Abstract
Cholecalciferol (D3) supplementation results in variable increases in serum 25(OH)D3 levels, however, the influence of genetic polymorphisms on these variable responses is unclear. We measured serum 25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3 and VDBP levels in 50 colorectal cancer (CRC) patients before and during 2,000 IU daily oral D3 supplementation for six months and in 263 archived CRC serum samples. Serum PTH levels and PBMC 24-OHase activity were also measured during D3 supplementation. TagSNPs in CYP2R1, CYP27A1, CYP27B1, CYP24A1, VDR, and GC genes were genotyped in all patients, and the association between these SNPs and serum vitamin D3 metabolites levels before and after D3 supplementation was analyzed. The mean baseline serum 25(OH)D3 level was less than 32 ng/mL in 65 % of the 313 CRC patients. In the 50 patients receiving D3 supplementation, serum levels of 25(OH)D3 increased (p = 0.008), PTH decreased (p = 0.036) and 24,25(OH)2D3, 1,25(OH)2D3, VDBP levels and PBMC 24-OHase activity were unchanged. GC SNP rs222016 was associated with high 25(OH)D3 and 1,25(OH)2D3 levels at baseline while rs4588 and rs2282679 were associated with lower 25(OH)D3 and 1,25(OH)2D3 levels both before and after D3 supplementation. CYP2R1 rs12794714 and rs10500804 SNPs were significantly associated with low 25(OH)D3 levels after supplementation but not with baseline 25(OH)D3. Our results show that D3 supplementation increased 25(OH)D3 levels in all patients. GC rs4588 and rs2283679 SNPs were associated with increased risk of vitamin D3 insufficiency and suboptimal increase in 25(OH)D3 levels after D3 supplementation. Individuals with these genotypes may require higher D3 supplementation doses to achieve vitamin D3 sufficiency.
Keywords: Single Nucleotide Polymorphism, Baseline Serum, Roswell Park Cancer Institute, Cholecalciferol Supplementation, CYP24A1 Rs111675277
Introduction
Serum 25(OH)D3 level is the accepted indicator of vitamin D3 sufficiency in humans. Since dietary sources of vitamin D3 are limited, the major cause of human vitamin D3 insufficiency (defined as serum 25(OH)D3 < 32 ng/mL) is inadequate exposure to the sun [1, 2]. Several factors including living at higher latitudes, cultural practices limiting skin exposure to sunlight, advanced age, obesity, and dark skin pigmentation are associated with low serum 25(OH)D3 levels [3–5]. We have reported that recent cancer chemotherapy may be a predisposing factor for vitamin D3 deficiency in CRC patients [6].
The clinical significance of vitamin D insufficiency in patients with established colorectal cancer, especially those with metastatic disease, is unclear. However, the association between vitamin D3 insufficiency and an increased risk of developing colorectal cancer is supported by several case–control studies [7, 8]. In a Norwegian study, higher colorectal cancer mortality was observed when the diagnosis of colon cancer was made during winter or spring (lower 25(OH)D3) compared to summer and autumn (higher 25(OH)D3) [9]. Furthermore, the Nurse’s Health Study (NHS) and Health Professionals follow-up study showed that the lowest risk of death (adjusted for other variables) from CRC disease was seen in patients in the highest quartile of serum 25(OH)D3 levels [10].
Polymorphisms in several vitamin D genes (CYP2R1, CYP27A1, CYP27B1, CYP24A1, VDBP and VDR) have been associated several human diseases, including CRC [11]. The proteins encoded by these genes include CYP2R1 and CYP27A1 which convert vitamin D to 25(OH)D3. 25(OH)D3 is then converted by CYP27B1 to 1,25(OH)2D3, the biologically most active form of vitamin D3. CYP24A1 converts 25(OH)D3 and 1,25(OH)2D3 to 24, 25(OH)2D3 and 1, 24, 25(OH)2D3, respectively, which are less active. Vitamin D binding protein (VDBP), encoded by GC (group-specific component), is the transporter of vitamin D3 metabolites in circulation while VDR, a nuclear hormone receptor, mediates the action of vitamin D3 by transcriptionally controlling the expression of hormone sensitive genes. Studies show significant association between single nucleotide polymorphisms (SNP) in the GC (rs2282679), DHCR7 (rs12785878), and CYP2R1 (rs10741657) genes and serum 25(OH)D3 levels [12, 13]. There are reported associations between 25(OH)D3 levels and SNPs in GC (rs4588 and rs7041), VDR (rs10735810) and CYP27B1 (rs10877012) genes [14, 15]. The AA genotype of VDR rs1544410 has been associated with higher serum 1,25(OH)2D3 levels compared to the GG genotype [16]. Reports on the effect of D3 supplementation on the associations between polymorphisms in the vitamin D3 pathway genes and serum 25(OH)D3, 24,25(OH)2D3 and 1,25(OH)2D3levels are few. A suboptimal response to D3 supplementation was associated with GC rs4588 SNP in healthy adults [17]. Comprehensive studies to identify SNPs in the vitamin D3 metabolizing and signaling pathway genes that are associated with response to D3 supplementation will provide insight into factors influencing vitamin D health and information on the planning of prospective cancer chemoprevention and therapeutic clinical trials.
In this study, we measured serum vitamin D metabolite levels (25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3) together with vitamin D binding protein (VDBP ) levels in 313 CRC patient samples and correlated these measures with SNPs in the major vitamin D3 metabolizing and signaling pathway genes at baseline and with vitamin D sufficiency. We also tested for associations between these SNPs and changes in the serum vitamin D3 metabolites levels in a subset of CRC patients receiving a fixed dose of 2,000 IU of oral cholecalciferol daily for 6 months.
Materials and Methods
Research Blood Sample Collections and Storage
Archived blood samples of 263 CRC patients were obtained from Roswell Park Cancer Institute (RPCI) Data Bank and BioRepository. Blood samples were also collected prospectively from RPCI CRC patients enrolled in a study of fixed oral dose of 2000 IU of cholecalciferol (D3) once daily for 6 months. Eligibility criteria, baseline and follow-up clinical evaluation of the patients enrolled in the D3 supplementation protocol has been published [18]. Protocols for these studies were both approved by the RPCI Institutional Review Board (IRB) prior to starting these studies.
Serum samples for vitamin D3 metabolite and PTH level measurements were collected before treatment and on days 14, 30, 60, 90, and 180 of D3 supplementation and stored at −80 °C until assayed. VDBP was measured in samples collected at baseline and on day 90 only. Baseline blood samples collected in EDTA tubes and stored at −80 °C was used for DNA isolation. Serum samples for PTH assay were stored at −80 °C and were assayed within 12 months of samples collection. PTH levels in archived serum samples were not measured.
Analytical Assays
Serum 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3 Assays
A high-performance liquid chromatographic assay with tandem mass spectrometric detection (LC/MS/MS) was used to simultaneously measure 24,25(OH)2D3 and 25(OH)D3 in 0.25 mL of human serum sample. Stable-isotope labeled 25-Hydroxyvitamin D3-(26,26,27,27,27-d6) was the internal standard [19, 20]. Sample preparation for LC/MS/MS analysis included liquid–liquid extraction using methanol and chloroform (1:3 v/v). The dried organic layer containing 24,25(OH)2D3 and 25(OH)D3 was reconstituted in 60 μL of methanol (MeOH)/water (60/40 %) and 25 μL volumes were injected and separated on reverse phase dC-18 column (Waters Corp) using MeOH/water gradient (0 min 80 % MeOH, 3 min 99 % MeOH, 10 min 99 % MeOH,10.1 min 80 % MeOH, 15 min 80 % MeOH at flow rate of 220 μL/min). TSQ Quantum ULTRA mass spectrometer with positive atmospheric pressure chemical ionization (APCI) source was used to monitor 24,25(OH)2D3, 25(OH)D3 and the internal standard at transitions of m/z 399 > 127, 383 > 365 and 389 > 371 respectively. The assay lower limit of detection were 0.25 ng/mL for both 24,25(OH)2D3 and 25(OH)D3. Serum 25(OH)D3 levels measured by our LC/MS/MS method were 10–17 % higher than the values obtained using DiaSorin 25-hydroxyvitamin D RIA kits. Serum 1,25(OH)2D3 levels were measured using DiaSorin RIA kits as previously described [21].
Plasma PTH and VDBP Assays
Serum PTH levels were measured using biologically intact hPTH 1–84 immunoradiometric Kits from DiaSorin (Stilwell, MN). Quantitative determination of total serum vitamin D binding proteins (VDBP) was performed using K2314 VDBP ELISA kits from ALPCO immunoassays (Salem, NH). Both assays were performed according to the instructions supplied with kits.
24-Hydroxylase Activity Assay
PBMC 24-OHase activity was assayed as previously described [22, 23]. The assay consisted of 2 × 106 PBMC (as the 24-OHase enzyme source), and 0.5 μCi (110,000 dpm) [3H]-25(OH)D3 as the substrate; total assay volume was 0.5 ml of buffer (0.19 M sucrose, 25 mM sodium succinate, 2 mM MgCl2, 1 mM EDTA and 20 mM HEPES), pH 7.4. The reaction was initiated by the addition of substrate followed by 30 min incubation at 37 °C. [3H]-25(OH)D3 oxidative metabolites were extracted by liquid–liquid partition, separated by HPLC and measured by liquid scintillation counting of collected HPLC fractions. Radioactivity in the fractions containing all 24-oxidative metabolites of 25(OH)D3 were pooled and 24-OHase activity, normalized for variations in extraction efficiency, was calculated using the radioactivity ratio of pooled 24-oxidative metabolites/total radioactivity recovered and expressed as femtomoles per hour per mg of protein.
Genotyping Assays
Genomic DNA was isolated from whole blood samples using Flexi Gene DNA kits (Qiagen Inc, Valencio, CA). The quality and quantity of DNA isolated was determined using both Nanodrop (Thermo Scientific, Waltham, MA) and PicoGreen (Molecular Probes Inc., Eugene, OR) methods.
TagSNPs in CYP2R1, CYP27A1, CYP27B1, CYP24A1, VDR and GC genes were selected from databases (NCBI; HapMap) and from CYP24A1 re-sequenced data generated in our laboratory from Caucasian DNA samples from the Coreil Cell Repository (unpublished). Briefly, SNP genotype datasets for Caucasians were loaded into the Haploview program (version 4.0) (Broad Institute, MIT and Harvard, Cambridge, MA, USA) and polymorphisms with frequencies greater than 5 % were selected for haplotype analysis at an r 2 threshold of 0.8. Haplotypes close to or greater than 2 % were organized into single blocks from which haplotype tagSNPs were derived. The tagger software was also used to generate tagSNPs. Other websites used for tagSNPs selection included NIEHS (http://snpinfo.niehs.nih.gov/snptag.htm) and GVS (http://gvs.gs.washington.edu/GVS/). A total of 80 tagSNPs were genotyped using the Sequenom MassArray platform (Sequenon, San Diego, CA) in accordance with the manufacturer’s instructions (http://www.sequenom.com/seq-genotyping). Controls were included to ensure genotyping accuracy in addition to genotyping approximately 10 % of the samples in duplicates.
Data Analysis
Patients with baseline serum 25(OH)D3 levels <20 ng/mL, 20–31.9 ng/mL and ≥32 ng/mL were considered to be vitamin D3 deficient, insufficient and sufficient, respectively. Free index (a measure of non-protein bound) 25(OH)D3, 24,25 (OH)2D3 and 1,25(OH)2D3 were calculated as a molar ratio of each vitamin D3 metabolite/VDBP. Frequencies and relative frequencies were computed for categorical variables and numeric variables were summarized using mean, standard deviation, standard error of mean, median and range as appropriate. Fisher’s exact test was used to study the association between categorical variables and the Wilcoxon rank sum test was used to compare the groups’ numeric variables.
The generalized linear models with corresponding link function and distribution of different response variables were used to investigate the association between study variables and a set of variables for multivariate analysis. P < 0.05 nominal significance level was used in all testing. All statistical analyses were done using SAS (version 9.1).
Association analysis of serum vitamin D3 metabolite concentrations with SNPs were performed using generalized linear models. Logistic regression models were used to measure SNP association analyses with sufficiency status (insufficient/deficient vs. sufficient). For the D3 supplementation population, vitamin D3 metabolite levels were log transformed and genotypes were coded as 0, 1, and 2 for homozygous major, heterozygous and homozygous minor genotypes, respectively. Due to small sample size of D3 supplementation group and rarity of minor genotypes, the minor and heterozygous genotypes were pooled and compared to major genotype (reference group) for all SNPS. Models were adjusted for age, sex, BMI, tumor stage, chemotherapy status and plasma VDBP levels. The β-coefficients from the linear models represent the quantitative change in plasma measures with the addition of the minor allele. The β coefficients from the logistic regression models reflect the increase in odds of deficiency (or sufficiency) with each additional copy of the variant allele.
Results
Patient Characteristics, Baseline Serum Vitamin D3 Metabolite and VDBP Levels
Characteristics, including self-reported racial background, of all 313 CRC patients, stratified into three vitamin status categories (deficient, insufficient, and sufficient, based on baseline serum 25(OH)D3 levels), are shown in Table 1; 69.1 % of the vitamin D3 sufficient patients had blood samples collected in summer/fall compared to 52.2 % and 39.8 % for the insufficient and deficient patients, respectively. Neither BMI nor other patient demographic characteristics (gender, age, race, and tumor stage) showed significant association with vitamin D3 status. Serum 24,25(OH)2D3 levels were significantly associated with baseline serum 25(OH)D3 levels (p < 0.0001), whereas serum 1,25(OH)2D3 and VDBP levels were not.
Table 1.
Demographic and serum vitamin D3 variables in CRC patients stratified by baseline serum vitamin D3 status
| Characteristics/variables | Baseline vitamin D3 status* | p value | |||
|---|---|---|---|---|---|
| Deficient (N = 88) | Insufficient (N = 115) | Sufficient (N = 110) | |||
| Age, years <40 | 4 | 4 | 9 | 0.44 | |
| 40–49 | 13 | 24 | 12 | ||
| 50–59 | 26 | 26 | 32 | ||
| 60–69 | 29 | 37 | 34 | ||
| ≥70 | 15 | 25 | 23 | ||
| Gender | F | 44 (50 %) | 48 (41.7 %) | 55 (50 %) | 0.37 |
| M | 44 (50 %) | 67 (58.3 %) | 55 (50 %) | ||
| Race | White | 76 (86.4 %) | 101 (87.8 %) | 100 (90.9 %) | 0.52 |
| Black | 8 (9.1 %) | 12 (10.4 %) | 6 (5.5 %) | ||
| Others | 4 (4.5 %) | 2 (1.7 %) | 4 (3.6 %) | ||
| Tumor stage | Early (I/II) | 8 (9.1 %) | 18 (15.8 %) | 16 (14.7 %) | 0.35 |
| Advanced (III/IV) | 80 (90.9 %) | 96 (84.2 %) | 93 (85.3 %) | ||
| Sample collection season | Summer–Fall | 35 (39.8 %) | 60 (52.2 %) | 76 (69.1 %) | 0.004 |
| Winter–Spring | 53 (60.2 %) | 55 (47.8 %) | 34 (30.9 %) | ||
| BMI (kg/m2) | <25 | 27 (30.7 %) | 23 (21.3 %) | 34 (33.0 %) | 0.065 |
| 25–30 | 25 (28.4 %) | 43 (39.8 %) | 42 (40.8 %) | ||
| >30 | 36 (40.9 %) | 42 (38.9 %) | 27 (26.2 %) | ||
| Vitamin D binding protein (VDBP) (μg/mL) | 354.1 ± 77.6 | 357.8 ± 75.0 | 387.3 ± 102.4 | 0.079 | |
| D3 metabolites | 25(OH)D3 (ng/mL) | 14.4 ± 4.0 | 25.7 ± 3.4 | 38.8 ± 5.9 | Inference |
| 24,25(OH)2D3(ng/ml) | 0.4 ± 0.3 | 1.0 ± 0.7 | 2.2 ± 2.1 | <0.0001 | |
| 1,25 (OH)2D3 (pg/mL) | 53.0 ± 16.2 | 53.5.4 ± 15.1 | 54.2 ± 15.4 | 0.46 | |
For numerical variables statistics represented as mean ± SD
For categorical variables statistics represented as frequency (% relative frequency)
*Definitions of vitamin D3 status categories are based on serum 25(OH)D3 levels
<20ng/mL = deficient, 20–31.9 ng/mL = insufficient and ≥32ng/mL = sufficient
Effect of D3 Supplementation on Serum Vitamin D3 Metabolites, PTH, VDBP Levels and PBMC 24-OHase Activity
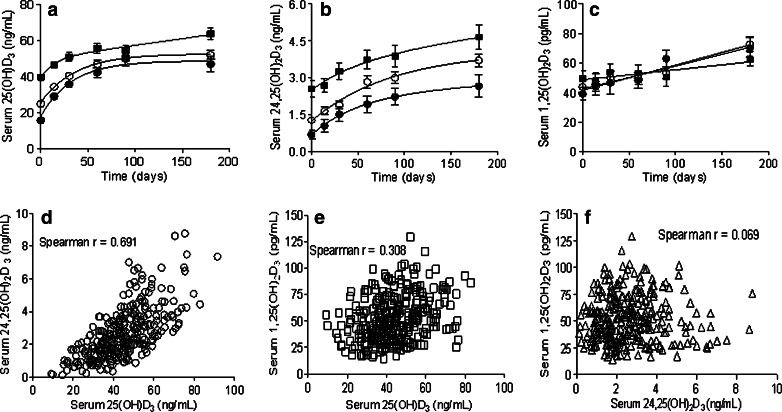
Time course of the changes in serum 25(OH)D3, 24,25(OH)2D3, 1,25(OH)2D3, PTH and VDBP levels and PBMC 24-OHase activity during D3 supplementation are shown in Fig. 1. The increase in 25(OH)2D3 attained equilibrium levels by day 90; the largest percentage increase in 25(OH)D3 levels was in the vitamin D3 deficient category (Fig. 1a). Similar increases in 24,25(OH)2D3 levels were observed (Fig. 1b). The 1,25(OH)2D3 levels also increased but remained within the physiologic range of 20–100 pg/mL (Fig. 1c). Serum 25(OH)D3 and 24,25(OH)2D3 levels at baseline and during D3 supplementation were linearly correlated (Fig. 1d). The correlation between 25(OH)D3 and 1,25(OH)2D3, and between 24,25(OH)2D3 and 1,25(OH)2D3 serum levels are shown in Fig. 1e and f, respectively.
Fig. 1.
Time course of the changes in serum 25(OH)D3 (panel a), 24,25(OH)2D3 (panel b), and 1,25(OH)2D3 (panel c) levels in vitamin D3 deficient (filled circle), insufficient (open circle), and sufficient (filled square) CRC patients receiving 2000IU of oral cholecalciferol daily. Vitamin D3 status stratification based on baseline serum 25(OH)D3 levels: deficient < 20 ng/mL (N = 9), insufficient 20 to 31.9 ng/mL (N = 24) and sufficient ≥ 32ng/mL (N = 17). Panels d, e, and f show plots (and correlation coefficients) of serum 25(OH)D3 versus 24,25(OH)2D3, 25(OH)D3 versus 1,25(OH)D3, and 24,25(OH)2D3 versus 1,25(OH)D3 levels at baseline and during D3 supplementation. Caption in panel a, also refers to panels b and c
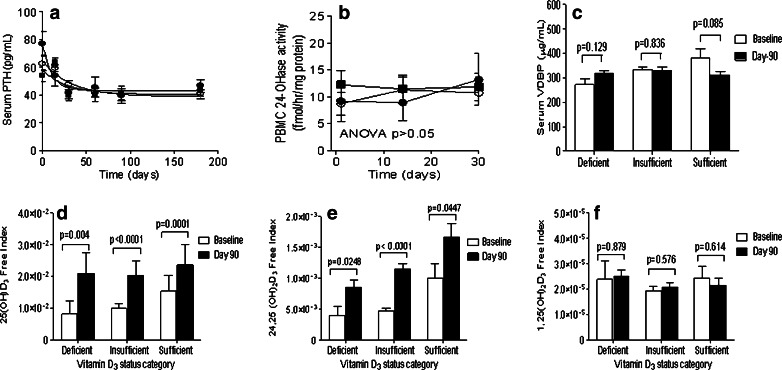
A decrease in serum PTH levels was observed within 30 days of starting D3 supplementation and no further decrease in PTH levels was observed from day 60 onwards (Fig. 2a). No significant changes in PBMC 24-OHase activity and serum VDBP levels were observed during D3 supplementation (Fig. 2b and c). Baseline and day 90 serum VDBP levels for all three vitamin D3 status categories were within the normal range (200–550 μg/mL). D3 supplementation increased the free indices of serum 25(OH)D3 and 24,25(OH)2D3 (Fig. 2d and e); 1,25(OH)2D3 free index did not change (Fig. 2f).
Fig. 2.
Time course of the change in serum PTH levels (panel a) and PBMC 24-hydroxylase activity (panel b) in vitamin D3 deficiency (filled circle, N = 9), insufficient (open circle, N = 24), and sufficient (filled square, N = 17) CRC patients receiving 2,000 IU of oral cholecalciferol daily; panel c shows the baseline and day 90 serum VDBP levels. Net effects of the cholecalciferol supplementation mediated changes in plasma vitamin D3 metabolite and VDBP levels were evaluated by calculating free index of 25(OH)D3 (panel d), 24,25(OH)2D3 (panel e) and 1,25(OH)2D3 (panel f) at baseline and on day 90 of the D3 supplementation. Free index = molar ratio of vitamin D3 metabolite/VDBP
In summary, the significant consequences of D3 supplementation were the decrease in serum PTH levels by day 30, and increase in 25(OH)D3 levels on days 30 and 90; the changes in serum of 24,24(OH)2D3, 1,25(OH)2D3 and VDBP levels and PBMC 24-OHase were not significant at any of the time points analyzed (Table 2).
Table 2.
Changes in vitamin D3 metabolites and other vitamin D3 related variables measured during D3 supplementation in 50 CRC patients categorized by vitamin D3 status
| Time (days) | Changes in serum D3 variables from baseline | Vitamin D3 status | p value | ||
|---|---|---|---|---|---|
| Deficient (N = 9) | Insufficient (N = 24) | Sufficient (N = 17) | |||
| 30 | Δ25(OH)D3 | 20.6 ± 5.9 | 15.5 ± 7.8 | 11.0 ± 9.6 | 0.009 |
| Δ24,25(OH)2D3 | 0.8 ± 0.6 | 0.6 ± 0.6 | 0.7 ± 1.2 | 0.80 | |
| Δ1,25(OH)2D3 | 7.5 ± 20.5 | 6.5 ± 15.1 | 4.4 ± 18.7 | 0.9 | |
| ΔPTH | −35.5 ± 18.2 | −15.4 ± 25.0 | −16.3 ± 19.9 | 0.04 | |
| ΔPBMC CYP24A1 activity | 3.5 ± 10.1 | 2.0 ± 6.0 | −0.3 ± 8.4 | 0.70 | |
| 90 | Δ25(OH)D3 | 34.5 ± 11.1 | 26.3 ± 11.6 | 13.8 ± 11.3 | 0.0007 |
| Δ24,25(OH)2D3 | 1.5 ± 1.0 | 1.7 ± 1.0 | 1.3 ± 1.7 | 0.30 | |
| Δ1,25(OH)2D3 | 23.7 ± 20.9 | 9.4 ± 20.5 | 1.0 ± 23.1 | 0.10 | |
| ΔPTH | −32.0 ± 14.0 | −21.6 ± 29.4 | −15.1 ± 23.4 | 0.20 | |
| ΔVDBP | 44.4 ± 71.2 | −3.4 ± 61.8 | −69.3 ± 166.1 | 0.09 | |
| 180 | Δ25(OH)D3 | 32.5 ± 14.6 | 27.5 ± 12.0 | 21.7 ± 15.4 | 0.20 |
| Δ24,25(OH)2D3 | 2.0 ± 1.3 | 2.3 ± 1.2 | 2.1 ± 2.0 | 0.60 | |
| Δ1,25(OH)2D3 | 31.1 ± 19.6 | 29.3 ± 27.4 | 12.0 ± 27.0 | 0.10 | |
| ΔPTH | −30.3 ± 23.4 | −24.1 ± 24.4 | −13.3 ± 21.7 | 0.20 | |
Statistics represent mean ± SD. Tests for equality for all the variables between baseline 25(OH)D3 groups at baseline, day 30, 90 and 180 done by Chi-square tests
Vitamin D SNPs and Serum Vitamin D3 Metabolite Levels and Risk of Vitamin D3 Insufficiency
Associations between the tag SNPs from the 6 genes (VDR, GC, CYP2R1, CYP27A1, CYP27B1, and CYP24A1) with baseline serum levels of 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3 were evaluated in the 313 CRC patients. At p < 0.05, two GC SNPs (rs4588 and rs2282679, in linkage disequilibrium [LD] with each other, r 2 = 0.98), two CYP24A1 SNPs (rs2762934 and rs2762939) and one VDR SNP (rs11168267) were associated with low baseline levels of at least one serum vitamin D3 metabolite; SNPs associated with high baseline levels of at least one serum vitamin D3 metabolite were GC rs222016, CYP24A1 rs111675277 and VDR rs11574077 (Table 3). After multiple testing, only GC rs4588 remained significantly associated with low baseline serum 1,25(OH)2D3 levels.
Table 3.
Associations between SNPs in the vitamin D3 pathway genes with serum 25(OH)D3, 24,25(OH)2D3 and 1,25(OH)2D3 levels at baseline and after 90-days of D3 supplementation
| D3 metabolites | Gene | SNP ID | Function | Baseline (N = 313) | After D3 supplementation (N = 50)a | ||
|---|---|---|---|---|---|---|---|
| β | p valueb | β | p valueb | ||||
| 25(OH)D3 | GC (VDBP) | rs4588 | Exon/Missense | −2.43 | 0.017 | −0.19 | 0.0009 |
| rs2282679 | Intron | −2.49 | 0.015 | −0.19 | 0.0008 | ||
| rs222016 | Intron | 2.24 | 0.035 | Not significant | |||
| CYP2R1 | rs10500804 | Intron | Not significant | −0.13 | 0.04 | ||
| rs12794714 | Exon/Synonymous | −0.13 | 0.04 | ||||
| rs7129781 | Intron | 0.23 | 0.04 | ||||
| CYP24A1 | rs111675277 | Intron | 5.15 | 0.028 | Not significant | ||
| VDR | rs11168267 | Intron | −3.59 | 0.030 | |||
| 24,25(OH)2D3 | CYP2R1 | rs7129781 | Intron | Not significant | 0.53 | 0.02 | |
| CYP24A1 | rs6022999 | Intron | −0.32 | 0.02 | |||
| VDR | rs11574077 | Intron | 0.71 | 0.023 | Not significant | ||
| 1,25(OH)2D3 | GC (VDBP) | rs4588 | Exon/Missense | −4.86 | 0.0008c | −0.28 | 0.01 |
| rs2282679 | Intron | −4.69 | 0.0012 | −0.27 | 0.02 | ||
| rs222016 | Intron | 3.40 | 0.025 | Not significant | |||
| CYP24A1 | rs2762934 | mRNA-untranslated | −4.01 | 0.010 | |||
| rs2762939 | Intron | −3.72 | 0.007 | ||||
| VDR | rs2525044 | Intron | Not significant | −0.34 | 0.007 | ||
| rs11574077 | Intron | 0.59 | 0.01 | ||||
| rs739837 | mRNA-untranslated | −0.42 | 0.002 | ||||
| rs7975232 | Intron | −0.43 | 0.001 | ||||
Only significant association (p < 0.05) between SNPs and either baseline or day-90 plasma vitamin D3 variables are shown. The only baseline and day-90 overlapping associations were between GC (rs4855 and rs2282679) with both 25(OH)D3 and 1,25(OH)2D3. SNPs in linkage disequilibrium [LD]: rs4855/rs2282679 (r2 = 0.98), rs10500804/rs12794714 (r2 = 1.0) and rs7975232/rs739837 (r 2 = 0.9). CYP2R1 rs7129781 was associated with high 25(OH)D3 and 24,25(OH)2D3 levels after D3 supplementation while VDR rs11574077 was associated with high 1,25(OH)2D3 levels
aMost frequent genotype was used as reference and compared with pooled heterozygous and rare genotypes because of small sample size
bRaw p values (not corrected for multiple testing)
cThe only significant association after multiple testing
The GC rs4588 and rs2282679 SNPs were associated with less increase in serum 25(OH)D3 and 1,25(OH)2D3 levels after 90 days of D3 supplementation in 50 patients (Table 3). Six other SNPs, rs10500804 and rs12794714 (CYP2R1), rs6022999 (CYP24A1) and rs2525044, rs739837, rs7975232 (VDR) were also associated with low levels of at least one vitamin D metabolite after supplementation (Table 3). Only two SNPs were associated with higher serum vitamin D3 metabolite levels after supplementation (Table 3).
The risk of vitamin D insufficiency in association with tagSNPs was evaluated in the 313 CRC patients stratified into 2 groups: vitamin D3 sufficient (reference, baseline serum 25(OH)D3 ≥ 32ng/ml) and vitamin D3 insufficient (baseline serum 25(OH)D3 < 32ng/mL). GC rs4588 and rs2282679 were associated with increased odds of vitamin D3 insufficiency while GC rs222014 and CYP24A1 rs4809958 and rs6013905 in LD (with r 2 = 1) were associated with reduced odds of insufficiency (Table 4).
Table 4.
Genotype association with vitamin D3 status at baseline
| Gene | SNPs ID | OR (95 % CI) | p value |
|---|---|---|---|
| GC | rs222014 | 0.476 (0.27–0.84) | 0.009 |
| CYP24A1 | rs4809958a | 0.570 (0.35–0.92) | 0.021 |
| CYP24A1 | rs6013905a | 0.580 (0.36–0.93) | 0.021 |
| GC | rs4588b,c | 1.65 (1.07–2.58) | 0.026 |
| GC | rs2282679b | 1.65 (1.07–2.58) | 0.026 |
25(OH)D3 reference group is sufficient (coded as zero), 1 = deficient + insufficient; increase in odds of deficient/insufficient for each additional copy of variant allele
aSNPs in linkage disequilibrium [LD] with each other
bSNPs in linkage disequilibrium [LD] with each other
cThis SNP is located in an exon, all others are in introns
Discussion
In this study, 64.9 % of CRC patients had baseline serum 25(OH)D3 levels less than 32 ng/mL. Similar high percentage of vitamin D3 deficient and insufficient individuals have been reported in healthy controls and patients with acute myeloid leukemia, colorectal, prostate, and breast cancer in Western New York [24–26]. Therefore, the high prevalence of vitamin D3 deficiency in these CRC patients simply reflects the vitamin D3 status of the general population in Western New York. As expected, 25(OH)D3 levels were higher in blood samples collected in summer/fall than in samples collected in winter/spring. There was no association between 25(OH)D3 levels and race perhaps because of the small sample size of Black Americans in this study.
The increase in 25(OH)D3 and decrease in PTH levels which we observed in all vitamin D3 status categories, is consistent with prior reports and were expected as physiologic response to D3 supplementation. However, other reports indicate that D3 supplementation has no effect on serum PTH levels [27, 28]. These contradictory observations may reflect differences in D3 supplementation doses utilized, calcium homeostasis related to dietary calcium intake and other calciotropic hormones. Our data suggest that serum 24,25(OH)2D3, 1,25(OH)2D3, and VDBP levels are of limited value as measures of response to D3 supplementation. Changes in serum 25(OH)D3 without associated change in serum 1,25(OH)2D3 levels during D3 supplements has been reported [29]. Moreover, there is no difference in baseline serum 24,25(OH)2D3 levels in these CRC patients (1.4 ± 0.5 ng/mL) and that reported in healthy controls [30, 31]. This finding suggests that 25(OH)D3-24-hydroxylation may not play a critical role in the pathogenesis of systemic vitamin D3 insufficiency in CRC patients. The use of serum 24,25(OH)2D3 levels in evaluating the role of 24-hydroxylase in vitamin D3 status and responses to cholecalciferol supplementation is, however, of limited value because of its further metabolism, by the same enzyme, to 25(OH)D3-23,26-lactone and cholecalcoic acid. Whereas, the decrease in 25(OH)D3 is not a useful measure of 24-hydroxylase because it is also a substrate of non 24-hydroxylase enzymes such as CYP11A1 [32, 33].
We found two major kinetic differences between the changes in total and free serum 25(OH)D3, 24,25(OH)2D3, and 1,25(OH)2D3 levels during D3 supplementation: First, while the increase in total serum 25(OH)D3 and 24,25(OH)2D3 levels was biphasic, the increase in total serum 1,25(OH)2D3 levels was linear. Second, free indices of both 25(OH)D3 and 24,25(OH)2D3 increased whereas 1,25(OH)2D3 free index did not change. These observations suggest that changes in total and free serum 1,25(OH)2D3 levels were insufficient to trigger changes in PBMC 24-OHase and serum calcium levels. Free 1,25(OH)2D3 index is the serum measure highly correlated with hypercalcemia [34]. Although underlying mechanisms of the kinetic differences in 25(OH)D3 and 1,25(OH)2D3 changes during D3 supplementation are unknown, we suggest tight reciprocal regulation of 1,25(OH)2D3 levels by CYP24A1 and CYP27B1 catalyzed metabolism prevents the increase serum 1,25(OH)2D3 levels. Differences in the VDBP capacity and binding affinity along with variations in adipose tissue storage may play a further role in regulating serum 1,25(OH)2D3 levels. The percentages of free forms of 25(OH)D3 and 1,25(OH)2D3 in circulation are 0.04 % and 0.4 %, respectively while VDBP affinity for 25(OH)D3 is 5 × 10−8–9.5 × 10−11 M compared to ∼4 × 10−7 M for 1,25(OH)2D3 [35, 36].
Our results show that genetic variants of GC SNPs rs4588 and rs2282679 were associated with low baseline levels of 25(OH)D3 and 1,25(OH)2D3, with increased odds of vitamin D3 insufficiency and with suboptimal response to D3 supplementation in CRC patients. These findings are consistent with previous reports where these same SNPs have been associated with low serum 25(OH)D3 in both healthy and patients with a variety of diseases including cancer [7–11] and reduced serum 25(OH)D3 changes after vitamin D3 supplementation in healthy volunteers [12]. Although, genome wide studies have associated CYP2R1 SNPs rs10500804 and rs12794714, with vitamin D levels and vitamin D insufficiency [7, 8] the present study only showed an association after supplementation.
GC SNP rs222014 and CYP24A1 SNPs rs4809958 and rs6013905 were associated with reduced risk of vitamin D3 insufficiency, while GC SNPs rs4588 and rs2282679 were associated with increased risk of insufficiency. However, it is unclear why GC rs222016 and CYP24A1 rs111675277, SNPs associated with high 25(OH)D3 levels, were not associated with reduced risk of D3 insufficiency. Possible explanations may include differences in statistical analysis (continuous vs. categorical OR), the character of the colorectal cancer disease itself and/or small sample size. Thus GC rs222014 and CYP24A1 rs4809958 and rs6013905 SNPs could be potential predictive markers for low risk vitamin D3 insufficiency, while GC rs4588 and rs2282679 may be both predictive of insufficiency and reduced response to D3 supplementation in CRC patients.
In summary, we found that baseline serum 25(OH)D3 levels and polymorphisms in the vitamin D3 pathway genes, especially in the GC and CYP24A1 genes affect response to D3 supplementation in CRC patients and are associated with increased risk of vitamin D3 insufficiency. Further studies, in large populations, are needed to confirm and determine if individuals with GC rs4588 or rs2282679 SNPs require higher doses of D3 supplementation to achieve vitamin D3 sufficiency.
Acknowledgments
We thank Dr. G.S. Reddy (Epimer LLC and Department of Chemistry, Brown University, Providence, RI, USA), and Dr. M. Uskokovic (Hoffmann LaRoche Inc. Nutley, NJ, USA) for supplying analytical grade authentic vitamin D3 oxidative metabolites for the LC/MS/MS assays. We are grateful to the following RPCI CSCG Shared Resources, Data Bank and BioRepository, Pharmacokinetics/Pharmacodynamic, Clinical Research Services and the Genomics Shared Resources for facilitating the collection and analytical aspects the study samples. We thank Ms. Jeanne McMahon and Michelle Panasiewicz for their excellent and tireless help in coordinating the clinical aspects of study and sample collection and processing, respectively.
Financial Support
This study was supported by NIH/NCI grant R21 CA-141187 and RPCI Alliance Foundation (JRM), ACS Grant MRSG-04-270-01 and Lisa Legacy Foundation (MGF) and NCI grant P30 CA47904.
Conflict of interest
The authors declare that they have no conflict of interest
References
- 1.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancer and cardiovascular diseases. Am J Clin Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 2.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 3.Goldner WS, Stoner JA, Thompson J, Taylor K, Larson L, Erickson J, McBride C. Prevalence of vitamin D insufficiency and deficiency in morbidly obese patients: a comparison with non-obese controls. Obes Surg. 2008;18:145–150. doi: 10.1007/s11695-007-9315-8. [DOI] [PubMed] [Google Scholar]
- 4.Linnebur SA, Vondracek SF, Vande Griend JP, Ruscin JM, McDermott MT. Prevalence of vitamin D insufficiency in elderly ambulatory outpatients in Denver, Colorado. Am J Geriatr Pharmacother. 2007;5:1–8. doi: 10.1016/j.amjopharm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakih MG, Trump DL, Johnson CS, Tian L, Muindi J, Sunga AY. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Color Dis. 2009;24:219–224. doi: 10.1007/s00384-008-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangrea J, Helzlsouer K, Pietinen P, Taylor P, Hollis B, Virtamo J, Albanes D. Serum levels of vitamin D metabolites and the subsequent risk of colon and rectal cancer in Finnish men. Cancer Causes Control. 1997;8:615–625. doi: 10.1023/A:1018450531136. [DOI] [PubMed] [Google Scholar]
- 8.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci E. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 9.Moan J, Porojnicu A, Lagunova Z, Berg JP, Dahlback A. Colon cancer: prognosis for different latitudes, age groups and seasons in Norway. J Photochem Photobiol. 2007;89:148–155. doi: 10.1016/j.jphotobiol.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, Giovannucci EL, Fuchs CS. Circulating 25-hydroxyvitamin D levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 11.Dong LM, Ulrich CM, Hsu L, Duggan DJ, Benitez DS, White E, Slattery ML, Farin FM, Makar KW, Carlson CS, Caan BJ, Potter JD, Peters U. Vitamin D related genes, CYP24A1 and CYP27B1, and colon cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:2540–2548. doi: 10.1158/1055-9965.EPI-09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TJ, Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M. Common genetic determinants of D insufficient; a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, Jacobs EJ, Ascherio A, Helzlsouer K, Jacobs KB, Li Q, Weinstein SJ, Purdue M, Virtamo J, Horst R, Wheeler W, Chanock S, Hunter DJ, Hayes RB, Kraft P, Albanes D. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–2745. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath JJ, Saha S, Burne TH, Eyles DW. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J Steroid Biochem Mol Biol. 2010;121:471–477. doi: 10.1016/j.jsbmb.2010.03.073. [DOI] [PubMed] [Google Scholar]
- 15.Bu FX, Armas L, Lappe J, Zhou Y, Gao G, Wang HW, Recker R, Zhao LJ. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum Genet. 2010;128:549–556. doi: 10.1007/s00439-010-0881-9. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Stampfer MJ, Gann PH, Hough HL, Giovannucci E, Kelsey KT, Hennekens CH, Hunter DJ. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer Epidemiol Biomarkers Prev. 1998;7:385–390. [PubMed] [Google Scholar]
- 17.Fu L, Yun F, Oczak M, Wong BY, Vieth R, Cole DE. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Fakih MG, Andrews C, McMahon J, Muindi JR. A prospective clinical trial of cholecalciferol 2000 IU/day in colorectal cancer patients: evidence of a chemotherapy-response interaction. Anticancer Res. 2012;32:1333–1338. [PubMed] [Google Scholar]
- 19.Chen H, Mccoy LF, Schleicher RL, Pfeiffer CM. Measurement of 25-hydroxyvitamin D3 (25OHD3) and 25-hydroxyvitamin D2 (25OHD2) in human serum using liquid chromatography-tandem mass spectrometry and its comparison to a radioimmunoassay method. Clinica Chimica Acta. 2008;391:6–12. doi: 10.1016/j.cca.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamin D2 and D3 . Clin Chem. 2005;51:1683–1690. doi: 10.1373/clinchem.2005.052936. [DOI] [PubMed] [Google Scholar]
- 21.Smith DC, Johnson CS, Freeman CC, Muindi J, Wilson JW, Trump DL. A Phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin Cancer Res. 1999;5:1339–1345. [PubMed] [Google Scholar]
- 22.Muindi JR, Peng Y, Wilson JW, Johnson CS, Branch RA, Trump DL. Monocyte fructose 1,6-bisphosphatase and cytidine deaminase enzyme activities: potential pharmacodynamic measures of calcitriol effects in cancer patients. Cancer Chemother Pharmacol. 2007;59:97–104. doi: 10.1007/s00280-006-0247-0. [DOI] [PubMed] [Google Scholar]
- 23.Chung I, Karpf AR, Muindi JR, Conroy JM, Nowak NJ, Johnson CS, Trump DL. Epigenetic silencing of CYP24 in tumor-derived endothelial cells contributes to selective growth inhibition by calcitriol. J Biol Chem. 2007;282:8704–8714. doi: 10.1074/jbc.M608894200. [DOI] [PubMed] [Google Scholar]
- 24.Lee HJ, Muindi JR, Tan W, Wilding G, Ford LA, Sait SNJ, Block AW, Barcos M, Vigil CE, Griffiths EA, Thompson JE, Wang ES, Johnson CS, Trump DL, Wetzler M (2010) Subnormal Vitamin D Levels Are Associated with Adverse Outcome In Newly-Diagnosed Similarly-Treated Adult Acute Myeloid Leukemia (AML) Patients Proc 52nd ASH meeting 2010 Abstract # 1041
- 25.Trump DL, Chadha MK, Sunga AY, Fakih MG, Ashraf U, Silliman CG, Hollis BW, Nesline MK, Tian L, Tan W, Johnson CS. Vitamin D deficiency and insufficiency among patients with prostate cancer. BJU Int. 2009;104:909–914. doi: 10.1111/j.1464-410X.2009.08531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao S, Sucheston LE, Millen AE, Johnson CS, Trump DL, Nesline MK, Davis W, Hong CC, McCann SE, Hwang H, Kulkarni S, Edge SB, O'Connor TL, Ambrosone CB. Pretreatment serum concentrations of 25-hydroxyvitamin D and breast cancer prognostic characteristics: a case–control and a case-series study. PLoS One. 2011;28(2):e17251. doi: 10.1371/journal.pone.0017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA, Vasikaran SD, Taranto M, Musk AA, Fraser WD. Serum 25-hydroxyvitamin D levels in vitamin D- insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone. 2009;45:870–875. doi: 10.1016/j.bone.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Bacon CJ, Gamble GD, Horne AM, Scott MA, Reid IR. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20:1407–1415. doi: 10.1007/s00198-008-0814-9. [DOI] [PubMed] [Google Scholar]
- 29.Himmelstein S, Clemens TL, Rubin A, Lindsay R. Vitamin D supplementation in elderly nursing home residents increases 25(OH)D but not 1,25(OH)2D. Amer J Clin Nutr. 1990;52:701–706. doi: 10.1093/ajcn/52.4.701. [DOI] [PubMed] [Google Scholar]
- 30.Masuda S, Okano T, Kamao M, Kanedai Y, Kobayashi T. A novel high-performance liquid chromatographic assay for vitamin D metabolites using a coulometric electrochemical detector. J Pharmaceut Biomedl Anal. 1997;15:1497–1502. doi: 10.1016/S0731-7085(96)02005-5. [DOI] [PubMed] [Google Scholar]
- 31.Higashi T, Awada D, Shimada K. Determination of 24,25-dihydroxyvitamin D3 in human plasma using liquid chromatography-mass spectrometry after derivatization with a Cookson-type reagent. Biomed Chromatogr. 2001;15:133–140. doi: 10.1002/bmc.43. [DOI] [PubMed] [Google Scholar]
- 32.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, Tuckey RC. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–4090. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pettifor JM, Bikle DD, Cavaleros M, Zachen D, Kamdar MC, Ross FP. Serum levels of free 1,25-dihydroxyvitamin D in vitamin D toxicity. Ann Intern Med. 1995;122:511–513. doi: 10.7326/0003-4819-122-7-199504010-00006. [DOI] [PubMed] [Google Scholar]
- 35.White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein. TEM. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- 36.Haddad JG. Plasma vitamin D-binding protein (GC-globulin): multiple tasks. J Steroid Biochem Mol Biol. 1995;53:579–582. doi: 10.1016/0960-0760(95)00104-8. [DOI] [PubMed] [Google Scholar]