Abstract
Delayed sleep phase syndrome (DSPS) is a circadian rhythm sleep disorder in which the timing of the sleep episode occurs later than desired and is associated with difficulty falling asleep, problems awakening on time (e.g., to meet work or school obligations), and daytime sleepiness. The phase relationship between the timing of sleep and endogenous circadian rhythms is critical to the initiation and maintenance of sleep, and significant alteration leads to impairment of sleep quality and duration. The aim of this retrospective study was to determine the phase relationship between sleep-wake times and physiological markers of circadian timing in clinic patients with DSPS. Objective and subjective measures of sleep timing and circadian phase markers (core body temperature and melatonin) were measured in patients with DSPS and compared with age-matched controls. As expected, significant delays in the timing of the major sleep episode and circadian phase of body temperature and melatonin rhythms were seen in the DSPS group when allowed to sleep at their own habitual schedules, but the phase relationship between sleep-wake times and circadian phase was similar between the two groups. These results suggest that the symptoms of insomnia and excessive daytime sleepiness in DSPS patients living under entrained real-life conditions cannot be explained by an alteration in the phase relationship between sleep-wake patterns and other physiological circadian rhythms.
Keywords: DSPS, circadian rhythm sleep disorder, circadian phase, circadian phase angle
Introduction
As a diurnal species, humans are generally awake and active during the daytime and asleep during a portion of the night, as work schedules allow. The timing and duration of sleep episodes, however, vary from individual to individual, and are dependent on numerous factors, including work/school schedule, lifestyle, health, age, and genetic predisposition. A misalignment of the habitual sleep schedule to the daily light/dark entrainment cycle may lead to sleep complaints of insufficient or non-restorative sleep. For some individuals, work schedules such as night work or shift work may cause these misalignments. In other cases, an individual’s sleep time is shifted relative to the environmental schedule, resulting in bedtimes and wake times that occur earlier or later than typical or desired times. These cases are examples of circadian rhythm sleep disorders in which there is an altered but stable temporal relationship of the sleep-wake rhythm to the environmental 24-hour day.
Advanced sleep phase syndrome (ASPS) and delayed sleep phase syndrome (DSPS) are circadian rhythm sleep disorders characterized by an alteration in the timing of the sleep episode. DSPS, the more commonly reported of these disorders (Ando et al., 2001; Schrader et al., 1993), with a particularly higher prevalence among adolescents and young adults (Pelayo et al., 1988; Regestein and Monk, 1995), is defined by a persistent inability to fall asleep and/or awaken as early as desired. An individual with DSPS is typically unable to fall asleep before 2 AM and if allowed to sleep and wake at will, awakens between 10 AM and 1 PM (Weitzman et al., 1981). In some cases, enforced morning awakenings imposed by work and school schedules result in chronic sleep loss. This schedule of sleep restriction could have serious consequences for health and performance (Banks and Dinges, 2007; Van Cauter et al., 2007; Van Dongen et al., 2003).
Various measures of sleep timing and circadian phase have been examined in individuals with DSPS (Ozaki et al., 1996; Rodenbeck et al., 1998; Uchiyama et al., 1999; Uchiyama et al., 2000; Watanabe et al., 2003; Wyatt et al., 2006). There has also been some progress made to identify the genetic basis underlying DSPS (Archer et al., 2003; Ebisawa et al., 1999; Ebisawa et al., 2000; Ebisawa et al., 2001; Hohjoh et al., 1999; Hohjoh et al., 2003; Takano et al., 2004), but there is still a great deal to learn about the cause and pathophysiology of the circadian rhythm sleep disorders. Several possible mechanisms could account for a persistently altered phase of sleep timing to the environment. These include a long endogenous circadian period, masking of the advanced region of the light phase response curve (PRC), an altered response to the phase-resetting effects of light, and an altered phase relationship between the circadian clock and the sleep-wake rhythm. A change in the phase relationship between habitual sleep timing and circadian phase may explain both the persistent delay in the sleep-wake times and difficulty initiating sleep at earlier times experienced by patients with DSPS.
The retrospective study presented here describes the phenotypic characterization of a large clinic based DSPS population. Our goal is to examine sleep parameters, circadian phase (melatonin and core body temperature rhythms), and phase angle between circadian markers and sleep schedule in DSPS patients and to determine if they are significantly different from age-matched control subjects. We hypothesized that, similar to what has been reported in the literature, the DSPS group will exhibit delays of their habitual sleep timing and circadian phase and that the phase relationship between sleep and circadian phase of melatonin and temperature rhythms will be altered in DSPS subjects, as has been previously reported in small samples (Ozaki et al., 1996; Rodenbeck et al., 1998; Uchiyama et al., 1999; Uchiyama et al., 2000; Watanabe et al., 2003). Our findings expand on these prior studies to include examination of sleep timing and circadian phase in a large number of DSPS patients living under their usual entrained conditions.
Methods
Subjects
Data were collected from a total of 122 subjects: 66 individuals diagnosed with DSPS (35 females) and 56 healthy control subjects (26 females) over a 7-year period between 1999 and 2006. The work was conducted at the Northwestern University Feinberg School of Medicine. The mean age ± standard deviation (SD) of the DSPS group was 30 ± 10 years with an age range of 16–62 years. Control subjects were age-matched to the DSPS group; mean age of 32 ± 13 years and age range of 19–74 years and there was no significant difference in age between the groups (p=0.41). Clinical diagnosis of circadian rhythm sleep disorder patients was determined by physician interview and according to The International Classification of Sleep Disorders Revised: Diagnostic and Coding Manual (2000) and The Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition TR (DSM-IV) (American Psychiatric Association, 2000). Collection of sleep and circadian measures in DSPS patients and analysis of these data for this retrospective study was approved by the Northwestern University Institutional Review Board. All control subjects were recruited for participation in unrelated sleep research studies and gave informed written consent prior to enrollment. Only baseline data collected from control subjects were analyzed in this study. A subset of the DSPS patients in this study gave informed written consent to participate in an unrelated study of oral melatonin administration as a therapeutic treatment (Mundey et al., 2005), and only baseline data collected in naïve patients, prior to intervention, were included in the present analysis
Monitoring Sleep-wake Activity
All subjects, DSPS and control, were asked to wear wrist activity monitors and maintain daily sleep diaries for a minimum of one week to determine habitual bedtimes and sleep times while living in their home environment. In order to detect any differences in sleep schedule between weekdays and weekends, sleep measures from at least 4 weekdays and 2 weekend days were analyzed. Weekdays included bedtime Sunday through wake time Friday and weekends included bedtime Friday through wake time Sunday. Bedtimes, wake times, estimated total sleep time, sleep latency, wake after sleep onset, naps, and any unusual events during the day or night were recorded in the sleep logs. From the reported sleep times, several measures were used for analysis: bedtime, wake time, and time in bed (wake time – bedtime). The Actiwatch system (Mini Mitter/Respironics, Bend, OR) was used to record activity. Each subject wore an actigraph continuously (except when bathing or swimming) on the wrist of the non-dominant hand. Daily activity data recorded via the wrist monitors were analyzed using Actiware© version 3.4 software (Mini Mitter/Respironics) to verify sleep periods reported in sleep logs and to determine sleep onset, sleep offset, and sleep duration objectively (sleep offset – onset) per nightly sleep episode. A combination of both sleep diary and actigraphic data were used to determine sleep latency (sleep onset – bedtime), and sleep efficiency (actual sleep/time in bed).
Measuring Melatonin and Determination of DLMO
Saliva samples were collected from a subset of DSPS participants (17) in their homes to determine dim light melatonin onsets (DLMO). Participants were instructed to collect samples using salivette tubes every 30 minutes, under dim light conditions (at most a side lamp or television in the room) beginning 4 hours prior to habitual bedtime until sleep time. Subjects were asked to refrain from eating or drinking 20 minutes prior to sample collection (Mundey et al., 2005). Salivary melatonin samples were measured using a Buhlmann Saliva-direct radioimmunoassay (ALPCO, Windham, NH). The lower limit of functional sensitivity is 0.9 pg/ml; the intra-assay coefficient of variance was 4.1% at 3.56 pg.ml and the inter-assay coefficient of variance was 7.5% at 3.39 pg/ml (Mundey et al., 2005).
Plasma melatonin was measured in a subset of DSPS patients (8) and a majority of control participants (31), from blood samples collected under dim light conditions (<20 lux) in the General Clinical Research Center (GCRC) at a 20–30 minute interval. Blood sampling began approximately 24-hours following admission to the GCRC during which participants were kept in modified entrainment conditions: dim light (<20 lux) during wake, darkness during sleep scheduled at habitual sleep/wake times, and restricted activity to avoid vigorous exercise. A sterile heparin-lock catheter was inserted in the forearm 2 hours before the beginning of sampling, and the IV line was kept patent by a slow drip of heparinized saline. Blood samples were collected using plastic syringes connected to the lateral arm of a three-way stopcock. During waking hours, the stopcock was directly attached to the antecubital catheter, and during sleep, the indwelling catheter was connected to plastic tubing extending to an adjacent room (Benloucif et al., 2005). Plasma melatonin samples from each individual were measured in the GCRC core laboratory using commercially available reagents (Stockgrand LTD, University of Surrey, Guilford, Surrey, UK) with a procedure developed by J. Arendt (University of Surrey) using an anti-melatonin antiserum raised in a rabbit by Dr. J.P. Ravault (Nouzilly, France). This antiserum has been validated for the assay of melatonin in human plasma using an iodinated melatonin tracer. The lower limit of sensitivity was 2.5 pg/ml. The intra-assay coefficient of variation averaged 15–17.5% for values <10 pg/ml, and 5–16% for values >10 pg/ml. The inter-assay coefficient of variation averaged ~20 for values <10 pg/ml and ~13.5% for values >10 pg/ml (Baehr et al., 2003; Benloucif et al., 2005).
Salivary melatonin has been validated as a circadian phase marker and the timing of different melatonin profile parameters determined from saliva samples was significantly correlated to those using plasma melatonin assay in a general subject population (Laakso et al., 1990; McIntyre et al., 1987; Voultsios et al., 1997) and specifically in DSPS patients (Nagtegaal et al., 1998). In order to determine the DLMO from salivary and plasma samples, onsets were calculated as the time of the first sample greater than 2 standard deviations above the basal mean (Lewy et al., 1999; Voultsios et al., 1997).
Measuring Core Body Temperature and Determination of Nadir
To determine the circadian phase of core body temperature (CBT), all participants (DSPS and control) were asked to wear a rectal thermistor for a minimum of 36 hours (Mini Logger, Mini Mitter/Respironics). This flexible rectal thermometer, connected to a lightweight data-recording unit, was used to sample CBT every minute over this period of time. DSPS patients were given a temperature-recording unit after detailed explanation of its use for home recording. Core body temperature recording in control subjects was done during their stay in the GCRC under ambulatory conditions. The 24-hour CBT profiles were quantitatively described using the Cleveland regression procedure to assess the phase of the rhythm (Cleveland, 1979). The phase of CBT was characterized by the timing of the fitted nocturnal minimum (CBTnadir).
Determining phase angle of entrainment
Four different phase angle measures were determined using the melatonin, temperature, and actigraphic data. The actigraphy data was usually collected for a minimum of 1 week before and during collection of melatonin and/or temperature data for phase determination in the GCRC studies and concomitantly (during the last 2 days of the actigraphy) for at home studies. This interval between actigraphy and circadian phase measure was typically 0–2 days. Phase angles were calculated as the difference between a circadian phase marker (DLMO or CBTnadir) and a mean sleep timing measure (sleep onset or offset). These 4 measures were defined as: DLMO-sleep onset, DLMO-sleep offset, CBTnadir-sleep onset, and CBTnadir-sleep offset.
Statistical tests
Unpaired t-tests were used for comparisons of sleep, circadian phase, and phase angle measures between the groups (DSPS vs. controls, SPSS 11). Paired t-tests were used for comparisons of sleep measures during weekdays and weekends for all subjects. A 2-way analysis of variance (ANOVA) was used to compare sleep measures during weekdays and weekends between DSPS and control groups (Prism 4.0c). Subjective measures of sleep schedules were correlated with objective actigraphy-derived sleep measures for all subjects. Differences with a p value less than 0.05 were considered significant.
Results
Habitual Sleep Schedule
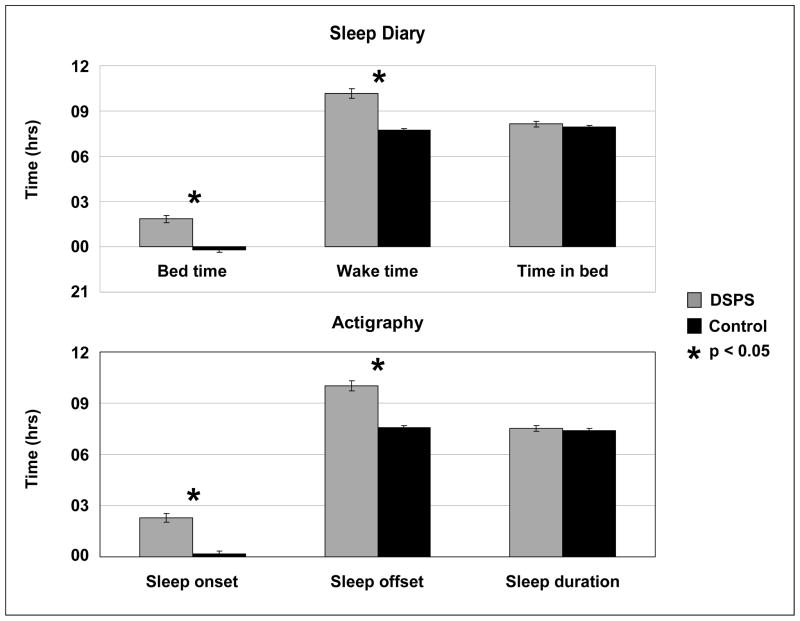
Wrist actigraphy and sleep diaries were collected in 122 subjects (66 DSPS and 56 controls). Six sleep measures were assessed for both participant groups. Actigraphic recordings correlated very highly with self-reported diaries for sleep measures: bedtime vs. sleep onset (r2 = 0.93, p<0.001), wake time vs. sleep offset (r2 = 0.99, p<0.001). DSPS patients had significantly later sleep-wake times when compared with controls (p<0.001), although they did not differ in the amount of time in bed or duration of sleep (see Figure 1). Furthermore, there was no difference in sleep latency or sleep efficiency between the 2 groups.
Figure 1.
Comparison of sleep measures between DSPS and control groups. Three subjective measures collected from sleep diaries are shown in the top panel and 3 objective sleep measures obtained from wrist actigraphy are shown in the bottom panel. Standard error (SE) bars are shown.
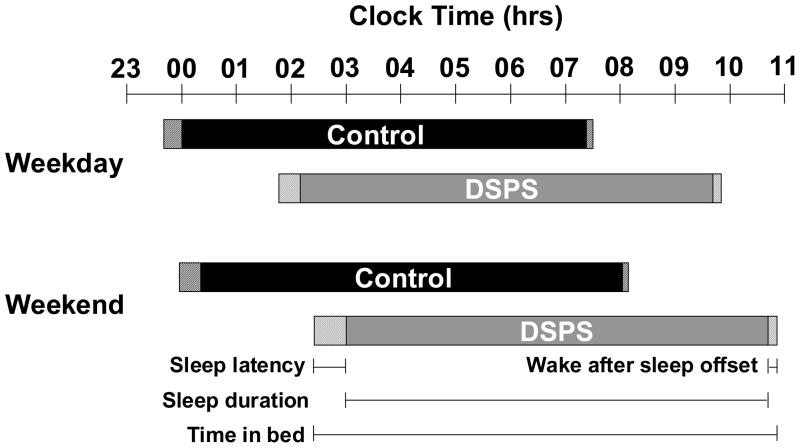
Habitual sleep measures from a minimum of 4 weekdays and 2 weekend days were collected from a total of 121 subjects (65 DSPS, and 56 controls). A comparison of sleep measures for weekdays and weekends for both subject groups is shown in Figure 2. On the weekends all subjects, DSPS and controls, regardless of diagnostic status, were going to bed later (by 29 minutes) and waking later (by 50 minutes) compared to the weekdays (p<0.05). Additionally, all subjects spent significantly more time in bed (26 minutes) and slept longer (21 minutes) on weekends (p<0.05). There was no difference in sleep latency or sleep efficiency between weekdays and weekends. Results from the 2-way ANOVA showed only significant differences in bedtime, wake time, sleep onset, and sleep offset between DSPS and controls (all p<0.0001).
Figure 2.
Sleep measures during the weekday and weekend for DSPS and control groups. Time in bed (solid + striped bars) is defined as the time between bedtime and wake time. Sleep duration (solid bars) is the time between sleep onset and offset. Sleep latency (striped bars preceding the solid bars) is the time between bedtime and sleep onset. Wake after sleep offset (striped bars following the solid bars) is the time between wake time and getting out of bed.
Circadian Phase of Melatonin and CBT Rhythms
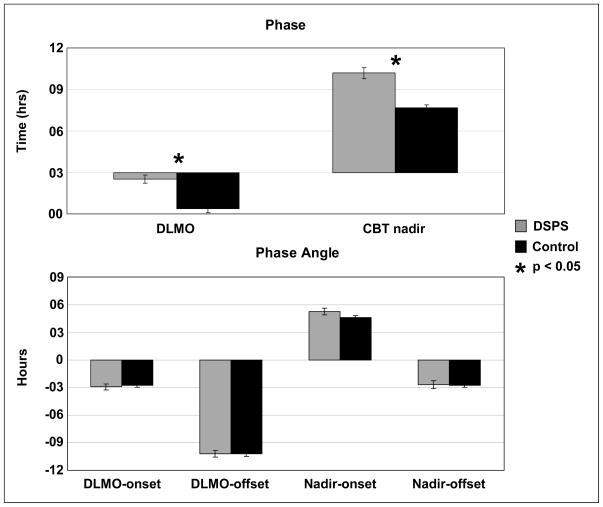
Salivary melatonin was collected in 17 DSPS patients and plasma melatonin was collected in 8 DSPS participants and 31 controls. CBT was collected in 82 subjects (40 DSPS and 42 controls). DSPS patients were significantly phase delayed in both the melatonin and CBT rhythms, relative to the age-matched control group (see Figure 3, top panel). The mean DLMO was delayed by more than 2 hours (p<0.001) and the mean CBTnadir occurred about 2.5 hours later (p<0.001) in the DSPS group. The salivary melatonin phase (DLMO) of the 17 DSPS patients was compared to the DLMO determined from plasma samples of the 8 DSPS participants in order to test for factors of sample type and the conditions under which they were collected that could influence the results. The DLMO was not significantly different in these 2 groups (p=0.81; t-test).
Figure 3.
Comparison of circadian phase and phase angle measures between DSPS and control groups. Mean times of 2 circadian phase markers, dim-light melatonin onset (DLMO), and core body temperature (CBT) nadir, are shown in the top panel. Four phase angle measures were calculated as the difference between a phase measure, DLMO or CBT nadir, and a sleep measure, sleep onset or offset, and are shown in the bottom panel. SE bars are shown.
Phase Angle: Relationship Between Circadian Phase Markers and Sleep Timing
Four phase angles between circadian phase markers (DLMO and CBTnadir) and habitual sleep schedule (sleep onset and offset) are were determined. Figure 3, bottom panel shows the comparison of DSPS subjects and age-matched controls. Although DSPS patients exhibit significantly later phase of both melatonin and temperature rhythms as well as later sleep and wake times when compared with controls, the circadian phase angle was not significantly different between the DSPS and control groups for any of the intervals measured (p = 0.63, 1.00, 0.10, and 0.89, respectively).
Discussion
The current study expands upon prior studies of DSPS in 2 important ways: the large sample size in which subjective and objective sleep measures and circadian phase markers were collected and the use of portable data collection of these measures from DSPS patients in their natural home environments. This DSPS population, as a group, demonstrated a consistent delay in the timing of the sleep episode as compared to controls, but based on sleep diaries and wrist actigraphy did not appear to have difficulty falling asleep. Furthermore, they showed no differences in amount of time in bed, sleep duration or sleep efficiency. These results are surprising given that one of the major complaints of DSPS subjects is sleep onset insomnia or an inability to fall asleep at the desired time, which may manifest as long sleep latencies. While there were some DSPS individuals exhibiting particularly long sleep latencies (>1 hour in 8 DSPS and 6 control subjects), the group on average took only 6 minutes longer to fall asleep than controls, a difference that is not significant. This suggests that these DSPS patients were maintaining a habitual sleep schedule that was later than they desired and significantly later than the control group. These patients came to the sleep center to seek treatment to advance the timing of their sleep and wake cycle. Maintaining a delayed bedtime did not appear to show, in our study, adverse consequences of longer sleep latency or decreased sleep efficiency.
A comparison of sleep schedules on weekdays and weekends showed that all participants were going to bed and waking at significantly later times on the weekend. Both DSPS and control groups spent significantly more time in bed and slept longer on weekends, presumably due to a less restricted schedule free from work/school obligations. This indicates that both groups were restricting sleep during weekdays and making up a “sleep deficit” during weekends. It is interesting to note that even though both groups slept significantly more on the weekends, there was no difference between DSPS and control groups in sleep duration for either weekends or weekdays. This result is in contrast to other published reports of DSPS patients having longer sleep durations than control subjects (Ozaki et al., 1996; Shibui et al., 1999; Uchiyama et al., 2000; Watanabe et al., 2003). Taken together, these results show that the DSPS population studied here had a persistent delay in sleep timing with no evidence for longer sleep duration, difficulty falling asleep or staying asleep. Our findings suggest that under entrained conditions, when allowed to sleep at their habitual sleep times, DSPS patients had normal sleep.
There were significant differences in the circadian phase markers of both temperature and melatonin rhythms with DSPS patients having a characteristically later CBTnadir and DLMO relative to controls. Examination of the phase relationship between sleep measures and the phase of the body temperature rhythm did not show an alteration between either sleep onset or offset and CBTnadir in DSPS patients compared with controls. These results differ from reports of a longer phase interval between CBTnadir and sleep offset in DSPS patients (Ozaki et al., 1996; Uchiyama et al., 2000; Watanabe et al., 2003). In these previous studies, there was no difference in the phase relationship between CBTnadir and sleep onset, only with sleep offset, which is most likely due to significantly longer sleep durations and therefore later sleep offset times reported in their DSPS patients compared with our population. We found no significant difference in the phase relationship between sleep time and DLMO in DSPS patients compared with controls, as had been previously reported (Shibui et al., 1999). Again, this may be due to longer sleep durations and subsequent longer interval between wake time and melatonin phase measures in the previously published DSPS group that we did not find in our study. Our findings are consistent with a more recent report (Wyatt et al., 2006) in which there was no difference in phase angle of DLMO and sleep between DSPS and control groups. Furthermore, it is possible that genetic differences in circadian and homeostatic regulation of sleep and wake may have accounted for the observed difference between the Japanese and US studies, particularly as there have been reported differences in the prevalence of polymorphisms in clock genes reported in different ethnic populations (Ciarleglio et al., 2008; Hawkins et al., 2008; Mishima et al., 2005).
The significant delay in both phase markers without an accompanying alteration in the phase angle of entrainment suggests that although DSPS subjects exhibit later sleep-wake times under entrained conditions, they are not sleeping at an “adverse” circadian phase for maximum sleep efficiency. This is consistent with our sleep timing results demonstrating that the DSPS group did not show greater sleep latencies than controls. Taken together, our findings suggest that when allowed to sleep at their own habitual schedules, patients with DSPS have both normal sleep parameters and circadian phase angle.
There have been a number of possible explanations proposed for the delayed timing of the sleep episode in DSPS patients, including a longer endogenous circadian period, which would require a greater daily phase advance to entrain to the 24-hour day. If DSPS individuals are not receiving light, the strongest entraining stimulus, at the early part of day where it will cause a phase advance, they may exhibit a delayed sleep schedule. Independent of circadian period length, the sleep-wake schedule for individuals with DSPS, on average, is considerably later than controls and suggests they may not be receiving light in the early portion of the day when they may be still asleep. Furthermore, since they are awake later in the evening/night, they may be receiving light in the delay region of the photic PRC, which would delay their sleep even more. The current study was conducted with individuals living in their home environments, presumably under 24-hour entrained conditions, and therefore endogenous circadian period was not assessed.
Alternatively, it has been proposed that DSPS individuals may have an altered response to light: altered sensitivity to light, smaller phase advance region, or larger phase delay region of the light PRC (Czeisler et al., 1981). Another possible explanation is that DSPS subjects have an altered phase relationship between their circadian phase and the light/dark cycle. In laboratory studies of young morning- and evening-type individuals, where the light/dark cycle and sleep-wake timing was controlled, there was a phase angle difference showing that morning types were waking at an earlier circadian phase than evening types (Duffy et al., 1999). While our results do not show an altered phase angle between sleep schedule and circadian phase, we do not know the light/dark conditions, which may differ somewhat from the sleep-wake times for these subjects.
These findings have implications for both diagnosis and treatment of this disorder. At the time of diagnosis of most of the DSPS subjects in this retrospective study, the clinical diagnostic criteria did not require physiologic measures of circadian phase. Since then, there have been revisions made to both the ICSD and DSM-IV criteria that include these measures. Additionally, actigraphy and/or polysomnography have been added to the criteria to confirm the delay of the sleep schedule. Ultimately, the single criterion that remains critical to a clinical diagnosis of DSPS is a self-reported sleep-related complaint by the individual. Results from actigraphic recordings, sleep diaries, and physiologic phase measures, while confirming the delayed timing of the sleep episode and circadian phase, do not support the maladaptive response to this pattern that forms the basis of the sleep complaint. We found no difference in sleep latency, sleep duration, or sleep efficiency between DSPS subjects and controls that would indicate difficulty initiating or maintaining sleep. The analysis of sleep on weekdays and weekends further showed that both groups were extending sleep when given the opportunity, but the DSPS group did not show a longer sleep episode that might indicate greater sleep pressure, relative to the control group. Because we found no significant difference in the phase relationship of sleep timing and circadian phase between the two groups, the DSPS group does not appear to be sleeping at an “adverse” circadian phase that could explain sleep problems. Again, the greater variability seen in the DSPS group suggests that although our measures from the large group, on average, do not differ from the controls, there are likely greater inter-individual differences and multiple symptoms leading to the sleep complaint, suggesting various mechanisms that could explain the consistent delayed sleep phase that is common to these individuals. To this end, objective measures obtained in the subjects’ home environment may be very useful in characterizing a more precise schedule of sleep and timing of circadian phase that may explain the sleep complaint and also help determine the proper manner and timing of treatment.
Acknowledgments
The authors thank the subjects for their participation in the study and the staff of the research laboratory and the sleep disorders clinic for their critical assistance with this work. We wish to also thank Lawrence I. Orbeta for his technical assistance. This work was supported by the Brookdale Foundation, Army Research Office grant DAAG55, and NIH grants HL67604, AG00810, and HL069988 and in part by NIH grant M01 RR-00048 from the National Center for Research Resources.
References
- The International Classification of Sleep Disorders. Revised: Diagnostic and coding manual. American Academy of Sleep Medicine; Rochester, Minnesota: 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 2000. (DSM-IV) [Google Scholar]
- Ando K, Kripke DF, Ancoli-Israel S. Delayed and advanced sleep phase symptoms. Isr J Psychiatry Relat Sci. 2001;39:81–88. [PubMed] [Google Scholar]
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von schantz M. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26:413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Eastman CI, Revelle W, Losee Olson SH, Wolfe LF, Zee PC. Circadian phase-shifting effects of nocturnal exercies in older compared to young adults. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2003 doi: 10.1152/ajpregu.00761.2002. [DOI] [PubMed] [Google Scholar]
- Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3:519–528. [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Guico MJ, Reid KJ, Wolfe LF, L’Hermite-Balériaux M, Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. Journal of Biological Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- Ciarleglio CM, Ryckman KK, Servick SV, Hida A, Robbins S, Wells N, Hicks J, Larson SA, Wiedermann JP, Carver K, Hamilton N, Kidd KK, Kidd JR, Smith JR, Friedlaender J, McMahon DG, Williams SM, Summar ML, Johnson CH. Genetic differences in human circadian clock genes among worldwide populations. Journal of Biological Rhythms. 2008;23:330–340. doi: 10.1177/0748730408320284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- Czeisler CA, Richardson GS, Coleman RM, Zimmerman JC, Moore-Ede MC, Dement WC, Weitzman ED. Chronotherapy: Resetting the circadian clocks of patients with delayed sleep phase insomnia. Sleep. 1981;4:1–21. doi: 10.1093/sleep/4.1.1. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T, Kajimura N, Uchiyama M, Katoh M, Sekimoto M, Watanabe T, Ozeki Y, Ikeda M, Jodoi T, Sugishita M, Iwase T, Kamei Y, Kim K, Shibui K, Kudo Y, Yamada N, Toyoshima R, Okawa M, Takahashi K, Yamauchi T. Alleic variants of human melatonin 1a receptor: function and prevalence in subjects with circadian rhythm sleep disorders. Biochem Biophys Res Commun. 1999;262:832–837. doi: 10.1006/bbrc.1999.1308. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Uchiyama M, Kajimura N, Kamei Y, Shibui K, Kim K, Kudo Y, Iwase T, Sugishita M, Jodoi T, Ikeda M, Ozeki Y, Watanabe T, Sekimoto M, Katoh M, Yamada N, Toyoshima R, Okawa M, Takahashi K, Yamauchi T. Genetic polymorphisms of human melatonin 1b receptor gene in circadian rhythm sleep disorders and controls. Neuroscience Letters. 2000;280:29–32. doi: 10.1016/s0304-3940(99)00981-7. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Uchiyama M, Kajimura N, Mishima K, Kamei Y, Katoh M, Watanabe T, Sekimoto M, Shibui K, Kim K, Kudo Y, Ozeki Y, Sugishita M, Toyoshima R, Inoue Y, Yamada N, Nagase T, Ozaki N, Ohara O, Ishida N, Okawa M, Takahashi K, Yamauchi T. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Journal. 2001;2:342–346. doi: 10.1093/embo-reports/kve070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins GA, Meyers DA, Bleecker ER, Pack AI. Identification of coding polymorphisms in human circadian rhythm genes PER1, PER2, PER3, CLOCK, ARNTL, CRY1, CRY2 and TIMELESS in a multi-ethnic screening panel. DNA Seq. 2008;19:44–49. doi: 10.1080/10425170701322197. [DOI] [PubMed] [Google Scholar]
- Hohjoh H, Takahashi Y, Hatta Y, Tanaka H, Akaza T, Tokunaga K, Honda Y, Juji T. Possible association of human leucocyte antigen DR1 with delayed sleep phase syndrome. Psychiatry Clin Neurosci. 1999;53:527–529. doi: 10.1046/j.1440-1819.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Hohjoh H, Takasu M, Shishikura K, Takahashi Y, Honda Y, Tokunaga K. Significant association of the arylalkylamine N-acetyltransferase (AA-NAT) gene with delayed sleep phase syndrome. Neurogenetics. 2003;4:151–153. doi: 10.1007/s10048-002-0141-9. [DOI] [PubMed] [Google Scholar]
- Laakso M-L, Porkka-Heiskanen T, Alila A, Stenberg D, Johansson G. Correlation between salivary and serum melatonin: Dependence on serum melatonin levels. Journal of Pineal Research. 1990;9:39–50. doi: 10.1111/j.1600-079x.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. Journal of Biological Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Melatonin rhythm in human plasma and saliva. Journal of Pineal Research. 1987;4:177–183. doi: 10.1111/j.1600-079x.1987.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. AmJ Med Genet B Neuropsychiatr Genet. 2005;133:101–104. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- Mundey K, Benloucif S, Harsanyi K, Dubocovich ML, Zee PC. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28:1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- Nagtegaal E, Peeters T, Swart W, Smits M, Kerkhof G, van der Meer G. Correlation between concentrations of melatonin in saliva and serum in patients with delayed sleep phase syndrome. Therapeutic Drug Monitoring. 1998;20:181–183. doi: 10.1097/00007691-199804000-00008. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Uchiyama M, Shirakawa S, Okawa M. Prolonged interval from body temperature nadir to sleep offset in patients with delayed sleep phase syndrome. Sleep. 1996;19:36–40. [PubMed] [Google Scholar]
- Pelayo RP, Thorpy MJ, Glovinsky P. Prevalence of delayed sleep phase syndrome among adolescents. Sleep Research. 1988;17:391–391. [Google Scholar]
- Regestein QR, Monk TH. Delayed sleep phase syndrome: A review of its clinical aspects. American Journal of Psychiatry. 1995;152:602–608. doi: 10.1176/ajp.152.4.602. [DOI] [PubMed] [Google Scholar]
- Rodenbeck A, Huether G, Ruther E, Hajak G. Altered circadian melatonin secretion patterns in relation to sleep in patients with chronic sleep-wake rhythm disorders. Journal of Pineal Research. 1998;25:201–210. doi: 10.1111/j.1600-079x.1998.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. Journal of Sleep Research. 1993;2:51–55. doi: 10.1111/j.1365-2869.1993.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Shibui K, Uchiyama M, Okawa M. Melatonin rhythms in delayed sleep phase syndrome. Journal of Biological Rhythms. 1999;14:72–76. doi: 10.1177/074873049901400110. [DOI] [PubMed] [Google Scholar]
- Takano A, Uchiyama M, Kajimura N, Mishima K, Inoue Y, Kamei Y, Kitajima T, Shibui K, Katoh M, Watanabe T, Hashimotodani Y, Nakajima T, Ozeki Y, Hori T, Yamada N, Toyoshima R, Ozaki N, Okawa M, Nagai K, Takahashi K, Isojima Y, Yamauchi T, Ebisawa T. A missense variation in human casein kinase I epsilon gene that induces functional alteration and shows an inverse association with circadian rhythm sleep disorders. Neuropsychopharmacology. 2004;29:1901–1909. doi: 10.1038/sj.npp.1300503. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Kim K, Kudo Y, Hayakawa T, Kamei Y, Urata J. Poor recovery sleep after sleep deprivation in delayed sleep phase syndrome. Psychiatry and Clinical Neurosciences. 1999;53:195–197. doi: 10.1046/j.1440-1819.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Okawa M, Shibui K, Kim K, Tagaya H, Kudo Y, Kamei Y, Hayakawa T, Urata J, Takahashi K. Altered phase relation between sleep timing and core body temperature rhythm in delayed sleep phase syndrome and non-24-hour sleep-wake syndrome in humans. Neuroscience Letters. 2000;294:101–104. doi: 10.1016/s0304-3940(00)01551-2. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Holmback U, Knutson K, Leproult R, Miller A, Nedeltcheva A, Pannain S, Penev P, Tasali E, Spiegel K. Impact of sleep and sleep loss on neuroendocrine and metabolic function. Horm Res. 2007;67(Suppl 1):2–9. doi: 10.1159/000097543. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. Journal of Biological Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kajimura N, Kato M, Sekimoto M, Nakajima T, Hori T, Takahashi K. Sleep and circadian rhythm disturbances in patients with delayed sleep phase syndrome. Sleep. 2003;26:657–661. doi: 10.1093/sleep/26.6.657. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement WC, Richardson GS, Pollak CP. Delayed sleep phase syndrome: A chronobiological disorder associated with sleep onset insomnia. Arch Gen Psychiatry. 1981;38:737–746. doi: 10.1001/archpsyc.1981.01780320017001. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Stepanski EJ, Kirkby J. Circadian phase in delayed sleep phase syndrome: predictors and temporal stability across multiple assessments. Sleep. 2006;29:1075–1080. doi: 10.1093/sleep/29.8.1075. [DOI] [PubMed] [Google Scholar]