Short abstract
In vitro cultures with insulin-like growth factor-1 (IGF-1) and transforming growth factor-β1 (TGF-β1) have previously been shown to differentially modulate the growth of immature bovine articular cartilage. IGF-1 stimulates expansive growth yet decreases compressive moduli and increases compressive Poisson’s ratios, whereas TGF-β1 maintains tissue size, increases compressive moduli, and decreases compressive Poisson’s ratios. The current study’s hypothesis was that sequential application of IGF-1 and TGF-β1 during in vitro culture produces geometric and compressive mechanical properties that lie between extreme values produced when using either growth factor alone. Immature bovine articular cartilage specimens were harvested and either untreated (D0, i.e., day zero) or cultured in vitro for either 6 days with IGF-1 (D6 IGF), 12 days with IGF-1 (D12 IGF), or 6 days with IGF-1 followed by 6 days with TGF-β1 (D12 SEQ, i.e., sequential). Following treatment, all specimens were tested for geometric, biochemical, and compressive mechanical properties. Relative to D0, D12 SEQ treatment enhanced volumetric growth, but to a lower value than that for D12 IGF. Furthermore, D12 SEQ treatment maintained compressive moduli and Poisson’s ratios at values higher and lower, respectively, than those for D12 IGF. Considering the previously described effects of 12 days of treatment with TGF-β1 alone, D12 SEQ induced both growth and mechanical property changes between those produced with either IGF-1 or TGF-β1 alone. The results suggest that it may be possible to vary the durations of select growth factors, including IGF-1 and TGF-β1, to more precisely modulate the geometric, biochemical, and mechanical properties of immature cartilage graft tissue in clinical repair strategies.
Introduction
Articular cartilage supports and distributes loads in synovial joints while providing a nearly frictionless contact surface during joint motion. Articular cartilage experiences a high level of mechanical stress and can tolerate decades of repetitive loading; however, damage, degeneration, and arthritis occur often with joint injury and aging at particular sites. Articular cartilage has a poor intrinsic healing capacity, likely related to its low metabolic activity and avascularity. The attainment of a number of specific design goals related to composition, structure, and function may be critical to a consistently successful articular cartilage repair strategy [1,2]. Current repair strategies include transplanted osteochondral auto- and allografts, autologous chondrocyte implantation, microfracturing, and tissue engineered constructs [1,3,4]. A major distinction between these strategies is the immediate load-bearing properties of the implant and, associated with that, the post-operative rehabilitation time. Implants with mechanical properties like those of mature tissue are needed to facilitate normal joint biomechanics. Thus, precise modulation of articular cartilage tissue explant properties in vitro may aid in the identification of a consistently successful repair strategy.
In vitro cultures with insulin-like growth factor-1 (IGF-1) and transforming growth factor-β1 (TGF-β1) regulate articular cartilage metabolism and resultant mechanical properties. Both IGF-1 and TGF-β1 stimulate glycosaminoglycan (GAG) and collagen (COL) synthesis in bovine articular cartilage explants [5–9] while producing differential effects on growth in terms of tissue size and mechanical properties. IGF-1 treatment enhances volumetric growth and degrades mechanical properties, as evidenced by reduced tensile and compressive moduli and increased compressive Poisson’s ratios [9–13]. Contrarily, TGF-β1 treatment inhibits tissue growth and maintains or enhances mechanical properties [5,9,12,13].
The long-term goal of this study is to identify in vitro growth protocols using IGF-1, TGF-β1, and possibly other regulatory agents that precisely modulate the geometric, biochemical, and mechanical properties of articular cartilage graft tissue in the context of improving clinical repair strategies. For example, if articular cartilage tissue transplantation is desired from a region of lower thickness and load bearing to a region of higher thickness and load bearing, IGF-1 treatment may first be used to increase tissue thickness. However, because that treatment may result in a decrease of mechanical integrity, subsequent TGF-β1 treatment may then be used to restore, and enhance, mechanical properties needed for a successful transplantation to a higher load-bearing region. Motivated by the differential effects that IGF-1 and TGF-β1 have on tissue properties, the current study’s hypothesis was that sequential application of IGF-1 and TGF-β1 during in vitro culture produces geometric and compressive mechanical properties that lie between extreme values produced when using either growth factor alone. If the results support this hypothesis, then various durations of growth factor application during culture may be used to produce a targeted articular cartilage graft tissue.
The specific aims of the current study are to determine geometric, biochemical, and compressive mechanical properties for bovine articular cartilage specimens either untreated or cultured in vitro, while treated with IGF-1 with and without sequential application with TGF-β1.
Methods
Harvest and Culture.
Harvest and culture methods are similar to those used previously [9]. Articular cartilage explants with intact articular surfaces were harvested from the medial and lateral ridges of the patellofemoral groove of six immature (1- to 3-week-old) bovine knees. The anterior-lateral corner was cut from each explant to track orientation throughout culture and testing. The axial direction was defined as normal to the articular surface and in the direction of loading during compression testing. A coordinate system was defined with 1-, 2-, and 3- directions in medial-lateral, proximal-distal, and axial directions, respectively. Explants were distributed into four experimental groups. Day 0 (D0) explants were immediately placed into phosphate buffered saline with protease inhibitors (PBS + PIs) at 4°C for 1 h after harvest and then frozen at -70°C. Other explants were placed into well plates with PBS + antibiotics and prepared for culture. 1-mm-thick slices were taken from the articular surface of each explant using a vibrating microtome and trimmed to 6 × 6 × 1-mm3 specimens for culture. Initial wet weight (WWi) was recorded and initial thickness was obtained as an average of three measurements using a laser micrometer. Throughout preparation, specimens were soaked in PBS + antibiotics. D6 IGF explants were cultured for 6 days in medium (Dulbecco’s modified Eagle’s medium with 100 μg/ml ascorbate, 0.01% bovine serum albumin, 0.1 mM nonessential amino acids, 0.4 mM l-proline, 2 mM l-glutamine, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B) with 50 ng/mL IGF-1. D12 IGF explants were cultured for 12 days in medium with 50 ng/mL IGF-1. D12 SEQ specimens were cultured for 6 days in medium with 50 ng/mL IGF-1 followed by 6 days in medium with 10 ng/mL TGF-β1. All specimens were kept in well plates with 1.4 mL/explant of medium at 37°C in humidified 5% CO2 incubators. Medium was changed every other day. Wet weight (WWf) and final thickness were obtained at the end of culture, and specimens were soaked in PBS + PIs for 1 h at 4°C before being frozen at -70°C.
Mechanical Testing.
Specimens were tested in confined compression (CC) and then unconfined compression (UCC) using established protocols [11,14,15]. For CC testing, specimens were cut into 4.8-mm-diameter disks using a stainless steel dermal punch, placed in a confining ring between permeable platens in a materials testing machine (Dynastat, Northern Industrial, Albany, NY), and continuously hydrated with PBS + PIs throughout testing. The CC test protocol involved consecutive 400-s ramps to 15%, 30%, and 45% compressive strains with stress relaxation to equilibrium following each ramp. Equilibrium was defined as a change in stress of <0.003 MPa over 180 s. Using force and displacement data from the Dynastat, stress and strain were calculated by normalizing load and displacement by original cross-sectional area and height, respectively, and secant CC modulus (H A) was calculated at each strain as total stress over total strain. Directly following CC testing, specimens were placed into well plates containing PBS + PIs and allowed to soak overnight at 4°C before UCC testing. As confirmed by pilot testing, the specimens swelled back to their original thicknesses during this time.
For UCC testing, each 4.8-mm-diameter disk was punched into a 3.2-mm-diameter disk using a stainless steel dermal punch, placed between smooth, impermeable platens in the same materials testing machine, and continuously hydrated with PBS + PIs throughout testing. The UCC test protocol involved consecutive 400-s ramps to 15%, 30%, and 45% compressive strains with stress relaxation to equilibrium following each ramp. Equilibrium was defined as a change in stress of <0.003 MPa over 180s. At each equilibrium strain, compression testing was paused while images were taken of the specimens using a Nikon D-80 camera from two orthogonal angles (anterior-lateral direction and posterior-medial direction) using mirrors [11]. These images were processed in a custom matlab (Mathworks, Natick, MA) edge detection script to determine lateral expansion, which was used to calculate Poisson’s ratios at each strain (ν31 and ν32). Secant UCC Young’s modulus (E) was calculated as described above for H A.
Biochemical Analyses.
Following mechanical testing, specimens were frozen at -70°C overnight until thawing in PBS + PIs, lyopholized, and measured for dry weight. Water content was calculated as the difference between final wet and dry weights as a percent of WWf. The tissue was digested in a solution of proteinase K, and assessed for DNA, hydroxyproline, and GAG content. Calculations for cells and COL used ratios of 7.7 pg DNA/chondrocyte [16] and 7.25 g COL/g hydroxyproline [17,18], respectively. All data (water, GAG, COL, and cells) were normalized to WWi to represent contents and to WWf to represent concentrations. For D0 specimens, WWf equals WWi.
Statistical Analyses.
The effects of culture condition on geometric, biochemical, and mechanical properties were assessed by single factor analysis of variance (ANOVA) with post hoc Tukey tests. The effects of direction on Poisson’s ratios at each strain level were assessed after pooling all specimens by paired t-tests. Significance levels were defined by p = 0.05. Data are presented as mean ± standard error of the mean (SE).
Each mechanical property (H A, E, ν31, and ν32) at the 30% strain level were plotted against each biochemical property (water, GAG, COL, cells) as percentages of WWf and analyzed with univariate linear regression followed by t-tests to assess if predicted regression slopes were significantly different than zero. Linear regression was analyzed for each group individually (D0, D6 IGF, D12 IGF, D12 SEQ) and for all groups combined (ALL). Additional linear regression analyses performed included mechanical properties at the 30% strain level against GAG/COL ratio and against GAG and COL combined (multivariate analysis). Significance levels were defined using p = 0.05. Coefficients of determination (R 2) were determined for each regression and reported for significant relationships.
Results
Geometric and Biochemical Properties.
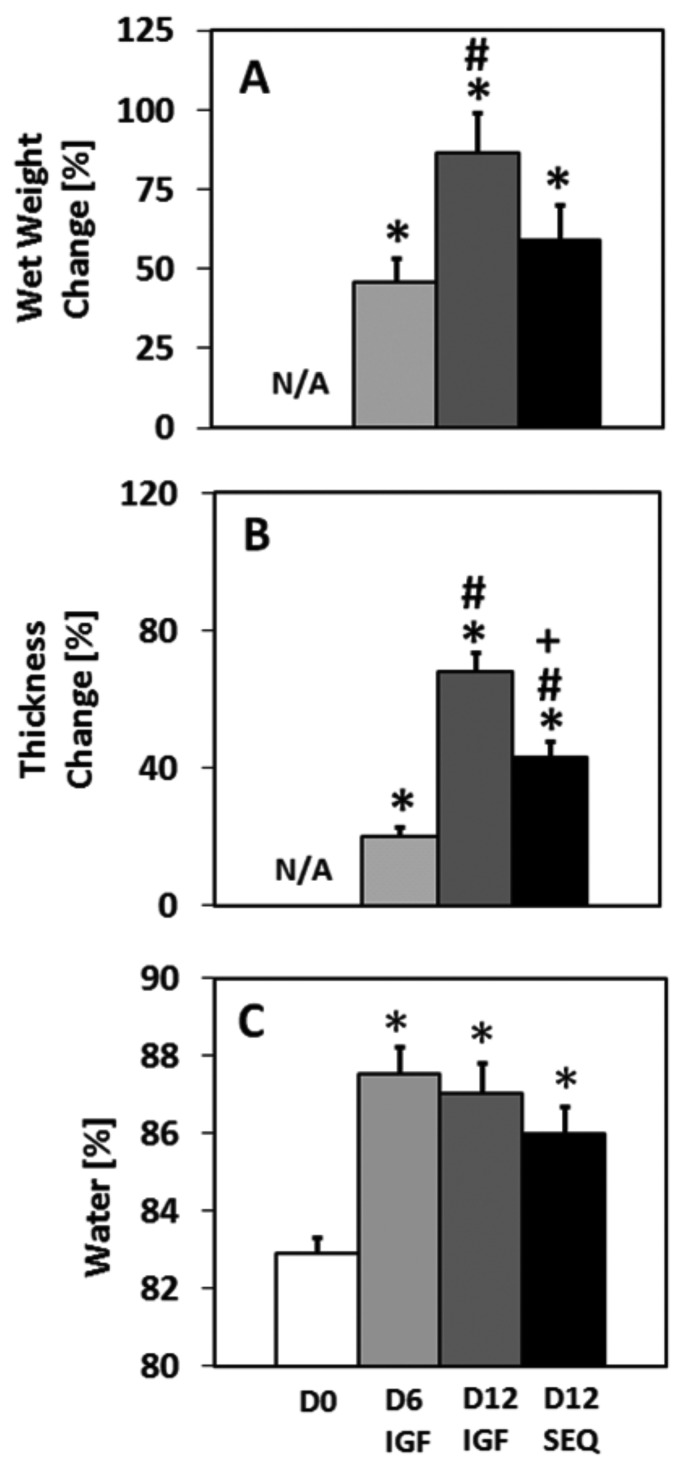
Tissue wet weights, thicknesses, and water contents varied among groups (Fig. 1). D6 IGF showed increases in wet weight (46%, p < 0.00001), thickness (20%, p < 0.01), and water content (5.6%, p < 0.001) relative to D0. D12 IGF showed increases in wet weight (86%, p < 0.05), thickness (68%, p < 0.00001), and water content (5.0%, p < 0.01) relative to D0. D12 SEQ showed increases in wet weight (59%, p < 0.05), thickness (43%, p < 0.00001), and water content (3.7%, p < 0.05) relative to D0. D12 SEQ thickness change was higher than that for D6 IGF (p < 0.0001) yet lower than that for D12 IGF (p < 0.001). D12 SEQ wet weight change trended to values between those for D6 IGF and D12 IGF.
Fig. 1.
Change in (a) wet weight, and (b) thickness for cultured explants. (c) Water content of D0 and cultured explants. Symbols indicate p < 0.05 versus *D0; #D6 IGF; + D12 IGF. Mean ± SE. n = 15.
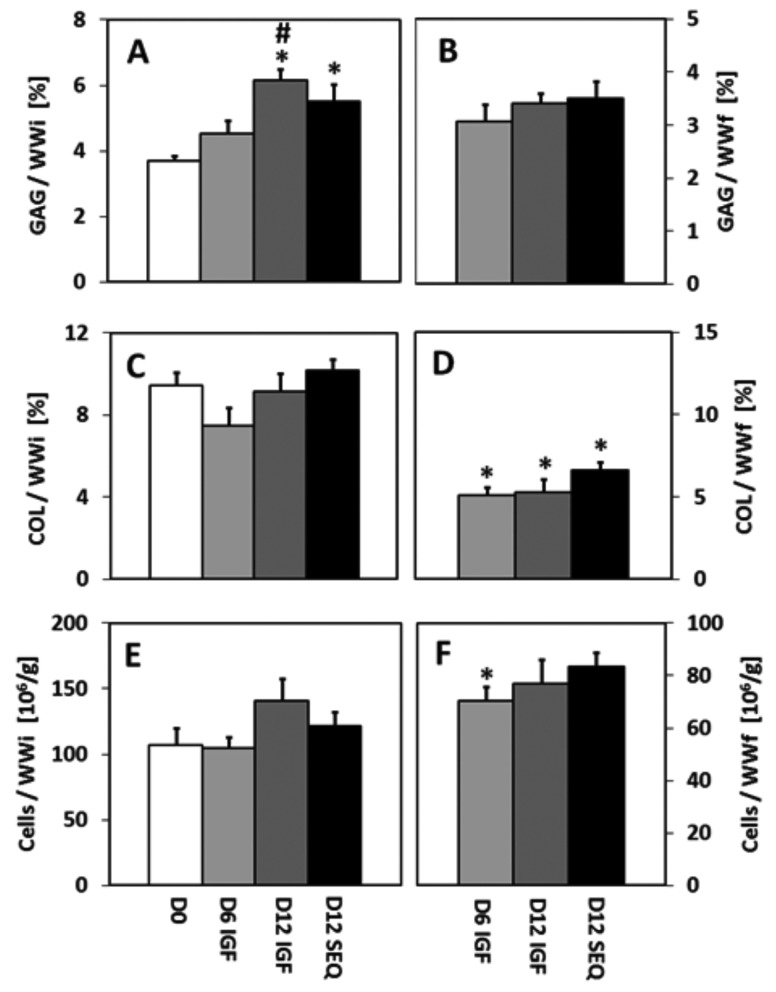
Biochemical contents, normalized by WWi to quantify changes in mass during culture, varied among groups (Figs. 2(a), 2(c), and 2(e)). GAG contents increased by 66% for D12 IGF (p < 0.01) and 49% for D12 SEQ (p < 0.05) relative to D0. GAG content for D12 IGF was also higher than D6 IGF (p < 0.05). COL and cell contents were similar for all groups.
Fig. 2.
(a, b) Glycosaminoglycan (GAG) content, (c, d) collagen (COL) content, and (e, f) cell content of D0 and cultured explants, normalized to initial wet weight WWi (a, c, e), and final wet weight WWf (b, d, f). For D0, WWi = WWf. Symbols indicate p < 0.05 versus *D0; #D6 IGF; + D12 IGF. Mean ± SE. n = 15.
Biochemical concentrations, normalized by WWf to account for wet weight changes during culture, also varied among groups (Figs. 2(b), 2(d), and 2(f)). GAG concentrations were similar for all groups. COL concentrations decreased by 46% for D6 IGF (p < 0.001), 44% for D12 IGF (p < 0.001), and 30% for D12 SEQ (p < 0.05) relative to D0. Cell concentration decreased by 34% for D6 IGF (p < 0.05) relative to D0.
Mechanical Properties.
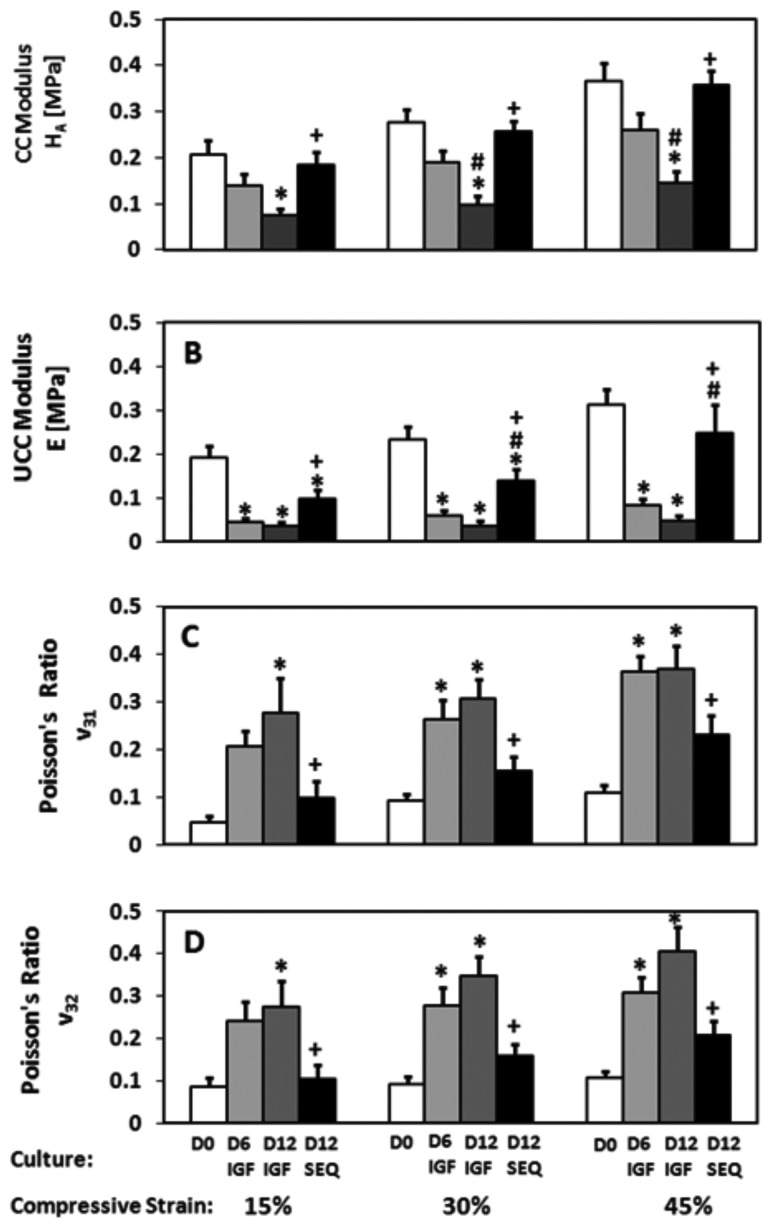
All D12 SEQ mechanical properties trended to values between those for D0 and both D6 IGF and D12 IGF (Fig. 3). CC H A values for D12 SEQ were higher than D12 IGF at all strains (p < 0.01) but not different than D0 and D6 IGF at any strains. H A values for D6 IGF were not different than D0 at any strains. H A values for D12 IGF were lower than D0 at all strains (p < 0.01) and lower than D6 IGF at 30% and 45% strains (p < 0.05).
Fig. 3.
Compressive mechanical properties of D0 and cultured explants measured at 15%, 30%, and 45% compressive strains. (a) Equilibrium confined compression (CC) modulus, H A, (b) unconfined compression (UCC) modulus, E, (c) UCC Poisson’s ratio, ν31, and (d) UCC Poisson’s ratio, ν32. Symbols indicate p < 0.05 versus *D0; #D6 IGF; + D12 IGF. Mean ± SE. n = 15.
UCC E values for D12 SEQ were higher than D12 IGF at all strains (p < 0.05), higher than D6 IGF at 30% and 45% strains (p < 0.05), and lower than D0 at 15% and 30% strains (p < 0.05). E values for both D6 IGF and D12 IGF were lower than D0 at all strains (p < 0.05).
UCC Poisson’s ratios in the two orthogonal directions (i.e., ν31 and ν32) were not different at any strains, but varied among culture groups. UCC ν31 and ν32 values for D12 SEQ were lower than D12 IGF at all strains (p < 0.05) but not different than D0 or D6 IGF at any strains. UCC ν31 and ν32 values for D6 IGF were higher than D0 at 30% and 45% strains (p < 0.05). UCC ν31 and ν32 values for D12 IGF were higher than D0 at all strains (p < 0.05).
Structure-Function Relations.
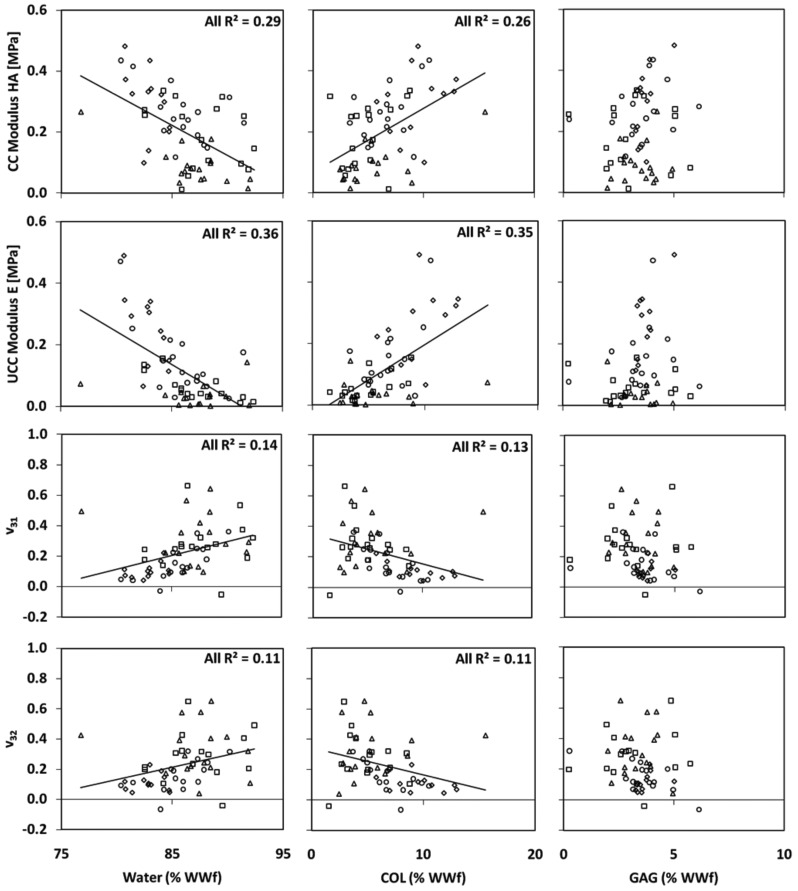
Linear regression of mechanical properties at 30% strain and biochemical concentrations indicated significant correlations between specific group comparisons (Fig. 4, Table 1). H A was negatively correlated with water for D12 IGF, D12 SEQ, and ALL; positively correlated with COL for D12 IGF and ALL; and positively correlated with GAG for D0. E was negatively correlated with water; positively correlated with COL for D6 IGF, D12 IGF, D12 SEQ, and ALL; and positively correlated with GAG for D0. ν31 was positively correlated with water for D12 SEQ and ALL; and negatively correlated with COL for D0, D12 SEQ, and ALL. ν32 was positively correlated with water for D12 SEQ and ALL; negatively correlated with COL for D12 SEQ and ALL; and negatively correlated with GAG for D12 SEQ. No mechanical properties at 30% strain were correlated with GAG/COL ratio or with GAG and COL combined (multivariate analysis).
Fig. 4.
Regression of (a-c) equilibrium CC modulus, (d-f) equilibrium UCC modulus, and (g-l) Poisson’s ratios at 30% with water, COL, and GAG concentrations (% of WWf). Data points correspond to individual D0 (), D6 IGF (), and D12 SEQ () specimens. Trendlines shown correspond to significant relationships for all data points combined (ALL). See Table 1 for more details.
Table 1.
Significant linear correlations between mechanical and biochemical propertiesa
| D0 | D6 IGF | D12 IGF | D12 SEQ | ALL | |
|---|---|---|---|---|---|
| H A versus water | – | – | A = -0.01 | A = -0.02 | A = -0.02 |
| B = 1.28 | B = 1.76 | B = 1.90 | |||
| R 2 = 0.52 | R 2 = 0.29 | R 2 = 0.29 | |||
| H A versus COL | – | – | A = 0.01 | – | A = 0.02 |
| B = 0.02 | B = 0.07 | ||||
| R 2 = 0.40 | R 2 = 0.26 | ||||
| H A versus GAG | A = 0.14 | – | – | – | – |
| B = −0.22 | |||||
| R 2 = 0.34 | |||||
| E versus water | A = −0.05 | A = −0.01 | A = −0.01 | A = −0.02 | A = −0.02 |
| B = 4.68 | B = 1.04 | B = .65 | B = 2.21 | B = 2.13 | |
| R 2 = 0.38 | R 2 = 0.63 | R 2 = 0.44 | R 2 = 0.39 | R 2 = 0.42 | |
| E versus COL | – | A = 0.01 | A = 0.01 | A = 0.03 | A = 0.03 |
| B = −0.00 | B = −0.01 | B = −0.05 | B = −0.05 | ||
| R 2 = 0.41 | R 2 = 0.47 | R 2 = 0.31 | R 2 = 0.41 | ||
| E versus GAG | A = 0.15 | – | – | – | – |
| B = −0.31 | |||||
| R 2 = 0.35 | |||||
| ν31 versus water | – | – | – | A = 0.03 | A = 0.02 |
| B = −2.82 | B = −1.66 | ||||
| R 2 = 0.61 | R 2 = 0.20 | ||||
| ν31 versus COL | A = −0.01 | – | – | A = −0.04 | A = −0.02 |
| B = 0.23 | B = 0.43 | B = 0.34 | |||
| R 2 = 0.41 | R 2 = 0.49 | R 2 = 0.11 | |||
| ν31 versus GAG | – | – | – | – | – |
| ν32 versus water | – | – | – | A = 0.03 | A = 0.02 |
| B = −1.98 | B = −1.19 | ||||
| R 2 = 0.37 | R 2 = 0.11 | ||||
| ν32 versus COL | – | – | – | A = −0.04 | A = −0.02 |
| B = 0.41 | B = 0.35 | ||||
| R 2 = 0.47 | R 2 = 0.11 | ||||
| ν32 versus GAG | – | – | – | A = −0.06 | – |
| B = 0.37 | |||||
| R 2 = 0.54 |
H A, CC modulus; E, UCC modulus; ν31 and ν32, UCC Poisson’s ratios; “-”, not significant. All mechanical properties correspond to 30% strain. Water, water % of WWf; COL, collagen % of WWf; GAG, glycosaminoglycan % of WWf. A and B are the slope and y intercept of the linear correlations y = A* x + B.
Discussion
Relative to D0, culture with application of IGF-1 alone enhanced volumetric growth and degraded mechanical properties, characterized by reduced compressive moduli and increased compressive Poisson’s ratios consistent with previous studies for this tissue source [9–12]. Relative to D0, culture with sequential application of IGF-1 and TGF-β1 also enhanced volumetric growth but to a lesser extent than that for IGF-1 treatment alone, as the D12 SEQ thickness increase of 43% was significantly less than the D12 IGF-1 value of 68%. Furthermore, culture with sequential application of IGF-1 and TGF-β1 maintained compressive moduli and Poisson’s ratios at values higher and lower, respectively, than those for IGF-1 treatment alone. More specifically, D12 SEQ CC and UCC moduli were significantly higher than D12 IGF values at each strain level and D12 SEQ UCC Poisson’s ratios were significantly lower than D12 IGF values at each strain level. Furthermore, most D12 SEQ mechanical properties were unchanged relative to D0 values, with exceptions that D12 SEQ UCC moduli at 15% and 30% strains decreased.
Upon considering the results of the current study with those for 12 days of treatment with TGF-β1 alone using the same tissue source [9], the results support the hypothesis that sequential application of IGF-1 and TGF-β1 produces geometric and compressive mechanical properties that lie between extreme values produced when using either growth factor alone. For ∼0.8–mm-thick superficial specimens tested in [9] (designated as the S layer in that study), 12 days of TGF-β1 treatment alone (i.e., D12 TGF) maintained both wet weight and thickness, increased CC modulus at 30% strain by 103%, increased UCC modulus at 30% strain by 65%, and maintained UCC Poisson’s ratios at D0 values. Thus, D12 SEQ property changes measured in the current study lie between the two extremes produced when using either IGF-1 or TGF-β1 alone.
Differences in final GAG and COL concentrations may explain the differential regulation of mechanical properties with IGF-1 treatment alone, sequential application of IGF-1 and TGF-β1, and TGF-β1 treatment alone. In the current study, GAG and COL concentrations (normalized by WWf) for D12 SEQ were 3% higher and 24% higher, respectively, than D12 IGF (those differences were not significant). In the previous study with S layer specimens [9], GAG and COL concentrations for D12 TGF were 37% higher and 63% higher, respectively, than D12 IGF (those differences were significant). It is noteworthy that COL concentrations varied more than GAG concentrations among D12 IGF, D12 SEQ, and D12 TGF groups, because the results of the current study and past studies with this immature tissue source [9,11] indicate that correlations of compressive mechanical properties with COL concentration are as strong, or stronger, than correlations with GAG concentration. When specimens from all groups were pooled together, the current study reveals that CC modulus and UCC modulus are positively correlated with COL but not GAG concentration, and that UCC Poisson’s ratios are negatively correlated with COL but not GAG concentration.
Inclusion of the D6 IGF group in the current study provides evidence that subsequent application of TGF-β1 reverses the mechanical property changes induced by IGF-1 treatment alone. More specifically, following 6 days of IGF-1 treatment alone (D6 IGF), continued IGF-1 treatment for 6 days (D12 IGF) further reduced CC and UCC moduli and further increased UCC Poisson’s ratios, whereas subsequent TGF-β1 treatment for 6 days (D12 SEQ) reverses those trends to restore all mechanical properties to values near D0. On the other hand, subsequent application of TGF-β1 did not arrest volumetric growth produced by IGF-1 alone, as thickness continued to increase with subsequent TGF-β1 treatment for 6 days to values significantly higher than D6 IGF.
UCC Poisson’s ratios in the two orthogonal directions (ν31 and ν32) were not significantly different at any of the strain levels, in agreement with recent results [9]. These UCC Poisson’s ratios may be a measure of COL network mechanical properties in the plane parallel to the articular surface, as several studies have suggested that COL network properties provide resistance to lateral expansion during UCC loading [9,11,19]. Consequently, these results suggest that COL structure and properties may be isotropic in the plane parallel to the articular surface, a conclusion supported by the results that tension modulus measured in two orthogonal directions in the plane parallel to the articular surface were not significantly different for this tissue source [20]. Although mature articular cartilage typically contains a classical Benninghoff structure with principal fiber orientations transitioning from a split-line direction parallel to the articular surface to a random direction in the middle zone, imaging studies with immature articular cartilage tissue from other species suggest that the Benninghoff structure is absent at birth [21–24]. Thus, immature tissue likely contains a different type of material symmetry as compared to mature articular cartilage, with symmetry in the plane parallel to the articular surface.
An interesting result emerged regarding the ability of TGF-β1 treatment to restore compressive moduli from decreased D6 IGF values back to native D0 values. Specifically, for the D12 SEQ group, subsequent TGF-β1 treatment restored CC, but not UCC, moduli to native D0 values (Figs. 3(a) and 3(b)). This discrepancy may be explained by the differential changes observed for GAG and COL concentrations (normalized by tissue WWf) (Figs. 2(a) and 2(b)–(d)) and the different structural roles that GAG and COL serve in CC and UCC. Compared to D0 values, GAG and COL concentrations did not change and decreased, respectively, for the D12 SEQ group. As evidenced in Fig. 4 and Table 1, correlations with COL concentration were higher for UCC than for CC modulus; thus, the observed decreases in COL affected UCC modulus more than CC modulus. These observations may be related to previous results, as discussed above, that suggest that in UCC, COL functions to restrain lateral expansion and thereby maintains GAGs at a higher density, and, consequently, a higher swelling pressure that resists compression. Furthermore, the suggestion that COL functions to restrain lateral expansion is consistent with the observed increases in Poisson’s ratios for all treatment groups (some that were significant) because of the observed decreases in COL concentration for all treatment groups.
The current study has several limitations. One limitation is that we did not consider an experimental group that reversed the order of growth factor treatment; i.e., TGF-β1 followed by IGF-1. However, this omission was motivated by the long-term aim of modulating tissue to enhance both volumetric expansion and mechanical integrity. Because of the effects of IGF-1 treatment observed in this and previous studies (e.g., Ref. [9]), a logical hypothesis is that applying IGF-1 treatment after TGF-β1 would lead to a decrease in mechanical integrity and thereby counteract any improvements in mechanical integrity resulting from TGF-β1 treatment.
Another limitation is that we did not include a control group with 12 days of incubation without growth factors; we did this for two reasons. First, a previous study with this tissue source and growth factors did include a basal control [12]; in that study, it was found that the selected growth factor concentrations produced distinct effects versus basal culture controls (i.e., no growth factor supplementation) with respect to explant metabolism, matrix composition, and mechanical function. For example, in that previous study, it was found that, relative to D0, the basal control group produced no thickness change, an increase in wet weight significantly less than that produced by IGF-1 (i.e., IGF-1 produced wet weight changes that were 27-61% higher than basal controls for this region), and a decrease in some tensile mechanical properties. Second, our results were sufficient to address the specific hypothesis tested here, i.e., that sequential application of IGF-1 and TGF-β1 produces geometric and compressive mechanical properties that lie between extreme values produced when using either growth factor alone.
However, there may be other treatment protocols following IGF-1 application, other than the sequential application of TGF-β1, that may produce the similar result of a restoration of mechanical integrity back to, or beyond, D0 values following IGF-1 induced volumetric expansion. One possibility is sequential treatment of IGF-1 followed by basal medium; however, in our previous study [12], it was found that basal control, but not TGF-β1, resulted in a decrease in some tensile mechanical properties. Because we aimed to restore mechanical properties back to, or better than, native levels, we chose TGF-β1 for the second treatment. Another possibility is sequential treatment of IGF-1 followed by a select, chemically defined serum-free medium, such as that studied in [25], which maintained explant volume while increasing mechanical integrity through 42 days in culture.
The results may have limited clinical significance because immature bovine articular cartilage explants were used, whereas tissue-grafting strategies involve mature human osteochondral tissue. Immature bovine articular cartilage from the patellofemoral groove was chosen as the model system because biomechanical properties of specimens both untreated and treated with IGF-1 and TGF-β1 have been extensively documented in previous studies [9–12,20]. Furthermore, use of immature articular specimens ensured that specimen properties were less heterogeneous than those of mature specimens [26,27], which is desirable because only averaged biochemical and mechanical properties were considered.
Although immature tissue was used in an attempt to limit heterogeneities, the use of ∼1-mm-thick specimens from this tissue source likely did involve substantial depth-dependent heterogeneities that may limit the study’s findings. From the articular surface to ∼1-mm deep, a previous study measured GAG, COL, and CC modulus increases of ∼80%, 90%, and 290%, respectively [27]. Consequently, measured biochemical and mechanical property changes must be interpreted as changes averaged through the depth of the tissue. For example, in some specimens, the digital images used for Poisson’s ratios analyses revealed that the superficial regions experienced greater lateral expansion than middle/deep regions, a phenomenon that was mostly observed in the “softer” D12 IGF samples. However, the observed depth-dependency of lateral expansion is likely not related to a depth-dependency in Poisson’s ratios, because our recent study revealed that for D0 and D12 IGF specimens, the averaged Poisson’s ratio for specimens harvested to a depth of 0.8 mm below the articular surface tended to lower values than for specimens harvested from 0.8 to 1.4 mm below the articular surface [9]. Rather, the observed depth-dependency of lateral expansion is likely related to the observed depth-dependency of compressive moduli, which has been shown to increase with depth from the articular surface [9,27]. Nevertheless, future experimental and modeling studies with depth-dependent properties may be useful in more accurately assessing the differential effects of IGF-1 and TGF-β1 treatment on tissue properties.
Regardless, the results of the current study may have important implications for modulating immature tissue-engineered constructs or mature graft tissue for implantation into defects surrounded by mature native tissue. Future studies can be more closely linked to clinical cartilage repair strategies by using adult articular tissue and full thickness osteochondral grafts in an attempt to increase both thickness and mechanical integrity for transplantation into regions that are both thicker and required to support higher loads. Inclusion of subchondral bone may lead to complications. For example, including the subchondral bone will greatly alter the structural integrity of the incubated material and, consequently, differences in geometric tissue growth may be produced. Furthermore, it may be beneficial to first remove the bone marrow before incubation to more precisely control osteochondral tissue remodeling [28]. An alternative strategy could be to remove the subchondral bone before incubation and then attempt to induce calcification via chemical treatment in the deep cartilage regions during incubation [29]. For use in allografting procedures, consideration should be given to how such treatment strategies can be integrated with current testing and storage protocols. For example, if integration of such protocols substantially increases the time from harvest to transplantation, concerns related to the maintenance of cell viability, especially in the superficial region, must be addressed [30,31]. On the other hand, because typical storage times are ∼3 weeks in current clinical use [31], the absolute duration of growth factor application studied here (12 days) should be extended in future studies. Further, for a specific allograft tissue implant, targeted properties may be difficult to achieve because of variability in outcome parameters (e.g., tissue thickness) that accompany treatment protocols, which may be exacerbated by tissue heterogeneities in full thickness specimens; this poses additional challenges for future studies. Finally, studies that determine the fate of constructs following implantation may be considered.
In summary, the D12 SEQ property changes measured in the current study reflect substantial volumetric growth with a maintenance of the most compressive mechanical properties, lying between the two extremes produced when using either IGF-1 or TGF-β1 alone. These results provide motivation for future studies aimed at adjusting the timing of the sequential application of select growth factors to more precisely modulate geometric and biomechanical properties. Thus, a novel conclusion of the current study is that in vitro growth with sequential application of IGF-1 and TGF-β1 may be used to produce a graft tissue with targeted properties. For example, the individual durations of IGF-1 and TGF-β1 treatment may be adjusted to obtain a thickness needed for graft implantation into a different location in the joint and to obtain mechanical properties that may enhance a graft’s survival following implantation from a low-weight-bearing region to a high-weight-bearing region.
Acknowledgment
This work is supported by grants from the National Institutes of Health (S.J.H., R.L.S., and S.M.K.), the National Science Foundation (R.L.S. and S.M.K.), and the Donald E. Bently Center for Engineering Innovation (S.M.K.). Individual support (to G.M.W.) was provided through an NSF Graduate Research Fellowship and a NIH Ruth L. Kirchstein Pre-Doctoral Fellowship.
Contributor Information
Jennifer R. Van Donk, Mechanical Engineering Department, California Polytechnic State University, San Luis Obispo, CA 93405
Albert C. Chen, Department of Bioengineering, University of California-San Diego, La Jolla, CA 92093
Scott J. Hazelwood, Biomedical and General Engineering Department, California Polytechnic State University, San Luis Obispo, CA 93405
Robert L. Sah, Department of Bioengineering, University of California-San Diego, La Jolla, CA 92093; Department of Orthopaedic Surgery, University of California-San Diego, La Jolla, CA 92093
Stephen M. Klisch, Mechanical Engineering Department, California Polytechnic State University, San Luis Obispo, CA 93405, e-mail: sklisch@calpoly.edu.
References
- [1]. Sah, R. L. , Chen, A. C. , Chen, S. S. , Li, K. W. , DiMicco, M. A. , Kurtis, M. S. , Lottman, L. M. , and Sandy, J. D. , 2001, “Articular Cartilage Repair,” Arthritis and Allied Conditions. A Textbook of Rheumatology, Koopman W. J., ed., Lippincott, Williams & Wilkins, Philadelphia, pp. 2264–2278. [Google Scholar]
- [2]. Darling, E. M. , and Athanasiou, K. A. , 2003, “Biomechanical Strategies for Articular Cartilage Regeneration,” Ann. Biomed. Eng., 31(9), pp. 1114–1124. 10.1114/1.1603752 [DOI] [PubMed] [Google Scholar]
- [3]. Hunziker, E. B. , 2002, “Articular Cartilage Repair: Basic Science and Clinical Progress. A Review of the Current Status and Prospects,” Osteoarthritis Cartilage, 10, pp. 432–463. 10.1053/joca.2002.0801 [DOI] [PubMed] [Google Scholar]
- [4]. Smith, G. D. , Knutsen, G. , and Richardson, J. B. , 2005, “A Clinical Review of Cartilage Repair Techniques,” J. Bone Joint Surg. Br., 87B(4), pp. 445–449.10.1302/0301- 20X.87B4.15971 [DOI] [PubMed] [Google Scholar]
- [5]. Morales, T. I. , and Roberts, A. B. , 1988, “Transforming Growth Factor-β Regulates the Metabolism of Proteoglycans in Bovine Cartilage Organ Cultures,” J. Biol. Chem., 263, pp. 12828–12831. Available at: http://www.jbc.org/content/263/26/12828.short.http://www.jbc.org/content/263/26/12828.short [PubMed] [Google Scholar]
- [6]. Schalkwijk, J. Joosten, L. A. B. , van den Berg, W. B. , van Wyk, J. J. , and van de Putte, L. B. A. , 1989, “Insulin-Like Growth Factor Stimulation of Chondrocyte Proteoglycan Synthesis by Human Synovial Fluid,” Arthritis Rheum., 32, pp. 66–71. 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- [7]. Morales, T. I. , and Hascall, V. C. , 1991, “Transforming Growth Factor-β1 Stimulates Synthesis of Proteoglycan Aggregates in Calf Articular Organ Cultures,” Arch. Biochem. Biophys., 286, pp. 99–106. 10.1016/0003-9861(91)90013-9 [DOI] [PubMed] [Google Scholar]
- [8]. Sah, R. L. , Trippel, S. B. , and Grodzinsky, A. J. , 1996, “Differential Effects of Serum, Insulin-Like Growth Factor-I, and Fibroblast Growth Factor-2 on the Maintenance of Cartilage Physical Properties during Long-Term Culture,” J. Orthop. Res., 14, pp. 44–52. 10.1002/(ISSN)1554-527X [DOI] [PubMed] [Google Scholar]
- [9]. Williams, G. M. , Dills, K. , Flores, C. , Stender, M. , Stewart, K. , Nelson, L. , Chen, A. , Masuda, K. , Hazelwood, S. , Klisch, S. M. , and Sah, R. L. , 2010, “Differential Regulation of Immature Articular Cartilage Compressive Moduli and Poisson’s Ratios by in vitro Stimulation with IGF-1 and TGF-β1,” J. Biomech., 43, pp. 2501–2507. 10.1016/j.jbiomech.2010.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Sah, R. L. , Chen, A. C. , Grodzinsky, A. J. , and Trippel, S. B. , 1994, “Differential Effects of bFGF and IGF-I on Matrix Metabolism in Calf and Adult Bovine Cartilage Explants,” Arch. Biochem. Biophys., 308, pp. 137–147. 10.1006/abbi.1994.1020 [DOI] [PubMed] [Google Scholar]
- [11]. Ficklin, T. P. , Thomas, G. C. , Barthel, J. C. , Asanbaeva, A. , Thonar, E. J. , Masuda, K. , Chen, A. C. , Sah, R. L. , Davol, A. , and Klisch, S. M. , 2007, “Articular Cartilage Mechanical and Biochemical Property Relations Before and After in vitro Growth,” J. Biomech., 40, pp. 3607–3614. 10.1016/j.jbiomech.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Asanbaeva, A. , Masuda, K. , Thonar, E. J.-M. A. , Klisch, S. M. , and Sah, R. L. , 2008, “Regulation of Immature Cartilage Growth by IGF-I, TGF-Beta 1, BMP-7, and PDGF-AB: Role of Metabolic Balance between Fixed Charge and Collagen Network,” Biomech. Model. Mechanobiol., 7, pp. 263–276. 10.1007/s10237-007-0096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Stender, M. , Balcom, N. , Berg-Johansen, B. , Dills, K. , Dyk, D. , Hazelwood, S. , Sah, R. , and Klisch, S. , 2011, “Differential Regulation of Articular Cartilage Tensile Properties by IGF-1 and TGF-B1 during in vitro Growth,” International Conference on the Mechanics of Biomaterials and Tissues Hawaii, December 11-15, 2011. [Google Scholar]
- [14]. Chen, A. C. , Bae, W. C. , Schinagl, R. M. , and Sah, R. L. , 2001, “Depth- and Strain-Dependent Mechanical and Electromechanical Properties of Full-Thickness Bovine Articular Cartilage in Confined Compression,” J. Biomech., 34, pp. 1–12. 10.1016/S0021-9290(00)00170-6 [DOI] [PubMed] [Google Scholar]
- [15]. Williamson, A. K. , Chen, A. C. , and Sah, R. L. , 2001, “Compressive Properties and Function-Composition Relationships of Developing Bovine Articular Cartilage,” J. Orthop. Res., 19, pp. 1113–1121. 10.1016/S0736-0266(01)00052-3 [DOI] [PubMed] [Google Scholar]
- [16]. Kim, Y. J. , Sah, R. L. Y. , Doong, J. Y. H. , and Grodzinsky, A. J. , 1988, “Fluorometric Assay of DNA in Cartilage Explants Using Hoechst 33258,” Anal. Biochem., 174, pp. 168–176. 10.1016/0003-2697(88)90532-5 [DOI] [PubMed] [Google Scholar]
- [17]. Herbage, D. , Bouillet, J. , and Bernengo, J.-C. , 1977, “Biochemical and Physicochemical Characterization of Pepsin-Solubilized Type-II Collagen from Bovine Articular Cartilage,” Biochem. J., 161, pp. 303–312. Available at: http://www.biochemj.org/bj/161/bj1610303.htm.http://www.biochemj.org/bj/161/bj1610303.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Pal, S. , Tang, L.-H. , Choi, H. , Habermann, E. , Rosenberg, L. , Roughley, P. , and Poole, A. R. , 1981, “Structural Changes during Development in Bovine Fetal Epiphyseal Cartilage,” Coll. Relat. Res., 1, pp. 151–176. [DOI] [PubMed] [Google Scholar]
- [19]. Kiviranta, P. , Rieppo, J. , Korhonen, R. K. , Julkunen, P. , Toyras, J. , and Jurvelin, J. S. , 2006, “Collagen Network Primarily Controls Poisson’s Ratio of Bovine Articular Cartilage in Compression,” J. Orthop. Res., 24, pp. 690–699. 10.1002/(ISSN)1554-527X [DOI] [PubMed] [Google Scholar]
- [20]. Williamson, A. K. , Chen, A. C. , Masuda, K. , Thonar, E. J.-M. A. , and Sah, R. L. , 2003, “Tensile Mechanical Properties of Bovine Articular Cartilage: Variations with Growth and Relationships to Collagen Network Components,” J. Orthop. Res., 21, pp. 872–880. 10.1016/S0736-0266(03)00030-5 [DOI] [PubMed] [Google Scholar]
- [21]. Rieppo, J. , Hyttinen, M. M. , Halmesmaki, E. , Ruotsalainen, H. , Vasara, A. , Kiviranta, I. , Jurvelin, J. S. , and Helminen, H. J. , 2009, “Changes in Spatial Collagen Content and Collagen Network Architecture in Porcine Articular Cartilage during Growth and Maturation,” Osteoarthritis Cartilage, 17(4), pp. 448–455. 10.1016/j.joca.2008.09.004 [DOI] [PubMed] [Google Scholar]
- [22]. Hyttinen, M. M. , Holopainen, J. R. , van Weeren, P. , Firth, E. C. , Helminen, H. J. , and Brama, P. A. J. , 2009, “Changes in Collagen Fibril Network Organization and Proteoglycan Distribution in Equine Articular Cartilage during Maturation and Growth,” J. Anat., 215(5), pp. 584–591. 10.1111/joa.2009.215.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Julkunen, P. , Iivarinen, J. , Brama, P. A. , Arokoski, J. , Jurvelin, J. S. , and Helminen, H. J. , 2010, “Maturation of Collagen Fibril Network Structure in Tibial and Femoral Cartilage of Rabbits,” Osteoarthritis Cartilage, 18(3), pp. 406–415. 10.1016/j.joca.2009.11.007 [DOI] [PubMed] [Google Scholar]
- [24]. van Turnhout, M. C. , Schipper, H. , Engel, B. , Buist, W. , Kranenbarg, S. , and van Leeuwen, J. L. , 2010, “Postnatal Development of Collagen Structure in Ovine Articular Cartilage,” BMC Dev. Biol., 10,p. 62. 10.1186/1471-213X-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Bonomi, M. , Branduardi, D. , Bussi, G. , Camilloni, C. , Provasi, D. , Raiteri, P. , Donadio, D. , Marinelli, F. , Pietrucci, F. , Broglia, R. A. , and Parrinello, M. , 2009, “PLUMED: A Portable Plugin for Free-Energy Calculations with Molecular Dynamics,” Comput. Phys. Commun., 180(10), pp. 1961–1972. 10.1016/j.cpc.2009.05.011 [DOI] [Google Scholar]
- [26]. Schinagl, R. M. , Gurskis, D. , Chen, A. C. , and Sah, R. L. , 1997, “Depth-Dependent Confined Compression Modulus of Full-Thickness Bovine Articular Cartilage,” J. Orthop. Res., 15, pp. 499–506. 10.1002/(ISSN)1554-527X [DOI] [PubMed] [Google Scholar]
- [27]. Klein, T. J. , Chaudhry, M. , Bae, W. C. , and Sah, R. L. , 2007, “Depth-Dependent Biomechanical and Biochemical Properties of Fetal, Newborn, and Tissue-Engineered Articular Cartilage,” J. Biomech., 40, pp. 182–190. 10.1016/j.jbiomech.2005.11.002 [DOI] [PubMed] [Google Scholar]
- [28]. Bian, L. , Stoker, A. M. , Marberry, K. M. , Ateshian, G. A. , Cook, J. L. , and Hung, C. T. , 2010, “Effects of Dexamethasone on the Functional Properties of Cartilage Explants During Long-Term Culture,” Am. J. Sports Med., 38(1), pp. 78–85. 10.1177/0363546509354197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Hwang, J. , Kyubwa, E. , Bae, W. , Bugbee, W. , Masuda, K. , and Sah, R. , 2010, “in vitro Calcification of Immature Bovine Articular Cartilage: Formation of a Functional Zone of Calcified Cartilage,” Cartilage, 1, pp. 287–297. 10.1177/1947603510369552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Pearsall, A. W. , IV, Tucker, J. A. , Hester, R. B. , and Heitman, R. J. , 2004, “Chondrocyte Viability in Refrigerated Osteochondral Allografts Used for Transplantation within the Knee,” Am. J. Sports Med., 32(1), pp. 125–131. 10.1177/0095399703258614 [DOI] [PubMed] [Google Scholar]
- [31]. Allen, R. T. , Robertson, C. M. , Pennock, A. T. , Bugbee, W. D. , Harwood, F. L. , Wong, V. W. , Chen, A. C. , Sah, R. L. , and Amiel, D. , 2005, “Analysis of Stored Osteochondral Allografts at the Time of Surgical Implantation,” Am. J. Sports Med., 33, pp. 1479–1484. 10.1177/0363546505275010 [DOI] [PubMed] [Google Scholar]