age groups as speed increased. The vastus lateralis showed a significant speed–age interaction (Fig. 2). Tukey’s post hoc test revealed age was only a factor at the faster walking speed, with the older adults displaying 22% less activity than the younger adults. The medial hamstrings also showed a speed–age interaction (Fig. 2). Age was only significant at the faster walking speed, where the older adults showed 36% more activity than the young adults.
Fig. 2.
Mean normalized electromyographic activities for the young and older adults during phases of the gait cycle where muscle activity showed a significant speed–age interaction. The asterisk (*) denote where age was a significant factor in post-hoc tests.
3.2. Mid-stance
Gastrocnemius and biceps femoris activities showed significant speed effects, whereby activity increased as speed increased. With the older adults showing significantly more activity across all three speeds, soleus (p = 0.001), vastus lateralis (p = 0.001), and rectus femoris (p = 0.006) showed significant age effects, along with a speed effect where activity increased as speed also increased. The tibialis anterior only showed an age effect where the older adults exhibited more activity than the younger adults across all three speeds (p = 0.000). Medial hamstrings activity showed a speed–age interaction (Fig. 2). Tukey’s post hoc analysis showed age was only significant at the faster walking speed, with older adults displaying 52% more medial hamstring activity than the young adults.
3.3. Terminal stance and pre-swing
Speed was the only significant effect in tibialis anterior, gastrocnemius, biceps femoris, vastus lateralis, medial hamstring, and rectus femoris activities. These activities significantly increased as speed increased. Soleus activity exhibited a speed–age interaction (Fig. 2). At the fast walking speed, older adults used 20% less soleus activity than the young adults.
3.4. Initial swing
Only rectus femoris activity showed a speed effect, with activity significantly increasing as speed increased.
3.5. Terminal swing
Biceps femoris and medial hamstring activity did not show any age effects but did show speed effects, with activity levels increasing with speed.
4. Discussion
We quantified lower-extremity EMG patterns in healthy young and older adults at three walking speeds. Our results show older adults adopt a neuromuscular activation pattern that seems to utilize greater coactivation of muscles about the ankle at all speeds and about the knee at fast speeds during mid-stance and depend less on soleus muscle activation to push off at faster walking speeds. Increased levels of hamstring activity in the older adults were also present during loading and mid-stance at faster walking speeds, which may contribute to the increased hip extensor power during early-stance commonly observed in older adult gait (DeVita and Hortobagyi, 2000; McGibbon and Krebs, 2004; Silder et al., 2008).
The differences in muscle activity between groups observed in this study cannot be attributed to gait speed differences, since both young and older subjects exhibited similar walking speeds. Prior studies have reported a slower walking speed in older adults of ~70 years of age (Kerrigan et al., 1998; McGibbon, 2003; McGibbon and Krebs, 1999; Winter, 1991). In this study, the older adults’ average preferred walking speed (1.32 m/s) was near the upper end of the range measured for this age group (1.03–1.30 m/s, Judge et al., 1996; Kang and Dingwell, 2008; Kerrigan et al., 1998; McGibbon and Krebs, 1999, 2004). The faster speeds seen in this study may, in part, reflect the high activity levels of the older adults tested (CHAMPS score = 11,772). Another recent cross-sectional study also found that active healthy older adults exhibited similar walking speeds to height and weight matched younger adults (Kang and Dingwell, 2008).
We observed a greater prevalence of age effects on uniarticular muscle activities (soleus, vastus lateralis), than on biarticular muscles, that cross the same joint (gastrocnemius, rectus femoris). For example, older adults exhibited comparable gastrocnemius activation to the young adults during push off at all speeds but showed 20% less soleus activity during push off at the faster walking speed. This difference may relate to the different biomechanical roles that these muscles play during normal gait. The results of a forward dynamic model suggest that the uniarticular soleus is more important in generating forward propulsion of the trunk, while the biarticular gastrocnemius plays a bigger role in initiating swing limb motion (Neptune et al., 2001). A recent empirical study has confirmed differential biomechanical function of the soleus and gastrocnemius during normal walking (Stewart et al., 2007). Thus, our results would suggest that in older adults, the gastrocnemius retains its role in swing initiation. Since older adults show less soleus activity during push off at the fast walking speed but retain similar walking speeds, the soleus seems to diminish its role in providing forward propulsion. In addition to the changes at the ankle, older adults showed 22% less activity in the uniarticular knee extensor (vastus lateralis) during the loading phase of fast walking while the biarticular rectus femoris exhibited no change in activation during loading. It has previously been observed that there is a slight reduction in the negative work done by the knee extensors during loading (DeVita and Hortobagyi, 2000), which this study would attribute to reduced vastus activation.
Prior studies have reported that older adults often exhibit increased hip extensor power and/or increased hip flexor power generation in conjunction with reduced ankle plantar flexor power, particularly at faster walking speeds (DeVita and Hortobagyi, 2000; Judge et al., 1996; McGibbon, 2003; McGibbon and Krebs, 2004; Winter et al., 1990). Similarly, we previously showed that the older adults analyzed in this study generated more peak hip extensor power and did significantly more positive work by both the hip extensors and flexors, when compared to the younger subjects (Silder et al., 2008). We did not record surface EMG activity from the uniarticular hip flexors and extensors and thus are unable to definitively assess how these muscles may have contributed to the biomechanical differences observed. However, at the fast walking speed the older adults showed an average of 36% more medial hamstring activity during loading and 52% more activity during mid-stance than the young adults. These results suggest that the hamstrings may contribute to the increased hip extensor power observed biomechanically and thus may serve to compensate for reduced soleus power output during plantar flexion (McGibbon, 2003). The older adults activated the rectus femoris more than the young adults during mid-stance across all three speeds but showed no difference in activity during terminal stance and pre-swing. Therefore, an increase in hip flexor work, if generated actively, likely must arise from modulation of uniarticular hip flexors such as the iliacus and psoas muscles.
Cocontraction has been observed as a task independent strategy that is believed to be used to stiffen the joint and enhance stability (Benjuya et al., 2004; Hortobagyi and DeVita, 2006; Manchester et al., 1989). Our results suggest that older adults may employ greater coactivation of the uniarticular soleus and tibialis anterior muscles during mid-stance at all three speeds, which may increase ankle stiffness and accommodate potential balance concerns. Increased activity levels were also present in the vastus lateralis (at all speeds) and hamstrings (fast speed) during mid-stance, suggesting that the joint stiffening strategy may extend to the knee when older adults increase their walking speed. However, it is interesting to note that none of the older adults exhibited lower-extremity sensory deficits as measured by the plantar monofilament test or great toe vibratory sensation testing. This suggests that sensory deficits are either not present or are not detectable via standard clinical means. Alternatively, cortical mechanisms may be responsible for the increased cocontraction commonly seen in older adults, with the scaling of flexion, extension, and coactivity commands in the cortex becoming more inaccurate with age (Hortobagyi and DeVita, 2006). A potential side-effect of increased cocontraction of opposing muscles would be higher metabolic costs, as discussed by Mian et al. (2006).
In this study, we normalized EMG signals to average activities measured at the preferred walking speed. Such a normalization approach was beneficial in assessing how EMG activity was distributed across the gait cycle and how activity levels were modulated with increasing and decreasing speed. However in using this approach, we did not attain a measure of the activity level with respect to maximum. Furthermore, while the young and older adults walked at the same preferred speeds, it is possible that they were operating at differing percentages of their aerobic capacities. As a result, it is not possible to determine whether the reduced soleus activity in older adults during terminal stance and pre-swing at the faster speed represented a saturation effect (i.e. near maximal activity) or alternatively represented a deliberate attempt to rely less on the soleus to push off. However, in either case, it is clear that reduced push off power must, in part, have a neural basis and thus, does not strictly represent a reduction in strength due to age-related sarcopenia (Frontera et al., 2000, 1991; Visser et al., 2002).
In summary, age-related changes in the neuromuscular activation of walking are clearly present, even among active healthy older adults. The changes in activity observed in this study would actively induce the distal-to-proximal shift in power production observed in the gait of older adults (DeVita and Hortobagyi, 2000) and would also act to enhance joint stiffness during single limb support.
Acknowledgements
This publication was made possible by Grant Number AG24276 from the National Institutes of Health and a National Science Foundation pre-doctoral fellowship (AS). We gratefully acknowledge the contribution of Ben Whittington, MS.
Biographies
Anne Schmitz received the BS degree in Mechanical Engineering at the University of Wisconsin-Madison in 2006. Currently, she is a Master’s candidate in Biomedical Engineering at UW-Madison. Her research interests include computational biomechanics, simulation of human gait, and finite element analysis. Clinical applications include knee surgery simulation and cerebral palsy gait analysis.

Amy Silder received the BS degree (2003) in Biosystems Engineering from Michigan State University and the MSE degree (2005) from the University of Wisconsin-Madison, where she is currently a doctoral student in Biomedical Engineering. Her research interests include imaging musculotendon mechanics via magnetic resonance imaging, running and walking mechanics, and injury mechanics and rehabilitation.

Bryan Heiderscheit, PT, PhD is an Assistant Professor in the Departments of Orthopedics & Rehabilitation and Biomedical Engineering at the University of Wisconsin-Madison. He completed his physical therapy training at the University of Wisconsin-La Crosse (1994) and his doctorate in biomechanics from the University of Massachusetts (2000). He is co-director of the Neuromuscular Biomechanics Laboratory at the University of Wisconsin-Madison and director of the Runners’ Clinic through the University of Wisconsin Sports Medicine Clinic. His research is aimed at understanding and enhancing movement coordination as it relates to injury and aging, with recent projects focused on the mechanisms of running-related injuries and falls-risk detection in older adults. He has received research support from the NIH, NFL Medical Charities and American Physical Therapy Association.

Jane Mahoney, MD, is an Associate Professor in the Geriatics Section of the Department of Medicine at the University of Wisconsin-Madison School of Medicine and Public Health, and the Medical Director of Care Wisconsin. She received her MD degree (1986) from the University of California – San Francisco. Her internship and residency (1986–1989) were done in Internal Medicine at the University of Wisconsin Hospitals and Clinics in Madison, WI; followed by a fellowship in Geriatrics (1989–1991) at the William S. Middleton Memorial Veterans Hospital, also in Madison, WI. Her research interests include epidemiologic, randomized trial, and dissemination research examining risk of falls in the elderly and interventions to decrease falls and improve mobility. She has received funding from the NIH, CDC, and American Physical Therapy Foundation.

Darryl G. Thelen received the BS degree in Mechanical Engineering from Michigan State University, in 1987 and the MSE and PhD degrees in Mechanical Engineering from the University of Michigan, Ann Arbor, MI, in 1988 and 1992, respectively. He is currently an Associate Professor in the Department of Mechanical Engineering at the University of Wisconsin-Madison. His research interests include computational biomechanics, simulation and control of human locomotion, and dynamic imaging of musculotendon mechanics.

References
- Basmajian JVe. Biofeedback: principles and practice for clinicians. 3rd ed 1989. [Google Scholar]
- Benjuya N, Melzer I, Kaplanski J. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J Gerontol A Biol Sci Med Sci. 2004;59A(2):166–71. doi: 10.1093/gerona/59.2.m166. [DOI] [PubMed] [Google Scholar]
- Cappozzo A, Catani F, Croce UD, Leardini A. Position and orientation in space of bones during movement: anatomical frame definition and determination. Clin Biomech (Bristol, Avon) 1995;10(4):171–8. doi: 10.1016/0268-0033(95)91394-t. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88(5):1804–11. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol. 1991;71(2):644–50. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88(4):1321–6. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, DeVita P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exercise Sport Sci Rev. 2006;34(1):29–35. doi: 10.1097/00003677-200601000-00007. [DOI] [PubMed] [Google Scholar]
- Huang RP, Rubin CT, McLeod KJ. Changes in postural muscle dynamics as a function of age. J Gerontol. 1999;54A(8):B352–7. doi: 10.1093/gerona/54.8.b352. [DOI] [PubMed] [Google Scholar]
- Judge JO, Davis R, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol Med Sci. 1996;51(6):M303–12. doi: 10.1093/gerona/51a.6.m303. [DOI] [PubMed] [Google Scholar]
- Kang H, Dingwell J. Separating the effects of age and walking speed on gait variability. Gait Posture. 2008;27(4):572–7. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehab. 1998;79(3):317–22. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- Konrad P. The ABC of EMG: a practical introduction to kinesiological electromyography. Scottsdale, AZ: 2005. [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50A(Spec):11–7. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor unites. Ann NY Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- Manchester D, Woollacott MH, Zederbauer-Hylton N, Marin O. Visual, vestibular, and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44(4):M118–27. doi: 10.1093/geronj/44.4.m118. [DOI] [PubMed] [Google Scholar]
- McGibbon CA. Toward a better understanding of gait changes with age and disablement: neuromuscular adaptation. Exercise Sport Sci Rev. 2003;31(2):102–8. doi: 10.1097/00003677-200304000-00009. [DOI] [PubMed] [Google Scholar]
- McGibbon CA, Krebs DE. Effects of age and functional limitation on leg joint power and work during stance phase of gait. J Rehab Res Dev. 1999;36(3):173–82. [PubMed] [Google Scholar]
- McGibbon CA, Krebs DE. Discriminating age and disability effects in locomotion: neuromuscular adaptations in musculoskeletal pathology. J Appl Physiol. 2004;96(1):149–60. doi: 10.1152/japplphysiol.00422.2003. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Narici MV, Minetti AE. Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiol (Oxf) 2006;186(2):127–39. doi: 10.1111/j.1748-1716.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34(11):1387–98. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol. 2000;83(2):128–43. doi: 10.1007/s004210000271. [DOI] [PubMed] [Google Scholar]
- Perry J. Gait analysis: normal and pathological function. 1992.
- Proctor DN, Balagopal P, Nair KS. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J Nutr. 1998;128(2):351S–5S. doi: 10.1093/jn/128.2.351S. [DOI] [PubMed] [Google Scholar]
- Silder A, Heiderscheit B, Thelen DG. Active and passive contributions to joint kinetics during walking in older adults. J Biomech. 2008;41(7):1520–7. doi: 10.1016/j.jbiomech.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exercise. 2001;33(7):1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stewart C, Postans N, Schwartz MH, Rozumalski A, Roberts A. An exploration of the function of the triceps surae during normal gait using functional electrical stimulation. Gait Posture. 2007;26(4):482–8. doi: 10.1016/j.gaitpost.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70–79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50(5):897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]
- Whitney SL, Hudak MT, Marchetti GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. J Vestibul Res. 2000;10(2):99–105. [PubMed] [Google Scholar]
- Whittington B, Silder A, Heiderscheit B, Thelen DG. The contribution of passive-elastic mechanisms to lower extremity joint kinetics during human walking. Gait Posture. 2008;27(4):628–34. doi: 10.1016/j.gaitpost.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter DA. The biomechanics and motor control of human gait: normal, elderly and pathological. 2nd ed 1991. [Google Scholar]
- Winter D, Patla A, Frank J, Walt S. Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther. 1990;70(6):340–7. doi: 10.1093/ptj/70.6.340. [DOI] [PubMed] [Google Scholar]