Abstract
Background and Purpose
Stroke is a common cause of neonatal brain injury. The subventricular zone (SVZ) is a lifelong source of newly generated cells in rodents, and erythropoietin (EPO) treatment has shown benefit in different animal models of brain injury. The purpose of this study is to investigate the specific role of exogenous EPO on SVZ progenitor cell populations in response to neonatal stroke.
Methods
Intraventricular injections of GFP-expressing lentivirus to label SVZ precursor cells were made in postnatal day 1 (P1) Long-Evans rats, which then underwent transient middle cerebral artery occlusion (MCAO) on P7. MCAO and sham rats were treated with either vehicle or EPO (1000 U/kg) at reperfusion, 24 hours, and 7 days later. The density of double-labeled DCx+/GFP+, NeuN+/GFP+, O4+/GFP+, GFAP+/GFP+, as well as single-labeled GFP+ and Ki67+ cells, was calculated to determine cell fate outcome in the striatum at 72 hours and 2 weeks after stroke.
Results
There was a significant increase in DCx+/GFP+, NeuN+/GFP+ neurons and O4+/GFP+ oligodendrocyte precursors, with decreased GFAP+/GFP+ astrocytes at both time points in EPO-MCAO animals. There was also a significant increase in GFP+ cells and Ki67+ proliferating cells in EPO compared to vehicle-MCAO animals.
Conclusions
These data suggest that SVZ neural progenitor cells proliferate and migrate to the site of injury after neonatal stroke and multiple doses of EPO, with a shift in cell fate toward neurogenesis and oligodendrogliosis at both early and late time points. The contribution of local cell proliferation and neurogenesis remains to be determined.
Keywords: Stroke, neurogenesis, neonate, erythropoietin
Introduction
Stroke is a major contributor to neonatal morbidity and mortality, making the identification of neuroprotective therapies vital. In rodents, the primary sources of postnatal neurogenesis are the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus1. The SVZ generates immature neurons (type A) that migrate tangentially via the rostral migratory stream (RMS) to the olfactory bulb (OB) where they mature2. The SVZ also generates astrocytes and oligodendrocytes that migrate radially toward overlying structures during early development1. Radial glia are stem cells that differentiate into neural stem cells (type B), characterized by maintenance of processes that maintain contact with the lateral ventricle wall during embryogenesis and the early postnatal period3. Type C transit-amplifying cells are generated by type B cells and have a high mitotic rate.
In animal models of hypoxia-ischemia (HI) and stroke, cells originating from the SVZ migrate and differentiate into region appropriate neurons, but do not appear to survive long-term4–6. A number of methods have been used to quantify the SVZ response to injury, and we have previously injected a lentiviral vector that expresses green fluorescent protein (GFP), directly labeling cells of the neural stem cell (NSC) lineage that maintain contact with ventricle wall after birth7. This infects both dividing and non-dividing cells and is expressed in their progeny. We previously saw decreased cell proliferation and increased astrocytosis of these labeled cells 2 weeks after stroke7.
Erythropoietin (EPO) is cytokine with a number of roles in addition to erythropoiesis, including cell death inhibition, immune modulation and angiogenesis8. EPO has been shown to preserve brain structure and function in several studies of rodent HI and stroke9–11, with long-term improvement seen with 3 doses of EPO12. There is also evidence of increased neurogenesis and oligodendrogliosis in different in vivo and in vitro models, although this may involve both a local and migratory response10, 13, 14. The specific contribution of SVZ NSCs to focal ischemic injury, and the effects of exogenous erythropoietin on cell fate outcome in this in vivo model, are not clear. The purpose of this study is to elucidate the effect of neonatal stroke and multiple dose EPO treatment on cell proliferation, differentiation and migration to the site of injury specifically in the SVZ NSC population.
Methods
All animal research was approved by the UCSF Institutional Animal Care and Use Committee, with all measures taken to reduce animal number and suffering.
GFP-Lentiviral Production
Packaging plasmid pCMV-dR8.91 and expressing envelope plasmid VSV-G were transfected with reporter plasmid FG12 into 293FT cells (supplemental methods)7. A lentivirus titer of 1×107 CFU/ml was used.
Intraventricular Injections
Timed-pregnant Long-Evans rats were obtained from Simonson Labs (Gilroy, CA, USA). Postnatal day 1 (P1, day of birth designated P0) rats were anesthetized by hypothermia and placed into a neonatal rodent stereotactic frame (Stoelting, Wood Dale, IL, USA). The right lateral ventricle was targeted at the following coordinates from bregma: 1.0-mm anterior, 1.2-mm lateral, 2-mm depth. Injections were made with a beveled pulled glass micropipette (Wiretrol 5-μl, Drummonds Scientific Company, Broomal, PA, USA) with a 50-μm diameter tip. 2-μl of lentivirus was injected at a rate of 0.5-μl/min. Animals were returned to the dam and monitored until they resumed nursing.
Middle Cerebral Artery Occlusion
Term equivalent P7 pups underwent middle cerebral artery occlusion (MCAO) or sham surgery. 2 pups died following the procedure and 4 did not satisfy injury criteria by diffusion-weighted (DW)-MRI, leaving 18 animals that underwent successful MCAO. Surgery was performed on spontaneously breathing pups anesthetized with 1.75% isoflurane in a mixture of 70% N2O/30% O2. Rectal temperature was monitored throughout the procedure to ensure normothermia. The internal carotid artery (ICA) was dissected and a temporary ligature applied at its origin using a 6-0 silk suture. A second suture was looped around the ICA above the pterygopalatine artery and retracted laterally to prevent retrograde blood flow. An arteriotomy was made in the proximal isolated ICA, and a coated 6-0 Dermalon filament was inserted and advanced 7.5–8.5-mm, depending on the animal’s weight, and secured with a temporary suture. Animals were reperfused after 90 minutes of occlusion by removing both sutures and the filament and covering the arteriotomy site with Surgicel. We previously demonstrated blood flow restoration in this model by contrast study15. Sham animals were anesthetized and the ICA exposed, then the skin incision was sutured closed.
Magnetic Resonance Imaging
MRI was performed using a 2-Tesla magnet (supplemental methods)7. Injury involving ipsilateral striatum and parieto-temporal cortex was verified during occlusion by DW-MRI (fig. 1B). Animals with subcortical injury were excluded.
Figure 1.

Experimental protocol (A). (B) Anterior to posterior coronal image slices of DW-MRI during occlusion demonstrate lesion (arrows) involving ipsilateral striatum and parieto-temporal cortex.
EPO Treatment
Immediately upon reperfusion or after sham surgery, vehicle [0.1% bovine serum albumin (Sigma, St. Louis, MO, USA) in saline] or recombinant human EPO (R&D Systems, Minneapolis, MN) at a dose of 1000 U/kg was injected intraperitoneally, as previously described12. Doses were repeated at 24 hours and 7 days after injury (fig. 1A). Following surgery, animals were returned to their dam, with daily weights measured for the first week to ensure adequate weight gain. For each group and time point (vehicle-sham, EPO-sham, vehicle-occluded, EPO-occluded) n=4, except for EPO-MCAO group at P21 (n=6). Animal sex was equally distributed between groups.
Immunohistochemistry
Animals were anesthetized with sodium pentobarbital (100 mg/kg; Abbot Labs, Abbot Park, Ill., USA), and brains were perfused, postfixed, and sectioned coronally at 50-μm intervals (supplemental methods)7. Free-floating sections were immunolabeled with the following antibodies: anti-doublecortin (DCx; rabbit polyclonal, 1:200; Cell Signaling, Beverly, MA, USA); anti-neuronal nuclei (NeuN; mouse monoclonal; 1:500; Chemicon, Temecula, CA, USA), anti-Ki67 (Ki67; rabbit monoclonal, 1:500; Abcam, Cambridge, MA, USA) anti-GFP (chicken polyclonal, 1:500; Abcam), anti-GFAP (mouse monoclonal, 1:500; Chemicon), anti-oligodendrocyte marker O4 (O4; mouse monoclonal, 1:250; Millipore, Billerica, MA, USA), and anti-cleaved caspase-3 (CC3, rabbit polyclonal, 1:400; Cell Signaling). Secondary antibodies were purchased from Jackson (1:500; Bar Harbor, ME, USA). Slides were coverslipped using Vectashield with DAPI (Vector Laboratories; Burlingame, CA, USA) to counterstain nuclei.
Cell Quantification
Fluorescently immunolabeled sections were analyzed on a Zeiss AxioScope Imager Z.2 with Apotome (Zeiss, Inc., Thornwood, NY, USA). Images were acquired and reconstructed using AxioVision Rel. 4.9 software. Quantitative analysis of DCx+/GFP+, NeuN+/GFP+, O4+/GFP+, GFAP+/GFP+, GFP+, Ki67+ and CC3+ cells was performed systematically in every 12th section after random selection of initial section. The striatum was analyzed from bregma +2.28-mm to −0.36-mm (Plates 14–30 of Paxinos Rat Brain Atlas) and defined by the corpus callosum, lateral ventricle and accumbens core. Double-immunostained sections were imaged by randomly placing the 20x objective (Plan-Apo lens, NA 0.8, camera resolution 1344×1024 pixels, field dimension 400×400×20-um) within the striatum. Following imaging of full thickness z-stack (1-um steps) of the 20x field, the field was manually moved a fixed distance of 600-μm in the horizontal and then vertical axis, resulting in 6–8 counting images per striatum per section. Single and double-labeled cells were quantified using Metamorph Offline (6.0, Universal Imaging Corporation, Downington, PA, USA). Cell density was calculated as the average number of cells per 20x HPF, and cell proportion was calculated as specific double-labeled cells per total GFP+ cells quantified.
Statistical Analysis
Results from cell counting were analyzed using ANOVA with Fisher’s PLSD post-hoc testing. All data are presented as means and standard deviation from the mean. Comparisons were interpreted as significant if p<0.05.
Results
EPO increases cells of SVZ NSC lineage in injured striatum after neonatal MCAO
Following MCAO, GFP-expressing cells were observed moving away from the SVZ toward the injured striatum, representing core and penumbra (fig. 2A,B). To examine the effect of stroke on SVZ NSC progeny in the striatum, the striatal density of GFP+ cells was determined at 72 hours (P10) and 2 weeks (P21) after MCAO. In the injured striatum, the majority of GFP+ cells were located near the ventricle at P10 (not shown), with increased migration at 2 weeks after stroke. At both time points, there were more GFP+ cells in the striatum in EPO-MCAO compared to vehicle-MCAO animals (13.0±2.9 vs. 10.4±0.83 at P10; 13.9±1.4 vs. 7.2±2.0 at P21, fig. 2C), with fewer cells remaining in SVZ of EPO-MCAO animals at P21 (fig. 2A,B; not quantified).
Figure 2.
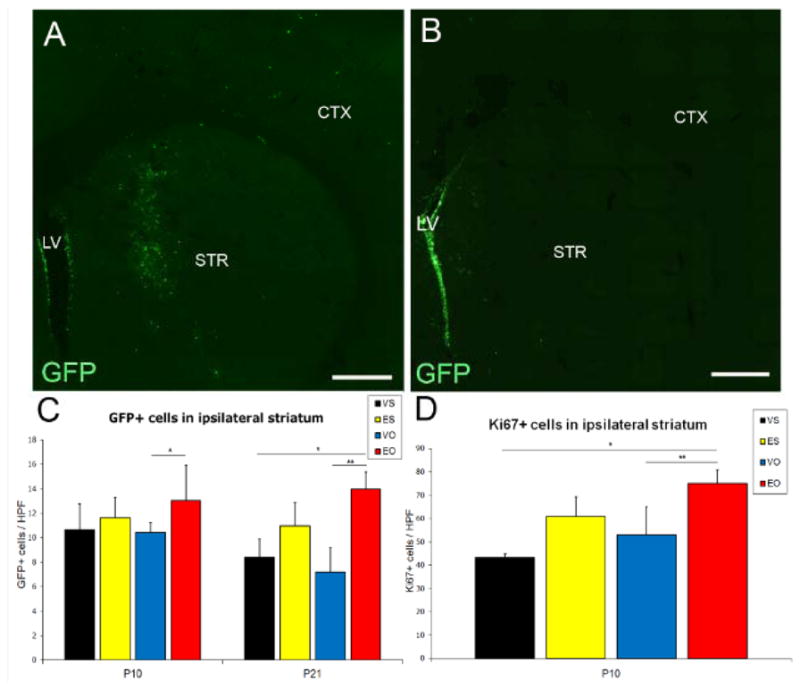
Intraventricular injection of GFP-lentivirus labels SVZ NSCs. Coronal sections of P21 rat forebrain (2 weeks after MCAO) in EPO-MCAO (A) and vehicle-MCAO (B) animal. LV: lateral ventricle, STR: striatum, CTX: cortex (tiled, 10x images). GFP+ cell density in striatum was increased in EPO-MCAO versus vehicle-MCAO animals at P10 (*p<0.05) and P21 (*p<0.05,**p<0.04) (C). Striatal density of Ki67+ cells also increased in EPO-MCAO versus vehicle-MCAO animals (*p<0.05**p<0.04). VS=vehicle-sham, ES=EPO-sham, VO=vehicle-MCAO, EO=EPO-MCAO. Scale bars: 250-μm.
To determine if EPO treatment affects cell proliferation, the density of proliferation marker Ki67 was calculated in the striatum at P10. There was an increased striatal density of Ki67+ cells in EPO-MCAO relative to vehicle-MCAO animals (75±5.9 vs. 53±12, fig. 2D). To determine if EPO also affects cell death, we examined CC3 expression, an apoptotic marker, in the striatum. EPO treatment reduced CC3 expression compared to vehicle-MCAO animals (supplemental fig. 1).
SVZ NSC-derived neurogenic activity increases with EPO treatment following neonatal stroke
To examine the effects of injury and treatment on neural cell fate commitment of SVZ NSC progeny, we first calculated the proportion of GFP+ cells in the striatum that co-labeled with DCx, a marker of immature neurons. There were more co-labeled DCx+/GFP+ cells at both time points in EPO-MCAO compared to vehicle-MCAO animals (0.12±0.028 vs. 0.09±.014 at P10; 0.14±.02 vs. 0.08±0.01 at P21, fig. 3A,C). As expected from the DCx data, there were more co-labeled NeuN+/GFP+ mature neurons in the EPO-MCAO group at P21 (0.085±0.01 vs. 0.056±0.01, fig. 3B,D), consistent with neuronal survival and maturation.
Figure 3.

Neuronal cell fate of GFP-labeled NSCs and progeny. Analysis of ipsilateral striatum in EPO-MCAO animal demonstrates co-expression of DCx (A, red, 20x) and NeuN (B, red, 20x) at P21. DAPI (blue) is used as a nuclear counterstain, double-labeled cells marked with arrows. There was an increased density of DCx+/GFP+ co-labeled cells (*p<0.02,**p<0.05,***p<0.01 at P10; *p<0.04,**p<0.05 at P21), and NeuN+/GFP+ cells (*p<0.05) in EPO-MCAO versus vehicle-MCAO animals. Y-axis shows proportion of GFP+ cells that co-labeled with specified marker. Scale bars: 100-μm.
Altered gliogenesis of SVZ-derived NSCs with EPO treatment following neonatal stroke
In prior studies, we identified increased generation of astrocytes following MCAO consistent with astrogliosis and a glial scar7. To determine if EPO treatment resulted in a favorable effect on the generation of oligodendrocytes versus astrocytes, we calculated the density of GFP+ cells that co-labeled with markers for oligodendrocyte precursors (O4+) and astrocytes (GFAP+) in the striatum. There was an increased striatal density of co-labeled O4+/GFP+ oligodendrocytes at both time points in both EPO-sham and EPO-MCAO animals (EPO-MCAO 0.36±0.01 vs. vehicle-MCAO 0.21±0.09 at P10; EPO-MCAO 0.33±0.03 vs. vehicle-MCAO 0.2±0.06 at P21, fig. 4A,C). There were more co-labeled GFAP+/GFP+ astrocytes in the striatum in the vehicle-MCAO compared to vehicle-sham group (0.6±0.08 vs. 0.37±0.03 at P10; 0.62±0.07 vs. 0.39 ±0.08 at P21; fig. 4B,D), which was significantly reduced in EPO-MCAO animals (0.4±0.07 at P10; 0.45±0.04 at P21), and also reduced in EPO-sham animals at both time points (0.27±0.03 at P10; 0.29±0.03 at P21).
Figure 4.

Glial cell fate of GFP-labeled cells. Analysis of ipsilateral striatum at P21 in EPO-MCAO animal demonstrates co-expression of O4 (A, red, 20x) and GFAP (B, red, 20x). Increased proportion of O4+/GFP+ co-labeled cells at both time points in EPO-treated animals (*p<0.05). Following MCAO, there were more GFAP+/GFP+ cells, which was reduced in both EPO-MCAO and EPO-sham animals (*p<0.02,**p<0.0005,***p<0.0001 at P10; *p<0.05,**p<0.005,***p<0.0005 at P21). Scale bars: 100-μm.
Discussion
This study demonstrates that in this model of neonatal stroke, multiple dose EPO treatment results in a shift in SVZ NSC cell fate that favors production of neurons and oligodendrocytes in injured tissue, with less astrogliosis, at both early (72 hours) and late (2 weeks) time points. We previously reported decreased labeled NPCs and progeny, with increased astrocyte production, after MCAO7. This change in neuronal, oligodendrocyte and astrocytic cell number originating from the SVZ to the site of focal ischemia after exogenous EPO treatment may result from increased progenitor proliferation, decreased precursor cell death or a change in cell fate choice, and we observed evidence for each of these pathways. The effects of EPO on SVZ cell fate and number in the current study may play a significant role in the long-term histological and functional improvement previously reported with this protocol of prolonged EPO treatment12.
In rodents, neurogenesis is thought to primarily occur during embryogenesis, except in the SVZ and SGZ where neurogenesis peaks during the first two postnatal weeks16. Previous studies have found SVZ hypertrophy in response to brain injury6, 17, with an increase in progenitor cells and neuroblast migration, which do not persist and drop to baseline levels 2–4 weeks later4–6, 17. In this study, we used a high-titer, highly efficient GFP-lentivirus that infects both rapidly and slowly dividing cells in contact with the lateral ventricle wall when administered by intraventricular injection, including type B NSCs18. We previously demonstrated labeled cells in the SVZ 2 days after injection that exhibit radial glia morphology, co-express vimentin, and co-express DCx and NeuN in the RMS and OB7. Here we see labeled striatal glia and neurons, with robust labeling of all cells known to emerge from SVZ type B cell lineage.
This model of MCAO is distinct from HI, or the Vannucci model, in that there is transient focal-ischemia without systemic hypoxia, followed by a reperfusion phase when the obstruction is removed and blood flow is restored. Reperfusion is an important part of injury progression in stroke, with increased excitotoxicity, free radical formation, and nitric oxide production leading to delayed cell death19. This is also similar to the etiology and pattern of injury seen in human neonatal stroke. Although others have described increased cell proliferation and DCx expression in the short-term following neonatal HI6, we did not see increased proliferation of SVZ NSCs by GFP+ staining, or increased co-labeled DCx+ immature neurons in vehicle-MCAO animals. This may be secondary to the more focal nature of injury with MCAO, and our examination of the core as opposed to more mildly injured tissue. In addition, while the lentivirus is efficient in labeling cells in contact with the lateral ventricle at P1, including anterior and posterior SVZ cells and ependymal cells, it still represents a subset of progenitor cells, some of which no longer maintain contact with the ventricle at the time of injection.
This model enables us to evaluate the effects of neuroprotective therapies, specifically multi-dose EPO treatment, on cell fate outcome primarily in the SVZ NSC population. EPO has shown promise as a trophic factor that may support cell differentiation, survival, and incorporation into neural networks8. EPO has previously been shown to preserve brain volume following neonatal HI and stroke9–11. Both in vitro and in vivo models have also demonstrated shifts in cell fate with EPO treatment following brain injury14, 20, but not in the SVC NSC population after neonatal focal ischemic injury. We previously reported increased neurogenesis and decreased astrogliosis in the injured striatum 6 weeks after stroke, but we could not determine whether this represented migration from the SVZ or local neurogenesis and repair10. The purpose of this study was to determine the specific fate of NSCs in injured tissue that originate from the SVZ following MCAO and EPO treatment. In this study, an earlier (72 hours) time point after stroke was chosen to examine the early proliferative response to injury and treatment, as well as production of immature neurons and oligodendrocyte precursors that migrate to the injured area. The later time point (2 weeks after stroke) was examined to verify survival and maturation of immature neurons at the later time point, and to evaluate the proportion of astrocytes and oligodendrocytes which was previously shown to be altered 2 weeks after neonatal stroke7.
While there were increased co-labeled neurons in EPO-MCAO animals, there were many single-labeled DCx+, NeuN+, and O4+ cells that did not co-label with GFP. While a subset of NSCs is labeled in this model, and cells in the outer SVZ or that have previously translocated to more superficial regions of the brain may not be labeled, it is also possible that there is a significant local response to injury. It is known that some reactive astrocytes and progeny of radial glia may retain neurogenic potential21, and local production of neurotrophic factors by astrocytes and other cell types may influence cell survival and fate in the penumbra. The role of local neurogenesis and repair still needs to be clarified.
We did not previously see long-term improvement with single-dose EPO after MCAO, but 3 doses administered over a 1-week period did result in long-term histological and behavioral improvement. This may indicate the need for additional booster doses, such as the 7-day dose in this protocol, for long-term improvement. Interestingly, delayed initiation of prolonged EPO treatment following neonatal HI resulted in increased oligodendrogliosis and short-term behavioral improvement, despite lack of change in gross histology22. This may be secondary to the cell-specific effects of EPO, including increased or improved myelination. While there was an increased density of immature neurons in EPO-MCAO animals, others have seen maturational delay or block of cell types following injury5, 23. For this reason, we examined neuronal maturation of GFP+ cells in the striatum by calculating the density of NeuN+/GFP+ mature neurons at P21. In this study, there were increased co-labeled mature neurons in EPO- versus vehicle-MCAO animals, with these cells originating from the SVZ.
EPO and EPO receptor (EPOR) expression is elevated during gestation but declines rapidly after birth24, with cell-specific increased expression after injury25. Following hypoxia, neuronal transcription factors HIF-1 and HIF-2 are stabilized, with increased expression of downstream targets (including EPO and VEGF) that initiate pathways for neuroprotection, angiogenesis, and repair8. These pathways have been shown to have anti-apoptotic, anti-inflammatory, and pro-angiogenic effects, as well as shifting cell fate toward a neurogenic outcome in adult brain injury models26,27. Post-injury angiogenesis may also be necessary for long-term survival of injured or newly generated cells, which may be enhanced by EPO and its interaction with VEGF. For example, EPO-treated mature rats have increased VEGF and brain-derived neurotrophic factor levels after stroke, as well as enhanced angiogenesis and neuroblast proliferation/migration to these regions13. However, the time course of activation of these pathways in the immature brain following stroke, and the role of early versus delayed EPO therapy on different signaling pathways is not clear.
To help clarify the effects of stroke and prolonged EPO treatment on cell proliferation and cell survival in the injured striatum, we calculated the relative density of single labeled Ki67+ in the striatum. Ki67 is expressed in all phases of the cell cycle, with rapidly decreasing expression after exiting the cell cycle28. There were more newly generated Ki67+ cells at 72 hours in EPO-MCAO compared to vehicle-MCAO animals, consistent with an overall increase in cell proliferation following injury. Additionally, there were fewer CC3+ apoptotic cells in the injured striatum at 72 hours in EPO-MCAO animals. It was not possible to quantify co-labeled CC3+/GFP+ cells, as there were relatively few double-labeled cells at this time point. CC3 expression peaks at 18–24 hours after injury29, and 72 hours may represent a relatively late time point for the initial wave of apoptotic cell death. More definitive quantification of apoptosis and cell death will require double-labeling at earlier time points, in both the SVZ and the striatum. In addition, the role of early EPO on cell death can be further clarified by initiating EPO therapy 24 hours, or later, after stroke.
This study demonstrates the effects of prolonged exogenous EPO administration specifically on SVZ NSC fate outcome in the injured striatum after neonatal stroke. Additional studies are needed to determine the relative importance of neuroprotection and cell survival versus cell proliferation and repair after neonatal stroke, as well as alternative sources of new cells in the developing forebrain.
Supplementary Material
Acknowledgments
Funding: NIH K08 NS064094 (FG), NIH P50 BS35902 (DF), NIH NS 33997 (DF), NIH/NCRR University of California, San Francisco-Clinical & Translational Science Institute (UL1 RR024131) (FG).
Footnotes
Disclosures: None
References
- 1.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 2.Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 5.Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- 6.Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Spadafora R, Gonzalez FF, Derugin N, Wendland M, Ferriero D, McQuillen P. Altered fate of subventricular zone progenitor cells and reduced neurogenesis following neonatal stroke. Dev Neurosci. 2010;32:101–113. doi: 10.1159/000279654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong T, Qu Y, Mu D, Ferriero D. Erythropoietin for neonatal brain injury: Opportunity and challenge. Int J Dev Neurosci. 2011;29:583–591. doi: 10.1016/j.ijdevneu.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Chang YS, Mu D, Wendland M, Sheldon RA, Vexler ZS, McQuillen PS, et al. Erythropoietin improves functional and histological outcome in neonatal stroke. Pediatr Res. 2005;58:106–111. doi: 10.1203/01.PDR.0000163616.89767.69. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez FF, McQuillen P, Mu D, Chang Y, Wendland M, Vexler Z, et al. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev Neurosci. 2007;29:321–330. doi: 10.1159/000105473. [DOI] [PubMed] [Google Scholar]
- 11.Kumral A, Uysal N, Tugyan K, Sonmez A, Yilmaz O, Gokmen N, et al. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res. 2004;153:77–86. doi: 10.1016/j.bbr.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez FF, Abel R, Almli CR, Mu D, Wendland M, Ferriero DM. Erythropoietin sustains cognitive function and brain volume after neonatal stroke. Dev Neurosci. 2009;31:403–411. doi: 10.1159/000232558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 14.Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derugin N, Wendland M, Muramatsu K, Roberts TP, Gregory G, Ferriero DM, et al. Evolution of brain injury after transient middle cerebral artery occlusion in neonatal rats. Stroke. 2000;31:1752–1761. doi: 10.1161/01.str.31.7.1752. [DOI] [PubMed] [Google Scholar]
- 16.Levison SW, Chuang C, Abramson BJ, Goldman JE. The migrational patterns and developmental fates of glial precursors in the rat subventricular zone are temporally regulated. Development. 1993;119:611–622. doi: 10.1242/dev.119.3.611. [DOI] [PubMed] [Google Scholar]
- 17.Ong J, Plane JM, Parent JM, Silverstein FS. Hypoxic-ischemic injury stimulates subventricular zone proliferation and neurogenesis in the neonatal rat. Pediatr Res. 2005;58:600–606. doi: 10.1203/01.PDR.0000179381.86809.02. [DOI] [PubMed] [Google Scholar]
- 18.Geraerts M, Eggermont K, Hernandez-Acosta P, Garcia-Verdugo JM, Baekelandt V, Debyser Z. Lentiviral vectors mediate efficient and stable gene transfer in adult neural stem cells in vivo. Hum Gene Ther. 2006;17:635–650. doi: 10.1089/hum.2006.17.635. [DOI] [PubMed] [Google Scholar]
- 19.Perlman JM. Intervention strategies for neonatal hypoxic-ischemic cerebral injury. Clin Ther. 2006;28:1353–1365. doi: 10.1016/j.clinthera.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Osredkar D, Sall JW, Bickler PE, Ferriero DM. Erythropoietin promotes hippocampal neurogenesis in in vitro models of neonatal stroke. Neurobiol Dis. 2011;38:259–265. doi: 10.1016/j.nbd.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori T, Buffo A, Gotz M. The novel roles of glial cells revisited: The contribution of radial glia and astrocytes to neurogenesis. Curr Top Dev Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 22.Iwai M, Stetler RA, Xing J, Hu X, Gao Y, Zhang W, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41:1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kako E, Kaneko N, Aoyama M, Hida H, Takebayashi H, Ikenaka K, et al. Subventricular zone-derived oligodendrogenesis in injured neonatal white matter in mice enhanced by a nonerythropoietic erythropoietin derivative. Stem Cells. 2012;30:2234–2247. doi: 10.1002/stem.1202. [DOI] [PubMed] [Google Scholar]
- 24.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–49. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Sola A, Rogido M, Lee BH, Genetta T, Wen TC. Erythropoietin after focal cerebral ischemia activates the janus kinase-signal transducer and activator of transcription signaling pathway and improves brain injury in postnatal day 7 rats. Pediatr Res. 2005;57:481–487. doi: 10.1203/01.PDR.0000155760.88664.06. [DOI] [PubMed] [Google Scholar]
- 27.Yin D, Kawabata H, Tcherniamtchouk O, Huynh T, Black KL, Koeffler HP. Glioblastoma multiforme cells: Expression of erythropoietin receptor and response to erythropoietin. Int J Oncol. 2007;31:1193–1198. [PubMed] [Google Scholar]
- 28.Mori T, Wakabayashi T, Takamori Y, Kitaya K, Yamada H. Phenotype analysis and quantification of proliferating cells in the cortical gray matter of the adult rat. Acta Histochem Cytochem. 2009;42:1–8. doi: 10.1267/ahc.08037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Cooke MJ, Morshead CM, Shoichet MS. Hydrogel delivery of erythropoietin to the brain for endogenous stem cell stimulation after stroke injury. Biomaterials. 33:2681–2692. doi: 10.1016/j.biomaterials.2011.12.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


