Abstract
Genetic association studies of the CLOCK 3111C/T polymorphism and diurnal preference have yielded conflicting results since the first report that the 3111C allele was associated with eveningness. The goal of the present study was to investigate the association of this polymorphism with diurnal preference and circadian physiology in a group of 179 individuals, by comparing the frequency of the 3111C allele to diurnal preference, habitual sleep timing, circadian phase markers, and circadian period. We did not find a significant association between this allele and morningness/eveningness or any circadian marker.
Diurnal preference refers to an individual’s preference for timing daily activities. Diurnal preference is an individual trait that is normally distributed, and may be assessed subjectively through questionnaires such as the Horne-Östberg Morningness-Eveningness Questionnaire [MEQ; (Horne and Östberg, 1976)]. Based on the idea that the circadian timing system has a strong influence on diurnal preference, a series of recent investigations have studied the association of morningness/eveningness with circadian clock-related genes. The first such report (Katzenberg et al., 1998) described a single nucleotide polymorphism (SNP) in the human CLOCK gene (3111C/T) that was associated with diurnal preference, finding that the 3111C allele correlated with eveningness (Katzenberg et al., 1998). While one subsequent report replicated that finding (Mishima et al., 2005), several others have not (Johansson et al., 2003; Pedrazzoli et al., 2007; Robilliard et al., 2002). The purpose of the current study was to examine the association between this SNP and diurnal preference in a group of individuals who underwent circadian rhythm phenotyping in our laboratory, and to further examine whether there were differences in circadian phenotypic measures (circadian phase, phase angle, or period) between individuals of different CLOCK 3111C/T genotypes.
This prospective study included 179 healthy individuals who had previously completed a physiologic study in the Division of Sleep Medicine at Brigham and Women’s Hospital across all seasons in 2001–2010. The study was approved by the Partners Health Care Human Research Committee and conducted according to the principles stated in the Declaration of Helsinki. Subjects were recruited for participation if they had completed an in-patient phenotyping protocol, and separate written informed consent was obtained for enrollment in the genetic study. Each completed the MEQ, which was scored according to the categories defined in the original publication (Horne and Östberg, 1976); and donated a blood sample from which DNA was extracted and genotyped for the CLOCK 3111T/C polymorphism (rs10801260). In a subset of 41 subjects, the MEQ score was used as selection criterion for enrollment into the in-patient study, and those individuals were all morning or evening types. For 2–3 weeks prior to admission, subjects were required to maintain a stable sleep schedule, which was self-selected and based on their habitual sleep times. Subjects who participated in studies where circadian phase was measured were required to keep an 8h sleep schedule. Compliance was verified with daily timestamped calls and confirmed with wrist actigraphy for at least 1 week. Habitual bedtimes and wake times were calculated from the mean call-ins of the week prior to admission.
Some of the protocols included a constant routine procedure during which circadian phase was assessed using the core body temperature nadir [CBT (Brown and Czeisler, 1992)] and/or the dim-light melatonin onset (DLMO25%) as described elsewhere (Cain et al., 2010). Phase angle of entrainment was defined as the interval between CBT nadir and wake time/lights on and/or DLMO25% and bed time/lights off. For 35 subjects, their study also included a forced desynchrony procedure from which circadian period was determined using CBT and/or melatonin (Czeisler et al., 1999). For an additional 33 subjects, we could assess circadian period but not circadian phase (see Supplemental Online Materials Figure S1). Mean age ± SD of the group was 27.46 ± 11.80 years (range 18–70) and was 27.78 ± 12.15 in the 55 females and 27.32 ± 11.69 in the 124 males. The racial composition was 74% Caucasian, 12% Asian, 7% Black/African American, and 7% reporting more than 1 or unknown race. The frequencies of the 3111C (23%) and T (77%) alleles did not deviate significantly from the Hardy-Weinberg Equilibrium calculated for this allele (χ2, p=0.85). Frequencies of the C/C, C/T, and T/T genotypes were 3.9% (n=7), 37.4%, (n=70), and 58.7% (n=105), respectively, similar to a previous study with a similar racial composition (Pedrazzoli et al., 2007).
We compared MEQ score, age, habitual bedtime and wake time, circadian phase, phase angle of entrainment, and circadian period, where available, among genotypes (C/C, C/T, and T/T) and between allelic (C allele and non-C allele) groups using mixed model analysis of variance (ANOVA; SAS 9.1). Due to the small number of individuals with the C/C genotype, our analysis focused on comparing the two allelic groups, as was done in the original publication of this polymorphism (Katzenberg et al., 1998).
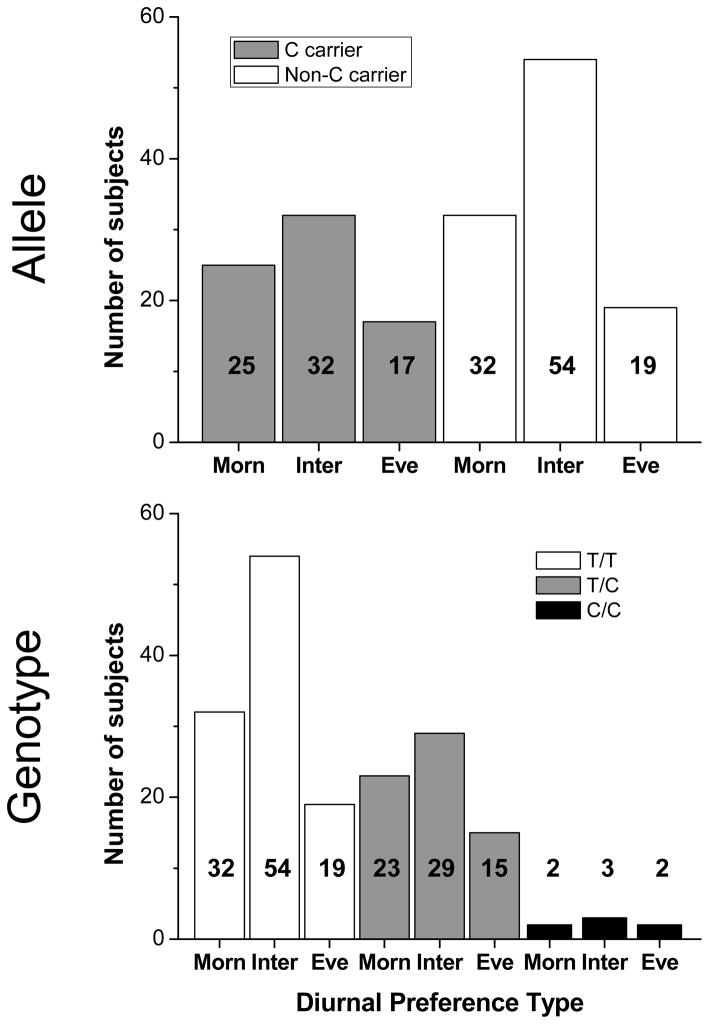
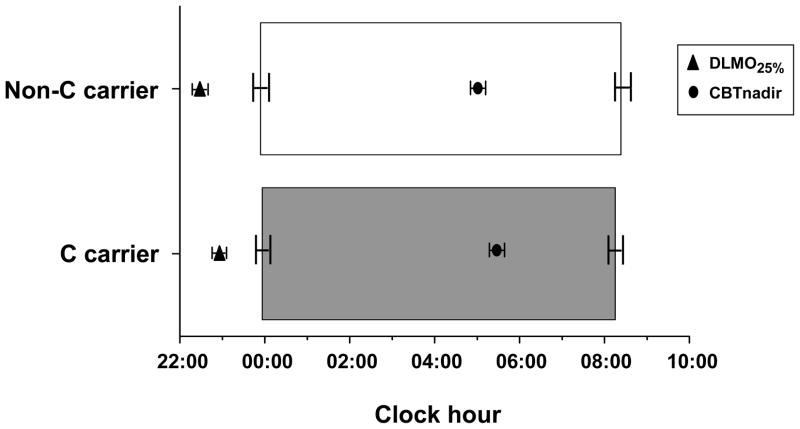
MEQ score, sleep timing and circadian measures are shown in Table 1. MEQ scores were normally distributed when grouped by C allele carriers (Figure 1 top panel) and by genotype (Figure 1 bottom panel) and did not vary among allelic (p=0.902) or genotypic (p=0.992) groups. Habitual bedtime and wake time did not differ between allelic groups (Table 1 and Figure 2) or between genotypes (Table 1). There were no differences in the timing of circadian phase measures between C allele and non-C allele carriers, nor between genotypes (Table 1). The phase angle between CBT nadir and habitual wake time/lights on was not different between allelic groups or by genotype (Table 1). The phase angle between DLMO25% and bedtime/lights off suggested a trend for a shorter interval in C allele carriers (p=0.053), but when the factors age and sex were included in our statistical model this was no longer the case (p=0.463). The phase angle between DLMO25% and bedtime/lights off was not different among the genotypes (Table 1). Circadian period did not differ between allelic groups (Table 1). We did not compare circadian period by genotype due to the small number in the C/C group from whom circadian period was collected (n=2).
Table 1.
Demographic data, MEQ score, habitual sleep timing, and chronobiologic measures for the two allelic groups: C and Non-C carriers; and the three genotypic groups: T/T, C/T, and C/C.
| p1 | n | C carriers (C/T and C/C) | n | Non-C carriers (T/T) | n | C/T | n | C/C | p2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.911 | 74 | 27.60 ± 12.60 | 105 | 27.40 ± 11.20 | 67 | 27.70 ± 13.00 | 7 | 26.30 ± 8.50 | 0.949 |
| ♂:♀ | na | 74 | 51:23 | 105 | 73:32 | 67 | 46:21 | 7 | 5:2 | na |
| Race (%Caucasian) | na | 74 | 77% | 105 | 73% | 67 | 73% | 7 | 100% | na |
| MEQ | 0.902 | 74 | 51.95 ± 12.80 | 105 | 52.20 ± 12.50 | 67 | 51.95 ± 13.00 | 7 | 52.00 ± 16.40 | 0.992 |
| Bedtime | 0.887 | 74 | 23:56 ± 1:30 | 105 | 23:54 ± 1:42 | 67 | 23:55 ± 1:30 | 7 | 24:11 ± 1:30 | 0.898 |
| Wake time | 0.543 | 74 | 8:15 ± 1:24 | 105 | 8:23 ± 1:30 | 67 | 8:18 ± 1:24 | 7 | 7:47 ± 1:12 | 0.557 |
| CBT phase | 0.334 | 42 | 5:19 ± 1:48 | 57 | 4:54 ± 2:18 | 37 | 5:28 ± 1:48 | 5 | 4:09 ± 1:47 | 0.263 |
| CBT phase angle | 0.174 | 42 | 2.90 ± 1.20 | 57 | 3.28 ± 1.50 | 37 | 2.80 ± 1.20 | 5 | 3.64 ± 0.55 | 0.171 |
| CBT period | 0.262 | 30 | 24.18 ± 0.20 | 39 | 24.13 ± 0.20 | 28 | 24.18 ± 0.20 | 2 | 24.21 ± 0.21 | na |
| Melatonin phase | 0.752 | 40 | 22:54 ± 1:42 | 48 | 22:30 ± 2:00 | 35 | 22:57 ± 1:42 | 5 | 22:35 ± 1:59 | 0.925 |
| Melatonin phase angle | 0.053 | 40 | 1.28 ± 0.80 | 48 | 1.72 ± 1.10 | 35 | 1.37 ± 0.80 | 5 | 1.78 ± 1.10 | 0.149 |
| Melatonin period | 0.203 | 29 | 24.21 ± 0.20 | 39 | 24.15 ± 0.20 | 27 | 24.21 ± 0.20 | 2 | 24.16 ± 0.20 | na |
NOTE: Mean ± standard deviation. Bedtimes, wake times, and phase measures for both CBT and melatonin are listed as clock hour. CBT phase angle is calculated as the difference between CBT nadir and habitual wake time/lights on; melatonin phase angle is the difference between DLMO25% and habitual bedtime/lights off. P1 values denote comparisons between allelic groups (C vs. Non-C carriers) and p2 values are from comparisons among the genotypes (T/T, C/T, and C/C).
Figure 1.
Distribution of diurnal preference in CLOCK 3111 C and non-C allele carriers and genotypes. The upper panel shows the distribution of morning, intermediate, and evening types by allelic group: C allele (gray bars), non-C allele (open bars) carriers; and by genotype in the lower panel: T/T (open bars), T/C (gray bars), and C/C (black bars). Numbers within/above the bars indicate number of subjects.
Figure 2.
Habitual sleep times and timing of circadian phases for CLOCK 3111C allele and non-C allele carriers. Mean +/− SEM. Horizontal bars: timing of the habitual sleep episode; triangles: timing of the circadian phase of melatonin onset (DLMO25%); circles: timing of the circadian phase of the core body temperature nadir (CBTnadir).
The goal of the current study was not simply to examine the association of the CLOCK 3111C allele with diurnal preference, but to extend previous findings by investigating the influence of this polymorphism on sleep timing and chronobiologic measures of circadian phase, phase angle of entrainment, and period. In our study population, MEQ score did not associate with the CLOCK 3111T/C genotype, and the frequency of the 3111C allele was not significantly different between morning and evening types. We found no association between the 3111C allele and habitual sleep timing, circadian phase, phase angle of entrainment, or circadian period. Given that none of the measures showed differences between the genotypic or allelic groups, this polymorphism does not appear to be a strong determinant of human diurnal preference.
We did not replicate the original finding of an association between the 3111C/C genotype with evening preference (Katzenberg et al., 1998), which has been replicated in only one subsequent report in a study of 421 Japanese subjects (Mishima et al., 2005). This may be related to differences in the study populations, selection criteria, and/or analytic methods. While our sample size was relatively small for a genetic study, it was similar to or larger than the other studies of this SNP (Johansson et al., 2003; Mishima et al., 2005; Pedrazzoli et al., 2007; Robilliard et al., 2002). Furthermore, our finding of no association between diurnal preference and the 3111C/T SNP is consistent with several other studies (Johansson et al., 2003; Lee et al., 2007; Pedrazzoli et al., 2007; Robilliard et al., 2002), and our phenotyping (which included documented sleep timing and circadian parameters) was more extensive than reliance on a onetime self-report of diurnal preference. Given the increasing number of studies seeking to find a genetic basis for individual differences in human sleep and circadian rhythmicity, our findings suggest that caution should be used when inferring phenotypic information from a questionnaire.
Acknowledgments
We would like to thank the study participants; A. O’Malley and J. Row for subject recruitment; Drs. D. Aeschbach, C. Anderson, S. Baddam, O.M. Buxton, S.W. Cain, D.A. Cohen, J.J. Gooley, J.T. Hull, E.B. Klerman, S.W. Lockley, M.A. Mograss, M.Y. Münch, S.M. Rajaratnam, M. Rüger, N. Santhi, K.D. Scheuermaier, and E. Van Reen, who served as Project Leaders for many of the inpatient physiologic studies. Those inpatient physiologic studies were supported by NIH grants AG06072, AG09975, AT002571, HL077399, HL077453, HL080978, MH045130, NS041886, NS054277; by NSBRI grants HFP00402 and HFP01601; by AFOSR grant FA9550; and by an investigator-initiated trial funded by Cephalon, Inc.
The current study was funded by NIH grants HL080978, AG09975, and HL078360. AMC was supported in part by a pilot grant from Harvard Catalyst [The Harvard Clinical and Translational Science Center, NIH grant UL1 RR025758 and financial contributions from participating institutions], and by F32 HL078360. Collection of blood samples was performed at the Brigham and Women’s Hospital Center for Clinical Investigation, part of the Harvard Clinical and Translational Science Center, formerly a General Clinical Research Center (M01 RR02635).
References
- Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. Journal of Biological Rhythms. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SS, Santhi N, Schoen M, Czeisler CA, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. Journal of Biological Rhythms. 2010;25:288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology. 1976;4:97–110. [PubMed] [Google Scholar]
- Johansson C, Willeit M, Smedh C, Ekholm J, Paunio T, Kieseppa T, Lichtermann D, Praschak-Rieder N, Neumeister A, Nilsson LG, Kasper S, Peltonen L, Adolfsson R, Schalling M, Partonen T. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003;28:734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- Katzenberg D, Young T, Finn L, Lin L, King DP, Takahashi JS, Mignot E. A clock polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Paik JW, Kang SG, Lim SW, Kim L. Allelic variants interaction of CLOCK gene and G-protein beta3 subunit gene with diurnal preference. Chronobiology International. 2007;24:589–597. doi: 10.1080/07420520701534632. [DOI] [PubMed] [Google Scholar]
- Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005;133:101–104. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli M, Louzada FM, Pereira DS, Benedito-Silva AA, Lopez AR, Martynhak BJ, Korczak AL, Koike, Bdel V, Barbosa AA, D’Almeida V, Tufik S. Clock polymorphisms and circadian rhythms phenotypes in a sample of the Brazilian population. Chronobiology International. 2007;24:1–8. doi: 10.1080/07420520601139789. [DOI] [PubMed] [Google Scholar]
- Robilliard DL, Archer SN, Arendt J, Lockley SW, Hack LM, English J, Leger D, Smits MG, Williams A, Skene DJ, von schantz M. The 3111 clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. Journal of Sleep Research. 2002;11:305–312. doi: 10.1046/j.1365-2869.2002.00320.x. [DOI] [PubMed] [Google Scholar]