Abstract
Background. In late 2011 and early 2012, 13 cases of human influenza resulted from infection with a novel triple reassortant swine-origin influenza virus, influenza A (H3N2) variant. This variant was notable for its inclusion of the matrix gene from the 2009 influenza A(H1N1) pandemic virus. While most of these confirmed cases were among children, the transmission potential and likely age-dependent susceptibility to the virus was unknown. Preliminary serologic studies indicated that very young children have less protection than older children and adults.
Methods. We construct a mathematical transmission model of influenza transmission that allows for external zoonotic exposure to infection and show how exposure and susceptibility-related factors contribute to the observed case distribution.
Results and Conclusions. Age-dependent susceptibility to infection strongly influences epidemic dynamics. The result is that the risk of an outbreak in a highly susceptible age group may be substantially higher than in an older age group with less susceptibility, but exposure-related factors must also be accounted for when interpreting case data.
Keywords: novel influenza, emerging infection, modeling, zoonosis
During the latter half of 2011 and early 2012, 13 cases of influenza were attributed to a novel triple reassortant swine-origin virus, influenza A (H3N2) variant (henceforth referred to as H3N2v) in the United States [1–3]. Most of these cases occurred in association with agricultural fairs, where swine (and other livestock) contact is common. This reassortant strain is notable for its acquisition of the matrix (M) gene from the influenza A(H1N1) 2009 pandemic virus (A(H1N1)pdm09). Studies are underway to determine whether the acquisition of the M gene from the A (H1N1)pdm09 virus enhanced the ability of H3N2v virus to spread in ferrets and infect humans [4, 5] . The number of H3N2v cases in the United States in 2011 and early 2012 (not counting the summer/fall 2012 cases in the United States) was uncertain because laboratory confirmation of influenza-like illness (ILI) was infrequently performed. After accounting for underreporting at each stage of surveillance, initial estimates of the H3N2v-attributable cases indicate that between 200 and 250 infections occurred for each reported case. Additionally, the ratio of H3N2v-attributable cases among those age >20 years to those age <20 years was approximately 1:6.5 [6].
Serological data shed some light on susceptibility, which is an important determinant of the incidence of infection by age. The proportion of an age group showing antibody titers above some threshold value (ie, the proportion “seropositive”) is expected to be correlated with the relative protection of that age group from infection, though the relationship is not straightforward. Serological studies of H3N2v have shown large differences in seropositivity with age. A study performed by the Centers for Disease Control and Prevention (CDC), using serum collected for a 2010–2011 trivalent influenza vaccine study, and the 2007–2008 National Health and Nutrition Examination Survey (2007–2008 samples used for 4–17 year old children), showed that no children age <4 years and only 5% of children aged 4–9 years sampled had levels of cross-reactive antibody to the H3N2v virus above the threshold of hemagglutination inhibition (HI) titer ≥40, whereas 20%–35% of children aged 10–17 years and adults had HI titers above the same threshold [7]. A serological study conducted in Canada also found a much lower proportion of seropositive children than adults. However, a study conducted in Norway found low seropositivity in the youngest children and adults aged 45–54 years (≤15% with HI ≥ 40) but higher seropositivity in other age groups (>40% with HI ≥ 40) [8, 9]. Interestingly, these levels of adult seropositivity were similar to what was seen against the (H1N1)pdm09 virus among individuals aged >60 years, an age group that was less likely to have influenza during the pandemic [7, 10, 11]. Therefore, we would expect the observed seropositivity to H3N2v seen in adults to reflect a decreased susceptibility to H3N2v in these older age groups, although the relationship between serological titer and the degree of protection against a specific virus subtype is not well understood and may be different between H3N2 and H1N1 subtypes [7, 12].
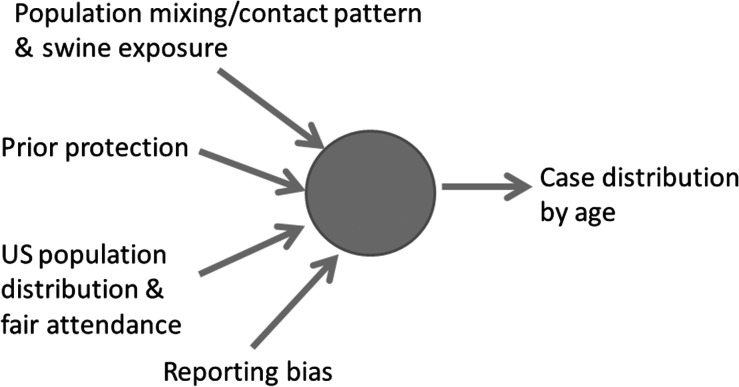
In addition to age-related variation in susceptibility, a number of other factors determine the observed age distribution of H3N2v cases found via surveillance. These include the relative exposure to infection (whether from swine or humans), the age distribution of the local population, and age-related variation in case ascertainment (Figure 1) .
Figure 1.
Illustration of the factors that influence the distribution of influenza cases by age .
Mathematical models can help to quantify the causal determinants of epidemics, both in the overall population and by allowing examination of the specific age-group contributions. Models require each factor with a role in transmission to be explicitly defined. For example, in representing exposure to infection, we need to distinguish between the proportion of transmission that is human-to-human (expressed by the reproduction number R, the number of secondary infections generated by a single infection) and the proportion that is due to exposure to the zoonotic reservoir (in this case, swine).
Here, we construct a simple susceptible-exposed-infected-recovered (SEIR) model of influenza transmission, with an additional external zoonotic source of infection, to illustrate how each factor listed above contributes to the observed case distribution and how age-stratified case data for emerging infections should be interpreted when transmission is part zoonotic and part human-to-human. Our analysis is presented in the context of the H3N2v cases observed in agricultural fair settings 2011 and early 2012 [6].
METHODS
We constructed a mathematical transmission model of an influenza outbreak, in the setting of a state agricultural fair, that includes 2 routes of transmission: swine-to-human and human-to-human (Figure 2). The human population consisted of 4 age groups (age 0–9, 10–19, 20–59, 60+ years), each with a size proportional to the current US population age distribution [13]. We assumed this population distribution was the same for fair-goers. The swine-to-human route of transmission was modeled as a constant, but age-dependent, force of infection on the human population, calibrated to generate 100 new human infections per week across all ages (approximately the incidence of infection that would result in the 13 observed cases). We allowed adults and children to be exposed to swine to different extents, with the ratio of adult-to-child exposure to infected swine to vary between 1:1 and 1:10 in the fair setting.
Figure 2.
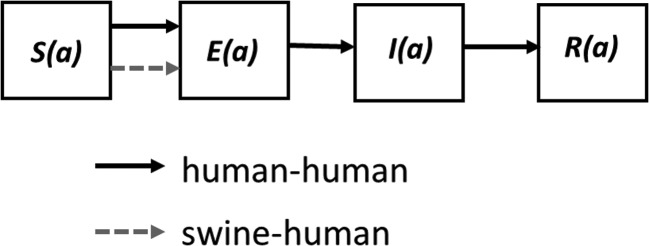
Model compartmental flow diagram illustrating the 4 infection states into which each age group, denoted a, is partitioned. Abbreviations: S, susceptible; E, exposed (incubating); I, infected and infectious; R, recovered.
Our model of human-to-human transmission has an SEIR structure [14]), with contact rates between individuals in each age group determined by the “all-contacts” data collected by the POLYMOD diary study of contact patterns in Great Britain (GB) [15]. In the absence of comparable contact data for US populations, we use GB data as a plausible proxy. We allow for age groups to have a level of prior protection, represented as a reduced susceptibility to infection. For simplicity, we assume children (aged 0–10 and 10–20 years) have susceptibility p = 1 and all other age groups have susceptibility p < 1. Details of the model structure are given in the Supplementary Materials.
Two related analyses were conducted, each for the range of possible values of the human-to-human reproduction number, R (namely, 0 < R < 2):
For 4 values of the adult-to-child exposure ratio in the fair setting, we fitted (using maximum likelihood estimation) the observed age distribution of cases by adjusting the relative susceptibility parameter p. This analysis shows how the combination of heterogeneous exposure by age, immunity, and contact patterns can give rise to the observed age distribution of cases.
For a given value of the susceptibility parameter p (0.2 here, so that the adult-to-child susceptibility ratio is 1:5), we calculated the final case distribution and the age-specific reproduction number (Ra). We define Ra as the relevant diagonal element of the next generation matrix [16–18]. This analysis gives insight into the age groups at most risk of self-sustaining outbreaks should the human-to-human transmissibility of H3N2v increase.
We represented the observed age distribution of cases as a single ratio: the ratio of the number of cases observed in children aged 0–19 years to the number observed in all older age groups. We used the value of 1:6.5 for this ratio, which is the (reporting rate-adjusted) value found among the first 13 cases.
RESULTS
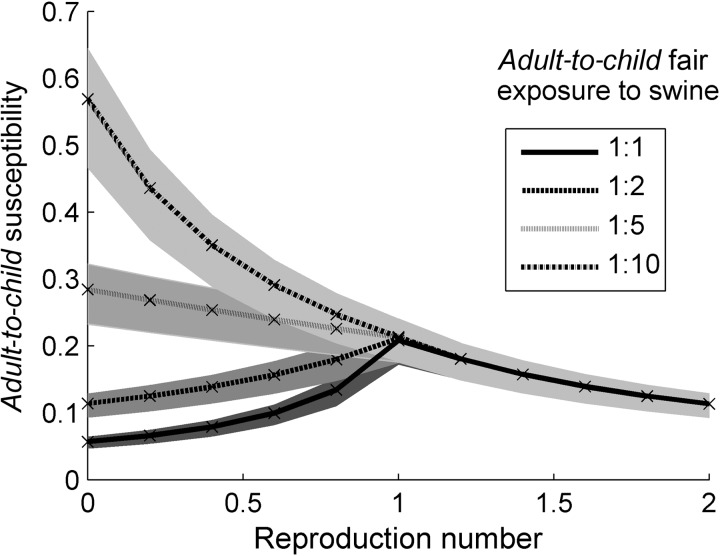
The results presented in Figure 3 were generated by assuming a particular value of the adult-to-child swine exposure ratio and the human-to-human transmissibility (as indicated by R) and calculating the value of p (the relative susceptibility of adults compared with children) necessary to reproduce the observed adult-to-child case ratio, that is, 1:6.5 (Figure 1).
Figure 3.
Variation in the calculated susceptibility ratio between adults and children. We set the model-generated adult-to-child case ratio to the observed case ratio of 1:6.5; the reproduction number is increased from zero for each of the 4 swine exposure scenarios (see legend inset). At R = 0, the case distribution is generated purely by swine exposure and the 4 curves corresponding to the different degrees of swine exposure are clearly demarcated. As R increases, swine exposure becomes less relevant until it is entirely irrelevant as R > 1, when cases are overwhelmingly generated through human-to-human transmission. Shaded regions indicate 95% confidence intervals.
Figure 3 shows how variation in the relative rate of exposure to the zoonotic source (indicated by the different plotted lines in the figure) requires different relative degrees of protection between the age groups to achieve the same age distribution of cases. The far left-hand side of this figure corresponds to entirely zoonotic transmission (ie, R = 0). Here, because swine exposure is the only transmission route, for each different rate of adult-to-child swine exposure, our model would calculate very different levels of adult-to-child susceptibility. For example, in order to explain the age distribution of H3N2v cases in 2011 (ie, adult-to-child = 1:6.5), if we assume that adults and children are equally likely to be exposed to swine in a fair setting and there is no human-to-human transmission, then adults would need to be 1/20th as susceptible as children to zoonotic transmission. The fact that this ratio is larger than 1:6.5 (the observed case ratio) reflects the underlying age distribution of the population (there are approximately 3 times the number of people age >20 years than those age <20 years in the US population).
As the degree of human-to-human transmission is increased (ie, increasing R), relative swine exposure becomes a less important factor in determining the observed age distribution of cases. The curves in Figure 3 that correspond to different assumed ratios of adult-to-child swine exposure merge into 1 curve at R = 1; at that point, transmission becomes self-sustaining in the human population, meaning a negligible fraction of cases are due to direct exposure to swine. Here and for values of R > 1, the age distribution of cases is solely determined by age-specific contact patterns and susceptibility.
Figure 3 also illustrates how sensitive the relative susceptibility of adults is to the dominant route of transmission for a given age distribution of cases. When R is less than 1, we need independent data, such as fair-attendance records, on the age distribution of human exposure to swine to determine which curve is the correct one. This simple figure therefore shows the challenges involved in quantitative estimation of the degree of prior protection in adults.
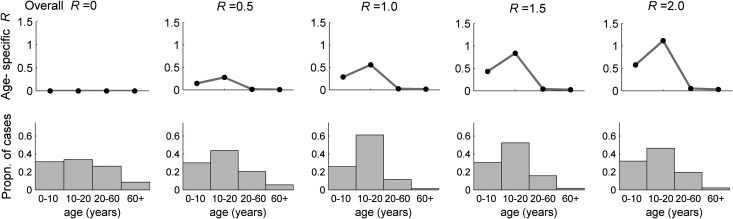
The converse of this analysis is that a given value of the relative susceptibility of adults compared with children can generate a variety of age distributions of H3N2v cases, depending on the degree of human-to-human transmission assumed. This is illustrated in Figure 4 (for which we assumed p = 0.2), where the observed age distribution of cases changes markedly with increasing R.
Figure 4.
The age-specific reproduction number Ra and the case distribution by age. In each subfigure we set the overall population R to the value shown, the ratio of susceptibility between adults (aged >20 years) and children (aged <20 years) to 1:5 and equal exposure of all age groups to swine. As the overall population R is raised from zero, in steps of 0.5 (from left to right), the figure shows the changing age distribution of cases and the age-specific reproduction number.
Figure 4 also shows how the age-specific reproduction number varies as the overall reproduction number increases. The much higher susceptibility and higher contact rates in those age <20 years leads to a very large difference in age-specific R. If the overall human-to-human transmissibility of H3N2v were to increase to close to 1, this analysis suggests we might start seeing self-sustaining outbreaks of transmission in school-age children first (the age group with the highest age-specific R value).
DISCUSSION
Here, we show how the age distribution of disease cases (ie, the proportion of total cases distributed by age) is dependent upon several factors, most notably age-specific variation in exposure to infection and susceptibility to infection (the latter determined by prior immunity to related viral subtypes). It is particularly difficult to estimate the relative susceptibility of different age groups in the emerging H3N2v context because there was both animal-to-human and human-to-human transmission. At the extremes of entirely animal-to-human (R = 0) or human-to-human (R > 1) transmission, the dominant exposures are clear. However, between those extremes, our analysis shows that care must be taken in interpreting age-specific incidence data.
Our analysis also illustrates the importance of jointly using models and survey data when an infection is emerging, most notably to determine the human-to-human reproduction number (eg, analysis of passive surveillance data, examining the ratio of swine-linked vs nonswine-linked cases [19, 20, 21] and the degree of human exposure to animals and how this varies with age (eg, surveys of populations visiting swine barns at state fairs [21]).
Because of the multiple factors determining observed case age distributions, the temptation to attribute these distributions to prior immunity alone should be avoided. The difficulty in resolving the separate effects of prior protection and exposure compounds the already complex problem of interpreting serological data directly as an indicator of protection. In the case of H3N2v, 3 studies have shown similar qualitative trends in seropositivity by age, though details of the numerical estimates vary considerably [7–9]. The experience of the 2009 H1N1 pandemic showed that very low disease attack rates in those age >65 years correlated with higher measured seropositivity in those age groups; however, the relationship was far from linear [23, 24]. The analysis presented here may contribute to a better understanding of the quantitative relationship between seroprevalence and epidemiological protection against infection (ie, as quantified by relative susceptibility by age).
This study is limited by our examination of the relative epidemiological effects in only 2 age groups, adults and children, which was necessitated by the small numbers of cases observed thus far. We also model an influenza outbreak in a deterministic framework, in which there is no randomness in the infection process. However, a better idea of epidemic risk, especially when R is very close to 1, can be gained from stochastic modeling, in which the random infection process is explicitly accounted for.
This work was initiated while the first zoonotic cases of H3N2v infection were being identified in the United States and demonstrates the usefulness of performing rapid mathematical modeling studies, alongside outbreak investigations and epidemiological studies, to help assess the risk associated with novel influenza strains. While early case clusters were still occurring, we were able to estimate the transmission potential of the new virus [21] and the age groups most at risk of infection and to determine that R was likely to be <1.
Prior protection remains a difficult quantity to measure. However, with good data on age-specific contact rates and zoonotic exposure, we show that, in principle, it can be estimated from data on the age distribution of cases during the emergence of an infection. If this is done in concert with serological studies, we can further improve our understanding of the relation between antibody levels and protection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Karen Wong, Dr Carrie Reed, others in the Influenza Division of the Centers for Disease Control and Prevention, and several members of the NIH-MIDAS network, for comments.
Financial support. This work was supported by funding from the UK Medical Research Council, the National Institutes of Health, the Bill and Melinda Gates Foundation, the European Union, and the US federal government.
Supplement sponsorship. This article was published as part of a supplement entitled “The Emergence of Influenza A (H3N2)v Virus: What We Learned From the First Wave, July 2011–April 2012,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Update: Influenza A (H3N2)v transmission and guidelines—five states, 2011. MMWR Morb Morta Wkly Rep. 2012;60:1741–4. [PubMed] [Google Scholar]
- 2.Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis. 2012;18:834–7. doi: 10.3201/eid1805.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q, Ma J, Liu H, et al. Emergence of novel reassortant H3N2 swine influenza viruses with the 2009 pandemic H1N1 genes in the United States. Arch Virol. 2012;157:555–62. doi: 10.1007/s00705-011-1203-9. [DOI] [PubMed] [Google Scholar]
- 4.Lakdawala SS, Lamirande EW, Suguitan AL, Jr., et al. Eurasian-origin gene segments contribute to the transmissibility, aerosol release, and morphology of the 2009 pandemic H1N1 influenza virus. PLoS Pathog. 2011;7:e1002443. doi: 10.1371/journal.ppat.1002443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearce MB, Jayaraman A, Pappas C, et al. Pathogenesis and transmission of swine origin A(H3N2)v influenza viruses in ferrets. Proc Natl Acad Sci U S A. 2012;109:3944–9. doi: 10.1073/pnas.1119945109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biggerstaff M, Epperson S, Jhung M, et al. Estimates of the prevalence of human infections with influenza A (H3N2) variant virus, United States, August 2011–April 2012. Clin Infect Dis. 2013;57(Suppl 1):S12–5. doi: 10.1093/cid/cit273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antibodies cross-reactive to influenza A (H3N2) variant virus and impact of 2010–11 seasonal influenza vaccine on cross-reactive antibodies - United States. MMWR Morb Mortal Wkly Rep. 2012;61:237–41. [PubMed] [Google Scholar]
- 8.Waalen K, Kilander A, Dudman S, Ramos-Ocao R, Hungnes O. Age-dependent prevalence of antibodies cross-reactive to the influenza A(H3N2) variant virus in sera collected in Norway in 2011. Eurosurveillance. 2012;17:5–9. [PubMed] [Google Scholar]
- 9.Skowronski DM, De Serres G, Janjua NZ, et al. Cross-reactive antibody to swine influenza A(H3N2) subtype virus in children and adults before and after immunisation with 2010/11 trivalent inactivated influenza vaccine in Canada, August to November 2010. Eurosurveillance. 2012;17:2–9. doi: 10.2807/ese.17.04.20066-en. [DOI] [PubMed] [Google Scholar]
- 10.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. New Engl J Med. 2009;361:1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 11.Cauchemez S, Donnelly CA, Reed C, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361:2619–27. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox JP, Cooney MK, Hall CE, Foy HM. Influenzavirus infections in Seattle families, 1975–1979. II. Pattern of infection in invaded households and relation of age and prior antibody to occurrence of infection and related illness. Am J Epidemiol. 1982;116:228–42. doi: 10.1093/oxfordjournals.aje.a113408. [DOI] [PubMed] [Google Scholar]
- 13.US Census. 2010 [Google Scholar]
- 14.Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton: Princeton University Press; 2008. [Google Scholar]
- 15.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5:e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glass K, Mercer GN, Nishiura H, McBryde ES, Becker NG. Estimating reproduction numbers for adults and children from case data. J R Soc Interface. 2011;8:1248–59. doi: 10.1098/rsif.2010.0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercer GN, Glass K, Becker NG. Effective reproduction numbers are commonly overestimated early in a disease outbreak. Stat Med. 2011;30:984–94. doi: 10.1002/sim.4174. [DOI] [PubMed] [Google Scholar]
- 18.Nishiura H, Hoye B, Klaassen M, Bauer S, Heesterbeek H. How to find natural reservoir hosts from endemic prevalence in a multi-host population: a case study of influenza in waterfowl. Epidemics. 2009;1:118–28. doi: 10.1016/j.epidem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson NM, Fraser C, Donnelly CA, Ghani AC, Anderson RM. Public health. Public health risk from the avian H5N1 influenza epidemic. Science. 2004;304:968–9. doi: 10.1126/science.1096898. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Smith JO, George D, Pepin KM, et al. Epidemic dynamics at the human-animal interface. Science. 2009;326:1362–7. doi: 10.1126/science.1177345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cauchemez S, Epperson S, Biggerstaff M, Swerdlow D, Finelli L, Ferguson NM. Using routine surveillance data to estimate the epidemic potential of emerging zoonoses: application to the emergence of US swine origin influenza A H3N2v virus. PloS Med. 2013;10:e1001399. doi: 10.1371/journal.pmed.1001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong K, Greenbaum A, Moll M, Lando J, Moore E, Ganatra R. Outbreak of influenza A (H3N2) variant virus infection among attendees of an agricultural fair, Pennsylvania, USA, 2011. Emerg Infect Dis. 2012;18:1937–44. doi: 10.3201/eid1812.121097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 24.Reed C, Katz JM. Serological surveys for 2009 pandemic influenza A H1N1. Lancet. 2010;375:1062–3. doi: 10.1016/S0140-6736(09)62194-2. [DOI] [PubMed] [Google Scholar]