Abstract
Slugs and snails (class Gastropoda) are the obligate intermediate hosts of the rat lungworm, Angiostrongylus cantonensis. This nematode is the causative agent of human angiostrongyliasis and the most common cause of human eosinophilic meningoencephalitis. Humans can become infected by accidental consumption of slugs or snails and possibly flatworms (or a portion of one of these animals) in fresh produce, but the slime from these animals can contain nematodes and may also constitute a disease risk. Gastropod carriers in Hawai‘i include, among other species, giant African snails, veronicellid slugs, and the semi-slug Parmarion martensi. This latter species was first discovered on the island of Hawai‘i in 2004 and is now common in the area where the majority of the state's documented cases of human angiostrongyliasis occurred between 2005 and 2011. This species is considered a high risk carrier of A. cantonensis because of its climbing behavior, abundance around human dwellings, and high worm burdens. One individual collected from east Hawai‘i Island contained >6,800 infective third stage A. cantonensis larvae. Common and efficient control methods for slugs and snails include sanitation (eg, removal of objects that serve as hiding places) and the use of poison food baits, such as those containing metaldehyde and iron. An iron-containing bait that is relatively safe to non-target organisms was effective in controlling semi-slugs in cage experiments, although it killed more slowly than a metaldehyde-containing bait and the majority of slugs affected did not die until 1–2 weeks following ingestion.
Keywords: Angiostrongylus cantonensis, Hawaii, Iron phosphate, Parmarion martensi, Rat lungworm, Semi-slug, Slug baits
Introduction
Angiostrongylus cantonensis was first discovered in rats in Canton, China, in 1935. It was recognized as causing human disease (meningitis) in 1944. However, the scientific report published the following year was in Japanese and was largely overlooked until reported by Beaver and Rosen in 1964.1 In 1955 the role of slugs and snails as intermediate hosts of Angiostrongylus cantonensis was reported.2 The information proved correct although it was later discovered that the authors were actually studying A. mackerrasae, a species almost identical to A. cantonensis.3 In the early 1960s, outbreaks of eosinophilic meningitis occurred in human populations in a number of Pacific islands. University of Hawai‘i parasitologist Joseph Alicata correctly inferred that A. cantonensis infection was the cause of those outbreaks, and it was subsequently demonstrated that A. cantonensis was the usual cause of eosinophilic meningitis in South-East Asia and the Pacific islands.4,5
Although slugs or snails are the obligatory intermediate hosts of A. cantonensis, first to third stage larvae can also be found in the tissues of other, paratenic, hosts that are passive carriers. Regarding risk to humans, the most important paratenic hosts are crustaceans (such as prawns and land crabs) and predacious land planarians (such as flatworms in the genus Platydemus).5 Platydemus spp. are predators of slugs and snails, excreting digestive juices that externally digest their prey. These flatworms are considered high risk carriers of nematodes because they are small, commonly found on lettuce, cabbage, and fruits, and easily overlooked while preparing food that is consumed without cooking.6–8 Hosts of A. cantonensis such as snails, slugs, and prawns are considered safe for human consumption if thoroughly cooked (heated to an internal temperature of 74°C or 165°F).9
A key unresolved question,5 is whether the mucus (slime) deposited by slugs and snails infected with A. cantonensis on fresh produce constitutes a human disease risk. Observations of veronicellid slugs showed that they rarely shed larvae in their mucus, and when they did, the larvae survived for only a few hours.7 Angiostrongylus cantonensis larvae were not detected in mucus rinsed off infected Lissachatina fulica (giant African snail; also referred to as Achatina fulica).10 Angiostrongylus larvae shed in the mucus of Microparmarion malayanus were viable and could be used to successfully infect rats, although the numbers of larvae were low.3,11 Angiostrongylus costaricensis, the cause of human abdominal angiostrongyliasis, has been found in slug mucus but only in very small numbers.12 A study using PCR (polymerase chain reaction) and microscopic analysis found that 3 of 25 naturally infected Parmarion martensi shed A. cantonensis larvae in their mucus when prodded, although only one to four larvae per individual were observed.13 In a different study, levels of parasite DNA detected by quantitative PCR in the mucus of a naturally-infected P. martensi individual were low compared with levels in various parts of the semi-slug body. An equivalent of 0.83 larvae were detected per mg of mucus compared with 41.3 larvae per mg of tail tissue and 49.3 larvae per mg of midsection tissue.14 As all these studies detected only very low numbers of larvae, mucus probably poses minimal risk of human infection.
The prevalence of A. cantonensis in intermediate hosts can be assessed by digesting slug or snail tissue in artificial gastric juice (1% pepsin with 1% HCl) and the third-stage larvae in the sediment identified morphologically under the microscope using the key of Ash.15 PCR tests have now been developed to identify A. cantonensis in tissues of slugs and snails and quantify the parasite load.14,16
Parmarion martensi as a Host in Hawai‘i
In 2004, the semi-slug Parmarion martensi (family Helicarionidae; Figure 1), native to southeast Asia and introduced to Hawai‘i, was discovered on a residential property in the Puna district of Hawai‘i Island.17 The three residents at this property had developed symptoms of angiostrongyliasis (infection by A. cantonensis) after consuming home-grown lettuce reportedly contaminated by immature semi-slugs.17 Semi-slugs from the property were heavily infected with A. cantonensis larvae. An island-wide survey in 2005 found that P. martensi was common in residential properties throughout the lower Puna area.
Figure 1.

Parmarion martensi. (A) eggs and neonates. Eggs are about 2.5 mm in diameter. (B) Adult with yellowish-brown flattened fingernail-shaped shell visible on the dorsum. The distinct keel along the posterior dorsal midline helps distinguish this species from similar-looking species in Hawai‘i. (C) Adult with shell covered by mantle folds. Adults are about 5 cm in length.
An average of 78% of semi-slugs collected from residential properties were infected with A. cantonensis, compared with only 24% of Cuban slugs (Veronicella cubensis, family Veronicellidae) collected from the same properties. Residents reported that semi-slugs were more often found climbing on structures such as exterior house walls, drain pipes, and water tanks than other slug species. Some residents reported finding semi-slugs in outdoor sinks, on dishes, and in food preparation areas. They were frequently abundant under plastic and in piles of compost, fallen palm leaves, and in other types of rotting organic matter.17
There is both direct evidence17 and circumstantial evidence that P. martensi has been responsible for an increase in the number of human cases of rat lungworm disease on Hawai‘i Island. The State of Hawai‘i Department of Health investigated 38 cases between 2005 and 2011, many of which were considered as probable cases only, as they were not confirmed by visualization of the larvae in the cerebrospinal fluid. The majority of these cases occurred on the east side of Hawai‘i Island (M. Dixon, State of Hawai‘i, Department of Health, letter to SIJ, August 24, 2012). This area includes the center of the distribution of P. martensi as determined in the 2005 survey.17 Since that time, the distribution of P. martensi has expanded and the Hilo area is now also infested (RGH, personal observations). Isolated populations are also present along the Hamakua coast north of Hilo (RGH, personal observations) and in Waimea and Kailua-Kona.17
Based on the biology and behavior of this species, it appears to represent an unusually high risk for infecting people and animals with A. cantonensis relative to other slug and snail species. Very high parasite loads have been found in some individuals. For example, over 6,800 larvae were extracted from an individual semi-slug from Puna (SIJ, unpublished). This, combined with their common climbing behavior and ability to move quickly and cover large distances across dry substrates (including wood, concrete, tree bark) to locate food sources such as bird food, dog food, cat food, fish entrails, and papaya fruits17 increases the likelihood that people and pets will come into contact with them and the parasitic nematodes they carry.
The environmental fate of infective (third-stage) larvae of A. cantonenis in individuals of P. martensi after death is poorly known. Angiostrongylus cantonensis larvae can exit the bodies of drowned snails and live up to 72 h in water.18,19 Both field observations and cage studies (RGH, unpublished) suggest that P. martensi may have an annual life cycle. Observations on semi-slugs held individually revealed that an egg clutch is sometimes produced by a semi-slug a day or two before it dies. In the field, clutches have been found mainly during spring or summer, sometimes in the immediate vicinity of dead adults. Larvae of A. cantonensis in the bodies of the dead semi-slug adults could be acquired by immature semi-slugs directly feeding on the former or via contact of immature semi-slugs with materials in the environment contaminated by A. cantonensis larvae passively liberated or exiting the tissues of the dead semi-slugs.
In Okinawa, P. martensi and a flatworm predator of slugs and snails, Platydemus manokwari, were carriers of A. cantonensis associated with an outbreak of human cases of rat lungworm disease in 2000, the flatworms presumably having become infected by eating infected slugs or snails.8 Both species are found in Hawai‘i, sometimes in association with one another (RGH, personal observation).
Management and Control of Slugs and Snails around Homes and Gardens
In parts of the world, including Hawai‘i, where slugs and snails are carriers of Angiostrongylus cantonensis, controlling these animals around homes, gardens, and in the landscape is recommended and should reduce disease risk. Control is most easily accomplished using a combination of sanitation and chemical control.9,20
Sanitation and Non-chemical Control
Slugs and snails are mainly active at night, which helps preserve water balance, a critical survival factor for these animals. Slugs in particular can quickly become dehydrated and die if they forage on a hot, sunny day and are unable to find cover. Therefore, a good method for reducing their populations is to limit the number of moist hiding places. This may include removing unnecessary ground cover, cutting back vegetation, removing rocks and fallen wood, and raising items in the landscape off the ground. For example, plant pots and storage sheds can be placed on blocks instead of directly on the ground.
Conversely, a home owner, gardener, or farmer might want to provide hiding places along the perimeter of the area to be protected in order to lure and trap the slugs or snails so that they can be more easily disposed of. Some slugs and snails show homing behavior, returning to the same resting sites each day after a night's feeding.21 Lures may be as simple as boards or pieces of plywood placed on the surface of the soil. Other lures include overturned flowerpots, overturned melon rinds, or orange peels. These lures can be checked frequently to collect slugs and snails for disposal. It is important to use gloves or a tool (such as disposable chopsticks or tweezers) to handle the slugs and snails, as they carry other parasites besides rat lungworms. Hand-picking of snails and slugs when they are out of their hiding places can be effective and supplement other methods if the species is relatively large. It is best done at night or early in the morning following rains or watering, which increase moisture levels in the ground, foliage, and air. Burch22 considered hand-picking impractical for most species, yet the only satisfactory method for reducing populations of the giant African snail (Lissachatina fulica), partly because these snails sometimes feed high in foliage where they would not encounter poison bait pellets applied on the ground.
Drowning slugs or snails for several days in a covered bucket filled with soapy water or a 15% solution of salt water is a convenient and safe way to kill slugs and snails. In the case of salt water, this treatment would also be expected to kill any A. cantonensis larvae that might exit the bodies or remain within them (SIJ, unpublished). Simply smashing slugs and snails and leaving them on the ground is not recommended, as the disease-causing nematodes might be eaten by other animals (such as pets or surviving slugs and snails).
Chemical Control
In Hawai‘i, the most common formulations of molluscicides (chemicals used to control slugs and snails) around homes and gardens are food bait pellets or granules that can be broadcast over the area to be protected, or selectively placed in crop borders or underneath objects where slugs and snails take refuge (Figure 2). These typically contain metaldehyde or iron phosphate as the active ingredient. Placing the baits underneath boards or other objects protects them from the direct effects of rain (which can cause baits to fall apart) and may slow the development of mold, which can make baits unpalatable. It also reduces the chance that domestic animals will consume them. Using molluscicides as the only control measure seldom produces adequate results. Frequently a bait application might kill only half of the slugs or snails in the treated area. Others will survive the treatment because either they were buried or hidden at the time of treatment, they were not attracted to the bait, or they did not eat enough to kill them.
Figure 2.

Common products used to control slugs and snails in Hawai‘i in commercial and residential settings. From left to right: Sluggo® (food bait containing 1% iron phosphate) (Neudorff, Emmerthal, Germany), Durham® metaldehyde granules 7.5 (AMVAC Chemical Corp., Los Angeles, CA), Metarex® (metaldehyde food bait) (De Sangosse, Pont Du Casse, France) and Deadline® (metaldehyde food bait) (AMVAC Chemical Corp., Los Angeles, CA).
Food bait pellets attract snails and slugs, but they may also attract domestic pets. If consumed, metaldehyde products are very toxic to dogs, cats, and other animals, and poisoning incidents are common. Partly for this reason, metaldehyde products are also available as granules without the food attractants. Granular formulations are finer than the pellets. Slugs or snails accidentally contacting granules will respond by producing copious amounts of slime. Under the right environmental conditions (hot and dry), the associated loss of moisture leads to death of the snail or slug.23 Liquid forms of metaldehyde are used as a foliar spray or pot drench but are not allowed on edible crops. Metaldehyde products must be kept out of waterways and cannot be used legally around water or in swampy areas. For all pesticide products, directions for use are indicated on product labels, and directions must be strictly followed as a matter of federal law.
Alternatives to metaldehyde products are food baits with 1% iron phosphate or a chelated form of iron (sodium ferric EDTA) as the active ingredient. These products are considered generally safer for use around domestic animals and wildlife than those containing metaldeyde. Slugs or snails that feed on iron phosphate baits stop feeding immediately but may not die for several days, so the control will not be immediately apparent. Iron-containing baits can be just as effective, or more so, as metaldehyde baits. However, the scientific consensus is that metaldehyde baits outperform iron phosphate baits under normal conditions.20 Relative performance will vary by pest species (which can affect bait acceptance), bait formulation, and environmental conditions following application (eg, temperature and moisture), which affect survival associated with contact exposure to metaldehyde and mold growth on bait.
Experimental Assessment of Baits for Chemical Control of Semi-slugs
As a preliminary step in determining the acceptability and efficacy of three food bait products for control of Parmarion martensi, experiments were conducted in which semi-slugs were exposed to baits in mesh cages (∼46 × 46 × 48 cm high). Each cage had a plastic pot as a hiding place, a stick to elevate the lip of the pot to allow access, and a piece of cardboard moistened by spraying with water every 1–3 days (Figure 3). Ten adult semi-slugs were placed in each cage. The cages were set up on tables in a covered outdoor area. Each experiment used four cages, one as the control (no bait or other food provided), while the other three were provisioned with 140 pellets each of one of three types of bait pellets. Additional pellets were added as necessary to ensure that bait was always available. The tests were replicated three times.
Figure 3.

Inside view of cage in which semi-slugs were offered food baits.
Tests were set up on a Monday, and mortality and bait consumption were monitored usually each weekday following test set up for a period of two weeks (two replicates) or three weeks (one replicate). Products tested were Deadline® MP's™ (4% metaldehyde, AMVAC Chemical Corp., Los Angeles, CA), Sluggo® (for details see Figure 2), and Ferroxx®, a newly available iron-containing product (5% sodium ferric EDTA, Neudorff, Emmerthal, Germany). For each of the products, almost all bait consumption occurred within the first two days. Although feeding by individual semi-slugs was not tracked, the results suggested that all three products were generally accepted as food. The average number (± SE) of pellets consumed by the ten semi-slugs was 17.7 (± 1.4), 41.7 (± 5.5) and 75.7 (± 15.9), corresponding to 0.48, 0.88 and 0.91 g dry weight for Deadline, Sluggo, and Ferroxx, respectively Cumulative mortality of semi-slugs after 7 and 14 days is shown in Table 1. Deadline performed best, killing an average of 90% of semi-slugs within 7 days and 100% by 14 days. Ferroxx performed better than Sluggo, killing an average of 63% (compared to 30% for Sluggo) by the end of the second week. Results for the test monitored over a three-week period are shown in Figure 4. Although all semi-slugs were eventually killed in the Ferroxx treatment in this test, 100% mortality was not observed until 16 days after the start of the trial. These results were in sharp contrast to results obtained in a single test using Cuban slugs (Veronicella cubensis), in which Deadline and Ferroxx produced 100% mortality by day 4 and Sluggo produced 100% mortality by day 7 (Figure 5).
Table 1.
Cumulative percent mortality of Parmarion martensi semi-slugs exposed to poisoned food baits.
| Daysa | Replicate | Deadline | Sluggo | Ferroxx | Control |
| 7 | 1 | 100 | 0 | 0 | 0 |
| 7 | 2 | 100 | 30 | 10 | 10 |
| 7 | 3 | 70 | 10 | 10 | 0 |
| 14 | 1 | 100 | 10 | 70 | 0 |
| 14 | 2 | 100 | 70 | 60 | 20 |
| 14 | 3 | 100 | 10 | 60 | 0 |
The number of days post treatment.
Figure 4.
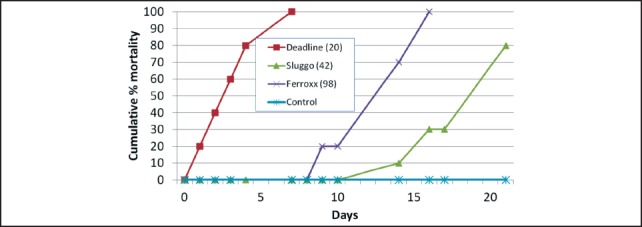
Mortality of semi-slugs (Parmarion martensi) offered different types of food baits. Numbers in parentheses indicate the number of bait pellets consumed by the 10 semi-slugs.
Figure 5.
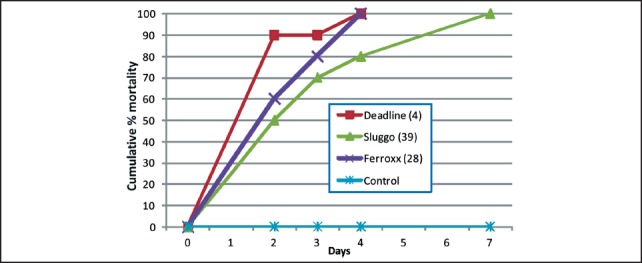
Mortality of Cuban slugs (Veronicella cubensis) exposed to different types of food baits. Numbers in parentheses indicate the number of bait pellets consumed by the 10 slugs.
While Deadline was the most effective product tested, it would also be the most toxic to domestic animals and must be used with discretion. The choice of bait for home users should take into consideration efficacy, cost, and the potential risks of each bait. Given favorable weather, a sufficient amount of bait, and repeated treatments, any of the three products could result in complete control of semi-slugs over time.
Acknowledgements
We thank Glenn Asmus of the United States Department of Agriculture, Agricultural Research Service (USDA-ARS) for technical assistance. This work was funded by USDA-ARS and the University of Hawai‘i, Hilo, College of Pharmacy. This paper represents a contribution to the Rat Lungworm Disease Scientific Workshop held at the Ala Moana Hotel, Honolulu, Hawai‘i in August 2011. Funding for the workshop and for this publication was provided by the National Institute of Food and Agriculture, United States Department of Agriculture, through Award No. 2011-65213-29954. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. Opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA. USDA is an equal opportunity provider and employer.
Conflict of Interest
None of the authors identifies any conflict of interest.
References
- 1.Beaver PC, Rosen L. Memorandum on the first report of Angiostrongylus in Man, by Nomura and Lin, 1945. Am J Trop Med Hyg. 1964;13:589–590. doi: 10.4269/ajtmh.1964.13.589. [DOI] [PubMed] [Google Scholar]
- 2.Mackerras MJ, Sandars DF. The life history of the rat lungworm, Angiostrongylus cantonensis (Chen) (Nematoda: Metastrongylidae) Aust J Zool. 1955;3:1–21. [Google Scholar]
- 3.Prociv P, Spratt DM, Carlisle MS. Neuro-angiostrongyliasis: unresolved issues. Int J Parasitol. 2000:1295–1303. doi: 10.1016/s0020-7519(00)00133-8. [DOI] [PubMed] [Google Scholar]
- 4.Alicata JE. The discovery of Angiostrongylus cantonensis as a cause of human eosinophilic meningitis. Parasitol Today. 1991;7:151–153. doi: 10.1016/0169-4758(91)90285-v. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth RG, Cowie RH. Apple snails as disease vectors. In: Joshi R, editor. Global Advances in Ecology and Management of Golden Apple Snails. Muñoz, Nueva Ecija, Philippines: Philippine Rice Research Institute; 2006. pp. 121–132. [Google Scholar]
- 6.Alicata JE, Jindrak K. Angiostrongylosis in the Pacific and Southeast Asia. Springfield, Illinois: Charles C. Thomas; 1970. [Google Scholar]
- 7.Ash LR. Observations on the role of mollusks and planarians in the transmission of Angiostrongylus cantonensis infection to Man in New Caledonia. Rev Biol Trop. 1976;24:163–174. [Google Scholar]
- 8.Asato R, Taira K, Nakamura M, Kudaka J, Itokazu K, Kawanaka M. Changing Epidemiology of Angiostrongyliasis cantonensis in Okinawa Prefecture, Japan. Jpn J Infect Dis. 2004;57:184–186. [PubMed] [Google Scholar]
- 9.Hollyer JR, Troegner VA, Cowie RH, et al. Best On-Farm Food Safety Practices: Reducing Risks Associated with Rat Lungworm Infection and Human Eosinophilic Meningitis. Food Safety and Technology 39. Honolulu, Hawai‘i: College of Tropical Agriculture and Human Resources, University of Hawai‘i; 2010. [Google Scholar]
- 10.Chen XG, Li H, Lun ZR. Angiostrongyliasis, mainland China. Emerg Infect Dis. 2005;11:1645–1647. doi: 10.3201/eid1110.041338. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heyneman D, Lim BL. Angiostrongylus cantonensis: proof of direct transmission with its epidemiological implications. Science. 1967;158:1057–1058. doi: 10.1126/science.158.3804.1057. [DOI] [PubMed] [Google Scholar]
- 12.Bonetti VCDB de O, Graeff-Teixeira C. Angiostrongylus costaricensis and the intermediate hosts: observations on elimination of L3 in the mucus and inoculation of L1 through the tegument of mollucs [sic] Rev Soc Bras Med Trop. 1998;31:289–294. doi: 10.1590/s0037-86821998000300006. [DOI] [PubMed] [Google Scholar]
- 13.Qvarnstrom Y, Sullivan JJ, Bishop HS, Hollingsworth RG, da Silva AJ. PCR-based detection of Angiostrongylus cantonensis in tissue and mucus secretions from molluscan hosts. Appl Environ Microbiol. 2007;73:1415–1419. doi: 10.1128/AEM.01968-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvi SI, Farias EM, Howe K, Jacquier S, Hollingsworth R, Pitt W. Quantitative PCR estimates Angiostrongylus cantonensis (rat lungworm) infection levels in semi-slugs (Parmarion martensi) Mol Biochem Parasitol. 2012;185(2):174–176. doi: 10.1016/j.molbiopara.2012.08.002. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ash LR. Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea) J Parasitol. 1970;56:249–253. [PubMed] [Google Scholar]
- 16.Qvarnstrom Y, da Silva ACA, Teem JL, et al. Improved molecular detection of Angiostrongylus cantonensis in mollusks and other environmental samples with a species-specific internal transcribed spacer 1-based TaqMan assay. Appl Environ Microbiol. 2010;76:5287–5289. doi: 10.1128/AEM.00546-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollingsworth RG, Kaneta RK, Sullivan JJ, et al. Distribution of Parmarion cf. martensi (Pulmonata: Helicarionidae), a new semi-slug pest on Hawai‘i Island, and its potential as a vector for human angiostrongyliasis. Pac Sci. 2007;61:457–467. [Google Scholar]
- 18.Cheng TC, Alicata JE. Possible role of water in the transmission of Angiostrongylus cantonensis (Nematoda: Metastrongylidae) J Parasitol. 1964;50:39–40. [Google Scholar]
- 19.Ubelaker JE, Bullick GR, Caruso J. Emergence of third-stage larvae of Angiostrongylus costaricensis Morera and Cespedes 1971 from Biomphalaria glabrata (Say) J Parasitol. 1980;66:856–856. [PubMed] [Google Scholar]
- 20.Hollingsworth RG. Methods for excluding slugs and snails on exported horticultural commodities. In: Benkeblia N, editor. Postharvest Technologies for Horticultural Crops. Vol. 2. Kerala, India: Research Signpost; 2009. pp. 93–119. [Google Scholar]
- 21.Port CM, Port GR. The biology and behavior of slugs in relation to crop damage and control. In: Russell GE, editor. Management and Control of Invertebrate Crop Pests. Andover, UK: Intercept; 1989. pp. 243–287. [Google Scholar]
- 22.Burch JB. Some Snails and Slugs of Quarantine Significance to the United States. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service 82-1; 1960. p. 70. [Google Scholar]
- 23.South A. Terrestrial Slugs: Biology, Ecology and Control. London: Chapman & Hall; 1992. [Google Scholar]


