Abstract
The freshwater apple snail Pomacea canaliculata was introduced to Taiwan then to mainland China in the early 1980s from Argentina, its native region, for the purpose of aquaculture. Because of the lack of natural enemies and its tolerance of a wide range of environmental conditions, both its abundance and distribution have dramatically increased and it has become a harmful species to local agriculture and other native species in many areas of China. Unfortunately, the snail also acts as an intermediate host of Angiostrongylus cantonensis, and has been implicated in transfer of the parasite to people, resulting in angiostrongyliasis manifested as eosinophilic meningitis. Efforts to prevent its further spread and population expansion were initiated many years ago, including the use of chemicals and biological control agents to control the snail.
Keywords: Angiostrongyliasis, Apple snail, China, Control, Eosinophilic meningitis, Host, Introduced species, Nematode, Parasite, Pest, Pomacea canaliculata
Introduction to China
The native range of Pomacea canaliculata, one of two species commonly known in Asia as the golden apple snail, is Argentina and Uruguay. It is an invasive species and is now widespread in many countries of eastern and southern Asia, including the Philippines, Vietnam, Thailand, Japan, and Korea.1 A cluster of eggs of P. canaliculata was brought to Taiwan at the end of the 1970s from Argentina by a Chinese person resident in Argentina. In 1981, P. canaliculata was intentionally introduced to Zhongshan, Guangdong province, China, as an aquaculture species and bred successfully.2,3 Subsequently, during the 1980s, aquaculture of this snail rapidly expanded to many regions of 17 provinces, including Guangdong, Guangxi, Fujian, Sichuan, Shanghai, Hubei, Guizhou, Zhejiang, Jiangsu, Anhui, Beijing, and even to Gansu and Liaoning. During this time, many government sponsored technical training programs and publications greatly boosted the spread and culture of this snail in China. In the 1990s, the impetus for development of aquaculture gradually faded because of the distastefulness of the snail to many people. However, the species had established many natural populations in many areas where it had never been artificially bred and cultured, expansion of its distribution being facilitated by its wide tolerance of environmental conditions and broad food preferences. Gradually it became a devastating agricultural pest of wetland crops, most notably rice, in various Southeast Asian countries2,4 and Japan.5 Pomacea canaliculata was subsequently found to be an important intermediate host of Angiostrongylus cantonensis, the rat lungworm, which can infect humans, causing angiostrongyliasis manifested as eosinophilic meningitis.6–7 In 2003, P. canaliculata was included in a blacklist of 16 invasive pests by the State Environmental Protection Administration (SEPA) of China.
Spread in Mainland China
There are no accurate data on the abundance and spread of P. canaliculata since its introduction to China in about 1980. However, based on reports from various parts of China, it has been suggested that the spread of P. canaliculata in mainland China took place in two main phases, a first phase of rapid spread during the 1980s resulting from domestic introduction for aquaculture, and a second phase of slow spread in the subsequent 20 years or so once major control measures against the snail were widely implemented (Figure 1).8 Pomacea canaliculata is now well established in waters of southern China, in a band spanning from northeast to southwest. It has also been observed in mountainous areas at high elevations in Yunnan province. And it has crossed from the Pearl River valley into the Yangtze River valley, and now occurs in the southeast section of the latter.9 In a separate area in the Sichuan basin, there is an independent population of Pomacea canaliculata that may be associated with the its earlier introduction for aquaculture.9
Figure 1.
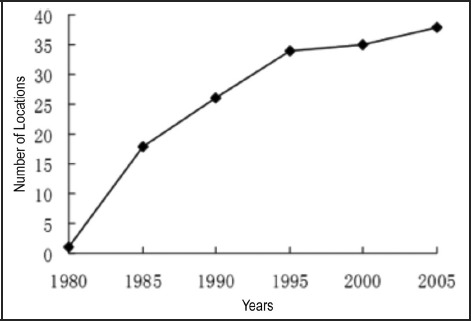
The number of locations in which Pomacea canaliculata was recorded in China from 1980 to 2005. Modified from Yang et al.8
The most important factor driving the invasion of P. canaliculata is considered to be the environmental temperature, although many other variables, such as the level of dissolved oxygen, the pH of the water, and soil moisture during dormancy, are associated with overwintering success.10 Experimental studies on P. canaliculata have found that the threshold temperature and effective accumulated temperature for its development were 11.4 ° and 1309 degree days respectively. Based on these results, it was predicted that this snail can overwinter in nearly all areas in China as long as they are in unfrozen water. In all provinces there are some natural waters that could permit it to complete one generation in two years, and in southern provinces such as Guangdong and Hainan that could allow it to complete three generations per year.11 This potential is confirmed by the existence of the natural population in Sichuan province, despite many waters in this province being iced up in winter.12 In Wenzhou, Zhejiang Province, the natural population of P. canaliculata moved northward about 10 km per year.13 Based on its current distribution in China and the effective temperature accumulation for its development, it has been predicted that its range will expand into large areas further northwards by the 2030s, including the entire Chongqing municipality, Hunan, Hubei, Jiangxi, and Zhejiang provinces.14 The predicted increase in the distribution between the 2020s and 2030s is 378,700 km2. The region in which it becomes established will move further northeast, mainly including the Huaihe River valley in southern Henan and Anhui provinces, and central Jiangsu province.14 Considering uncertainties regarding the rate of global climate change, the future distribution may be even greater than these predictions suggested.
Host of Angiostrongylus cantonensis
Angiostrongylus cantonensis, known as the rat lungworm, is a nematode parasite, with the adult worms being found in the pulmonary arteries of rats. Molluscs, ie, freshwater and terrestrial snails, serve as the intermediate hosts. By ingestion of uncooked or undercooked foods (eg snails) containing live third stage larvae of A. cantonensis, the parasite causes an infection in humans known as angiostrongyliasis, with the symptoms of eosinophilic meningitis, although humans are only accidental hosts of this species of nematode and in which it does not complete its life cycle. Eosinophilic meningitis caused by Angiostrongylus cantonensis, a potentially fatal disease, is considered an emerging infectious disease in mainland China.12 Thirty-two species of molluscs in China have been screened for A. cantonensis; 22 of them (69%) harbored the parasite.12 The highest rate of infection was recorded in the giant African snail, Achatina fulica (97%), followed by slugs (up to 100% in some species, ie, Philomycus bilineatus and Vaginulus spp.), and Pomacea canaliculata (69.4%). Although terrestrial snails and slugs showed higher rates and intensities of infections than freshwater molluscs overall, at least one freshwater snail, P. canaliculata, plays an important role in the epidemiology of angiostrongyliasis.
Extensive epidemiological evidence indicates that P. canaliculata is becoming the most important natural intermediate host for A. cantonensis in mainland China because of its high susceptibility to the parasite and its wide environmental tolerance. Third stage larvae of A. cantonensis were first detected in P. canaliculata in China in Wenzhou, Zhejiang province, from examination of 361 individuals of this snail species.6 The prevalence (proportion of the sample that were infected) was 69.4% and mean intensity (number of larvae per snail) was about 32.6. Subsequently, there have been many other reports from other parts of China.15–17 The broad and rapid expansion of the distribution of P. canaliculata due to its dispersal both on the land and in the water, the high prevalence and intensity of infection by A. cantonensis,9 and the fact that it is still being collected as food by some people and offered in some restaurants, have contributed to it being the most important intermediate host not only in the regions where it now occurs, but also in provinces where it is not found, having been transported for food to such locations.7 Pomacea canaliculata is a somewhat amphibious snail. Females emerge from the water to lay their eggs attached to emergent vegetation, rocks, logs, and other rigid surfaces. In the absence of water (eg, drained rice paddies) they survive by estivation in the mud. Thus, although the primary route of infection by A. cantonensis larvae is from ingestion of rat feces washed into the water, their ability to survive out of the water enhances their likely contact with rat feces and increases their chance of infection.
The biggest outbreak of eosinophilic meningitis caused by A. cantonensis in Beijing, where P. canaliculata does not occur, resulted from consuming undercooked P. canaliculata imported from more southerly regions where it does occur.3 Based on reports of outbreaks of eosinophilic meningitis in China caused by A. cantonensis, 8 of 9 outbreaks resulted from consumption of undercooked P. canaliculata and only one from eating Achatina fulica (Table 1).16 Thus, the emergence of angiostrongyliasis has largely been attributed to the spread of P. canaliculata.14
Table 1.
Reported outbreaks of eosinophilic meningitis caused by Angiostrongylus cantonensis infection in mainland China (from Zhang, et al.)16
| Year | Location (city, province) | Number of people infected | Source of infection | Reference |
| 1997 | Wenzhou, Zhejiang | 65 | Pomacea canaliculata | 22 |
| 2002 | Changle, Fujian | 8 | Pomacea canaliculata | 15 |
| 2002 | Fuzhou, Fujian | 9 | Pomacea canaliculata | 23 |
| 2002 | Fuzhou, Fujian | 13 | Achatina fulica | 24 |
| 2004 | Kunming, Yunnan | 25 | Pomacea canaliculata | 25 |
| 2005 | Kunming, Yunnan | 9 | Pomacea canaliculata | 26 |
| 2006 | Beijing | 160 | Pomacea canaliculata | 27 |
| 2007 | Zhaoqing, Guangdong | 6 | Pomacea canaliculata | 28 |
| 2008 | Dali, Yunnan | 41 | Pomacea canaliculata | 29 |
Control of P. canaliculata in China
Since in China Pomacea canaliculata appears to be the main vector of angiostrongyliasis to humans, and because it is a major pest of crops in many regions of mainland China, widespread efforts have been implemented in attempts to control it and limit its further spread. These control measures can generally be divided into physical, chemical, biological, and agricultural methods.18 Most simply, farmers hand-pick egg masses of the apple snails or collect the snail in the ditches and paddy fields.19 Many commercial molluscicides, primarily niclosamide, crystal copper sulfate, sodium pentachlorophenate and fentin acetate, have proven to be effective, but with environmental side effects.18 Many by-products and extracts of plants, such as tea seed cake, aqueous saponins, nicotine, and extract of Eynedrella nodiflora have been used to kill or control the snails.20,21 Animals used for biological control of the snails mainly include ducks and snail eating fish such as black carp (Mylopharyngodon piceus). It has even been reported that ducks cultured in paddy fields could eliminate 99% of the adult snails and 92% of egg masses in rice fields.18 Mechanized farming and rotation of aquatic and xeromorphic crops can kill some of the snails and prevent major outbreaks of the snail in crop fields.10,19
Conclusion
To reduce the chance of human infection by the parasite, one of the most effective methods would be interrupting its life cycle by controlling the intermediate hosts. The current wide distribution of P. canaliculata in China and the lack of a powerful, specific, and environmentally safe molluscicide make it extremely difficult to control populations of this snail in wild. Furthermore, the wide environmental tolerance exhibited by P. canaliculata facilitates its rapid spread, and its potential spread to all parts of China requires the highest attention. Global warming will assuredly facilitate its northward spread. Due to the close connection of this snail with outbreaks of eosinophilic meningitis, the abundance, distribution, and spread of this snail should be closely monitored. However, the most effective method to prevent human infection is to educate people to maintain good sanitation in food preparation areas, not to eat raw or undercooked snails, and to avoid eating raw vegetables that may harbor inconspicuous (eg juvenile) snails or slugs in regions where A. cantonensis is present.
Acknowledgments
This work was supported in part by a grant from a collaborative project on tropical diseases between the National Science Foundation of China and Taiwan. We are especially grateful to Dr Robert H. Cowie, University of Hawai‘i at Manoa, Honolulu, Hawai‘i, USA, for his help in editing our manuscript. The authors thank all the members working in the laboratories for their contribution to this work. Dr. Zhao-Rong Lun's laboratory was supported in part by the National Basic Research Program of China (973 Program) (No. 2010CB530000). This paper represents a contribution to the Rat Lungworm Disease Scientific Workshop held at the Ala Moana Hotel, Honolulu, Hawai‘i, in August 2011. Funding for the workshop and for this publication was provided by the National Institute of Food and Agriculture, United States Department of Agriculture, through Award No. 2011-65213-29954.
Conflict of Interest
None of the authors identifies any conflict of interest.
References
- 1.Hayes KA, Joshi RC, Thiengo SC, Cowie RH. Out of South America: multiple origins of non-native apple snails in Asia. Divers Distrib. 2008;14:701–712. [Google Scholar]
- 2.Joshi RC, Sebastian LS. Global Advances in Ecology and Management of Golden Apple Snails. Nueva Ecija: PhilRice; 2006. [Google Scholar]
- 3.Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostronyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 4.Halwart M. The golden apple snail, Pomacea canaliculata in Asian rice farming systems: present impact and future threat. Int J Pest Manage. 1994;40:199–206. [Google Scholar]
- 5.Yusa Y, Wada T. Impact of the introduction of apple snails and their control in Japan. Naga, ICLARM Quarterly. 1999;22:9–13. [Google Scholar]
- 6.Pan CW, Xing WL, Liang SH, et al. First finding of the larvae of Angiostrongylus cantanensis in the snail, Pomacea canaliculata in Wenzhou. Chin J Parasit Dis Control. 1998;11(1):78. [Google Scholar]
- 7.Wang QP, Chen XG, Lun ZR. Invasive freshwater snail, China. Emerg Infect Dis. 2007;13:1119–1120. doi: 10.3201/eid1307.061360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YX, Hu YC, Li XH, et al. Historical invasion, expansion process and harm investigation of Pomacea canaliculata in China. Chin Agr Sci Bull. 2010;26(5):245–250. [Google Scholar]
- 9.Lv S, Zhang Y, Liu HX, et al. Invasive snails and an emerging infectious disease: results from the first national survey on Angiostrongylus cantonensis in China. PLoS Neglect Trop Dis. 2009;3(2):e368. doi: 10.1371/journal.pntd.0000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K. Environmental factors influencing overwintering success of the golden apple snail, Pomacea canaliculata (Gastropoda: Ampullariidae), in the northern most population of Japan. App Ent Zool. 2002;37:655–661. [Google Scholar]
- 11.Zhou WC, Wu YF, Yang JQ. Viability of the ampullaria snail in China. Fujian J Agric Sci. 2003;18(1):25–28. [Google Scholar]
- 12.Lv S, Zhang Y, Steinmann P, Zhou XN. Emerging angiostrongyliasis in mainland China. Emerg Infect Dis. 2008;14(1):161–164. doi: 10.3201/eid1401.061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu XP, He TJ, Li ZF, et al. Occurrence of golden apple snail, Pomacea canaliculata (Lamarck), in paddy fields and its management. Acta Agr Zhejiangensis. 2001;13(5):247–252. [Google Scholar]
- 14.Lv S, Zhang Y, Steinmann P, et al. The emergence of angiostrongyliasis in the People's Republic of China: the interplay between invasive snails, climate change and transmission dynamics. Freshw Biol. 2011;56:717–734. [Google Scholar]
- 15.Lin JX, Li YS, Zhu K, et al. Epidemiological study on group infection of Angiostrongylus cantonensis in Changle. Chin J Parasitol Parasit Dis. 2003;21(2):110–112. [PubMed] [Google Scholar]
- 16.Zhang Y, Lv S, Yang K, et al. The first national survey on natural nidi of Angiostrongylus cantonensis in China. Chin J Parasitol Parasit Dis. 2009;27(6):508–512. [PubMed] [Google Scholar]
- 17.Gen YJ, Huang DN, Xie X, et al. Investigation on infection status of the animal host of Angiostrongylus cantonensis in Shenzhen City. Chin J Dis Control Prev. 2011;15(9):801–805. [Google Scholar]
- 18.Wang ZG, Tan JC, Liu J, Zhong L. Research progress on integrated control of Pomacea canaliculata (Lamarck) Chin Agr Sci Bull. 2009;25(12):201–205. [Google Scholar]
- 19.Lin WQ, Zhou XY. Occurrence rule and integrated countermeasures of Pomacea canaliculata in rice field. Chin Rice. 2005;2005(2):27–28. [Google Scholar]
- 20.Chen JM, Yu XP, Zhen XS, Xu HX, LV ZX, Zhang JF. Biological characteristics of golden apple snail, Pomacea canaliculata (Lamarck) in Jiaobai field and its integrated managements strategies. Acta Agr Zhejiangensis. 2003;15(3):154–160. [Google Scholar]
- 21.Zhang JE, Fang L. Ecological issues of research for biological invasion of Ampullaria gigas Spix in China. Chin J Eco-Agr. 2008;16(6):1585–1589. [Google Scholar]
- 22.Zheng RY, Jin RL, Lin BC, Pan CW, Xue DY. Probing and demonstrating etiological factor for outbreak of angiostrongyliasis cantonensis in Wenzhou. Sh J Prev Med. 2001;13(3):105–107. [Google Scholar]
- 23.Wu CH, Yan XH. Angiostrongyliasis endemic on small scale. Chin J Zoonoses. 2004;20(5):454. [Google Scholar]
- 24.Yang FZ, Zhang YZ, Tu ZP, Xu NS. Survey on angiostrongyliasis cantonensis due to snail meal. Strait J Prev Med. 2004;10(1):44–45. [Google Scholar]
- 25.Han JF, Zhu YH, Ji WZ, et al. Clinical analysis of 25 cases of eosinophilic meningitis. Chin J Epidem. 2005;26(9):679. [Google Scholar]
- 26.Wei LP, Zheng KW, Wei Y. Case report: clinical nursing of 9 angiostrongyliasis cases. J Clin Nurs. 2005;4(6):21–22. [Google Scholar]
- 27.He ZY, Jia L, Huang F, et al. Investigation on outbreak of angiostrongyliasis cantonensis in Beijing. Chin J Public Health. 2007;23(10):1241–1242. [Google Scholar]
- 28.Deng ZH, Cai JS, Lin RX, et al. The first local outbreak of Angiostrongylus cantonensis infection in Guangdong Province. South China J Prev Med. 2007;33(4):17–20. [Google Scholar]
- 29.Chen SR, Lv S, Wang LP, et al. An outbreak of angiostrongyliasis in Dali. Parasitoses Inf Dis. 2008;6(6):137–138. [Google Scholar]


