Abstract
Objective
The purpose of this study was to conduct a systematic review of the literature of cardiovascular factors pertaining to incident Alzheimer disease (AD).
Methods
A systematic literature review was conducted of all studies of cardiovascular risk factors for incident AD listed in PUBMED in English from 2000–2007. Risk factors included hypertension, diabetes, exercise, alcohol intake, smoking, B complex vitamins, homocysteine, stroke, atrial fibrillation, APOE, lipids, and diet. Inclusion criteria consisted of diagnoses of incident AD and longitudinal studies with cohorts of 500 or more.
Results
Individual clinically defined risk factors such as hypertension and diabetes were not significantly associated with increased risk for AD. The strength of the association for hypertension could be considerably strengthened by changing criteria such as midlife measurements or using higher cutoffs for systolic blood pressure. APOE ε4 was the most consistent risk factor. Interactions between risk factors modify risk particularly for hypertension and diabetes. Interactions modifying risk were also found for exercise and physical function, APOE ε4, diabetes and cholesterol.
Conclusions
In this review the evidence that single clinically defined cardiovascular risk factors are significantly associated with incident Alzheimer disease is inconsistent at best. The strength of the association of cardiovascular risk factors and AD can be influenced greatly by changing the parameters of measurement of risk factors and by identifying interactions between the factors.
Keywords: incident Alzheimer disease, cardiovascular risk factors, interactions
Introduction
There is now a great deal of interest in the prevention of Alzheimer Disease. Ideally prevention strategies should be driven by the results of large scale primary prevention trials. However, few if any of these trials are being conducted at present. Designing such a trial is not a simple issue and, even if initiated today, results might not be available for decades. Prevention strategies require the identification of a suitable intervention approach derived from systematic reviews of observational studies. The intervention must be considered together with potential modifiers such as subject characteristics. It also requires the identification of a target population based on risk factor characteristics and the risks and benefits of the prevention strategy.
Recent reviews of observational studies do not consistently focus on AD as the outcome of interest. Rather, investigators have reported on risk factors for multiple different cognitive outcomes including cognitive impairment, cognitive decline, and dementia as well as AD1–3. These studies have identified multiple potentially preventable risk factors. The most consistent finding in these prior reviews is the association of cognitive impairment with cardiovascular disease4.
It has been suggested that researchers focusing on different cognitive outcomes may identify a different set of risk factors (or place different weights on known risk factors). Risk factors might logically differ across different cognitive outcomes because different cognitive outcomes may emanate from different pathophysiological processes2. This concern appears to be supported by the work of Bennett et al who have reported that many risk factors that have been identified using other clinical outcomes become non significant when AD pathology is used as the outcome5.
At the last Alzheimer's Association conference on prevention, a symposium on potential primary prevention trials was held. In preparation for this symposium, we undertook a systematic review of the literature on all risk factors focusing only on incident AD. This paper reports on only the results pertaining to cardiovascular risk factors for incident AD.
Methods
We conducted a systematic literature search in PubMed of all published articles in the English language from 2000 to April of 2007 in order to capture the most recent publications. Search terms used included combinations of Alzheimer disease, risk factors, and words including and related to the following: hypertension, lipids, diabetes, metabolic syndrome, cerebrovascular, education, social networks, depression, APOE, exercise, diet, BMI, atrial fibrillation, smoking, and alcohol. We excluded all meta-analyses and reviews. We included longitudinal studies enrolling 500 or more subjects and reporting on incident Alzheimer disease. We selected studies with sample sizes of 500 or more because studies with these large sample sizes are more likely to have the power to detect meaningful relationships. Included papers also had to describe a formal clinical assessment procedure and use the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised Third Edition, or Fourth Edition, or the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria for Alzheimer's disease for diagnosis of AD.
The initial search returned 1926 articles. By applying the criteria described above and excluding for example imaging studies and in vitro studies, we selected 197 with potentially relevant citations for review of the abstracts; 76 articles met the criteria for data abstraction. This paper reports on the results of 61 papers from 23 studies, all observational, that pertain to the cardiovascular risk factors. We defined cardiovascular risk factors to include hypertension, diabetes, exercise, alcohol intake, smoking, B complex vitamins, stroke, atrial fibrillation, APOE, and lipids. APOE was included although it only has a modest effect on lipid regulation, because it also has a major effect on AD incidence. Hypertension was defined by self-reported diagnosis or treatment, observed participant medications, measurements, or a computerized inpatient register system. Diabetes was defined by self-reported diagnosis or treatment, observed patient medication, documented use of insulin or an oral hypoglycemic drug, measured blood glucose, or health records. Exercise was determined by self-report from various questionnaires, as was alcohol intake and smoking status. A study participant's level of B complex vitamins were determined by blood samples or estimated from self-report of diet. The diagnosis of stroke was based on self-report, informant report, or medical records, and sometimes supplemented by a neurological examination. APOE genotype was determined using DNA from blood samples. Lipid levels were determined by using self-reported diagnosis or treatment, observed patient medication, venous blood specimen, or a laboratory database.
For each paper reviewed, we noted study cohort size, the number of incident AD cases, and the years of follow up. We extracted from the multivariate model reported in each paper the estimated Hazard Ratio (HR), Relative Risk (RR) or Odds Ratio (OR), respective 95% confidence intervals and p-values. We also extracted information regarding interactions of a risk factor with other factors. All studies included were from observational studies that varied considerably on exposure measures. In addition, a wide range of statistical models were used in these papers and covariates included in these models also differed substantially. To conduct a meta-analysis on these risk factors would require uniform criteria resulting in the exclusion of many studies. We chose to present a systematic review of all literature so that possible sources of heterogeneity could be examined instead of focusing on a statistical combination of data6. In presenting the results, we combined all effect estimates into one column without specifically noting whether they were HR, RR or OR estimates. For incident AD rates, the three measures of association (HR, RR, and OR) can be regarded as approximately the same7.
Unlike the common approach in meta-analysis where one publication from a study is usually included, we allowed multiple papers from the same studies in order to show the wide variations in defining risk exposures even within the same study.
There were 12 articles from 8 studies reporting on dietary constituents and dietary supplements as a risk factor for incident AD8–19. The reported analyses included consideration of intake of vitamin B complex, niacin, vitamin C, vitamin E, combinations of B, C and E, multiple vitamins, antioxidants, fat including saturated and unsaturated, DHA and α-linolenic acid, carbohydrates, protein, tryptophan, tea and juice, and Mediterranean diet. Many studies reported an association between the particular dietary constituent and altered risk for AD. However, definitions and criteria for the constituents of diet varied considerably from paper to paper as did the statistical analytic models employed. This variability in the methodological approach precludes a simple summary of the evidence regarding AD risk and diet. For this reason, we include these papers in the reference list but do not further detail their results.
Results
Hypertension
Sixteen papers from ten studies reported on hypertension as a risk factor for AD. Nine papers from seven studies reported that hypertension was not a risk factor for AD20–28. According to two articles from the Kungsholmen Project, however, hypertension had the potential to become a risk factor depending on the parameters. The first article reported that a systolic blood pressure equal to or greater than 160 mm Hg did not affect risk significantly29, while the second reported that a systolic blood pressure greater than 180 mm Hg significantly increased risk30. Additionally, the first paper reported that a low diastolic blood pressure (less than 70 mm Hg)29 increased risk and the second reported that a very low diastolic blood pressure (less than or equal to 65 mm Hg) also increased risk30 (see Table 1).
Table 1.
Summary of results from the systematic review on the association between hypertension and incident AD. Interacting factors with hypertension are also included
| Article | Study Name | Sample Size | Number of Incident AD Cases | Years of Follow Up | Risk Factor | Interaction | OR/RR/HR | 95% Confidence Interval | P value |
|---|---|---|---|---|---|---|---|---|---|
| Blood Pressure Parameters | |||||||||
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | SBP 140–159 | not analyzed | 1.3 | 0.8–2.0 | n.s. |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | high SBP (≥ 160) | not analyzed | 1.4 | 0.9–2.2 | n.s. |
| Qiu/200330 | Kungsholmen Project | 1270 | 256 | Max: 6 | very high SBP (above 180) | not analyzed | 1.5 | 1.0–2.3 | 0.07 |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | DBP ≥ 90 | not analyzed | 1.0 | 0.7–1.4 | n.s. |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | low DBP (less than 70) | not analyzed | 1.9 | 1.2–3.0 | not given |
| Qiu/200330 | Kungsholmen Project | 1270 | 256 | Max: 6 | very low DBP (≤65) | not analyzed | 1.7 | 1.1–2.4 | not given |
| Midlife SBP | |||||||||
| Launer/200031 | HHP/HAAS | 3703 | 118 | Max: 28 | Midlife borderline high SBP (140–159) | not analyzed | 1.23 | 0.63–2.43 | n.s. |
| Kivipelto/200221 | CAIDE | 1449 | 48 | 21 | midlife SBP (140–159) | not analyzed | 1.6 | 0.7–4.2 | n.s. |
| Launer/200031 | HHP/HAAS | 3703 | 118 | Max: 28 | high midlife SBP (≥ 160) high midlife | not analyzed | 1.22 | 0.37–4.04 | n.s. |
| Kivipelto/200221 | CAIDE | 1449 | 48 | 21 | SBP (≥160) | not analyzed | 2.6 | 1.1–6.6 | not given |
| Midlife DBP | |||||||||
| Launer/200031 | HHP/HAAS | 3703 | 118 | Max: 28 | DBP (below 80) | not analyzed | 1.86 | 1.01–3.46 | not given |
| Kivipelto/200221 | CAIDE | 1449 | 48 | 21 | Midlife DBP (90–95) | not analyzed | 1.2 | 0.4–3.3 | n.s. |
| Launer/200031 | HHP/HAAS | 3703 | 118 | Max: 28 | borderline high DBP (between 90–94 | not analyzed | 5.1 | 1.7–15.2 | not given |
| Kivipelto/200221 | CAIDE | 1449 | 48 | 21 | high midlife DBP (≥95) | not analyzed | 2.0 | 0.9–4.6 | n.s. |
| Launer/200031 | HHP/HAAS | 3703 | 118 | Max: 28 | DBP (≥95) | not analyzed | 6.6 | 2.0–21.4 | not given |
| Interactions | |||||||||
| Luchsinger/200528 | WHICAP | 1138 | 176 | 5.5 | hypertension | diabetes | 3.3 | 1.9–5.9 | 0.03 |
| Honig/200332 | WHICAP | 1766 | 181 | Max: 7 | hypertension severe | stroke | 2.14 | 1.40–3.27 | not given |
| Xu/200733 | Kungsholmen Project | 1173 | 307 | Max: 9 | systolic hypertension (≥180) | borderline diabetes | 4.89 | 1.07–22.42 | 0.04 |
| Xu/200434 | Kungsholmen Project | 1301 | 260 | Max: 6 | severe systolic hypertension (≥180) | diabetes | 2.6 | 1.0–6.8 | 0.05 |
| Borenstein/200535 | Kame Project | 1859 | 90 | Max: 9 | high SBP (≥140) | APOE ε4 non carriers | 1.79 | 0.82–3.89 | 0.15 |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | SBP | APOE ε4 | not given | no interaction | n.s. |
| Kivipelto/200221 | CAIDE | 1449 | 48 | 21 | SBP | APOE ε4 | not given | no interaction | n.s. |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | SBP ≥140 | antihypertensive drug use | 0.5 | 0.2–1.1 | 0.09 |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | SBP ≥140 | APOE ε4 carrier and antihypertensive drug use | 0.5 | 0.1–1.5 | n.s. |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | low DBP (less than 70) | APOE ε4 carrier | 4.5 | 2.6–8.0 | not given |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | low DBP (less than 70) | APOE ε4 non carriers | 1.3 | 0.7–2.5 | n.s. |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | DBP | antihypertensive drug use | not given | no interaction | not given |
| Qiu/200329 | Kungsholmen Project | 966 | 204 | Max: 6 | DBP (less than 70) | APOE ε4 carrier and antihypertensive drug use | 3.2 | 0.9–11.6 | 0.07 |
AD-Alzheimer disease; OR-odds ratio; RR-relative risk; HR-hazard ratio; SBP-systolic blood pressure; DBP-diastolic blood pressure; HHP/HAAS-Honolulu Heart Program/Honolulu-Asia Aging Study; CAIDE-Cardiovascular Risk Factors, Aging, and Dementia; WHICAP-Washington Heights-Inwood Columbia Aging Project
Two articles from two studies reported on hypertension at midlife. One of these papers from the Cardiovascular Risk Factors, Aging, and Dementia study (CAIDE) reported that midlife systolic blood pressure in the range of 140–159 mm Hg did not affect risk significantly, while high midlife systolic blood pressure (equal to or greater than 160 mm Hg) significantly increased risk21. A paper from the Honolulu Heart Program/Honolulu-Asia Aging Study (HHP/HAAS) reported that low midlife diastolic blood pressure (below 80 mm Hg) increased risk31. These two papers from different studies reported inconsistent findings regarding midlife borderline high and midlife high diastolic blood pressure21, 31 (see Table 1). An article from the East Boston Established Populations for Epidemiologic Studies of the Elderly (EPESE) reporting systolic blood pressure and diastolic blood pressure at various years before and after AD diagnosis was inconclusive24.
Seven papers from four studies reported on the effect various interactions have on the risk for AD. One article from the Washington Heights-Inwood Columbia Aging Project (WHICAP) reported an interaction between hypertension and diabetes28 and a second article from the same study reported an interaction between hypertension and stroke that increased risk32. Two additional papers from the Kungsholmen Project reported that the interaction between severe systolic hypertension (greater than or equal to 180 mm Hg) and borderline diabetes or diabetes both significantly increased risk33, 34. Three articles from three separate studies, the Kame Project, the Kungsholmen Project, and the CAIDE study, reported no interaction between systolic blood pressure and the APOE ε4 allele21, 29, 35. The article from the Kungsholmen Project also reported no significant interaction between systolic blood pressure of greater than or equal to 140 mm Hg and antihypertensive drug use or between a systolic blood pressure of greater than or equal to 140 mm Hg, APOE ε4, and antihypertensive drug use29. Regarding diastolic blood pressure, the same paper reported an interaction between a low diastolic blood pressure (less than 70 mm Hg) and the APOE ε4 carrier that increased risk, but reported no increased risk for low diastolic blood pressure in the APOE ε4 non-carrier29. No interactions were reported between diastolic blood pressure and antihypertensive drug use or between low diastolic blood pressure, an APOE ε4 carrier, and antihypertensive drug use29 (see Table 1).
One paper reporting that hypertension increased risk in the Yoruba was discarded because the results were based on unreliable self report. The authors indicated that self report of hypertension was unreliable in this population because of their limited access to healthcare25.
Synthesis of Hypertension
The results from this review of hypertension as a risk factor illustrate the problems in interpreting results from observational studies. All of the studies reporting significant associations in one way or another changed the criteria for defining hypertension in their models in order to obtain significance for incident AD. Presumably this was because using generally accepted clinical criteria did not, as was the case for the few studies that reported associations using them. The manipulations included altering the criteria levels for systolic (higher than 140) and diastolic (both lower and higher) or changing the length of the observation (mid life). Thus the often repeated simple statement that hypertension is a risk factor for incident AD does not capture the complexities of the association as described here and may have implications for both prevention strategies and prevention trials.
Diabetes
Seventeen articles from nine studies reported on diabetes as a risk factor for AD. Nine articles from seven studies reported that diabetes was not a significant risk factor20, 23, 26, 27, 34–38. Only one paper from the Religious Orders Study reported that diabetes increased risk39. A paper from the Kungsholmen Project reported that borderline diabetes increased risk33, while an article from HHP/HAAS found that type 2 diabetes increased risk40 (see Table 2).
Table 2.
Summary of results from the systematic review on the association between diabetes and incident AD. Interacting factors with diabetes are also included.
| Article | Study Name | Sample Size | Number of Incident AD Cases | Years of Follow Up | Risk Factor | Interactions | OR/RR/HR | 95% Confidence Interval | P value |
|---|---|---|---|---|---|---|---|---|---|
| Hayden/200620 | CCSMHA | 3264 | 104 | Max: 5 | diabetes | not analyzed | 1.33 | 0.66–2.46 | n.s. |
| Akomolafe/200636 | Framingham | 2210 | 237 | 12.7 | diabetes | not analyzed | 1.15 | 0.65–2.05 | n.s. |
| Borenstein/200535 | Kame Project | 1859 | 90 | Max: 9 | diabetes | not analyzed | not given | not given | 0.35 |
| Xu/200434 | Kungsholmen Project | 1301 | 260 | Max: 6 | diabetes | not analyzed | 1.3 | 0.9–2.1 | n.s. |
| Lindsay/200223 | CSHA | 4615 | 194 | Max: 5 | diabetes | not analyzed | 1.03 | 0.58–1.84 | n.s. |
| MacKnight/200238 | CSHA | 5574 | 267 | Max: 5 | diabetes | not analyzed | 1.3 | 0.83–2.03 | n.s. |
| Posner/200226 | WHICAP | 1259 | 157 | Max: 7 | diabetes | not analyzed | 1.3 | 0.7–2.6 | n.s. |
| Luchsinger/200137 | WHICAP | 1262 | 157 | 4.3 | diabetes | not analyzed | 1.3 | 0.8–1.9 | n.s. |
| Tyas/200127 | MSHA | 694 | 36 | Max: 5 | diabetes | not analyzed | 2.7 | 0.85–8.52 | n.s. |
| Arvanitakis/200439 | Religious Orders Study | 824 | 151 | 5.5 | diabetes | not analyzed | 1.65 | 1.10–2.47 | not given |
| Xu/200733 | Kungsholmen Project | 1173 | 307 | Max: 9 | borderline diabetes | not analyzed | 1.77 | 1.06–2.97 | not given |
| Peila/200240 | HHP/HAAS | 2574 | 76 | 2.91 | diabetes, type 2 | not analyzed | 1.9 | 1.1–3.2 | not given |
| Honig/200332 | WHICAP | 1766 | 181 | Max: 7 | diabetes | stroke | 4.12 | 2.35–7.23 | not given |
| Luchsinger/200528 | WHICAP | 1138 | 176 | 5.5 | diabetes | hypertension | 3.3 | 1.9–5.9 | 0.03 |
| Xu/200733 | Kungsholmen Project | 1173 | 307 | Max: 9 | borderline diabetes | severe systolic hypertension (≥180 mm Hg) | 4.89 | 1.07–22.42 | 0.04 |
| Xu/200434 | Kungsholmen Project | 1301 | 260 | Max: 6 | diabetes | severe systolic hypertension (≥180 mm Hg) | 2.6 | 1.0–6.8 | 0.05 |
| Borenstein/200535 | Kame Project | 1859 | 90 | Max: 9 | diabetes | APOE ε4 non carriers | 2.68 | 1.20–6.00 | 0.02 |
| Borenstein/200535 | Kame Project | 1859 | 90 | Max: 9 | diabetes | APOE ε4 carriers | 0.45 | 0.11–1.96 | 0.29 |
| Xu/200733 | Kungsholmen Project | 1173 | 307 | Max: 9 | borderline diabetes | APOE-ε4 | not given | not given | n.s. |
| Xu/200434 | Kungsholmen Project | 1301 | 260 | Max: 6 | diabetes | APOE ε4 carriers | 1 | 0.3–2.9 | n.s. |
| Peila/200240 | HHP/HAAS | 2574 | 76 | 2.91 | diabetes, type 2 | APOE ε4 | not given | not given | p>0.2 |
AD-Alzheimer disease; OR-odds ratio; RR-relative risk; HR-hazard ratio; CCSMHA-Cache County Study of Memory, Health, and Aging; CSHA-Canadian Study of Health and Aging; WHICAP-Washington Heights-Inwood Columbia Aging Project; MSHA-Manitoba Study of Health and Aging; HHP/HAAS-Honolulu Heart Program/Honolulu- Asia Aging Study
Six papers from four studies reported on interactions that affected risk. One paper from WHICAP reported that the interaction between diabetes and stroke increased risk significantly32. A second paper from WHICAP reported an interaction between diabetes and hypertension28. Two articles from the Kungsholmen Project also reported interactions between borderline diabetes and severe systolic hypertension (greater than or equal to 180 mm Hg)33 and diabetes and severe systolic hypertension that increased risk significantly34.
Figure 1 illustrates the effect of diabetes/hypertension interactions on AD risk.
Figure 1.
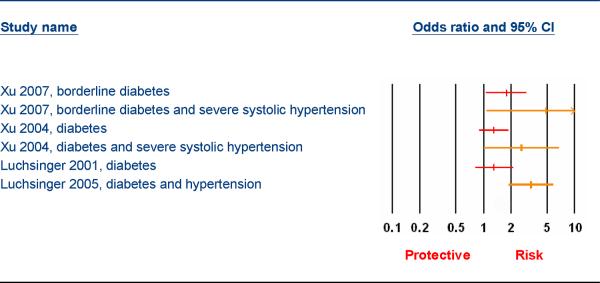
Hypertension, Diabetes Interactions and Alzheimer Disease Risk
Only one paper reported significant interaction between diabetes and APOE ε4. This paper from the Kame Project reported that APOE ε4 non-carriers with diabetes had an increased risk for AD, while APOE ε4 carriers with diabetes had no significant change in risk35. The two articles from the Kungsholmen Project reported no interaction between borderline diabetes and APOE ε433 or between diabetes and APOE ε4 carriers34. A fourth article from the HHP/HAAS study found no interaction between type 2 diabetes and APOE ε440 (see Table 2).
Additionally, one paper from WHICAP reported on the related disorder of hyperinsulinemia and found that increasing insulin levels significantly increased risk41. Two papers, one from a study done in Kuopio, Finland and another from the HHP/HAAS, reporting on metabolic syndrome were inconsistent42, 43.
Synthesis of Diabetes
The situation with regard to the association of diabetes with incident AD is similar in many respects to that described for hypertension. Most studies report no significant association between diabetes and incident AD and those few that do report only a modest effect.
It is only when diabetes is considered in association with other factors, particularly hypertension, that significant associations are reported. The magnitude of these interactions suggest a process which is not simply additive in nature, but an association beyond that expected from its component parts, hypertension and diabetes. (See table 2 and figure 1). This phenomenon has also been proposed for the association of the metabolic syndrome and heart disease risk but is not as yet uniformly accepted. The two papers reporting on the association of metabolic syndrome and incident AD produced inconsistent results however. Again, reporting simply that diabetes increases risk for AD does not fully describe the complexity of the relationship.
Lipids
Ten papers from eight studies reported on lipids as a risk factor for AD. Six papers from five studies reported on the effect high levels of cholesterol had on risk20–22, 44–46. Three papers from the Cache County, National Health and Nutrition Examination Survey I Epidemiologic Followup Study (NHEFS), and Adult Changes in Thought (ACT) studies reported that high cholesterol had no affect20, 44, 45. One article from the CAIDE study reported that high total cholesterol levels greater than 251 mg/dl increased risk significantly22. An additional paper from this study also looked at high midlife cholesterol effects and reported that a high midlife total cholesterol of greater than or equal to 251 mg/dl increased risk significantly21. Alternatively, an article from WHICAP reported that a total cholesterol of greater than or equal to 229.01 mg/dl decreased risk46.
Five articles from four studies reported on the affect interactions between cholesterol and other risk factors have on risk for AD. One paper from the NHEFS examined the interaction between high total cholesterol and high transferrin saturation and reported that the interaction between cholesterol greater than 261 mg/dl and transferrin saturation greater than 34.9% did not affect risk, while cholesterol greater than 268 mg/dl in combination with a transferrin saturation greater than 37% increased risk45. A paper from the CAIDE study reported an interaction between high midlife serum cholesterol greater than or equal to 251 mg/dl and high midlife systolic blood pressure (greater than or equal to 160 mm Hg) that significantly increased risk47. Two articles, from the CAIDE and ACT studies, reported no interaction between total cholesterol and APOE ε421, 44. A fifth paper from the Indianapolis-Ibadan study reported that increasing levels of cholesterol in an APOE ε4 non-carrier increased risk in African Americans, whereas in those with APOE ε4 there was no affect48.
Smoking
Four papers from four separate studies reported on the effect of smoking on incident AD. Three studies, the Chicago Health and Aging Project (CHAP), WHICAP, and a study from Chongqing City, reported that current smoking increased the risk of incident AD28, 49, 50. An additional article from the Canadian Study of Health and Aging (CSHA) recorded only smoking and found that it was not significant23.
B Complex Vitamins and Stroke
Three articles from three studies reported on B complex vitamins as a risk factor for AD. Of these, one from the Conselice study reported that serum levels of folate below 8.9 nmol/L increased risk51, while another from the Framingham study reported that low serum folate levels did not affect risk52. The third paper from WHICAP found that high levels of total folate intake (greater than or equal to 487.9 μg) significantly decreased risk53. All three articles reported that vitamins B6 and B12 had no effect on risk51℃53. These papers also looked at high levels of homocysteine, but the results were inconsistent.
Five papers, each from different studies, reported on stroke as a risk factor. Four of these reported that it did not increase risk20, 23, 32, 54. One of these papers from WHICAP, however, found that when stroke was combined with either hypertension or heart disease, the interactions significantly increased risk32. The fifth article from the Kame Project reported that a history of transient ischemic attack in an APOE ε4 non-carrier also increased risk35.
Exercise
Five papers from four studies reported on exercise as a risk factor for AD. One paper from the CSHA reported that regular physical activity decreased risk23. Two articles from the CHAP and ACT studies reported that physical activity had no effect on AD risk55, 56. The article from the ACT study also reported that the interaction between exercise and a low performance based physical function score decreased risk55. The higher performance based physical function score combined with exercise had no affect on risk55. Additionally, a different paper from the CSHA looked at the interaction between a high level of physical activity and gender and reported that females with a high level of physical activity had a significantly decreased risk57. Males with a high level of physical activity had no significant change in risk57. A paper from the Cardiovascular Health Cognition Study (CHCS) reported an interaction between leisure-time energy expenditure/activity index and APOE ε458. Those without APOE ε4 had a decreased risk while those with APOE ε4 had no significant change in risk58 (see Table 3). This paper and the previous paper also reported on the effect of varying levels of activity. Both found that at a lower level of activity the risk was not affected, but at higher levels of activity the risk decreased57, 58. The article from the CSHA also looked at various levels of activity combined with gender and reported that an increase in physical activity decreased risk significantly only in females57. The other article from the CHCS reported no correlation between increasing leisure-time energy expenditure and risk58.
Table 3.
Summary of results from the systematic review on the association between exercise and incident AD. Interacting factors with exercise are also included.
| Article | Study Name | Sample Size | Number of Incident AD Cases | Years of Follow Up | Risk Factor | Interaction | OR/RR/HR | 95% Confidence Interval | P value |
|---|---|---|---|---|---|---|---|---|---|
| Lindsay/200223 | CSHA | 4615 | 194 | Max: 5 | regular physical activity | not analyzed | 0.69 | 0.50–0.96 | not given |
| Wilson/200256 | CHAP | 842 | 139 | Max: 4 | physical activity (measured in time spent) | not analyzed | 1.04 | 0.98–1.10 | n.s. |
| Larson/200655 | ACT | 1740 | 107 | 6.2 | exercise (3x/week) | not analyzed | 0.69 | 0.45–1.05 | 0.081 |
| Larson/200655 | ACT | 1740 | 107 | 6.2 | exercise | performance based physical function score of 10 | 0.58* | 0.39–0.84* | 0.004* |
| Larson/200655 | ACT | 1740 | 107 | 6.2 | exercise | performance based physical function score of 11 | 0.66* | 0.46–0.94* | 0.023* |
| Larson/200655 | ACT | 1740 | 107 | 6.2 | exercise | performance based physical function score of 12 | 0.75* | 0.51–1.09* | 0.126* |
| Laurin/200157 | CSHA | 4615 | 194 | Max: 5 | high level of physical activity | female gender | 0.27 | 0.08–0.90 | 0.05 |
| Laurin/200157 | CSHA | 4615 | 194 | Max: 5 | high level of physical activity | male gender | 0.73 | 0.27–1.98 | 0.62 |
| Podewils/200558 | CHCS | 3375 | 245 | 5.4 | leisure-time energy expenditure/activity index | APOE ε4 non carriers | 0.44* | 0.28–0.69* | p<0.001* |
| Podewils/200558 | CHCS | 3375 | 245 | 5.4 | leisure-time energy expenditure/activity index | APOE ε4 carriers | 1.2* | 0.63–2.29* | 0.68* |
AD-Alzheimer disease; OR-odds ratio; RR-relative risk; HR-hazard ratio; CSHA-Canadian Study of Health and Aging; CHAP-Chicago Health and Aging Project; ACT-Adult Changes in Thought; CHCS-Cardiovascular Health Cognition Study
Data is from incident dementia, but authors reported observing the same pattern for incident AD.
Alcohol
Six articles from five studies reported on alcohol intake as a risk factor for AD. Four papers, all from different studies, reported that alcohol intake had no significant effect on risk8, 10, 59, 60. An article from the Indianapolis-Ibadan study looked at regular alcohol consumption in an African American population and reported a decreased risk for AD25. A sixth article from WHICAP reported that light to moderate intake of wine was the only type of alcohol intake that significantly decreased risk61. Furthermore, this study found that light to moderate intake of wine only decreased the risk in ApoE ε4 non-carriers61.
APOE ε4
Thirteen papers from eight studies analyzed APOE ε2 or APOE ε4 as risk factors for AD. Seven of these papers from five of the studies reported that APOE ε4 significantly increased risk21, 23, 29, 54, 62–64. One article from the Indianapolis-Ibadan Project reported that the presence of APOE ε4 in the Yoruba population in Nigeria had no affect on risk65. Two articles from two studies reported different results regarding the affect of APOE ε4 in African Americans. An article from the Indianapolis-Ibadan Project reported that APOE ε4 in African Americans increased the risk significantly66, while an article from a study done in Chicago, Illinois reported that ApoE ε4 had no affect67.
Three articles from two studies looked at APOE ε2 in three different populations. One article from the Indianapolis-Ibadan Project reported that APOE ε2 in African Americans significantly decreased risk66 and another article from the same study reported it had no affect on risk in the Yoruba65. The third paper from the Kungsholmen Project reported that APOE ε2 also did not affect risk in a Swedish population except in females between the ages of 75 and 8464. In this segment of the population the presence of APOE ε2 decreased risk significantly.
Synthesis of Other Risk Factors
From this review the evidence of an association between single risk factors and incidence AD, with the exception of the possession of the ε4 allele of APOE and current smoking, is inconsistent at best. As is the case with hypertension and diabetes, attaining significance often involves varying the criteria or the measurements of the risk factors in question (for example lipids or the B complex) or the consideration of interactions (e.g. exercise and physical function, cholesterol levels and hypertension, stroke and hypertension). These results again highlight the complexities of the relationships between cardiovascular risk factors and AD.
Discussion
In this review of cardiovascular risk factors, with the exception of APOE ε4 and possibly current smoking, the evidence for a significant association between single risk factors and incidence AD is mostly negative or inconsistent at best. This includes risk factors such as hypertension and diabetes when standard clinical criteria are employed. For example, in nine of nine papers, hypertension did not significantly increase AD risk, and diabetes did not significantly increase AD risk in 9 of 12 articles. This conclusion is at variance with other recent reviews of the association between risk factors and cognitive outcomes that reported a more consistent association2, 3 but these incorporated broader outcomes, cognitive decline2 or a mix of AD, dementia and cognitive decline3. It is possible that these outcomes included a wider range of pathological processes than those involved with incident AD.
However, the strength of the association between hypertension and AD risk could be modified considerably by changing the criteria for hypertension. This included either increasing or decreasing the cutoff point for systolic or diastolic blood pressure or by including observations at midlife. Thus the statement that hypertension is associated with incident AD does not capture the complexities of this relationship.
The results regarding the effect on AD risk of altering definitions of what constitutes hypercholesterolemia were more ambiguous. One reported that increasing cutoffs to greater than 251 mg/dl particularly when measured from midlife increased risk for AD but one other study reported that total cholesterol levels greater than or equal to 229 mg/dl actually decreased risk. The review indicates that the parameters in measuring the risk factors (both length and quantity of exposure) should be taken into consideration when considering risk for AD.
This review also points to the fact many clinically defined medical conditions such as hypertension may need further characterization to inform prevention strategy. For example, many studies measure blood pressure without further indicating whether subjects were already taking anti-hypertensive medication. The proportion of subjects on anti-hypertensive medicine may impact the strength of the association between blood pressure and AD risk.
A very striking feature of this review is the influence that interactions between risk factors have for modifying risk for AD. For example, although hypertension and diabetes individually were often reported as being non significant, the results from three papers indicated that there was an interaction between diabetes, hypertension and AD risk and this interaction dramatically increased the association between these risk factors and AD (Odds Ratios 2.6, 3.3, and 4.89 respectively)(Figure 1). The possession of APOE ε4 was reported to modify risk for diabetes and AD risk in one study. High cholesterol levels increased the risk for AD in one study of elderly African Americans but only for those individuals without APOE ε4. An interaction between cholesterol levels, transferrin saturation, and incident AD was reported in one study. While exercise was reported to have no effect in two of five reports a very intriguing interaction was reported by Larson et al. In their study on the effects of exercise on the risk for dementia and AD, they also directly measured performance based physical function. They reported an interaction between history of exercise and physical function. The association between exercise and dementia and AD risk was strongest in those participants with the lower performance based physical scores.
In particular this review highlights the sometimes highly significant effects of discovering interactions between individual risk factors and AD risk. The presence of a significant interaction between two risk factors may result in an altered risk for AD when both risk factors are present an order of magnitude greater than the risk associated with either risk factor alone or which could be explained by a simple additive effect. It strongly suggests that all analyses of risk factors from observational studies should include consideration of interactions.
Well designed prevention trials remain the most definitive method for identifying intervention strategies. Consideration of interactions might also play a significant role in identifying a vulnerable population for these trials. It does suggest for example that individuals with both hypertension and diabetes may be at greater risk for incident AD than hypertension and diabetes alone and perhaps should be the target group for an intervention strategy. It suggests also that exercise intervention may have its greatest effect on individuals with low physical function.
The information regarding potential prevention strategies with the possible exception of exercise is suggestive but not comprehensive enough to propose a specific strategy. The protective effects of antihypertensive medication would appear to depend upon the targeted levels of both systolic and diastolic blood pressures and also possibly the APOE ε4 allele. Within the B complex vitamins, only folate was identified as modifying risk for AD in one study. Four of six papers reported no effect of mild to moderate alcohol consumption on AD risk. Current smokers appeared at greater risk in 3 of 4 studies.
There are a number of limitations in this review. This review was limited only to papers published from 2000 to 2007 in order to capture the most recent publications. Thus pertinent articles published before 2000 were not included.
Systematic review of observational studies is a difficult task because of the various definitions and categories used in reporting risk factors in different studies. We have attempted to compare reports involving similar measurements of risk factors. However this was not always possible. In the case of hypertension, for example, self-report, medication use and/or actual blood pressure measurements were used to define hypertension in different studies. A major finding of our review is the identification of interactions between risk factors. However many studies did not report whether interactions were examined, making it difficult to confirm whether significant interactions reported in other studies are consistent findings or whether they are the result of publication bias.
Publication bias is a major problem with all systematic reviews. Most large cohort observational studies measure multiple potential risk factors. It is likely that positive correlates would receive greater publication priority than negative findings from these studies. Thus the non-significant associations we identified in this review such as hypertension and diabetes may be even more frequent had all negative results received publication. Similarly many studies did not report whether or not interventions were evaluated. It is possible that this is also the result of non publication of negative findings thus making it difficult to confirm whether or not the reported significant associations are consistent findings.
Although this review was undertaken in part in an effort to minimize the heterogeneity of pathological pathways which may be present in studies involving broader cognitive outcomes such as cognitive decline and dementia, several reports suggest that the clinical manifestations of AD itself is the result of a combination of AD pathology and cerebrovascular pathology. The apparent association between cardiovascular risk and incident AD may be through the cerebrovascular pathway rather than a direct effect on AD pathology68. The biological explanation for the interactive effects of hypertension and diabetes on AD risk is unclear. It may be similar to the somewhat controversial reported synergistic effects of several components of the metabolic syndrome such as insulin resistance, obesity and hypertension on cardiovascular risk69. One common pathway proposed for this cluster effect is microvascular dysfunction70.
In conclusion, there is limited evidence that single cardiovascular risk factors affect AD risk, but the strength of the association is influenced greatly by changing the parameters of the risk factors and in particular by identifying interactions between the factors. If the results of these significant interactions are confirmed by more studies, they may help identify both potential prevention strategies and study populations for future prevention trials.
Acknowledgement
The content of this manuscript has not been published or presented.
Dr. Sujuan Gao and Dr. Hugh C. Hendrie receive support from grant P30 AG10133 from the Alzheimer's Disease Center. Dr. Christopher M. Callahan is supported by NIA grants P30 AG024967 and K24 AG024078.
References
- 1.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3:343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 2.Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project Report of the Critical Evaluation Study Committee. Alzheimer's & Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Jedrziewski MK, Lee VM-Y, Trojanowski JQ. Lowering the risk of Alzheimer's disease: Evidence-based practices emerge from new research. Alzheimer's & Dementia. 2005;1:152–60. doi: 10.1016/j.jalz.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Grodstein F. Cardiovascular risk factors and cognitive function. Alzheimer's & Dementia. 2007;3:S16–22. doi: 10.1016/j.jalz.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA. Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord. 2006;20:S63–8. doi: 10.1097/00002093-200607001-00009. [DOI] [PubMed] [Google Scholar]
- 6.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. BMJ. 1998;316:140–4. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewell NP. Statistics for Epidemiology. Chapman & Hall/CRC; Boca Raton: 2004. [Google Scholar]
- 8.Scarmeas N, Stern Y, Tang MX, et al. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–21. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laitinen MH, Ngandu T, Rovio S, et al. Fat intake at midlife and risk of dementia and Alzheimer's disease: a population-based study. Dement Geriatr Cogn Disord. 2006;22:99–107. doi: 10.1159/000093478. [DOI] [PubMed] [Google Scholar]
- 10.Dai Q, Borenstein AR, Wu Y, et al. Fruit and vegetable juices and Alzheimer's disease: the Kame Project. Am J Med. 2006;119:751–9. doi: 10.1016/j.amjmed.2006.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang TL, Zandi PP, Tucker KL, et al. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65:1409–14. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 12.Zandi PP, Anthony JC, Khachaturian AS, et al. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: the Cache County Study. Arch Neurol. 2004;61:82–8. doi: 10.1001/archneur.61.1.82. [DOI] [PubMed] [Google Scholar]
- 13.Morris MC, Evans DA, Bienias JL, et al. Dietary niacin and the risk of incident Alzheimer's disease and of cognitive decline. J Neurol Neurosurg Psychiatry. 2004;75:1093–9. doi: 10.1136/jnnp.2003.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurin D, Masaki KH, Foley DJ, et al. Midlife dietary intake of antioxidants and risk of late-life incident dementia: the Honolulu-Asia Aging Study. Am J Epidemiol. 2004;159:959–67. doi: 10.1093/aje/kwh124. [DOI] [PubMed] [Google Scholar]
- 15.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–6. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 16.Morris MC, Evans DA, Bienias JL, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Luchsinger JA, Tang MX, Shea S, et al. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–8. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 18.Luchsinger JA, Tang MX, Shea S, et al. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59:1258–63. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 19.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Diet and risk of dementia: Does fat matter?: The Rotterdam Study. Neurology. 2002;59:1915–21. doi: 10.1212/01.wnl.0000038345.77753.46. [DOI] [PubMed] [Google Scholar]
- 20.Hayden KM, Zandi PP, Lyketsos CG, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 21.Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay J, Laurin D, Verreault R, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 24.Morris MC, Scherr PA, Hebert LE, et al. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. 2001;58:1640–6. doi: 10.1001/archneur.58.10.1640. [DOI] [PubMed] [Google Scholar]
- 25.Ogunniyi A, Hall KS, Gureje O, et al. Risk factors for incident Alzheimer's disease in African Americans and Yoruba. Metab Brain Dis. 2006;21:235–40. doi: 10.1007/s11011-006-9017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posner HB, Tang MX, Luchsinger J, et al. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. 2002;58:1175–81. doi: 10.1212/wnl.58.8.1175. [DOI] [PubMed] [Google Scholar]
- 27.Tyas SL, Manfreda J, Strain LA, et al. Risk factors for Alzheimer's disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. 2001;30:590–7. doi: 10.1093/ije/30.3.590. [DOI] [PubMed] [Google Scholar]
- 28.Luchsinger JA, Reitz C, Honig LS, et al. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–51. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu C, Winblad B, Fastbom J, et al. Combined effects of APOE genotype, blood pressure, and antihypertensive drug use on incident AD. Neurology. 2003;61:655–60. doi: 10.1212/wnl.61.5.655. [DOI] [PubMed] [Google Scholar]
- 30.Qiu C, von Strauss E, Fastbom J, et al. Low blood pressure and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Arch Neurol. 2003;60:223–8. doi: 10.1001/archneur.60.2.223. [DOI] [PubMed] [Google Scholar]
- 31.Launer LJ, Ross GW, Petrovitch H, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 32.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–12. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Qiu C, Winblad B, et al. The effect of borderline diabetes on the risk of dementia and Alzheimer's disease. Diabetes. 2007;56:211–6. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 34.Xu WL, Qiu CX, Wahlin A, et al. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63:1181–6. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- 35.Borenstein AR, Wu Y, Mortimer JA, et al. Developmental and vascular risk factors for Alzheimer's disease. Neurobiol Aging. 2005;26:325–34. doi: 10.1016/j.neurobiolaging.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham Study. Arch Neurol. 2006;63:1551–5. doi: 10.1001/archneur.63.11.1551. [DOI] [PubMed] [Google Scholar]
- 37.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 38.MacKnight C, Rockwood K, Awalt E, et al. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 39.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 40.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–62. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 41.Luchsinger JA, Tang MX, Shea S, et al. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–92. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 42.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20:2255–60. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 43.Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67:843–7. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 44.Li G, Shofer JB, Kukull WA, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology. 2005;65:1045–50. doi: 10.1212/01.wnl.0000178989.87072.11. [DOI] [PubMed] [Google Scholar]
- 45.Mainous AG, 3rd, Eschenbach SL, Wells BJ, et al. Cholesterol, transferrin saturation, and the development of dementia and Alzheimer's disease: results from an 18-year population-based cohort. Fam Med. 2005;37:36–42. [PubMed] [Google Scholar]
- 46.Reitz C, Tang MX, Luchsinger J, et al. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–14. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. Bmj. 2001;322:1447–51. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–7. doi: 10.1212/01.wnl.0000194507.39504.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal NT, Bienias JL, Bennett DA, et al. The relation of cigarette smoking to incident Alzheimer's disease in a biracial urban community population. Neuroepidemiology. 2006;26:140–6. doi: 10.1159/000091654. [DOI] [PubMed] [Google Scholar]
- 50.Juan D, Zhou DH, Li J, et al. A 2-year follow-up study of cigarette smoking and risk of dementia. Eur J Neurol. 2004;11:277–82. doi: 10.1046/j.1468-1331.2003.00779.x. [DOI] [PubMed] [Google Scholar]
- 51.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–43. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 52.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 53.Luchsinger JA, Tang MX, Miller J, et al. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol. 2007;64:86–92. doi: 10.1001/archneur.64.1.86. [DOI] [PubMed] [Google Scholar]
- 54.Qiu C, Winblad B, Fratiglioni L. Cerebrovascular disease, APOE epsilon4 allele and cognitive decline in a cognitively normal population. Neurol Res. 2006;28:650–6. doi: 10.1179/016164106X130443. [DOI] [PubMed] [Google Scholar]
- 55.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 56.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–4. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 57.Laurin D, Verreault R, Lindsay J, et al. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 58.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 59.Fujishima M, Kiyohara Y. Incidence and risk factors of dementia in a defined elderly Japanese population: the Hisayama study. Ann N Y Acad Sci. 2002;977:1–8. doi: 10.1111/j.1749-6632.2002.tb04793.x. [DOI] [PubMed] [Google Scholar]
- 60.Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–6. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 61.Luchsinger JA, Tang MX, Siddiqui M, et al. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–6. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 62.Heun R, Kolsch H, Jessen F. Risk factors and early signs of Alzheimer's disease in a family study sample. Risk of AD. Eur Arch Psychiatry Clin Neurosci. 2006;256:28–36. doi: 10.1007/s00406-005-0596-4. [DOI] [PubMed] [Google Scholar]
- 63.Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E epsilon4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Aging. Cmaj. 2004;171:863–7. doi: 10.1503/cmaj.1031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qiu C, Kivipelto M, Aguero-Torres H, et al. Risk and protective effects of the APOE gene towards Alzheimer's disease in the Kungsholmen project: variation by age and sex. J Neurol Neurosurg Psychiatry. 2004;75:828–33. doi: 10.1136/jnnp.2003.021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gureje O, Ogunniyi A, Baiyewu O, et al. APOE epsilon4 is not associated with Alzheimer's disease in elderly Nigerians. Ann Neurol. 2006;59:182–5. doi: 10.1002/ana.20694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murrell JR, Price B, Lane KA, et al. Association of apolipoprotein E genotype and Alzheimer disease in African Americans. Arch Neurol. 2006;63:431–4. doi: 10.1001/archneur.63.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–9. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 68.Stampfer MJ. Cardiovascular disease and Alzheimer's disease: common links. J Intern Med. 2006;260:211–23. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 69.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–14. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 70.Serne EH, de Jongh RT, Eringa EC, et al. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension. 2007;50:204–11. doi: 10.1161/HYPERTENSIONAHA.107.089680. [DOI] [PubMed] [Google Scholar]


